Synthesis and biological evaluation against Leishmania donovani of novel hybrid molecules containing indazole-based 2-pyrone scaffolds†
M.
El Ghozlani
a,
L.
Bouissane
 a,
M.
Berkani
a,
S.
Mojahidi
a,
A.
Allam
a,
C.
Menendez
b,
S.
Cojean
c,
P. M.
Loiseau
c,
M.
Baltas
b and
E. M.
Rakib
a,
M.
Berkani
a,
S.
Mojahidi
a,
A.
Allam
a,
C.
Menendez
b,
S.
Cojean
c,
P. M.
Loiseau
c,
M.
Baltas
b and
E. M.
Rakib
 *a
*a
aLaboratoire de Chimie Organique et Analytiques, Faculté des Sciences et Techniques, Université Sultan Moulay Slimane, B.P. 523, Béni-Mellal, Morocco. E-mail: elmostapha1@ymail.com
bLaboratoire de Synthèse et Physico-Chimie de Molécules d'Intérêt Biologique, Université Paul Sabatier, UMR-CNRS 5068, 118 route de Narbonne, 31062 Toulouse cedex 9, France
cChimiothérapie Antiparasitaire, UMR 8076 CNRS Faculté de Pharmacie, Université Paris-Saclay, Rue Jean-Baptiste Clément, F-92290 Chatenay-Malabry, France
First published on 19th November 2018
Abstract
A series of novel indazole–pyrone hybrids were synthesized by a one pot reaction between N-alkyl-6(5)-nitroindazoles and 2-pyrone (4-hydroxy-6-methyl-2H-pyran-2-one) using indium or stannous chloride as the reducing system in the presence of acetic acid in tetrahydrofuran. The hybrid molecules were obtained in good to excellent yields (72–92%) and characterized by NMR and single crystal X-ray diffraction. Nineteen compounds were tested in vitro against both Leishmania donovani (MHOM/ET/67/HU3, also called LV9) axenic and intramacrophage amastigotes. Among all, five compounds showed anti-leishmanial activity against intracellular L. donovani with an IC50 in the range of 2.25 to 62.56 μM. 3-(1-(3-Chloro-2-ethyl-2H-indazol-6-ylamino)ethylidene)-6-methyl-3H-pyran-2,4-dione 6f was found to be the most active compound for axenic amastigotes and intramacrophage amastigotes of L. donovani with IC50 values of 2.48 ± 1.02 μM and 2.25 ± 1.89 μM, respectively. However, the cytotoxicity of the most promising compound justifies further pharmacomodulations.
Introduction
Hybridization of two different bioactive molecules with different mechanisms of action is one of the methods that are being adopted to treat different diseases. From the last few decades, the synthesis of hybrid molecules by the combination of different biologically relevant moieties has been under intense research along with their evaluation as pharmacological agents and potent drugs. A number of reports are focused on the biological potential of hybrid molecules with particular mention of those exhibiting anti-inflammatory, anti-cancer, and anti-infectious activities, such as anti-fungal, anti-tuberculosis, anti-malarial, and antileishmanial activities.1–5 For hybrid molecules containing indazole-based heterocycle scaffolds, only a few examples have been developed and reported in the literature as versatile chemotherapeutic agents and have found wide biological applications such as kinase inhibitors (pazopanib)6 and antibacterial,7 receptor antagonist,8 and anticancer agents.9 Three papers have reported on the antileishmanial activity of 5-nitroindazole derivatives.10–12 Biological evaluation of the studied indazoles has shown that some compounds exhibit adequate in vitro leishmanicidal activity against different parasite strains.2-Pyrone is an important class of heterocyclic scaffold. Due to its pharmacophore properties, the synthesis of hybrid derivatives bearing a 2-pyrone moiety has attracted much attention; most of the compounds display strong biological activities such as cytotoxic, antibiotic and antifungal activities.13–16 It is also known that some pyrones exhibit antileishmanial activity.17,18 In that context, we planned to explore the possibility of introducing a 2-pyrone into an indazole ring. We wish to report here the reductive coupling of N-alkyl-6(5)-nitroindazoles and 4-hydroxy-6-methyl-2-pyrone in order to obtain indazole/pyrone hybrid compounds and their inhibition activity against the axenic and intramacrophage amastigotes of Leishmania donovani.
Results and discussion
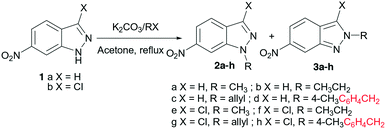
Chemistry
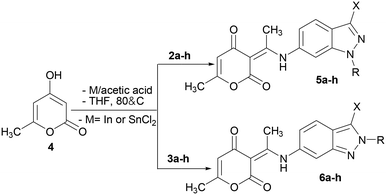
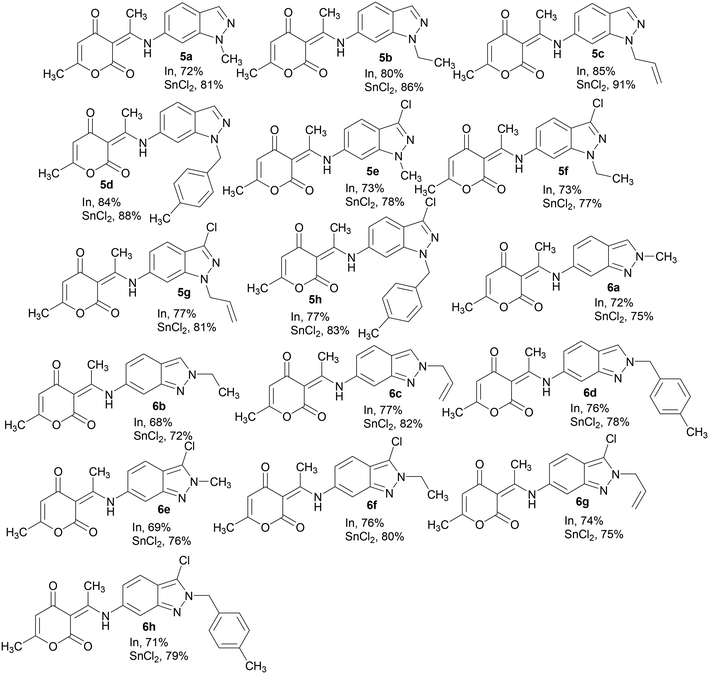
After chromatographic separation and isolation of each N-alkyl-6-nitroindazole isomer, we examined their reactivity and that of their chlorinated derivatives 2a–h and 3a–h with 4-hydroxy-6-methyl-2-pyrone 4 in the presence of different reducing agents (SnCl2/AcOH and In/AcOH in THF). By using excess anhydrous indium and acetic acid, respectively, in comparison with reagents 2/3 and 4, we obtained after reflux in THF the corresponding (3E)-3-(1-((N-alkyl-1H-indazol-6yl)amino)ethylidene)-6-methyl-3H-pyran-2,4-diones 5a–h and 6a–h in good yields 68–85% (Scheme 2). When we used SnCl2 as the reductive system for the nitroindazole derivatives, the yields of compounds 5a–h and 6a–h were slightly improved (72–91%).
All the structures of the obtained products 5a–h and 6a–h were determined by a detailed examination of their NMR data.
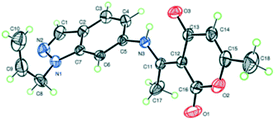
Furthermore, the structure of product 5c was unambiguously confirmed through X-ray crystallographic analysis (Fig. 1).20
Looking at the influence of the structure of the starting 6-nitro-N-alkyl-indazoles on the reduction/coupling yields in the presence of stannous chloride, we observed, in all cases, that the alkylated indazoles in the N-1 position 5a–h show better yields (78–91%) than the alkylated indazoles in the N-2 position 6a–h (72–82%). Compounds 2c and 2d (substituted at N-1 with an allyl or a benzyl group) gave the hybrid molecules 5c and d with excellent yields (91% and 88%, respectively).
