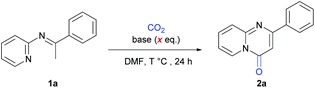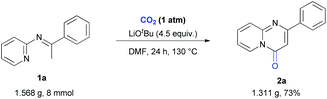Transition-metal-free lactamization of C(sp3)–H bonds with CO2: facile generation of pyrido[1,2-a]pyrimidin-4-ones†
Zhen
Zhang‡
*a,
Xiao-Yu
Zhou‡
b,
Jin-Gui
Wu
b,
Lei
Song
b and
Da-Gang
Yu
 *bc
*bc
aKey laboratory of Coarse Cereal Processing of Ministry of Agriculture and Rural Affairs, College of Pharmacy and Biological Engineering, Chengdu University, Chendu 610106, P. R. China. E-mail: zhangzhen1@cdu.edu.cn
bKey Laboratory of Green Chemistry & Technology of Ministry of Education, College of Chemistry, Sichuan University, Chengdu 610064, P. R. China. E-mail: dgyu@scu.edu.cn
cBeijing National Laboratory for Molecular Sciences, Beijing 100190, P. R. China
First published on 28th November 2019
Abstract
A novel carbonylation of C(sp3)–H bonds in pyridylamines with one atmosphere of CO2 is reported to synthesize important pyrimidinones in good yields. This transition-metal-free and redox-neutral process features the use of a nontoxic carbonyl source, broad substrate scope, good functional group tolerance, facile scalability and easy product derivatization.
The development of green and sustainable strategies for organic synthesis has received increasing attention. A green and recyclable building block may play a key role in these strategies. As an ideal C1 building block, CO2 is nontoxic, abundant, and recyclable. Therefore, it is highly important to utilize CO2 to synthesize high-value-added chemicals in a sustainable way.1 Among the diverse organic transformations of CO2,2 the carbonylation with CO2 to synthesize carbonyl-containing heterocycles has attracted increasing attention,3 replacing highly toxic and user-unfriendly CO and phosgene. Recently, significant progress has been achieved in the carbonylation of C–H with CO2 due to its high atom- and step-economy.4 Importantly, as it bears carbon with a higher valence than that of CO, CO2 can act ideally as the combination of CO and oxidants (CO2 = CO + [O]), realizing such carbonylations under redox-neutral reaction conditions and reducing the cost and heavy metal residues.5 However, most of the research in this field is focused on the carbonylation of C(sp2)–H bonds.4a–n In contrast, there are only few examples of carbonylation of C(sp3)–H bonds with CO2.4o–q Notably, to the best of our knowledge, only one example of lactamization of C(sp3)–H with CO2 was reported by Zhang and Lv,4p who realized the efficient generation of 4-hydroxy-2(1H)-quinolinones from o-acetamidoacetophenones, albeit under a high pressure of CO2 (3 MPa). Herein, we report a novel carbonylation of C(sp3)–H bonds in pyridylamines with one atmosphere of CO2 to synthesize important pyrimidinones under transition-metal-free and redox-neutral conditions (Scheme 1B).
Pyrimidinones are important motifs in many drug molecules and widely investigated in medicinal chemistry.6 Therefore, many groups have developed synthesis methods to generate such a structure efficiently.7 It is worth noting that Zeng and co-workers developed an elegant Pd-catalyzed lactamization of ketoimines with CO (Scheme 1A), which displays broad substrate scope and high step economy.8 However, the use of toxic CO and stoichiometric Cu(OAc)2 as the oxidant increases the risk for heavy metal residues and hampers its applications in industry. With our continuous interest in the sustainable organic synthesis with CO2,4b,9 we wondered whether we could realize the efficient carbonylation of the C(sp3)–H bonds in ketoimines with CO2 to obtain pyrimidinones under redox-neutral conditions. Such a scenario faces several challenges. First, the thermodynamic stability and kinetic inertness of CO2 render it difficult to realize efficient transformations, especially under low pressure. Second, the dearomatization of pyridines would occur during the reaction, which make such processes even more challenging (Fig. 1).
