Fast continuous alcohol amination employing a hydrogen borrowing protocol†
Ricardo
Labes
 a,
Carlos
Mateos
a,
Carlos
Mateos
 *b,
Claudio
Battilocchio‡
*b,
Claudio
Battilocchio‡
 a,
Yiding
Chen
a,
Yiding
Chen
 a,
Paul
Dingwall§
a,
Paul
Dingwall§
 a,
Graham R.
Cumming
a,
Graham R.
Cumming
 b,
Juan A.
Rincón
b,
Juan A.
Rincón
 b,
Maria José
Nieves-Remacha
b,
Maria José
Nieves-Remacha
 b and
Steven V.
Ley
b and
Steven V.
Ley
 *a
*a
aInnovative Technology Centre, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: svl1000@cam.ac.uk
bCentro de Investigación Lilly S.A., Avda. de la Industria 30, Alcobendas-Madrid 28108, Spain. E-mail: c.mateos@lilly.com
First published on 16th November 2018
Abstract
A continuous flow method for the direct conversion of alcohols to amines via a hydrogen borrowing approach is reported. The method utilises a low loading (0.5%) of a commercial catalyst system ([Ru(p-cymene)Cl2]2 and DPEPhos), reagent grade solvent and is selective for primary alcohols. Successful methylation of amines using methanol and the direct dimethylamination of alcohols using commercial dimethylamine solution are reported. The synthesis of two pharmaceutical agents Piribedil (5) and Buspirone (25) were accomplished in good yields employing these new methods.
Nitrogen containing compounds constitute the majority of pharmaceutical and agrochemical agents and feature in a vast range of natural products and chemical building blocks used for synthesis. As a consequence, their preparation is important as is the discovery of new, more sustainable routes to these types of molecules. The community is fairly well served with classical alkylation and arylation methods to form substituted amines, which are particularly relevant in an industrial settings.1 However, to convert alcohols to amines usually requires multiple steps via activation through halides or sulfonates followed by nucleophilic displacement with amines, or alternatively via oxidation and reductive amination. These multistep methods, although successful, often use quite aggressive reagents, produce excessive waste and are not always amenable to scale.
As an alternative approach, a hydrogen borrowing technique is becoming increasingly attractive. This process represents excellent atom efficiency and generates only water as by-product during the overall conversion of alcohol to amine. Reported first in 19812–4 and recently reviewed,5 direct alcohol amination through a variety of alcohol amination systems generally consists of an oxidation phase to generate a carbonyl species using a suitable hydrogen atom catalytic carrier, followed by rapid imine formation and final return of hydrogen to effect formation of the product amine (Scheme 1).
Many advances in this field have been made with varying degrees of success. All these approaches strive to obtain a robust practical sequence in terms of catalyst loading, reaction time and scalability.6–14 For example, an attractive application of hydrogen borrowing method was reported by Pfizer, using an Iridium catalyst originally developed by Fujita15 to prepare a GlyT1 inhibitor on kilogram scales.16 In the same report, the authors describe that the reaction works best in a pressurized vessel, heating above the boiling point of the reaction mixture suggesting its suitability for continuous flow development.16 In other work, the method was extended for other pharmaceutically relevant substrates, where potential catalyst poisoning can be a undesired side effect.17
Our recent findings on the efficient use of ruthenium catalysed continuous transfer hydrogenation reactions18,19 led us to study the benefits of these hydrogen borrowing methods in similar systems. Continuous flow hydrogen-borrowing methods have been reported in the past, but not always without issues, for example, low space–time yield and catalyst leaching.20,21 Nevertheless there is still scope to develop greater process windows (high temperatures and pressures) and provide a practical continuous method that can deliver the product quickly and safely on a reasonable scale (higher process intensification).