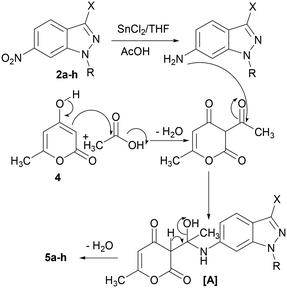
A plausible mechanism was proposed to explain the formation of (3E)-3-(1-((N-alkyl-1H-indazol-6yl)amino)ethylidene)-6-methyl-3H-pyran-2,4-diones 5a–h. First, the usual reduction of the nitro group generates the corresponding amine. Thereafter, the attack of the amine on the carbonyl group of the dehydroacetic acid formed by acylation of the 4-hydroxy-6-methyl-2-pyrone with the acetic acid gave intermediate [A]. The dehydration of H2O from [A], afforded the desired compounds 5a–h (Scheme 3). It should be noted that, we performed the reaction between 1-methyl-6-nitroindazole 2a with dehydroacetic acid in the presence of SnCl2 in THF with acetic acid; we isolated compound 5a in good yield.
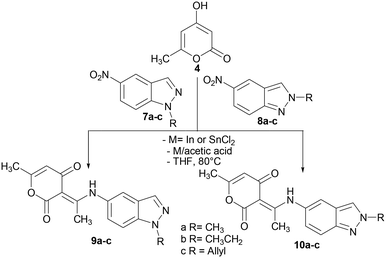
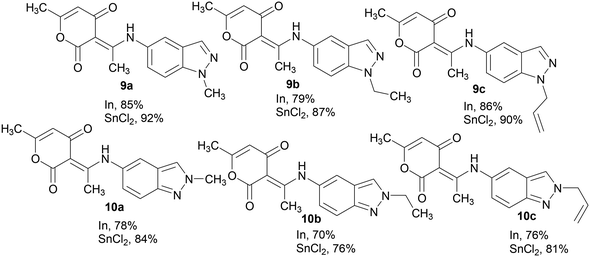
Encouraged by this result, we decided to extend the scope of this methodology to the synthesis of other hybrid molecules containing indazole and 2-pyrone moieties. The reaction of N-alkyl-5-nitroindazoles 7a–c and 8a–c with 4-hydroxy-6-methyl-2-pyrone 4 using the optimized reaction conditions (Scheme 4), afforded as expected compounds 9a–c and 10a–c with yields varying between 76–92%. We obtained the (3E)-3-(1-((1-alkyl-indazol-5-yl)amino)ethylidene)-6-methyl-3H-pyran-2,4-diones 9a–c in good yields (87–92%), while the N-2 alkylated indazoles 10a–c were obtained in 76–84% yields. The highest yields were obtained by the reduction of the nitroindazole derivatives with stannous chloride (Charts 1 and 2).
 | ||
| Chart 1 Yields of compounds 5a–h and 6a–h in the presence of In or SnCl2 as a reductive system of N-alkyl-6-nitroindazole derivatives. Isolated yield after column chromatography. | ||
Biological evaluation against L. donovani
The synthesized compounds were tested first for their cytotoxicity. Among the 19 compounds, only three of them exhibited cytotoxicity with CC50 values of around 6.25 μM, and all the others were not cytotoxic at all at 100 μM. The compounds were then evaluated in vitro for antileishmanial activity against axenic and intramacrophage amastigotes of L. donovani. In this evaluation, the activity was expressed in IC50 (concentration inhibiting the parasite growth by 50%).Among the compounds 5a–h possessing various substituents at the N-1 position and a hydrogen or chlorine atom at the C-3 position of the indazole ring, only 5f, and to a lesser extent 5h, exhibited interesting activities. As shown in Table 1, 5h showed moderate antileishmanial activity against both axenic and intramacrophage amastigotes (IC50 of 62.56 ± 1.25 and 49.89 ± 4.69 μM) whereas derivative 5f (substituted at C-3 with a chlorine atom and at N-1 with an ethyl group) showed the greatest activity (IC50 of 10.99 ± 1.79 and 6.50 ± 1.25 μM).
| Compounds | IC50 ± SD μM | CC50 ± SD (μM) | |
|---|---|---|---|
| L. d. LV9 axenic amastigotes | L. d. LV9 intramacrophage amastigotes | Cytotoxicity on macrophages | |
| Amphotericin B (AmB) was used as the reference drug. | |||
| 5a | >100 | >100 | >100 |
| 5b | >100 | >100 | >100 |
| 5c | >100 | >100 | >100 |
| 5d | >100 | >100 | >100 |
| 5e | >100 | >100 | >100 |
| 5f | 10.99 ± 1.79 | 6.50 ± 1.25 | 6.23 ± 0.74 |
| 5g | >100 | >100 | >100 |
| 5h | 62.56 ± 1.25 | 49.89 ± 4.69 | >100 |
| 6b | >100 | >100 | >100 |
| 6c | >100 | >100 | >100 |
| 6d | 49.64 ± 3.19 | 57.38 ± 6.75 | >100 |
| 6e | 11.89 ± 1.28 | 3.25 ± 1.99 | 6.26 ± 0.39 |
| 6f | 2.48 ± 1.02 | 2.25 ± 1.89 | 6.25 ± 0.47 |
| 6h | >100 | >100 | >100 |
| 9a | >100 | >100 | >100 |
| 9b | >100 | >100 | >100 |
| 9c | >100 | >100 | >100 |
| 10a | >100 | >100 | >100 |
| 10b | >100 | >100 | >100 |
| AmB | 0.201 ± 0.080 | 0.115 ± 0.090 | 4.23 ± 0.32 |
Regarding the series of compounds 6b–h (N-2 alkylated), we observed that three compounds 6d–f exhibited fair to very good activities on both intramacrophage and axenic amastigotes with IC50 values about ten times higher than those of amphotericin B, the reference compound. The most active compound 6f (substituted at C-3 with a chlorine atom and at N-2 with an ethyl group) displayed an IC50 of 2.48 ± 1.02 μM against L. donovani axenic amastigotes and, interestingly, an IC50 of 2.25 ± 1.89 μM against the intramacrophage ones. Compound 6e which is alkylated at N-2 with a methyl group, exhibited an almost four-fold better activity against the intramacrophage amastigotes than the axenic ones (IC50 of 3.25 ± 1.99 vs. 11.89 ± 1.28 μM).
Comparing the two series 5a–h/6b–h we can observe that N-2 alkylated derivatives are the most active, and among them are those bearing a chlorine atom at the C-carbon atom of the indazole ring. Nevertheless, the cytotoxicity on the macrophages of the active compounds tested remains quite strong and in a log range of their antileishmanial activities (CC50 = 6.25 μM).
Finally, looking at the influence of the position of pyrone attached to the indazole on the structure activity relationship, we observed that compounds 9a–c and 10a–c (N-alkylated and indazole substituted at position 5 with pyrone) did not exhibit any antileishmanial activity (IC50 > 100 μM). When looking at the selectivity index (SI) values, defined as the ratio between CC50/IC50 in the intracellular amastigotes, the most promising compound is compound 6f (SI = 2.77), followed by compound 6e (SI = 1.92), then 5f (SI = 0.96), this last compound exhibiting no margin between the activity and cytotoxicity.
