Fabrication of 2D metal–organic framework nanosheets with tailorable thickness using bio-based surfactants and their application in catalysis†
Xiudong
Zhang
ab,
Pei
Zhang
*a,
Chunjun
Chen
ab,
Jianling
Zhang
 ab,
Guanying
Yang
a,
Lirong
Zheng
ab,
Guanying
Yang
a,
Lirong
Zheng
 c,
Jing
Zhang
c and
Buxing
Han
c,
Jing
Zhang
c and
Buxing
Han
 *ab
*ab
aBeijing National Laboratory for Molecular Sciences, Key Laboratory of Colloid and Interface and Thermodynamics, Institute of Chemistry, CAS Research/Education Center for Excellence in Molecular Sciences, Chinese Academy of Sciences, Beijing 100190, China. E-mail: hanbx@iccas.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
cBeijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 1st November 2018
Abstract
Ultrathin metal–organic framework (MOF) nanosheets were fabricated by a surfactant-mediated coordination strategy through a pseudo-assembly process. The thickness of the nanosheets could be tuned from 3 nm to 60 nm by varying the hydrophobic chain length of the surfactant. Ru nanoparticles supported on the nanosheets exhibited superior catalytic activity for hydrogenation of levulinic acid to γ-valerolactone.
Two-dimensional (2D) nanomaterials with atomic or molecular thickness have received broad interest in recent years because of their unique dimension-related properties in energy storage, separation and catalysis compared to their bulk counterparts.1 2D metal–organic frameworks (MOFs) have emerged as a new member of the 2D family and received extensive attention.2 2D MOF nanosheets with extended lateral dimensions and nanometre thickness possess rich accessible active sites on their surface, and are expected to exhibit excellent performance in energy conversion and catalysis.3 However, rational synthesis of 2D MOF nanosheets with controllable thickness and diverse structures still remains a challenge. The top-down method involving exfoliation of bulk MOF materials has been demonstrated to be a simple and efficient approach for preparing 2D MOF nanosheets,4 but the exfoliation process by weakening interlayer interactions in MOF crystals via mechanical or solvent-mediated strategy often requires multistep approaches, leading to nanosheets with various thicknesses and low yields (typically < 15%).5 Therefore, it is highly desired to develop other facile approaches for preparing nanosheets in high yield.
Zirconium-based MOFs (Zr-MOFs) have attracted great attention because of their high thermal stabilities and rich structure types compared to those of other common MOFs.6 As a prototypical member of Zr-MOFs, UiO-66, which is synthesized from ZrCl4 and terephthalic acid (BDC), exhibits versatile tailored properties due to the missing-linker defects, opening up novel opportunities not only in absorption and catalysis, but also in controlling more challenging physical characteristics.7 Many efforts have been devoted to building 2D MOF nanosheets with various thicknesses, and exploring their chemical functionalities in a range of applications. The reports on Zr-MOF nanosheets are limited compared to many other MOF nanosheets, due to the strong metal–ligand bonds of Zr-MOFs.8
The combination of metal nanoparticles (MNPs) and MOFs for enhanced properties, particularly in catalysis has aroused great interest. MNPs have been incorporated into MOF crystal materials to develop MOF-based composites, generating a synergetic effect of individual components and offsetting their drawbacks for enhanced catalysis.9 As we know, the catalytic activity of MNPs is highly dependent on the nature of the support. However, the activity of 3D MOFs is often limited by the diffusion rates of substrates and products within the frameworks.10 2D materials can provide surfaces on which various steps of the reaction occur near active sites without geometric constraints. Therefore, incorporating 2D MOF nanosheets with other active components is an efficient way for the development of supported MNPs as catalysts.