With such challenges in mind, we began to investigate the reaction using N-(2-pyridyl) ketoimine 1a as the substrate under one atmosphere of CO2 (Table 1). To our delight, the desired product 2a was obtained in 61% yield with lithium t-butoxide as the base in DMF at 120 °C for 24 h (Table 1, entry 1). Other bases, such as NaOtBu, KOtBu and Cs2CO3, were also tested but gave lower yields (Table 1, entries 2–4). When using NaOtBu or KOtBu as the base, the reaction system was very viscous and 1a was transformed to a byproduct (see the ESI† for details), both of which might cause a lower yield of 2a. The screening of the amount of LiOtBu demonstrated that 4.5 equivalents was the best choice (Table 1, entries 5–10). Similar to our previous reports,4b the desired product was not detected when 1 equivalent of LiOtBu was used, indicating that LiOtBu not only acts as a base but also participates in the formation of intermediates. Subsequently, we evaluated the reaction temperature and found that 130 °C gave the highest yield (Table 1, entries 9 and 11–14). Although 2a was generated in very low yield at 80 °C, the carboxylative product could be detected obviously by ESI-MS (Table 1, entry 11), which indicates that it might be an intermediate for this transformation and the cyclization might not occur easily at lower temperatures. Other solvents, such as DMA, DMSO, diglyme, and THF, were also tested (Table 1, entries 15–19). However, no better results were obtained, indicating the unique role of DMF for this reaction. No desired product was obtained in the absence of CO2, demonstrating its crucial role as a carbonylative source (Table 1, entry 20).
| Entry | Base | x | T/°C | Yieldb (%) | Entry | Base | x | T/°C | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 1 atm of CO2, 2 mL of DMF, 24 h. b GC yields are given with dodecane as an internal standard and the isolated yield is given in parentheses. c DMA (2 mL). d DMSO (2 mL). e Diglyme (2 mL). f THF (2 mL). g 1,4-Dioxane (2 mL). h Under the atmospheric of N2 instead of CO2. | |||||||||
| 1 | LiOtBu | 3 | 120 | 61 | 11 | LiOtBu | 4.5 | 80 | <5 |
| 2 | NaOtBu | 3 | 120 | 35 | 12 | LiOtBu | 4.5 | 110 | 67 |
| 3 | KOtBu | 3 | 120 | 22 | 13 | LiO t Bu | 4.5 | 130 | 87 (86) |
| 4 | Cs2CO3 | 3 | 120 | 12 | 14 | LiOtBu | 4.5 | 140 | 86 |
| 5 | LiOtBu | 1 | 120 | N.D. | 15c | LiOtBu | 4.5 | 130 | 23 |
| 6 | LiOtBu | 2 | 120 | 35 | 16d | LiOtBu | 4.5 | 130 | 47 |
| 7 | LiOtBu | 2.5 | 120 | 56 | 17e | LiOtBu | 4.5 | 130 | 35 |
| 8 | LiOtBu | 3.5 | 120 | 63 | 18f | LiOtBu | 4.5 | 130 | 17 |
| 9 | LiOtBu | 4 | 120 | 74 | 19g | LiOtBu | 4.5 | 130 | 5 |
| 10 | LiOtBu | 4.5 | 120 | 77 | 20h | LiOtBu | 4.5 | 130 | 0 |
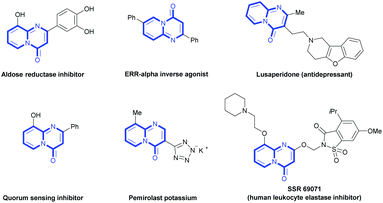
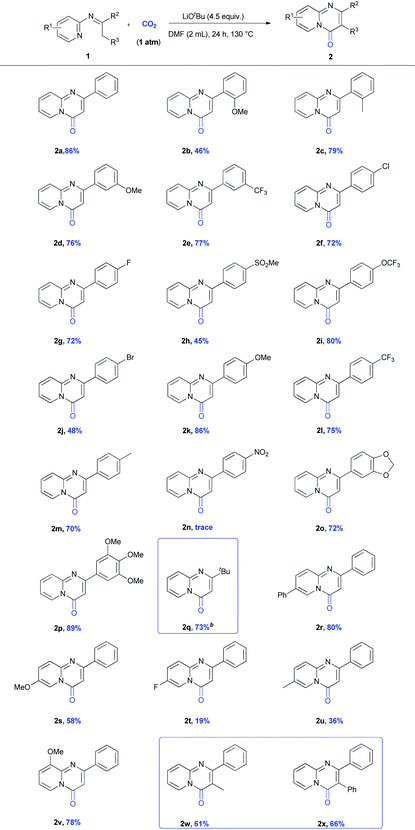