Conclusions
We have developed a simple and efficient synthesis of (3E)-3-(1-((N-alkyl-indazol-6-yl)amino)ethylidene)-6-methyl-3H-pyran-2,4-diones and (3E)-3-(1-((N-alkyl-indazol-5-yl)amino)ethylidene)-6-methyl-3H-pyran-2,4-diones by reductive coupling of N-alkyl-6(5)-nitroindazoles with 2-pyrone using indium or stannous chloride in THF in the presence of acetic acid. By using SnCl2/AcOH in THF at 80 °C with a cheaper cost, we realized a methodology which provided high yields, short reaction times, easy work-up, and purification of products by the chromatographic method and a clean reaction. The antileishmanial activity of 19 indazole-pyrone hybrid derivatives was studied, with compounds efficient against the intramacrophage and axenic forms of the parasite. The remarkable antileishmanial activity obtained for 6f (IC50 values of 2.48 ± 1.02 μM and 2.25 ± 1.89 μM, respectively, on axenic and intramacrophage amastigotes) is moderated by its cytotoxicity. Considering the mechanism of action of both series, the results obtained in this paper prompted us to develop further pharmacomodulations in order to diminish the cytotoxicity and enhance the antileishmanial activity. Therefore, compound 6f, the most promising compound from this series, will be the basis for further pharmacomodulations.Experimental
Chemistry
Melting points were determined using a Büchi-Tottoli apparatus. 1H and 13C NMR spectra were recorded in CDCl3, DMSO-d6 and solution (unless otherwise specified) with TMS as the internal reference using a Bruker AC 300 (1H) or 75 MHz (13C) instrument. Chemical shifts are given in δ parts per million (ppm) downfield from TMS. Multiplicities of 13C NMR resources were assigned by distortionless enhancement by polarization transfer (DEPT) experiments. Low-resolution mass spectra (MS) were recorded on a Perkin-Elmer Sciex API 3000 spectrometer. Column chromatography was carried out on SiO2 (silica gel 60 Merck 0.063–0.200 mm). Thin-layer chromatography (TLC) was carried out on SiO2 (silica gel 60, F 254 Merck 0.063–0.200 mm), and the spots were located with UV light. Commercial reagents were used without further purification unless stated.General procedure for the synthesis of compounds 5a–h, 6a–h, 9a–c and 10a–c
>N-alkyl-6(5)-nitroindazoles (1.0 mmol) were added to a mixture of anhydrous SnCl2 powder (460 mg, 4.0 mmol) and acetic acid (0.572 mL, 10 mmol) in tetrahydrofuran (10 mL) followed by the addition of 2-pyrone (1.0 mmol) in THF (15 mL). The reaction mixture was stirred at 80 °C for 4 to 6 h. After the reaction was completed, the mixture was diluted with ethyl acetate (30 mL), poured into 10% NaHCO3 (30 mL), and then extracted with ethyl acetate (50 mL × 3). The combined organic extracts were dried over MgSO4, filtered, and concentrated. The residue was purified by column chromatography on silica gel using ethyl acetate/hexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) to afford the desired products in good yields. 1H NMR, 13CNMR and DEPT copies of selected compounds are given in the ESI.†
7) to afford the desired products in good yields. 1H NMR, 13CNMR and DEPT copies of selected compounds are given in the ESI.†
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.08 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.73 (d, 1H, J = 1.2 Hz), 7.84 (d, 1H, J = 8.4 Hz), 8.10 (s, 1H, H-3), 15.83 (s, 1H, NH); 13C NMR (DMSO-d6): δ 19.8 (CH3), 20.6 (CH3), 36.0 (NCH3), 97.1 (C), 107.2 (CH), 118.9 (CH), 122.4 (CH), 123.0 (C), 133.2 (CH-3), 134.4 (C), 139.9 (C), 162.8 (C), 163.9 (C), 175.7 (CO), 184.4 (CO); EI-MS (m/z) = 298 [M + 1]+, anal. calcd for C16H15N3O3; C, 64.64; H, 5.09; N, 14.13. Found: C, 64.71; H, 5.02; N, 14.24.
CH), 7.08 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.73 (d, 1H, J = 1.2 Hz), 7.84 (d, 1H, J = 8.4 Hz), 8.10 (s, 1H, H-3), 15.83 (s, 1H, NH); 13C NMR (DMSO-d6): δ 19.8 (CH3), 20.6 (CH3), 36.0 (NCH3), 97.1 (C), 107.2 (CH), 118.9 (CH), 122.4 (CH), 123.0 (C), 133.2 (CH-3), 134.4 (C), 139.9 (C), 162.8 (C), 163.9 (C), 175.7 (CO), 184.4 (CO); EI-MS (m/z) = 298 [M + 1]+, anal. calcd for C16H15N3O3; C, 64.64; H, 5.09; N, 14.13. Found: C, 64.71; H, 5.02; N, 14.24.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.08 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.71 (d, 1H, J = 1.2 Hz), 7.80 (d, 1H, J = 8.4 Hz), 8.21 (s, 1H, H-3), 15.81 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 20.7 (CH3), 46.7 (NCH3), 97.9 (C), 107.0 (CH), 112.0 (CH), 120.4 (C), 121.8 (CH), 122.8 (CH), 123.7 (C), 125.2 (CH-3), 139.0 (C), 164.0 (C), 175.1 (CO), 184.9 (CO); EI-MS (m/z) = 298 [M + 1]+, anal. calcd for C16H15N3O3; C, 64.64; H, 5.09; N, 14.13. Found: C, 64.75; H, 5.05; N, 14.18.
CH), 7.08 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.71 (d, 1H, J = 1.2 Hz), 7.80 (d, 1H, J = 8.4 Hz), 8.21 (s, 1H, H-3), 15.81 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 20.7 (CH3), 46.7 (NCH3), 97.9 (C), 107.0 (CH), 112.0 (CH), 120.4 (C), 121.8 (CH), 122.8 (CH), 123.7 (C), 125.2 (CH-3), 139.0 (C), 164.0 (C), 175.1 (CO), 184.9 (CO); EI-MS (m/z) = 298 [M + 1]+, anal. calcd for C16H15N3O3; C, 64.64; H, 5.09; N, 14.13. Found: C, 64.75; H, 5.05; N, 14.18.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.96 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.27 (d, 1H, J = 1.2 Hz), 7.81 (d, 1H, J = 8.4 Hz), 8.07 (s, 1H, H-3), 15.57 (s, 1H, NH); 13C NMR (CDCl3): δ 14.9 (CH3), 20.1 (CH3), 21.0 (CH3), 44.1 (NCH2), 98.2 (C), 106.1 (CH), 106.5 (CH), 118.7 (CH), 122.9 (CH), 123.4 (C), 132.7 (CH-3), 134.4 (C), 138.7 (C), 160.1 (C), 164.0 (C), 175.8 (CO), 184.0 (CO); EI-MS (m/z) = 312 [M + 1]+, anal. calcd for C17H17N3O3; C, 65.58; H, 5.50; N, 13.50. Found: C, 65.67; H, 5.42; N, 13.64.