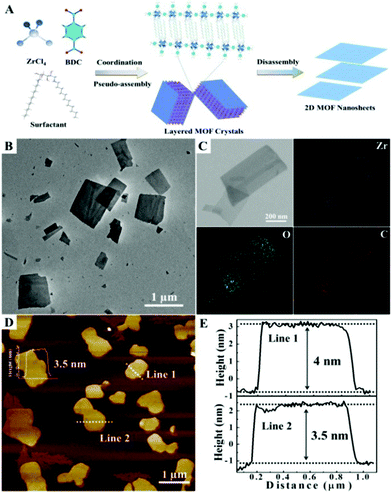
Herein, we demonstrate a facile strategy for the fabrication of ultrathin 2D MOF nanosheets via surfactant-mediated synthesis. The fabrication process is schematically illustrated in Fig. 1A. In consideration of the missing linker defects in UiO-66, we chose a bio-based surfactant sorbitol-alkylamine which possesses polyhydroxy and amine groups in the headgroup (SAAS-Cm, Scheme S1†), serving as competitive coordinating agents to chemically attach to the defects of Zr-BDC MOF, leading to the anisotropic growth of MOFs.11 Through precisely controlling the ratio of the surfactant and ligand, a pseudo-assembly process occurs in the MOF formation and intercalated MOFs are generated. The intercalated bulk MOFs assemble with the surfactant due to the interaction of the hydrophobic chains, which could weaken the interactions of interlayers and disassemble to form new ultrathin nanosheets (3–4 nm) in high yield (67%). Moreover, the thickness of the 2D nanosheets could be facilely tuned from 3 nm to 60 nm by varying the alkyl chain length of the applied surfactants. Furthermore, using the obtained ultrathin nanosheets as substrates to immobilize Ru nanoparticles, a very high efficiency in the hydrogenation of levulinic acid (LA) to γ-valerolactone (GVL) was realized compared with the corresponding hybrid 3D Zr-BDC MOF.
In the experiment, the bio-based surfactant SAAS-C12 was used to mediate the preparation of 2D Zr-BDC MOF nanosheets. And ultrathin nanosheets were easily obtained through mixing ZrCl4, BDC, acetic acid and SAAS-C12 into N,N-dimethyl formamide in one pot with heating at 120 °C for 24 h (see the experimental details in the ESI†). The transmission electron microscopy (TEM) and scanning electron microscopy (SEM) images of the 2D nanosheets are shown in Fig. 1B and S1,† illustrating the sheet-like morphology. The corresponding energy dispersive X-ray spectroscopy (EDX) elemental mapping analysis showed that Zr, O and C elements were well dispersed in the nanosheets (Fig. 1C). The 2D layered structure was further confirmed by atomic force microscopy (AFM), which exhibited a homogeneous thickness of ∼4 nm and a lateral size distribution of 0.5–2.0 μm, as shown in Fig. 1D and E.
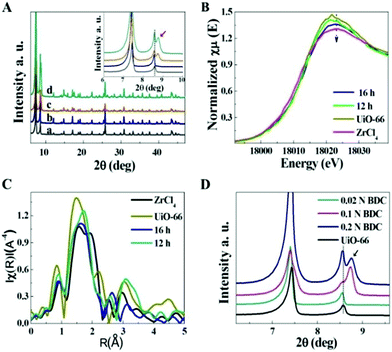
To reveal the possible formation mechanism of the nanosheets, we carried out experiments to investigate the growth process by examining the samples obtained at different reaction times using XRD and SEM. Meanwhile, the contrast experiment for the synthesis of Zr-MOF (UiO-66) in the absence of a surfactant was also performed parallelly, which did not lead to the exfoliation process, and the XRD spectrum matched well with that of reported UiO-66,12 as shown in Fig. 2A. However, the typical peaks of the Zr-BDC MOF in the XRD spectrum changed obviously with time in the presence of SAAS-C12. Before nanosheet formation (reaction time shorter than 12 h), the structure was almost the same as that of UiO-66. After prolonging the reaction time from 12 to 24 h, the intensity of peaks in the 2θ range of 6°–9° increased, suggesting an enhancement of crystallinity in the presence of the surfactant. Considering the XRD profiles, it was also found that Zr-BDC MOF samples obtained after 16 h generally presented broadening and splitting of the reflections, indicating the reduced particle size compared with UiO-66.13 Obviously, the formation of 2D nanosheets was observed by SEM and the bulk particles of the Zr-BDC MOF disappeared with increasing reaction time (Fig. S2†). X-ray absorption near-edge structure (EXAFS) spectroscopy is a very useful method for elucidating the local structure of inorganic cornerstones or inorganic backbones for different MOF materials.14 In the XANES region, a small decrease of the white line (first resonance after the edge) was observed compared to UiO-66, due to a partial loss of the ligand with the addition of the surfactant (Fig. 2B, 12 h). After the formation of 2D nanosheets (prolonging the reaction time to 16 h), there was a distinct decrease for the first resonance after the edge, indicating a greater loss of ligand. In the R-space (Fig. 2C), the two main peaks corresponding to oxygen and zirconium backscatters at 1.6 and 3.2 Å were higher shifted compared to that of UiO-66, which could be attributed to the new coordination with the surfactant. It follows that the surfactant provides a competitive ligand in the synthesis of 2D Zr-BDC MOF nanosheets. To verify this argument, the influence of the amount of surfactant on the structures of the Zr-BDC MOF was further investigated. As shown in Fig. 2D and S3,† the crystal structure and morphology were obviously affected by variable ratios of SAAS-C12 and BDC. The results indicated that 2D nanosheets could be formed only with an appropriate proportion of the surfactant and ligand, which also demonstrated the competitive coordination effects.