With the optimal reaction conditions in hand, we began to expand the substrate scope of N-(2-pyridyl) ketoimines 1 (Table 2). First, we examined the substrate bearing mono-substituents on the phenyl ring (1a–n). As shown in Scheme 2, various functional groups, such as electron-donating groups (EDGs, –OMe, –OCF3) and electron-withdrawing groups (EWGs, –CF3, –Cl, –SO2Me) at the ortho (1c), meta (1d–e), and para (1f–m) positions of the phenyl ring, did not affect the reaction. However, a substrate bearing a strong EWG, such as the nitro group (1n), at the para position showed low reactivity and was not suitable for this reaction. Besides the mono-substituents, the substrates bearing di- (1o) or tri-substituents (1p) on the phenyl ring can also undergo this transformation to provide the desired products in good yields. To our delight, our protocol was also suitable for the alkyl-substituted N-(2-pyridyl) ketoimine substrate 1q, which has not been reported via Pd-catalysis.8 Furthermore, substrates bearing substituents on the pyridine ring were also investigated. Similarly, the substrate with EDGs on the pyridine ring (1s and 1u) showed better reactivities than those with EWGs (1t) and electron-neutral groups (1r). Notably, the product 2r shows significant bioactivity and acts as an ERR-alpha inverse agonist.6c Besides the mono-substituted pyrimidinones, to our delight, we could also generate the di-substituted ones (2w and 2x) in good yields.
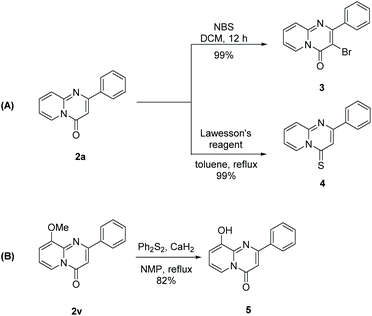
After developing this method, we further demonstrated its utility in organic synthesis. First, we conducted the gram-scale reaction of 1a and obtained the target product 2a in 73% yield (Scheme 2). Moreover, product 2a could be functionalized to give both 3 and 4 in quantitative yields (Scheme 3A). These results indicated the great potential application of this transformation in organic synthesis. In addition to 2r, a quorum sensing inhibitor 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6d could be rapidly accessed in good yield via the carbonylation of 1v followed by demethylation (Scheme 3B).
6d could be rapidly accessed in good yield via the carbonylation of 1v followed by demethylation (Scheme 3B).
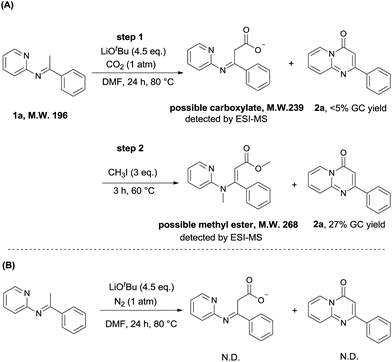
To gain more insight into the lactamization reaction, we did some mechanistic studies (Scheme 4). To confirm the carboxylate as the intermediate (Table 1, entry 11), we added CH3I to quench the reaction which was performed at 80 °C. We detected the formation of the corresponding methyl ester by ESI-MS and obtained product 2a in a higher yield (27% GC yield, Scheme 4A). In contrast, neither 2a nor the intermediate could be detected by ESI-MS in the control experiments under N2 (Scheme 4B). Therefore, we speculate that both the carboxylate intermediate and the ester could undergo the cyclization reaction to provide the desired product.
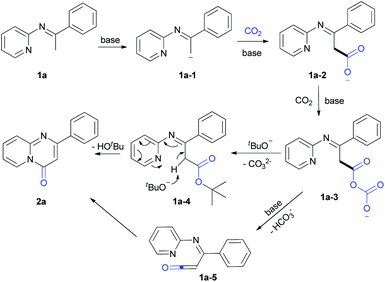
Based on the results and previous reports,7e,10 we proposed the following possible pathway (Scheme 5). 1a underwent deprotonation in the presence of the strong base LiOtBu to form 1a-1, which can further react with CO2 to generate intermediates 1a-2 and 1a-3. Under standard conditions, 1a-3 might react with tBuO− to generate 1a-4, which further transforms to 2a in the presence of a base. In addition, 1a-3 might also transform to 1a-5, which undergoes a cyclization reaction to provide the desired product 2a.