CH), 6.96 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.27 (d, 1H, J = 1.2 Hz), 7.81 (d, 1H, J = 8.4 Hz), 8.07 (s, 1H, H-3), 15.57 (s, 1H, NH); 13C NMR (CDCl3): δ 14.9 (CH3), 20.1 (CH3), 21.0 (CH3), 44.1 (NCH2), 98.2 (C), 106.1 (CH), 106.5 (CH), 118.7 (CH), 122.9 (CH), 123.4 (C), 132.7 (CH-3), 134.4 (C), 138.7 (C), 160.1 (C), 164.0 (C), 175.8 (CO), 184.0 (CO); EI-MS (m/z) = 312 [M + 1]+, anal. calcd for C17H17N3O3; C, 65.58; H, 5.50; N, 13.50. Found: C, 65.67; H, 5.42; N, 13.64.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.03 (dd, 1H, J = 9.0 Hz, J = 1.2 Hz), 7.65 (d, 1H, J = 1.2 Hz), 7.83 (d, 1H, J = 9.0 Hz), 8.23 (s, 1H, H-3), 15.88 (s, 1H, NH); 13C NMR (CDCl3): δ 15.6 (CH3), 20.0 (CH3), 20.7 (CH3), 49.0 (NCH2), 98.2 (C), 106.8 (CH), 111.4 (CH), 120.2 (C), 121.8 (CH), 122.6 (CH), 123.4 (C), 125.6 (CH-3), 138.7 (C), 164.2 (C), 175.6 (CO), 184.5 (CO); EI-MS (m/z) = 312 [M + 1]+, anal. calcd for C17H17N3O3; C, 65.58; H, 5.50; N, 13.50. Found: C, 65.71; H, 5.38; N, 13.58.
CH), 7.03 (dd, 1H, J = 9.0 Hz, J = 1.2 Hz), 7.65 (d, 1H, J = 1.2 Hz), 7.83 (d, 1H, J = 9.0 Hz), 8.23 (s, 1H, H-3), 15.88 (s, 1H, NH); 13C NMR (CDCl3): δ 15.6 (CH3), 20.0 (CH3), 20.7 (CH3), 49.0 (NCH2), 98.2 (C), 106.8 (CH), 111.4 (CH), 120.2 (C), 121.8 (CH), 122.6 (CH), 123.4 (C), 125.6 (CH-3), 138.7 (C), 164.2 (C), 175.6 (CO), 184.5 (CO); EI-MS (m/z) = 312 [M + 1]+, anal. calcd for C17H17N3O3; C, 65.58; H, 5.50; N, 13.50. Found: C, 65.71; H, 5.38; N, 13.58.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.97–6.06 (m, 1H,
CH2), 5.97–6.06 (m, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.13 (s, 1H,
CH), 6.13 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.97 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.27 (d, 1H, J = 1.2 Hz), 7.82 (d, 1H, J = 8.4 Hz), 8.07 (s, 1H, H-3), 15.48 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 21.0 (CH3), 52.1 (NCH2), 98.2 (C), 106.5 (CH), 110.5 (CH), 118.5 (
CH), 6.97 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.27 (d, 1H, J = 1.2 Hz), 7.82 (d, 1H, J = 8.4 Hz), 8.07 (s, 1H, H-3), 15.48 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 21.0 (CH3), 52.1 (NCH2), 98.2 (C), 106.5 (CH), 110.5 (CH), 118.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 118.8 (CH), 122.7 (CH), 123.7 (C), 132.1 (CH), 133.3 (CH), 134.3 (C), 139.2 (C), 163.9 (C), 164.7 (C), 176.0 (CO), 184.0 (CO); EI-MS (m/z) = 324 [M + 1]+, anal. calcd for C18H17N3O3; C, 66.86; H, 5.30; N, 13.00. Found: C, 66.74; H, 5.42; N, 13.08.
CH2), 118.8 (CH), 122.7 (CH), 123.7 (C), 132.1 (CH), 133.3 (CH), 134.3 (C), 139.2 (C), 163.9 (C), 164.7 (C), 176.0 (CO), 184.0 (CO); EI-MS (m/z) = 324 [M + 1]+, anal. calcd for C18H17N3O3; C, 66.86; H, 5.30; N, 13.00. Found: C, 66.74; H, 5.42; N, 13.08.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.83 (s, 1H,
CH2), 5.83 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.09–6.18 (m, 1H,
CH), 6.09–6.18 (m, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.90 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.54 (d, 1H, J = 1.0 Hz), 7.74 (d, 1H, J = 8.4 Hz), 8.10 (s, 1H, H-3), 15.71 (s, 1H, NH); 13C NMR (CDCl3): δ 19.9 (CH3), 20.6 (CH3), 56.3 (NCH2), 97.4 (C), 107.2 (CH), 113.5 (CH), 120.5 (CH), 120.7 (
CH), 6.90 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.54 (d, 1H, J = 1.0 Hz), 7.74 (d, 1H, J = 8.4 Hz), 8.10 (s, 1H, H-3), 15.71 (s, 1H, NH); 13C NMR (CDCl3): δ 19.9 (CH3), 20.6 (CH3), 56.3 (NCH2), 97.4 (C), 107.2 (CH), 113.5 (CH), 120.5 (CH), 120.7 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 122.1 (CH), 122.7 (C), 123.9 (CH), 131.3 (CH), 147.3 (C), 163.4 (C), 163.8 (C), 175.6 (CO), 184.6 (CO); EI-MS (m/z) = 324 [M + 1]+, anal. calcd for C18H17N3O3; C, 66.86; H, 5.30; N, 13.00. Found: C, 66.72; H, 5.38; N, 13.15.
CH2), 122.1 (CH), 122.7 (C), 123.9 (CH), 131.3 (CH), 147.3 (C), 163.4 (C), 163.8 (C), 175.6 (CO), 184.6 (CO); EI-MS (m/z) = 324 [M + 1]+, anal. calcd for C18H17N3O3; C, 66.86; H, 5.30; N, 13.00. Found: C, 66.72; H, 5.38; N, 13.15.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.94 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.11–7.15 (m, 5H), 7.82 (d, 1H, J = 8.4 Hz), 8.11 (s, 1H, H-3), 15.37 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 21.1 (2CH3), 53.3 (NCH2), 98.2 (C), 106.0 (CH), 106.6 (CH), 118.7 (CH), 122.9 (CH), 123.8 (C), 127.3 (2CH), 129.5 (C), 129.7 (2CH), 132.7 (C), 133.2 (CH), 134.2 (C), 138.1 (C), 139.2 (C), 163.4 (C), 175.1 (CO), 184.1 (CO); EI-MS (m/z) = 388 [M + 1]+, anal. calcd for C23H21N3O3; C, 71.30; H, 5.46; N, 10.85. Found: C, 71.18; H, 5.57; N, 10.74.
CH), 6.94 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.11–7.15 (m, 5H), 7.82 (d, 1H, J = 8.4 Hz), 8.11 (s, 1H, H-3), 15.37 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 21.1 (2CH3), 53.3 (NCH2), 98.2 (C), 106.0 (CH), 106.6 (CH), 118.7 (CH), 122.9 (CH), 123.8 (C), 127.3 (2CH), 129.5 (C), 129.7 (2CH), 132.7 (C), 133.2 (CH), 134.2 (C), 138.1 (C), 139.2 (C), 163.4 (C), 175.1 (CO), 184.1 (CO); EI-MS (m/z) = 388 [M + 1]+, anal. calcd for C23H21N3O3; C, 71.30; H, 5.46; N, 10.85. Found: C, 71.18; H, 5.57; N, 10.74.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.85 (dd, 1H, J = 8.4 Hz, J = 1.0 Hz), 7.17 (d, 1H, J = 7.8 Hz), 7.23 (d, 1H, J = 7.8 Hz), 7.52 (d, 1H, J = 1.0 Hz), 7.68 (d, 1H, J = 8.4 Hz), 7.95 (s, 1H, H-3), 15.72 (s, 1H, NH); 13C NMR (CDCl3): δ 19.9 (CH3), 20.5 (CH3), 21.2 (CH3), 57.5 (NCH2), 97.5 (C), 107.2 (CH), 113.2 (CH), 120.6 (C), 120.7 (CH), 122.2 (CH), 124.3 (CH), 128.5 (2CH), 129.8 (2CH), 131.4 (C), 135.1 (C), 138.9 (C), 163.5 (C), 163.7 (C), 175.5 (CO), 184.6 (CO); EI-MS (m/z) = 388 [M + 1]+, anal. calcd for C23H21N3O3; C, 71.30; H, 5.46; N, 10.85. Found: C, 71.21; H, 5.61; N, 10.78.