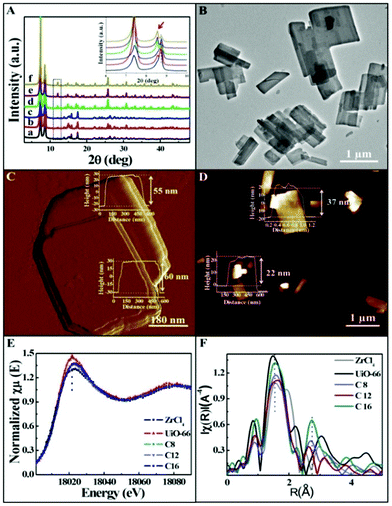
In consideration of the pseudo-assembly process, the alkyl chains of the surfactant played a key role in the formation of ultrathin 2D nanosheets. The properties of the nanosheets could be modified by changing the alkyl chain length. Thus, other surfactants of SAAS-Cm with different alkyl chains (m = 6, 8, 10, 14, 16) were used in this approach to synthesize 2D MOFs. Fortunately, longer alkyl chains of the surfactant could facilitate the formation of nanosheets with a thickness of 3–4 nm, such as SAAS-C14 and SAAS-C16. However, nanosheets of 28–60 nm were obtained using surfactants with shorter alkyl chains such as SAAS-C10 and SAAS-C8. And nanosheets failed to form when the alkyl chain length was shorter than C8. Remarkably, a broadening and splitting of the reflections in the XRD patterns occurred during ultrathin nanosheet formation when the alkyl chain length of the surfactant was longer than C10, accompanied by the appearance of a new peak at 12° (2θ), as shown in Fig. 3A. The TEM and AFM images clearly illustrated the variable thickness in 3 to 60 nm with varying alkyl chain lengths of the surfactant from C16 to C8 (Fig. 3B–D and S4†). It was found that almost the same reduction of the white line occurred in the XANES region (Fig. 3E), and the shift of peaks corresponding to Zr–O backscatters at 1.6 Å was not considerable with the changing alkyl chain length of the surfactant (Fig. 3F), indicative of little effect on the loss of ligand. In other words, the thickness of the nanosheets could be tuned by varying the length of the alkyl chain in a broad range without altering the crystalline structure. It is worth noting that the amount of surfactant together with the alkyl chain length plays a crucial role in the formation of ultrathin nanosheets.
Subsequently, to further validate the versatility of this method, other dicarboxylate ligands including electron-donating groups such as 2,5-dihydroxyterephthalic acid (BDC-(OH)2), 2-aminoterephthalic acid (BDC-NH2), 2-bromoterephthalic acid (BDC-Br), and dicarboxylate ligands with electron withdrawing groups such as nitroterephthalic acid (BDC-NO2) and 1,2,4-benzene tricarboxylic acid (BDC-COOH) were also employed to synthesize 2D MOF nanosheets in the presence of SAAS-C12. The results showed that 2D Zr-MOFs could be formed using dicarboxylate ligands with electron-donating groups, whose crystalline nature and sheet-like morphology were confirmed, as shown in Fig. S5A–D,† but nanosheets were not obtained using dicarboxylate ligands with electron withdrawing groups (Fig. S5E and 5F†).