Conclusions
In summary, we have developed an efficient transition metal-free and external oxidant-free strategy to generate valuable pyrimidinones via the carbonylation of C(sp3)–H bonds with an atmospheric pressure of CO2, which is the more environment-friendly carbonylation source. This protocol features broad substrate scope, good functional group tolerance, facile scalability and easy product derivatization, thus providing potential application in organic synthesis and the pharmaceutical industry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the National Natural Science Foundation of China (21822108, 21801025, and 21502124), the “973” Project from the MOST of China (2015CB856600), the “1000-Youth Talents Program”, Sichuan Science and Technology Program (2019YJ0669), the National Key Research and Development Program of China (2018YFC1602101) and the Fundamental Research Funds for the Central Universities for financial support. We also thank the comprehensive training platform of the Specialized Laboratory in the College of Chemistry at Sichuan University for compound testing.Notes and references
-
(a)
Carbon Dioxide as Chemical Feedstock, ed. M. Aresta, Wiley-VCH, Weinheim, Germany, 2010 Search PubMed
; (b) Carbon Dioxide Chemistry, ed. L.-N. He, Science Press, Beijing, 2013 Search PubMed
.
- Selected reviews on CO2 utilization, see:
(a) T. Sakakura, J.-C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365 CrossRef CAS
; (b) K. Huang, C.-L. Sun and Z.-J. Shi, Chem. Soc. Rev., 2011, 40, 2435 RSC
; (c) R. Martin and A. W. Kleij, ChemSusChem, 2011, 4, 1259 CrossRef CAS
; (d) Y. Tsuji and T. Fujihara, Chem. Commun., 2012, 48, 9956 RSC
; (e) M. He, Y. Sun and B. Han, Angew. Chem., Int. Ed., 2013, 52, 9620 CrossRef CAS
; (f) L. Zhang and Z. Hou, Chem. Sci., 2013, 4, 3395 RSC
; (g) M. Aresta, A. Dibenedetto and A. Angelini, Chem. Rev., 2014, 114, 1709 CrossRef CAS
; (h) C. Maeda, Y. Miyazaki and T. Ema, Catal. Sci. Technol., 2014, 4, 1482 RSC
; (i) Q. Liu, L. Wu, R. Jackstell and M. Beller, Nat. Commun., 2015, 6, 5933 CrossRef
; (j) G. Fiorani, W. Guo and A. W. Kleij, Green Chem., 2015, 17, 1375 RSC
; (k) S. Wang, G. Du and C. Xi, Org. Biomol. Chem., 2016, 14, 3666 RSC
; (l) L. Zhang, Z. Han, L. Zhang, M. Li and K. Ding, Chin. J. Org. Chem., 2016, 36, 1824 CrossRef CAS
; (m) Q. Zhu, L. Wang, C. Xia and C. Liu, Chin. J. Org. Chem., 2016, 36, 2813 CrossRef CAS
; (n) X.-F. Wu and F. Zheng, Top. Curr. Chem., 2017, 375, 4 CrossRef
; (o) Q.-W. Song, Z.-H. Zhou and L.-N. He, Green Chem., 2017, 19, 3707 RSC
; (p) Y.-Y. Gui, W.-J. Zhou, J.-H. Ye and D.-G. Yu, ChemSusChem, 2017, 10, 1337 CrossRef CAS
; (q) J. Luo and I. Larrosa, ChemSusChem, 2017, 10, 3317 CrossRef CAS
; (r) G. Yuan, C. Qi, W. Wu and H. Jiang, Curr. Opin. Green Sustain. Chem., 2017, 3, 22 CrossRef
; (s) Y. Zhao and Z. Liu, Chin. J. Chem., 2018, 36, 455 CrossRef CAS
; (t) Y. Cao, X. He, N. Wang, H.-R. Li and L.-N. He, Chin. J. Chem., 2018, 36, 644 CrossRef CAS
; (u) A. Tortajada, F. Juliá-Hernández, M. Börjesson, T. Moragas and R. Martin, Angew. Chem., Int. Ed., 2018, 57, 15948 CrossRef CAS
; (v) J. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow and W. Leitner, Chem. Rev., 2018, 118, 2434 CrossRef
; (w) S.-S. Yan, Q. Fu, L.-L. Liao, G.-Q. Sun, J.-H. Ye, L. Gong, Y.-Z. Bo-Xue and D.-G. Yu, Coord. Chem. Rev., 2018, 374, 439 CrossRef CAS
; (x) J. Hou, J.-S. Li and J. Wu, Asian J. Org. Chem., 2018, 7, 1439 CrossRef CAS
; (y) S. Wang and C. Xi, Chem. Soc. Rev., 2019, 48, 382 RSC
; (z) Z. Zhang, L. Gong, X.-Y. Zhou, S.-S. Yan, J. Li and D.-G. Yu, Acta Chim. Sin., 2019, 77, 783 CrossRef
.