CH), 6.85 (dd, 1H, J = 8.4 Hz, J = 1.0 Hz), 7.17 (d, 1H, J = 7.8 Hz), 7.23 (d, 1H, J = 7.8 Hz), 7.52 (d, 1H, J = 1.0 Hz), 7.68 (d, 1H, J = 8.4 Hz), 7.95 (s, 1H, H-3), 15.72 (s, 1H, NH); 13C NMR (CDCl3): δ 19.9 (CH3), 20.5 (CH3), 21.2 (CH3), 57.5 (NCH2), 97.5 (C), 107.2 (CH), 113.2 (CH), 120.6 (C), 120.7 (CH), 122.2 (CH), 124.3 (CH), 128.5 (2CH), 129.8 (2CH), 131.4 (C), 135.1 (C), 138.9 (C), 163.5 (C), 163.7 (C), 175.5 (CO), 184.6 (CO); EI-MS (m/z) = 388 [M + 1]+, anal. calcd for C23H21N3O3; C, 71.30; H, 5.46; N, 10.85. Found: C, 71.21; H, 5.61; N, 10.78.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.05 (d, 1H, J = 8.4 Hz), 7.20 (d, 1H, J = 1.0 Hz), 7.81 (d, 1H, J = 8.4 Hz), 15.65 (s, 1H, NH); 13C NMR (CDCl3): δ 20.0 (CH3), 20.7 (CH3), 47.6 (NCH3), 97.8 (C), 106.8 (CH), 107.1 (CH), 119.0 (CH), 121.0 (C), 121.4 (CH), 134.1 (C), 136.0 (C), 140.8 (C), 163.7 (C), 164.9 (C), 175.8 (CO), 184.6 (CO); EI-MS (m/z) = 332 (35Cl) [M + 1]+, 334 (37Cl) [M + 3]+ anal. calcd for C16H14ClN3O3; C, 57.93; H, 4.25; N, 12.67. Found: C, 57.82; H, 4.36; N, 12.54.
CH), 7.05 (d, 1H, J = 8.4 Hz), 7.20 (d, 1H, J = 1.0 Hz), 7.81 (d, 1H, J = 8.4 Hz), 15.65 (s, 1H, NH); 13C NMR (CDCl3): δ 20.0 (CH3), 20.7 (CH3), 47.6 (NCH3), 97.8 (C), 106.8 (CH), 107.1 (CH), 119.0 (CH), 121.0 (C), 121.4 (CH), 134.1 (C), 136.0 (C), 140.8 (C), 163.7 (C), 164.9 (C), 175.8 (CO), 184.6 (CO); EI-MS (m/z) = 332 (35Cl) [M + 1]+, 334 (37Cl) [M + 3]+ anal. calcd for C16H14ClN3O3; C, 57.93; H, 4.25; N, 12.67. Found: C, 57.82; H, 4.36; N, 12.54.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.88 (dd, 1H, J = 8.7 Hz, J = 1.5 Hz), 7.42 (d, 1H, J = 1.5 Hz), 7.62 (d, 1H, J = 8.7 Hz), 15.88 (s, 1H, NH); 13C NMR (CDCl3): δ 19.9 (CH3), 20.5 (CH3), 37.8 (NCH3), 97.5 (C), 107.2 (C), 114.2 (CH), 111.8 (C), 118.1 (C), 120.1 (CH), 120.6 (CH), 135.0 (C), 147.1 (C), 163.4 (C), 175.5 (CO), 184.8 (CO); EI-MS (m/z) = 332 (35Cl) [M + 1]+, 334 (37Cl) [M + 3]+ anal. calcd for C16H14ClN3O3; C, 57.93; H, 4.25; N, 12.67. Found: C, 57.78; H, 4.34; N, 12.52.
CH), 6.88 (dd, 1H, J = 8.7 Hz, J = 1.5 Hz), 7.42 (d, 1H, J = 1.5 Hz), 7.62 (d, 1H, J = 8.7 Hz), 15.88 (s, 1H, NH); 13C NMR (CDCl3): δ 19.9 (CH3), 20.5 (CH3), 37.8 (NCH3), 97.5 (C), 107.2 (C), 114.2 (CH), 111.8 (C), 118.1 (C), 120.1 (CH), 120.6 (CH), 135.0 (C), 147.1 (C), 163.4 (C), 175.5 (CO), 184.8 (CO); EI-MS (m/z) = 332 (35Cl) [M + 1]+, 334 (37Cl) [M + 3]+ anal. calcd for C16H14ClN3O3; C, 57.93; H, 4.25; N, 12.67. Found: C, 57.78; H, 4.34; N, 12.52.
(3E)-3-(1-(3-Chloro-1-ethyl-1H-indazol-6-ylamino)ethylidene)-6-methyl-3H-pyran-2,4-dione (5f)
Yield: 77%; mp 67–69 °C; 1H NMR (CDCl3): δ 1.51 (t, 3H, CH3, J = 7.2 Hz), 2.26 (s, 3H, CH3), 2.58 (s, 3H, CH3), 4.40 (q, 2H, NCH2, J = 7.2 Hz), 5.81 (s, 1H,![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.90 (dd, 1H, J = 8.7 Hz, J = 1.5 Hz), 7.45 (d, 1H, J = 1.5 Hz), 7.66 (d, 1H, J = 8.7 Hz), 15.82 (s, 1H, NH); 13C NMR (CDCl3): δ 14.8 (CH3), 20.2 (CH3), 21.0 (CH3), 44.5 (NCH2), 97.8 (C), 107.4 (C), 114.1 (CH), 111.9 (C), 118.5 (C), 120.3 (CH), 120.8 (CH), 135.1 (C), 146.9 (C), 163.8 (C), 175.6 (CO), 184.2 (CO); EI-MS (m/z) = 346 (35Cl) [M + 1]+, 348 (37Cl) [M + 3]+ anal. calcd for C16H14ClN3O3; C, 59.05; H, 4.66; N, 12.15. Found: C, 59.18; H, 4.54; N, 12.22.
CH), 6.90 (dd, 1H, J = 8.7 Hz, J = 1.5 Hz), 7.45 (d, 1H, J = 1.5 Hz), 7.66 (d, 1H, J = 8.7 Hz), 15.82 (s, 1H, NH); 13C NMR (CDCl3): δ 14.8 (CH3), 20.2 (CH3), 21.0 (CH3), 44.5 (NCH2), 97.8 (C), 107.4 (C), 114.1 (CH), 111.9 (C), 118.5 (C), 120.3 (CH), 120.8 (CH), 135.1 (C), 146.9 (C), 163.8 (C), 175.6 (CO), 184.2 (CO); EI-MS (m/z) = 346 (35Cl) [M + 1]+, 348 (37Cl) [M + 3]+ anal. calcd for C16H14ClN3O3; C, 59.05; H, 4.66; N, 12.15. Found: C, 59.18; H, 4.54; N, 12.22.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.91 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.45 (d, 1H, J = 1.5 Hz), 7.68 (d, 1H, J = 8.4 Hz), 15.78 (s, 1H, NH); 13C NMR (CDCl3): δ 15.7 (CH3), 20.0 (CH3), 20.6 (CH3), 48.8 (NCH2), 97.8 (C), 107.1 (C), 113.9 (CH), 112.0 (C), 118.2 (C), 120.4 (CH), 120.8 (CH), 134.9 (C), 146.8 (C), 163.5 (C), 175.7 (CO), 184.5 (CO); EI-MS (m/z) = 346 (35Cl) [M + 1]+, 348 (37Cl) [M + 3]+ anal. calcd for C16H14ClN3O3; C, 59.05; H, 4.66; N, 12.15. Found: C, 59.24; H, 4.50; N, 12.28.
CH), 6.91 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.45 (d, 1H, J = 1.5 Hz), 7.68 (d, 1H, J = 8.4 Hz), 15.78 (s, 1H, NH); 13C NMR (CDCl3): δ 15.7 (CH3), 20.0 (CH3), 20.6 (CH3), 48.8 (NCH2), 97.8 (C), 107.1 (C), 113.9 (CH), 112.0 (C), 118.2 (C), 120.4 (CH), 120.8 (CH), 134.9 (C), 146.8 (C), 163.5 (C), 175.7 (CO), 184.5 (CO); EI-MS (m/z) = 346 (35Cl) [M + 1]+, 348 (37Cl) [M + 3]+ anal. calcd for C16H14ClN3O3; C, 59.05; H, 4.66; N, 12.15. Found: C, 59.24; H, 4.50; N, 12.28.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.82–6.02 (m, 1H,
CH2), 5.82–6.02 (m, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.14 (s, 1H,
CH), 6.14 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.02 (d, 1H, J = 8.4 Hz), 7.24 (d, 1H, J = 1.0 Hz), 7.77 (d, 1H, J = 8.4 Hz), 15.54 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 21.1 (CH3), 52.5 (NCH2), 97.1 (C), 106.3 (CH), 106.9 (CH), 118.9 (
CH), 7.02 (d, 1H, J = 8.4 Hz), 7.24 (d, 1H, J = 1.0 Hz), 7.77 (d, 1H, J = 8.4 Hz), 15.54 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 21.1 (CH3), 52.5 (NCH2), 97.1 (C), 106.3 (CH), 106.9 (CH), 118.9 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 119.3 (CH), 121.0 (C), 121.6 (CH), 131.7 (CH), 133.5 (C), 135.3 (C), 140.5 (C), 163.8 (C), 165.0 (C), 176.2 (CO), 184.0 (CO); EI-MS (m/z) = 358 (35Cl) [M + 1]+, 360 (37Cl) [M + 3]+ anal. calcd for C18H16ClN3O3; C, 60.43; H, 4.51; N, 11.74. Found: C, 60.32; H, 4.64; N, 11.63.
CH2), 119.3 (CH), 121.0 (C), 121.6 (CH), 131.7 (CH), 133.5 (C), 135.3 (C), 140.5 (C), 163.8 (C), 165.0 (C), 176.2 (CO), 184.0 (CO); EI-MS (m/z) = 358 (35Cl) [M + 1]+, 360 (37Cl) [M + 3]+ anal. calcd for C18H16ClN3O3; C, 60.43; H, 4.51; N, 11.74. Found: C, 60.32; H, 4.64; N, 11.63.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.86 (s, 1H,
CH2), 5.86 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.10–6.17 (m, 1H,
CH), 6.10–6.17 (m, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.92 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 6.91 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.45 (d, 1H, J = 1.5 Hz), 7.61 (d, 1H, J = 8.4 Hz), 15.77 (s, 1H, NH); 13C NMR (CDCl3): δ 20.0 (CH3), 20.6 (CH3), 56.4 (NCH2), 97.6 (C), 106.8 (C), 113.6 (CH), 112.0 (C), 118.2 (C), 120.4 (CH), 120.7 (
CH), 6.92 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 6.91 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.45 (d, 1H, J = 1.5 Hz), 7.61 (d, 1H, J = 8.4 Hz), 15.77 (s, 1H, NH); 13C NMR (CDCl3): δ 20.0 (CH3), 20.6 (CH3), 56.4 (NCH2), 97.6 (C), 106.8 (C), 113.6 (CH), 112.0 (C), 118.2 (C), 120.4 (CH), 120.7 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 121.0 (CH), 131.5 (CH), 135.0 (C), 146.2 (C), 164.0 (C), 175.4 (CO), 184.8 (CO); EI-MS (m/z) = 358 (35Cl) [M + 1]+, 360 (37Cl) [M + 3]+ anal. calcd for C18H16ClN3O3; C, 60.43; H, 4.51; N, 11.74. Found: C, 60.28; H, 4.60; N, 11.61.
CH2), 121.0 (CH), 131.5 (CH), 135.0 (C), 146.2 (C), 164.0 (C), 175.4 (CO), 184.8 (CO); EI-MS (m/z) = 358 (35Cl) [M + 1]+, 360 (37Cl) [M + 3]+ anal. calcd for C18H16ClN3O3; C, 60.43; H, 4.51; N, 11.74. Found: C, 60.28; H, 4.60; N, 11.61.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.20 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.53 (d, 1H, J = 8.4 Hz), 7.57 (d, 1H, J = 1.2 Hz), 8.05 (s, 1H, H-3), 15.22 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 21.0 (CH3), 35.9 (NCH3), 98.2 (C), 106.2 (CH), 110.5 (CH), 118.1 (CH), 123.8 (C), 124.1 (CH), 128.8 (C), 133.0 (CH-3), 138.9 (C), 164.1 (C), 164.9 (C), 176.3 (CO), 183.6 (CO); EI-MS (m/z) = 298 [M + 1]+, anal. calcd for C16H15N3O3; C, 64.64; H, 5.09; N, 14.13. Found: C, 64.75; H, 5.18; N, 14.20.
CH), 7.20 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.53 (d, 1H, J = 8.4 Hz), 7.57 (d, 1H, J = 1.2 Hz), 8.05 (s, 1H, H-3), 15.22 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 21.0 (CH3), 35.9 (NCH3), 98.2 (C), 106.2 (CH), 110.5 (CH), 118.1 (CH), 123.8 (C), 124.1 (CH), 128.8 (C), 133.0 (CH-3), 138.9 (C), 164.1 (C), 164.9 (C), 176.3 (CO), 183.6 (CO); EI-MS (m/z) = 298 [M + 1]+, anal. calcd for C16H15N3O3; C, 64.64; H, 5.09; N, 14.13. Found: C, 64.75; H, 5.18; N, 14.20.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.09 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.48 (d, 1H, J = 1.8 Hz), 7.77 (d, 1H, J = 8.4 Hz), 8.02 (s, 1H, H-3), 15.59 (s, 1H, NH); 13C NMR (CDCl3): δ 19.9 (CH3), 20.5 (CH3), 40.6 (NCH3), 97.3 (C), 107.3 (CH), 116.8 (CH), 118.6 (CH), 121.4 (C), 124.9 (CH), 125.5 (CH-3), 130.5 (C), 146.8 (C), 163.4 (C), 163.8 (C), 175.7 (CO), 184.6 (CO); EI-MS (m/z) = 298 [M + 1]+, anal. calcd for C16H15N3O3; C, 64.64; H, 5.09; N, 14.13. Found: C, 64.72; H, 5.21; N, 14.24.