2D nanosheets possess unique properties such as rich exposed active sites compared to the bulk MOF crystals. Inspired by the properties, ultrasmall Ru nanoparticles (NPs) supported on the nanosheets were prepared to form 2D MOF based hybrid nanomaterials (Ru/nanosheets). Ru nanoparticles with a size of sub-2 nm were uniformly distributed in the nanosheets, and the characterization is presented in Fig. S6.† To determine the properties, we studied the catalytic performance of Ru/nanosheets in the hydrogenation of levulinic acid (LA) to γ-valerolactone (GVL), which is an important reaction in biomass conversion,15 and the results are given in Table 1. For comparison, the catalytic performance of bulk MOF loaded with Ru NPs was also studied, and the characterization of Ru/bulk Zr-BDC is provided in Fig. S7.† Although the obtained Zr-BDC nanosheets had a smaller BET surface area (451 m2 g−1) than the bulk Zr-BDC MOF (872 m2 g−1), as shown in Fig. S8,† the Ru/Zr-BDC nanosheets demonstrated much better catalytic performances, which exhibited nearly 10 times higher activity than that of the bulk Zr-BDC MOF loaded with Ru NPs. The activity was also much higher than commercial Ru/C and the reported Ru/ZrO2 catalysts.16 The enhanced catalytic activity may result mainly from the abundant active sites exposed and avoidable geometric constraints in the 2D structure. Meanwhile, the reusability of Ru/Zr-BDC nanosheets was studied under the conditions of entry 2 of Table 1, and no change was observed in the catalytic performance as well as the structure and morphology after being used six times (Fig. S9†), indicating their excellent stability.
| Entry | T (°C) | t (h) | C (%) | Y (%) | TOFg (h−1) |
|---|---|---|---|---|---|
| a Reaction conditions: 15 mg catalyst, 0.86 mmol LA, 3 MPa H2. b Ru loading 1.66 wt%. c Ru loading 1.72 wt%. d Ref 17. e Commercial Ru/C catalyst with 5 wt% Ru loading. f Ref 16; metal loadings were determined by ICP-AES; the conversion (C) and yield (Y) were determined by gas chromatography. g TOF denotes moles of GVL per mole of Ru per hour. | |||||
| Ru/Zr-BDC nanosheetsb | 60 | 3 | >99 | >99 | 116 |
| Ru/Zr-BDC nanosheetsb | 90 | 1 | >99 | >99 | 349 |
| Ru/Zr-BDC bulkc | 90 | 3 | >99 | 28.0 | 38.1 |
| Zr-BDC nanosheets | 90 | 3 | <1 | <1 | — |
| UiO-66d | 130 | 3 | 28.1 | 12.2 | — |
| Ru/Ce | 90 | 3 | >99 | 90.8 | 34.7 |
| Ru/ZrO2f | 130 | 2 | 99.9 | 99.9 | 178 |
Conclusions
In conclusion, we have developed a strategy to synthesize ultrathin 2D Zr-MOF nanosheets with a thickness of 3–4 nm in high yield (67%). This strategy relies on the interaction of a surfactant with defects in MOF crystals, providing a pseudo-assembly process to exfoliate 3D MOFs and generate 2D nanosheets. Moreover, the thickness of the nanosheets could be controlled in the range of 3–60 nm by varying the hydrophobic chain length of the surfactant. The obtained Ru/nanosheet hybrid nanomaterials exhibited high catalytic activity in the hydrogenation of levulinic acid (LA) to γ-valerolactone (GVL) under mild conditions. The Ru NPs on the nanosheets were much more active than Ru NPs loaded on 3D bulk MOFs and commercial Ru/C catalysts. The enhanced performance of 2D hybrid nanomaterials mainly resulted from the well-dispersed Ru active sites, and surfaces on which various steps of the reaction occurred near active sites without geometric constraints. We believe that the method can also be used to prepare other 2D MOF nanosheets with controlled structures and functions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the support from the National Key Research and Development Program of China (2017YFA0403003, 2017YFA0403103) and Beijing Municipal Science & Technology Commission (Z181100004218004). The XANES experiments were carried out at Beijing Synchrotron Radiation Facility.Notes and references
- (a) K. S. Novoselov, V. I. Fal'ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, Nature, 2012, 490, 192 CrossRef CAS PubMed; (b) R. Lv, J. A. Robinson, R. E. Schaak, D. Sun, Y. Sun, T. E. Mallouk and M. Terrones, Acc. Chem. Res., 2015, 48, 56 CrossRef CAS PubMed; (c) C. Tan, X. Cao, X. Wu, Q. He, J. Yang, X. Zhang, J. Chen, W. Zhao, S. Han, G. Nam, M. Sindoro and H. Zhang, Chem. Rev., 2017, 117, 6225 CrossRef CAS PubMed.
- (a) M. T. Zhao, Y. Huang, Y. W. Peng, Z. Q. Huang, Q. L. Ma and H. Zhang, Chem. Soc. Rev., 2018, 47, 6267 RSC; (b) Y. Peng, Y. S. Li, Y. J. Ban, H. Jin, W. M. Jiao, X. L. Liu and W. S. Yang, Science, 2014, 346, 1356 CrossRef CAS PubMed.
- (a) R. Makiura, S. Motoyama, Y. Umemura, H. Yamanaka, O. Sakata and H. Kitagawa, Nat. Mater., 2010, 9, 565 CrossRef CAS PubMed; (b) L. Cao, Z. Lin, F. Peng, W. Wang, R. Huang, C. Wang, J. Yan, J. Liang, Z. Zhang, T. Zhang, L. Long, J. Sun and W. Lin, Angew. Chem., Int. Ed., 2016, 55, 4962 CrossRef CAS PubMed.
- M. Naguib and Y. Gogotsi, Acc. Chem. Res., 2015, 48, 128 CrossRef CAS PubMed.
- Y. Ding, Y. P. Chen, X. Zhang, L. Chen, Z. Dong, H. L. Jiang, H. Xu and H. C. Zhou, J. Am. Chem. Soc., 2017, 139, 9136 CrossRef CAS PubMed.
- (a) M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632 CrossRef CAS; (b) X. Liu, N. K. Demir, Z. T. Wu and K. Li, J. Am. Chem. Soc., 2015, 137, 6999 CrossRef CAS PubMed; (c) Y. Bai, Y. B. Dou, L. H. Xie, W. Rutledge, J. R. Li and H. C. Zhou, Chem. Soc. Rev., 2016, 45, 2327 RSC.
- (a) S. He, Y. F. Chen, Z. C. Zhang, B. Ni, W. Heb and X. Wang, Chem. Sci., 2016, 7, 7101 RSC; (b) Z. L. Fang, B. Bueken, D. E. D. Vos and R. A. Fischer, Angew. Chem., Int. Ed., 2015, 54, 7234 CrossRef CAS PubMed.
- T. He, B. Ni, S. M. Zhang, Y. Gong, H. Q. Wang, L. Gu, J. Zhuang, W. P. Hu and X. Wang, Small, 2018, 14, 1703929 CrossRef PubMed.
- (a) Q. H. Yang, Q. Xu and H. L. Jiang, Chem. Soc. Rev., 2017, 46, 4774 RSC; (b) S. He, Y. F. Chen, Z. C. Zhang, B. Ni, W. He and X. Wang, Chem. Sci., 2016, 7, 7101 RSC.
- Z. Z. Zheng, H. T. Xu, Z. L. Xu and J. P. A. Ge, Small, 2018, 14, 1702812 CrossRef PubMed.
- M. T. Zhao, Y. X. Wang, Q. L. Ma, Y. Huang, X. Zhang, J. F. Ping, Z. C. Zhang, Q. P. Lu, Y. F. Yu, H. Xu, Y. L. Zhao and H. Zhang, Adv. Mater., 2015, 27, 7372 CrossRef CAS PubMed.
- M. J. Katz, Z. J. Brown, Y. J. Colon, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449 RSC.
- H. R. Abid, H. M. Ang and S. B. Wang, Nanoscale, 2012, 4, 3089 RSC.
- (a) J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850 CrossRef PubMed; (b) L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700 CrossRef CAS.
- (a) A. M. R. Galletti, C. Antonetti, V. D. Luise and M. Martinelli, Green Chem., 2012, 14, 688 RSC; (b) D. Albani, Q. Li, G. Vilé, S. Mitchell, N. Almora-Barrios, P. T. Witte, N. López and J. Pérez-Ramírez, Green Chem., 2017, 19, 2361 RSC.
- B. Coskuner Filiz, E. S. Gnanakumar, A. Martínez-Arias, R. Gengler, P. Rudolf, G. Rothenberg and N. R. Shiju, Catal. Lett., 2017, 147, 1744 CrossRef CAS.
- A. H. Valekar, K.-H. Cho, S. K. Chitale, D.-Y. Hong, G.-Y. Cha, U.-H. Lee, D. W. Hwang, C. Serre, J.-S. Chang and Y. K. Hwang, Green Chem., 2016, 18, 4542 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8gc02835d |
| This journal is © The Royal Society of Chemistry 2019 |