- W.-Z. Zhang, N. Zhang, C.-X. Guo and X.-B. Lu, Chin. J. Org. Chem., 2017, 37, 1309 CrossRef CAS
.
- For selected C(sp2)–H carbonylations with CO2:
(a) K. Sasano, J. Takaya and N. Iwasawa, J. Am. Chem. Soc., 2013, 135, 10954 CrossRef CAS
; (b) Z. Zhang, L.-L. Liao, S.-S. Yan, L. Wang, Y.-Q. He, J.-H. Ye, J. Li, Y.-G. Zhi and D.-G. Yu, Angew. Chem., Int. Ed., 2016, 55, 7068 CrossRef CAS
; (c) S. Wang, P. Shao, G. Du and C. Xi, J. Org. Chem., 2016, 81, 6672 CrossRef CAS
; (d) S. Sun, W.-M. Hu, N. Gu and J. Cheng, Chem. – Eur. J., 2016, 22, 18729 CrossRef CAS
; (e) W.-Z. Zhang, M.-W. Yang and X.-B. Lu, Green Chem., 2016, 18, 4181 RSC
; (f) Z. Zhang, T. Ju, M. Miao, J.-L. Han, Y.-H. Zhang, X.-Y. Zhu, J.-H. Ye, D.-G. Yu and Y.-G. Zhi, Org. Lett., 2017, 19, 396 CrossRef CAS
; (g) L. Fu, S. Li, Z. Cai, Y. Ding, X. Guo, L. Zhou, D. Yuan, Q. Sun and G. Li, Nat. Catal., 2018, 1, 469 CrossRef CAS
; (h) Z. Cai, S. Li, Y. Gao and G. Li, Adv. Synth. Catal., 2018, 360, 4005 CrossRef CAS
; (i) R. Huang, S. Li, L. Fu and G. Li, Asian J. Org. Chem., 2018, 7, 1376 CrossRef CAS
; (j) L. Song, G.-M. Cao, W.-J. Zhou, J.-H. Ye, Z. Zhang, X.-Y. Tian, J. Li and D.-G. Yu, Org. Chem. Front., 2018, 5, 2086 RSC
; (k) L. Song, L. Zhu, Z. Zhang, J.-H. Ye, S.-S. Yan, J. Han, Z.-B. Yin, Y. Lan and D.-G. Yu, Org. Lett., 2018, 20, 3776 CrossRef CAS PubMed
; (l) W. Li, C. Li and Y. Lyu, Org. Chem. Front., 2018, 5, 2189 RSC
; (m) Z. Zhang, C.-J. Zhu, M. Miao, J.-L. Han, T. Ju, L. Song, J.-H. Ye, J. Li and D.-G. Yu, Chin. J. Chem., 2018, 36, 430 CrossRef CAS
; (n) Y. Gao, Z. Cai, S. Li and G. Li, Org. Lett., 2019, 21, 3663 CrossRef CAS
. For selected C(sp3)–H carbonylations with CO2: (o) T. M. Harris and C. M. Harris, J. Org. Chem., 1966, 31, 1032 CrossRef CAS
; (p) W.-Z. Zhang, S. Liu and X.-B. Lu, Beilstein J. Org. Chem., 2015, 11, 906 CrossRef CAS PubMed
; (q) W.-Z. Zhang, M.-W. Yang and X.-B. Lu, Green Chem., 2016, 18, 4181 RSC
.
- Z. Zhang, T. Ju, J.-H. Ye and D.-G. Yu, Synlett, 2017, 28, 741 CrossRef CAS
.