CH), 7.09 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.48 (d, 1H, J = 1.8 Hz), 7.77 (d, 1H, J = 8.4 Hz), 8.02 (s, 1H, H-3), 15.59 (s, 1H, NH); 13C NMR (CDCl3): δ 19.9 (CH3), 20.5 (CH3), 40.6 (NCH3), 97.3 (C), 107.3 (CH), 116.8 (CH), 118.6 (CH), 121.4 (C), 124.9 (CH), 125.5 (CH-3), 130.5 (C), 146.8 (C), 163.4 (C), 163.8 (C), 175.7 (CO), 184.6 (CO); EI-MS (m/z) = 298 [M + 1]+, anal. calcd for C16H15N3O3; C, 64.64; H, 5.09; N, 14.13. Found: C, 64.72; H, 5.21; N, 14.24.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.19 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.51 (d, 1H, J = 8.4 Hz), 7.58 (d, 1H, J = 1.5 Hz), 8.06 (s, 1H, H-3), 15.25 (s, 1H, NH); 13C NMR (CDCl3): δ 14.9 (CH3), 20.1 (CH3), 20.9 (CH3), 44.2 (NCH2), 96.8 (C), 106.3 (CH), 110.4 (CH), 118.2 (CH), 123.9 (C), 124.1 (CH), 128.8 (C), 133.0 (CH-3), 138.0 (C), 164.1 (C), 164.8 (C), 176.2 (CO), 183.7 (CO); EI-MS (m/z) = 312 [M + 1]+, anal. calcd for C17H17N3O3; C, 65.58; H, 5.50; N, 13.50. Found: C, 65.75; H, 5.41; N, 13.64.
CH), 7.19 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.51 (d, 1H, J = 8.4 Hz), 7.58 (d, 1H, J = 1.5 Hz), 8.06 (s, 1H, H-3), 15.25 (s, 1H, NH); 13C NMR (CDCl3): δ 14.9 (CH3), 20.1 (CH3), 20.9 (CH3), 44.2 (NCH2), 96.8 (C), 106.3 (CH), 110.4 (CH), 118.2 (CH), 123.9 (C), 124.1 (CH), 128.8 (C), 133.0 (CH-3), 138.0 (C), 164.1 (C), 164.8 (C), 176.2 (CO), 183.7 (CO); EI-MS (m/z) = 312 [M + 1]+, anal. calcd for C17H17N3O3; C, 65.58; H, 5.50; N, 13.50. Found: C, 65.75; H, 5.41; N, 13.64.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.13 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.51 (d, 1H, J = 1.0 Hz), 7.82 (d, 1H, J = 8.4 Hz), 8.10 (s, 1H, H-3), 15.61 (s, 1H, NH); 13C NMR (CDCl3): δ 15.7 (CH3), 19.9 (CH3), 20.5 (CH3), 48.9 (NCH2), 97.3 (C), 107.2 (CH), 117.0 (CH), 118.3 (CH), 121.0 (C), 124.0 (CH), 125.5 (CH-3), 130.7 (C), 145.7 (C), 163.5 (C), 163.8 (C), 175.7 (CO), 184.6 (CO); EI-MS (m/z) = 312 [M + 1]+, anal. calcd for C17H17N3O3; C, 65.58; H, 5.50; N, 13.50. Found: C, 65.72; H, 5.38; N, 13.60.
CH), 7.13 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.51 (d, 1H, J = 1.0 Hz), 7.82 (d, 1H, J = 8.4 Hz), 8.10 (s, 1H, H-3), 15.61 (s, 1H, NH); 13C NMR (CDCl3): δ 15.7 (CH3), 19.9 (CH3), 20.5 (CH3), 48.9 (NCH2), 97.3 (C), 107.2 (CH), 117.0 (CH), 118.3 (CH), 121.0 (C), 124.0 (CH), 125.5 (CH-3), 130.7 (C), 145.7 (C), 163.5 (C), 163.8 (C), 175.7 (CO), 184.6 (CO); EI-MS (m/z) = 312 [M + 1]+, anal. calcd for C17H17N3O3; C, 65.58; H, 5.50; N, 13.50. Found: C, 65.72; H, 5.38; N, 13.60.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.97–6.08 (m, 1H,
CH2), 5.97–6.08 (m, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.19 (s, 1H,
CH), 6.19 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.17 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.50 (d, 1H, J = 9.0 Hz), 7.58 (d, 1H, J = 1.8 Hz), 8.07 (s, 1H, H-3), 15.27 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 20.8 (CH3), 52.1 (NCH2), 96.9 (C), 106.5 (CH), 110.7 (CH), 118.1 (CH), 118.4 (
CH), 7.17 (dd, 1H, J = 8.4 Hz, J = 1.8 Hz), 7.50 (d, 1H, J = 9.0 Hz), 7.58 (d, 1H, J = 1.8 Hz), 8.07 (s, 1H, H-3), 15.27 (s, 1H, NH); 13C NMR (CDCl3): δ 20.1 (CH3), 20.8 (CH3), 52.1 (NCH2), 96.9 (C), 106.5 (CH), 110.7 (CH), 118.1 (CH), 118.4 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 124.1 (CH), 124.3 (C), 128.9 (C), 132.2 (CH), 133.5 (CH), 138.5 (C), 164.1 (C), 164.7 (C), 176.2 (CO), 183.8 (CO); EI-MS (m/z) = 324 [M + 1]+, anal. calcd for C18H17N3O3; C, 66.86; H, 5.30; N, 13.00. Found: C, 66.70; H, 5.37; N, 13.16.
CH2), 124.1 (CH), 124.3 (C), 128.9 (C), 132.2 (CH), 133.5 (CH), 138.5 (C), 164.1 (C), 164.7 (C), 176.2 (CO), 183.8 (CO); EI-MS (m/z) = 324 [M + 1]+, anal. calcd for C18H17N3O3; C, 66.86; H, 5.30; N, 13.00. Found: C, 66.70; H, 5.37; N, 13.16.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 5.92 (s, 1H,
CH2), 5.92 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.05–6.20 (m, 1H,
CH), 6.05–6.20 (m, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.15 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.53 (d, 1H, J = 1.0 Hz), 7.68 (d, 1H, J = 8.4 Hz), 8.12 (s, 1H, H-3), 15.56 (s, 1H, NH); 13C NMR (CDCl3): δ 19.9 (CH3), 20.6 (CH3), 56.2 (NCH2), 97.6 (C), 106.9 (CH), 117.2 (CH), 118.3 (CH), 121.4 (
CH), 7.15 (dd, 1H, J = 8.4 Hz, J = 1.5 Hz), 7.53 (d, 1H, J = 1.0 Hz), 7.68 (d, 1H, J = 8.4 Hz), 8.12 (s, 1H, H-3), 15.56 (s, 1H, NH); 13C NMR (CDCl3): δ 19.9 (CH3), 20.6 (CH3), 56.2 (NCH2), 97.6 (C), 106.9 (CH), 117.2 (CH), 118.3 (CH), 121.4 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2), 124.8 (CH), 121.7 (C), 128.8 (CH), 130.7 (CH), 132.4 (C), 163.8 (C), 163.9 (C), 175.8 (CO), 184.4 (CO); EI-MS (m/z) = 324 [M + 1]+, anal. calcd for C18H17N3O3; C, 66.86; H, 5.30; N, 13.00. Found: C, 66.77; H, 5.43; N, 13.21.
CH2), 124.8 (CH), 121.7 (C), 128.8 (CH), 130.7 (CH), 132.4 (C), 163.8 (C), 163.9 (C), 175.8 (CO), 184.4 (CO); EI-MS (m/z) = 324 [M + 1]+, anal. calcd for C18H17N3O3; C, 66.86; H, 5.30; N, 13.00. Found: C, 66.77; H, 5.43; N, 13.21.
Biology
Concerning the evaluation on the intramacrophage amastigotes, RAW 264.7 macrophages were plated in 96-well microplates at a density of 2 × 104 cells per well and incubated for 24 h at 37 °C with 5% CO2. The axenic amastigotes were differentiated as described above, centrifuged at 2000g for 10 min, resuspended in DMEM complete medium, and added to each well to reach a 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 parasite to macrophage ratio. After 24 h of infection at 37 °C with 5% CO2, the extracellular parasites were removed, and DMEM complete medium (100 μL) containing two-fold serial dilutions of the compounds from a maximal concentration of 100 μM was added to each well. After 48 h of treatment, the medium was removed and replaced with Direct PCR Lysis Reagent (100 μL; Euromedex) before 3 freeze–thaw cycles at room temperature, addition of 50 μg mL−1 proteinase K, and a final incubation at 55 °C overnight to allow cell lysis. 10 μL of each cell extract was then added to 40 μL of Direct PCR Lysis Reagent containing Sybr Green I (0.05%; Invitrogen). DNA fluorescence was monitored using a Mastercycler® realplex (Eppendorf). The activity of the compounds was expressed as IC50 in μM. Amphotericin B (AmB) was used as the reference drug.
1 parasite to macrophage ratio. After 24 h of infection at 37 °C with 5% CO2, the extracellular parasites were removed, and DMEM complete medium (100 μL) containing two-fold serial dilutions of the compounds from a maximal concentration of 100 μM was added to each well. After 48 h of treatment, the medium was removed and replaced with Direct PCR Lysis Reagent (100 μL; Euromedex) before 3 freeze–thaw cycles at room temperature, addition of 50 μg mL−1 proteinase K, and a final incubation at 55 °C overnight to allow cell lysis. 10 μL of each cell extract was then added to 40 μL of Direct PCR Lysis Reagent containing Sybr Green I (0.05%; Invitrogen). DNA fluorescence was monitored using a Mastercycler® realplex (Eppendorf). The activity of the compounds was expressed as IC50 in μM. Amphotericin B (AmB) was used as the reference drug.
Conflicts of interest
The authors declare no competing interests.Acknowledgements
We thank the University of Sultan Moulay Slimane, Beni-Mellal, for financial support and the National Centre for Scientific and Technical Research (CNRST), Rabat, for providing the NMR and X-ray crystallography results of our compounds.Notes and references
- A. Shaveta, S. Mishra and P. Singh, Eur. J. Med. Chem., 2016, 124, 500–536 CrossRef.
- A. Noor ul and A. Matloob, Turk. J. Chem., 2018, 42, 1–20 CrossRef.
- M. Matias, S. Silvestre, A. Falcao and G. Alves, Mini-Rev. Med. Chem., 2017, 17, 486–517 CrossRef CAS PubMed.
- C.-G. Wilson, F. Y. Andres and H.-R. Angie, Curr. Med. Chem., 2018, 25, 3637–3679 CrossRef PubMed.
- R. H. Dodd, C. Ouannès, M. Robert-Gèro and P. Potier, J. Med. Chem., 1989, 32, 1272–1276 CrossRef CAS.
- P. A. Harris, A. Boloor, M. Cheung, R. Kumar, R. M. Crosby, R. G. Davis-Ward, A. H. Epperly, K. W. Hinkle, R. N. Hunter, J. H. Johnson, V. B. Knick, C. P. Laudeman, D. K. Luttrell, R. A. Mook, R. T. Nolte, S. K. Rudolph, J. R. Szewczyk, A. T. Truesdale, J. M. Veal and L. Wang, J. Med. Chem., 2008, 51, 4632–4640 CrossRef CAS PubMed.
- J. Zhang, Q. Yang, J. A. C. Romero, J. Cross, B. Wang, K. M. Poutsiaka, F. Epie, D. Bevan, Y. Wu, T. Moy, A. Daniel, B. Chamberlain, N. Carter, J. Shotwell, A. Arya, V. Kumar, J. Silverman, K. Nguyen, C. A. Metcalf, D. Ryan, B. Lippa and R. E. Dolle, ACS Med. Chem. Lett., 2015, 6, 1080–1085 CrossRef CAS PubMed.
- K. G. Liu, A. J. Robichaud, R. C. Bernotas, Y. Yan, J. R. Lo, M.-Y. Zhang, Z. A. Hughe, C. Huselton, G. M. Zhang, J. Y. Zhang, D. M. Kowal, D. L. Smith, L. E. Schechter and T. A. Comery, J. Med. Chem., 2010, 53, 7639–7646 CrossRef CAS PubMed.
- (a) A. Takeuchi, M. Hori, S. Sato, H. S. Ban, T. Kuchimaru, S. Kizaka-Kondoh, T. Yamori and H. Nakamura, Med. Chem. Commun., 2012, 3, 1455–1461 RSC; (b) N. Abbassi, H. Chicha, E. M. Rakib, A. Hannioui, M. Alaoui, A. Hajjaji, D. Geffken, C. Aiello, R. Gangemi, C. Rosano and M. Viale, Eur. J. Med. Chem., 2012, 57, 240–249 CrossRef CAS PubMed.
- C. Fonseca-Berzal, A. Ibáñez-Escribano, N. Vela, J. Cumella, J. J. Nogal-Ruiz, J. A. Escario, P. B. da Silva, M. M. Batista, M. N. C. Soeiro, S. Sifontes-Rodríguez, A. Meneses-Marcel, A. Gómez-Barrio and V. J. Arán, ChemMedChem, 2018, 13, 1246–1259 CrossRef CAS PubMed.
- C. Marín, I. Ramírez-Macías, M. J. Rosales, B. Muro, F. Reviriego, P. Navarro, V. J. Arán and M. Sánchez-Moreno, Acta Trop., 2015, 148, 170–178 CrossRef PubMed.
- L. Boiani, A. Gerpe, V. J. Arán, S. Torres de Ortiz, E. Serna, N. Vera de Bilbao, L. Sanabria, G. Yaluff, H. Nakayama, A. Rojas de Arias, J. D. Maya, J. A. Morello, H. Cerecetto and M. González, Eur. J. Med. Chem., 2009, 44, 1034–1040 CrossRef CAS PubMed.
- J. M. Dickinson, Nat. Prod. Rep., 1993, 10, 71–98 RSC.
- G. P. McGlacken and I. J. S. Fairlamb, Nat. Prod. Rep., 2005, 22, 369–385 RSC.
- I. J. S. Fairlamb, L. R. Marrison, J. M. Dickinson, F.-J. Lu and J. P. Schmidt, Bioorg. Med. Chem., 2004, 12, 4285–4299 CrossRef CAS.
- A. Goel and V. J. Ram, Tetrahedron, 2009, 65, 7865–7913 CrossRef CAS.
- A. G. Tempone, D. D. Ferreira, M. L. Lima, T. A. Costa Silva, S. E. T. Borborema, J. Q. Reimão, M. K. Galuppo, J. M. Guerra, A. J. Russell, G. M. Wynne, R. Y. L. Lai, M. M. Cadelis and B. R. Copp, Eur. J. Med. Chem., 2017, 139, 947–960 CrossRef CAS.
- O. Kayser, A. F. Kiderlen and S. L. Croft, Acta Trop., 2003, 86, 105–107 CrossRef CAS.
- M. El Ghozlani, H. Chicha, N. Abbassi, M. Chigr, L. El Ammari, M. Saadi, D. Spinelli and E. M. Rakib, Tetrahedron Lett., 2016, 57, 113–117 CrossRef CAS.
- M. El Ghozlani, E. M. Rakib, A. Gamouh, M. Saadi and L. El Ammari, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2014, 70, o1256 CrossRef CAS.
- K. Balaraman, N. C. Vieira, F. Moussa, J. Vacus, S. Cojean, S. Pomel, C. Bories, B. Figadère, V. Kesavan and P. M. Loiseau, Biomed. Pharmacother., 2015, 76, 127–133 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8md00475g |
| This journal is © The Royal Society of Chemistry 2019 |