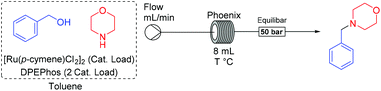
Previous studies have concentrated mainly on the multistep discovery of novel catalysts, however here we choose to focus on the use of a catalytic system that has well-recognised substrate compatibility and is commercially available. For this, the catalytic system developed by Williams and colleagues ([Ru(p-cymene)Cl2]2 and DPEPhos in toluene) was selected, as it is relatively inexpensive, air-stable and does not require extensive downstream processing.22
To accommodate the anticipated high temperatures and pressures needed for the reaction, a Phoenix flow reactor was selected. This device operates at up to 450 °C and 300 bar and has interchangeable 8 and 35 mL stainless steel flow coils.23 The initial evaluation began by flowing a solution of benzyl alcohol and morpholine similar to Williams’ original conditions but with 0.5 mol% of the ruthenium catalyst instead of 2.5 mol%,22 and varying the residence time and temperature of the reactor (Fig. 1). We were surprised by the strong positive effect the temperature had over the reaction turnover. In only 10 minutes of residence time in the reactor the product was obtained in 60% yield at 200 °C and fully converted at 250 °C, while only 12% of product was observed at 150 °C (Fig. 1). In the original work, the reaction mixture required reflux for 24h in the presence molecular sieves to obtain 84% yield.22 Here, non-dried and non-degassed toluene was used with no decrease in conversion. Interestingly at 250 °C no by-products, for example esters, were observed.
The equivalents of alcohol and phosphine were also varied and our results were in agreement with the original findings that DPEPhos and DPPF were the best performing phosphines. At 250 °C triphenylphosphine was also active, furnishing the product in 74%. Interestingly, more hindered tri-o-tolylphosphine resulted in only 12% conversion (see ESI†).
To better optimize and understand the reaction parameters a design of experiment (DoE) study was performed (see ESI†). The catalyst loading demonstrated the strongest effect, followed by time and then temperature. An increase in concentration marginally improved the reaction.
A selection of these DoE experiments is compiled in Table 1 to illustrate the versatility of the method. Thus, if lower temperature is required, higher catalyst loading and time can be used (Table 1, entry 1). If the catalyst loading was reduced, the conditions can be adjusted to work as low as 0.3 mol% of catalyst (Table 1, entry 2). On reducing the time scale, all other parameters can be adjusted to complete the reaction within 1 min (Table 1, entry 3). As expected, no reaction was observed without addition of the catalyst system (Table 1, entry 4).
One potential problem of hydrogen borrowing methods is that polar compounds, commonly encountered in pharmaceutical and agrochemical candidates, can suffer from low solubility in toluene/xylene, which constrains the applicability of the method. We therefore screened for alternative solvents with higher polarity and were pleased to find that the reaction worked equally well in trifluorotoluene(CF3Ph), CPME and THF (see ESI†). Here again non-anhydrous and non-degassed solvents were used to conform with the desired practicality. Differential Scanning Calorimetry (DSC) was conducted to confirm the reaction safety generating amines in THF at high temperatures (see ESI†).
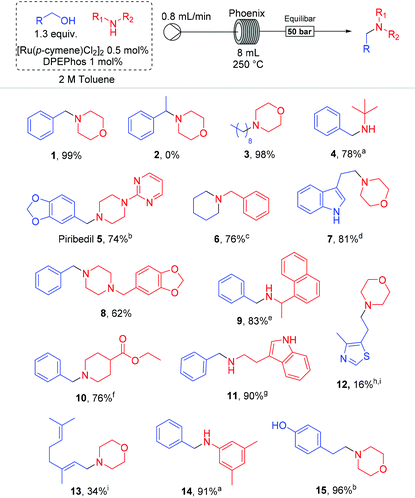
With a successful, robust and clean protocol in hand we next investigated if this could be applied more generally to other alcohol amination processes. The results of this brief reaction scope study are shown in Table 2.
| Reactions conditions are shown in the scheme. Yields are for the isolated compound.a 0.5 mol L−1, 2.5 mol% [Ru(p-cymene)Cl2]2, 5 mol% DPEPhos.b 1.1 equiv. of alcohol, 0.5 mol L−1 in THF, 1 mol% [Ru(p-cymene)Cl2]2, 2 mol% DPEPhos.c 1.3 equiv. of benzylamine, 1 mol L−1, 1 mol% [Ru(p-cymene)Cl2]2, 2 mol% DPEPhos.d 0.5 mol L−1 in CPME.e 0.5 mol L−1.f 1 mol L−1.g 1 mol L−1 in THF.h 2.5 mol% [Ru(p-cymene)Cl2]2, 5 mol% DPEPhos.i NMR yield using trimethoxybenzene as internal standard. |
|---|
|
The method suggests that high yields can be obtained with benzylic and aliphatic alcohols, while allylic alcohols suffered from additional reduction of the double bond, with decreased overall yield (13). Heterocycles such as morpholine (1, 3, 7, 15), piperidine (10), indole (7 & 11), methylenedioxy (5 & 8) and piperazine (the anti-Parkinson agent Piribedil 5 & 8) were all compatible, but thiazole 12 resulted in poor yield, although no further decomposition was observed and starting material was recovered in 83%, even using a higher 2.5 mol% loading of catalyst. tert-Butylamine (4) and aniline (14) required a higher catalyst loading to afford the corresponding benzylated products in higher yields. Cyclization of 1,5-pentanol afforded the piperidine 6 in 76% yield. Remarkably ester-containing products (10) were successfully reacted with benzyl alcohol without damage to the ester functionality. Phenolic substrate also remained unchanged in the process (15). We noticed too that with secondary alcohols, such as 1-phenylethanol (2), no amination occurred and starting material was recovered.
From the above results we speculated that this hydrogen borrowing method could be usefully applied to the direct methylation (or dimethylation) of amines with methanol. This would constitute an attractive greener approach towards certain active pharmaceutical ingredients (APIs). In an initial experiment using only methanol as solvent we noticed a colour change but no chemical reactivity. On the other hand, by using methanol in toluene useful reactivity was observed (Table 3).
Methylation of morpholine gave 93% yield of the corresponding N-methylmorpholine (16). Interestingly 2,4-dichloroaniline lead to the monomethylated product even when using 2.2 equivalents of methanol, while no substitution of chlorine observed (17). At the same time ester (19) was obtained with no significant transesterification with methanol. We believe that under the studied conditions the oxidation to formaldehyde is fast enough to avoid transesterification.
To complement these studies and further respond to the needs of industry, since dimethylamino derivatives occur in a range of bioactive materials, we chose to study the direct alcohol amination with dimethylamine as source of amine (Table 4). For this, different from previous reports,22 we used a commercial solution of dimethylamine in THF, which greatly improves the practicality of the method.
These illustrative examples show how these alcohols can be coupled with dimethylamine using the Phoenix flow reactor to afford the corresponding aminated products.
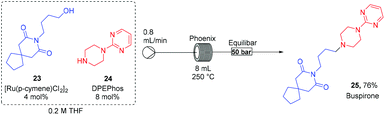
Finally, to demonstrate wider functional group tolerances we reacted the alcohol (23) with amine (24) to lead to the anxiolytic drug Buspirone (25) directly in 76% yield (Scheme 2). In doing so, we improved the synthesis from the commercial route by reducing the sequence by one step, avoiding the use of hydrogen gas and RANEY® Nickel as used in the established protocol.24
With few exceptions,16 most hydrogen borrowing alcohol amination methods reported to date lack the capability to produce material on larger scales. We therefore decided to scale up one experiment to validate the protocol in a larger reactor, during an extended reaction period (Scheme 3).
 | ||
| Scheme 3 Scale-up experiment. a500 mL of crude material from the first step (1.9 L over 9 hours) were processed in the downstream, value calculated back for the whole steady state collection. | ||
Therefore, with a larger coil reactor (35 mL) using 1.6 equivalents of benzyl alcohol and no solvent, it was possible to obtain 1.2 kg of product over 9 h of continuous processing time. The crude output was then processed continuously as its HCl salt delivering the product clean of ruthenium (<6 ppm) in 88% yield overall (Scheme 3, more details in ESI†).
Conclusions
In conclusion, we have developed a scalable high yielding continuous flow protocol, for the direct conversion of alcohols to amines, via a hydrogen borrowing approach, utilising simple experimental conditions, very low loading of a commercial catalyst system and selective for primary alcohols.Clearly, from the above reaction sequences, we can demonstrate new process opportunities under high temperatures and pressure conditions using flow reactor technology, which would be challenging to replicate under more traditional batch conditions. These hydrogen borrowing strategies add further to our synthesis repertoire and in particular conform to a more sustainable future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to Eli Lilly & Co. (RL) and the EPSRC (SVL, grants EP/K009494/1, EP/M004120/1 and EP/K039520/1) for financial support. This work was supported by Eli Lilly & Co. through the Lilly Research Award Program (LRAP).Notes and references
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337–2347 RSC.
- R. Grigg, T. R. B. Mitchell, S. Sutthivaiyakit and N. Tongpenyai, J. Chem. Soc., Chem. Commun., 1981, 611–612 RSC.
- B. T. Khai, C. Concilio and G. Porzi, J. Org. Chem., 1981, 46, 1759–1760 CrossRef.
- Y. Watanabe, Y. Tsuji and Y. Ohsugi, Tetrahedron Lett., 1981, 22, 2667–2670 CrossRef CAS.
- A. Corma, J. Navas and M. J. Sabater, Chem. Rev., 2018, 118, 1410–1459 CrossRef CAS PubMed.
- M. H. S. A. Hamid, P. A. Slatford and J. M. J. Williams, Adv. Synth. Catal., 2007, 349, 1555–1575 CrossRef CAS.
- R. Yamaguchi, K.-i. Fujita and M. Zhu, Heterocycles, 2010, 81, 1093–1140 CrossRef CAS.
- G. Guillena, D. J. Ramón and M. Yus, Chem. Rev., 2010, 110, 1611–1641 CrossRef CAS PubMed.
- R. H. Crabtree, Organometallics, 2011, 30, 17–19 CrossRef CAS.
- J. Schranck, A. Tlili and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 7642–7644 CrossRef CAS PubMed.
- C. Gunanathan and D. Milstein, Science, 2013, 341, 1229712–1229712 CrossRef PubMed.
- Q. Yang, Q. Wang and Z. Yu, Chem. Soc. Rev., 2015, 44, 2305–2329 RSC.
- S. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, ChemCatChem, 2011, 3, 1853–1864 CrossRef.
- G. E. Dobereiner and R. H. Crabtree, Chem. Rev., 2010, 110, 681–703 CrossRef CAS PubMed.
- K. Fujita, Z. Li, N. Ozeki and R. Yamaguchi, Tetrahedron Lett., 2003, 44, 2687–2690 CrossRef CAS.
- M. A. Berliner, S. P. A. Dubant, T. Makowski, K. Ng, B. Sitter, C. Wager and Y. Zhang, Org. Process Res. Dev., 2011, 15, 1052–1062 CrossRef CAS.
- J. Leonard, A. J. Blacker, S. P. Marsden, M. F. Jones, K. R. Mulholland and R. Newton, Org. Process Res. Dev., 2015, 19, 1400–1410 CrossRef CAS.
- R. Labes, D. González-Calderón, C. Battilocchio, C. Mateos, G. R. Cumming, O. de Frutos, J. A. Rincón and S. V. Ley, Synlett, 2017, 2855–2858 CAS.
- R. Labes, C. Battilocchio, C. Mateos, G. R. Cumming, O. de Frutos, J. A. Rincón, K. Binder and S. V. Ley, Org. Process Res. Dev., 2017, 21, 1419–1422 CrossRef CAS.
- S. Pei Shan, T. T. Dang, A. M. Seayad and B. Ramalingam, ChemCatChem, 2014, 6, 808–814 CrossRef.
- G. W. Lamb, F. A. Al Badran, J. M. J. Williams and S. T. Kolaczkowski, Chem. Eng. Res. Des., 2010, 88, 1533–1540 CrossRef CAS.
- M. H. S. A. Hamid, C. L. Allen, G. W. Lamb, A. C. Maxwell, H. C. Maytum, A. J. A. Watson and J. M. J. Williams, J. Am. Chem. Soc., 2009, 131, 1766–1774 CrossRef CAS PubMed.
- ThalesNano, Phoenix Reactor, http://thalesnano.com/phoenix-flow-reactor (accessed 2 June 2018).
- Y. H. Wu and J. W. Rayburn, US PatUS3976776, 1976 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8gc03328e |
| ‡ Current address: Syngenta Crop Protection, Process Research, Schaffhauserstrasse 101, Stein, CH-4332, Switzerland. |
| § Current address: Queen's University Belfast, University Rd, Belfast, BT7 1NN, UK. |
| This journal is © The Royal Society of Chemistry 2019 |