-
(a) F. Awouters, J. Vermeire, F. Smeyers, P. Vermote, R. Van Beek and C. J. E. Niemegeers, Drug Dev. Res., 1986, 8, 95 CrossRef CAS
; (b) Y. Yanagihara, H. Kasai, T. Kawashima and T. Shida, Jpn. J. Pharmacol., 1988, 48, 91 CrossRef CAS PubMed
; (c) R. L. Smith, R. J. Barrett and E. Sanders-Bush, J. Pharmacol. Exp. Ther., 1995, 275, 1050 CAS
; (d) H. Suga and J. Igarashi, (JP) PCT Int. Appl2009063901, 2009 Search PubMed
; (e) L. Peng, X. Gao, L. Duan, X. Ren, D. Wu and K. Ding, J. Med. Chem., 2011, 54, 7729 CrossRef CAS
.
-
(a) H. G. Bonacorso, F. J. Righi, I. R. Rodrigues, C. A. Cechinel, M. B. Costa, A. D. Wastowski, M. A. P. Martins and N. Zanatta, J. Heterocycl. Chem., 2006, 43, 229 CrossRef CAS
; (b) A. MoInár, A. Kapros, L. Párkányi, Z. Mucsi, G. Viád and I. Hermccz, Org. Biomol. Chem., 2011, 9, 6559 RSC
; (c) S. K. Guchhait and G. Priyadarshani, J. Org. Chem., 2015, 80, 6342 CrossRef CAS PubMed
; (d) S. N. Basahel, N. S. Ahmed, K. Narasimharao and M. Mokhtar, RSC Adv., 2016, 6, 11921 RSC
; (e) Z. Chen, Y. Wen, H. Ding, G. Luo, M. Ye, L. Liu and J. Xue, Tetrahedron Lett., 2017, 58, 13 CrossRef CAS
.
- Y. Xie, T. Chen, S. Fu, H. Jiang and W. Zeng, Chem. Commun., 2015, 51, 9377 RSC
.
- Selected examples, see:
(a) J.-H. Ye, L. Song, W.-J. Zhou, T. Ju, Z.-B. Yin, S.-S. Yan, Z. Zhang, J. Li and D.-G. Yu, Angew. Chem., Int. Ed., 2016, 55, 10022 CrossRef CAS
; (b) Y.-Y. Gui, N. Hu, X.-W. Chen, L.-L. Liao, T. Ju, J.-H. Ye, Z. Zhang, J. Li and D.-G. Yu, J. Am. Chem. Soc., 2017, 139, 17011 CrossRef CAS PubMed
; (c) J.-H. Ye, M. Miao, H. Huang, S.-S. Yan, Z.-B. Yin, W.-J. Zhou and D.-G. Yu, Angew. Chem., Int. Ed., 2017, 56, 15416 CrossRef CAS
; (d) L.-L. Liao, G.-M. Cao, J.-H. Ye, G.-Q. Sun, W.-J. Zhou, Y.-Y. Gui, S.-S. Yan, G. Shen and D.-G. Yu, J. Am. Chem. Soc., 2018, 140, 17338 CrossRef CAS
; (e) T. Ju, Q. Fu, J.-H. Ye, Z. Zhang, L.-L. Liao, S.-S. Yan, X.-Y. Tian, S.-P. Luo, J. Li and D.-G. Yu, Angew. Chem., Int. Ed., 2018, 57, 13897 CrossRef CAS PubMed
; (f) Q. Fu, Z.-Y. Bo, J.-H. Ye, T. Ju, H. Huang, L.-L. Liao and D.-G. Yu, Nat. Commun., 2019, 10, 3592 CrossRef PubMed
; (g) X.-W. Chen, L. Zhu, Y.-Y. Gui, K. Jing, Y.-X. Jiang, Z.-Y. Bo, Y. Lan, J. Li and D.-G. Yu, J. Am. Chem. Soc., 2019, 141, 18825 CrossRef CAS
.
-
(a) X. Zhang, B. Yang, G. Li, X. Shu, D. C. Mungra and J. Zhu, Synlett, 2012, 23, 622 CrossRef CAS
; (b) A. Fiksdahl, C. Plüg and C. Wentrup, Mesoions and Ketene Valence Isomers, J. Chem. Soc., Perkin Trans. 2, 2000, 1841 RSC
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9gc03659h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |