A solvent-free catalytic protocol for the Achmatowicz rearrangement†
Guodong
Zhao
a and
Rongbiao
Tong
 *ab
*ab
aDepartment of Chemistry, The Hong Kong University of Science and Technology, Clearwater Bay, Kowloon, Hong Kong, China. E-mail: rtong@ust.hk; Fax: +(852)23581594; Tel: +(852) 23587357
bHKUST Shenzhen Research Institute, Shenzhen 518057, China
First published on 19th November 2018
Abstract
Reported here is the development of an environmentally friendly catalytic (KBr/oxone) and solvent-free protocol for the Achmatowicz rearrangement (AchR). Different from all previous methods is that the use of chromatographic alumina (Al2O3) allows AchR to proceed smoothly in the absence of any organic solvent and therefore considerably facilitates the subsequent workup and purification with minimal environmental impacts. Importantly, this protocol allows for scaling up (from milligram to gram), recycling of the Al2O3, and integrating with other reactions in a one-pot sequential manner.
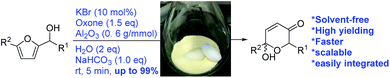
The Achmatowicz rearrangement (AchR) is the choice reaction for the synthesis of versatile dihydropyranone acetals,1–5 key synthetic precursors for functionalized tetrahydropyrans, dihydropyranones, oxidopyrylium, δ-lactones, and pyranoses.6–15 Therefore, the AchR continues to attract much attention from the synthetic and medicinal communities and to find wide applications in the total synthesis of natural products, including >17 natural products from our lab.16–25 The increasing importance of the AchR has led to the development of many new efficient methods by identifying new oxidants,26–41 and two oxidants, namely N-bromosuccinimide (NBS)42 and m-chloroperoxybenzoic acid (m-CPBA),43 were found to be among the most efficient ones. However, the use of these stoichiometric oxidants produces quantitative amounts of the organic byproduct of m-chlorobenzoic acid or succinimide, which usually requires immediate column chromatography for purification. To address this problem associated with the environmental concern from the organic byproducts, we previously developed a green catalytic protocol44 for the AchR by the identification of 5 mol% KBr as the catalyst and oxone as the stoichiometric terminal oxidant. In this new protocol, a THF/H2O (4/1) solution was identified as the most effective solvent system, partially due to the optimal solubility of both organic substrates (furfuryl alcohols) and inorganic reagents used (oxone and KBr). From the point of view of green chemistry, the THF/H2O solvent system is still not ideal and precludes subsequent transformations such as O-acetylation and O-Boc protection, in a one-pot manner. Herein, we report our recent efforts that led to the development of a solvent-free catalytic protocol for the AchR (Fig. 1). This new protocol represents the first example of a solvent-free catalytic AchR with broad substrate scope, and importantly enables us for the first time to further functionalize the AchR products in a one-pot sequential manner, which tremendously reduces the use of organic solvents in the liquid–liquid extraction workup and purification.
The development of a solvent-free45 process for organic reactions is a green chemistry approach because organic solvents are ecologically harmful and the largest contributors to the magnitude of the E factor.46 The additional advantages of solvent-free conditions include low cost and reduced reaction time.47,48 In particular, catalytic processes under solvent-free conditions greatly reduce the environmental impact and economic cost.49 Nevertheless, there are relatively few processes using catalysis under solvent-free conditions. This paucity is not unexpected because catalyst efficiency is usually very sensitive to the solvent properties and concentration.50 In this context, we sought a solid reaction medium that could support our solid catalyst (KBr) and oxidant (oxone) for the reaction with liquid substrates (furfuryl alcohols). A literature search and our initial experimental attempts led us to identify chromatographic neutral alumina (Al2O3, Merck KGaA, 70–230 mesh, and pH = 6.8–7.8) as the ideal medium.51,52
We first examined and optimized the AchR reaction of 1a using Al2O3 as the reaction medium (Table 1). To our delight, 82.7% yield of the AchR product 2a was obtained in only 5 min when 1a suspended in Al2O3 was mixed and stirred with KBr (10 mol%), oxone (1.5 equiv.), H2O (10 eq.) and NaHCO3 (6 eq.) at room temperature (entry 1). Control experiments suggested that Al2O3 (entry 2), H2O (entry 3), KBr (entry 4) and NaHCO3 (entry 5) were indispensable for good yields. Notably, the reaction time was significantly reduced from 30 min (THF/H2O/oxone/KBr) to 5 min (Al2O3/oxone/KBr). Further optimizations (entries 6–12) allowed for the substantial reduction of the quantity of oxone, Al2O3, NaHCO3 and H2O without compromising the high yield. The product 2a was isolated from the reaction mixture by simple filtration with ethyl acetate and the 1H-NMR spectrum of the concentrated filtrate (crude 2a) was very clean (Fig. 2). Therefore, this new protocol could eliminate the liquid–liquid extraction (and drying) used in the workup/purification of all prior methods.
| Entrya | Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (mg) b (mg) |
H2O (eq.)c | KBr (eq.)c | Oxone (eq.)c | NaHCO3 (eq.)c | Yieldd (2a, %) |
|---|---|---|---|---|---|---|
| a The reaction was carried out at room temperature: 1a (0.05 mmol) suspended in Al2O3 was mixed and stirred vigorously with H2O, KBr and NaHCO3 for 5 min. b Al2O3 was activated at 120 °C for 48 h. c The equivalent of different materials to 1a. d Yield was determined by 1H-NMR of the crude reaction mixture using CH2Br2 as the internal reference. | ||||||
| 1 | 100 | 10 | 0.1 | 1.5 | 6 | 82.7 |
| 2 | 0 | 10 | 0.1 | 1.5 | 6 | 20.7 |
| 3 | 100 | 0 | 0.1 | 1.5 | 6 | 0 |
| 4 | 100 | 10 | 0 | 1.5 | 6 | 31.9 |
| 5 | 100 | 10 | 0.1 | 1.5 | 0 | 50.7 |
| 6 | 100 | 10 | 0.1 | 1 | 6 | 81.4 |
| 7 | 100 | 10 | 0.1 | 0.6 | 6 | 55.9 |
| 8 | 30 | 10 | 0.1 | 1 | 6 | 82.3 |
| 9 | 20 | 10 | 0.1 | 1 | 6 | 56.7 |
| 10 | 30 | 2 | 0.1 | 1 | 6 | 84.3 |
| 11 | 30 | 1 | 0.1 | 1 | 6 | 78.4 |
| 12 | 30 | 2 | 0.1 | 1 | 1 | 91 |
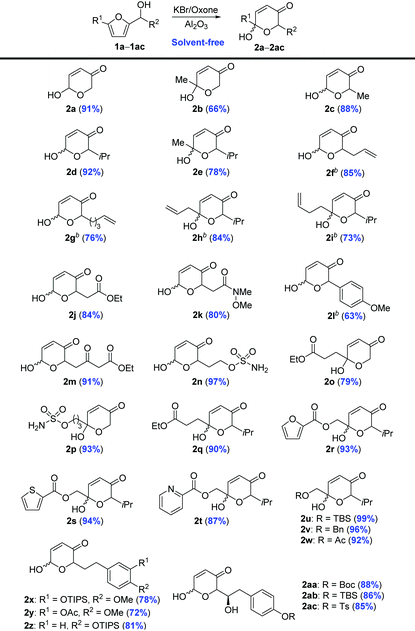
With the optimized conditions in hand, we set out to examine the substrate scope (Table 2). Generally, a variety of furfuryl alcohols could be employed for the AchR under these solvent-free conditions, which demonstrated comparable efficiency and generality to those solvent-mediated processes. Furfuryl alcohol derivatives bearing ester (1j, 1o, and 1w), alkene (1f–i), ketone (1m), Weinreb amide (1k), electron-rich arenes (1x–ac), and sulfonamide (1n and 1p) at the 2- or 5-position of furan were excellent substrates for the AchR to provide the corresponding dihydropyranone acetals in good to excellent yields (72–97%). Notably, there were no potentially competing side reactions, including arene bromination,53,54 alkene dibromination,55 and alcohol oxidation,56 all of which have been reported when the combination of oxone and stoichiometric alkali bromide was used. Heterocycles (1r–t) and the widely used protecting groups like TIPS (1z), TBS (1ab and 1u), Boc (1aa) and Bn (1v) were also well tolerated in our solvent-free, catalytic AchR to afford the desired products in excellent yields (81−96%).
| a Reaction conditions: Furfuryl alcohol (0.2 mmol), KBr (0.1 eq.), oxone (1.0 eq.), H2O (2.0 eq.), NaHCO3 (1.0 eq.), Al2O3 (120 mg), room temperature, 5 min, and isolated yield. b Reaction conditions: Furyl alcohol (0.2 mmol), KBr (0.4 eq.), oxone (2.0 eq.), H2O (8.0 eq.), NaHCO3 (2.0 eq.), Al2O3 (240 mg), room temperature, and 10 min. |
|---|
|
To demonstrate the additional value (environmental benefit) of our solvent-free catalytic protocol over the corresponding solvent-mediated process, we recycled the alumina (Al2O3) six times without any noticeable decrease of the efficiency for the gram-scale reaction (Table 3). It was noted, however, that more water and a longer reaction time (25–35 min) were needed to achieve the high yield for the recycling system. Remarkably, the recycling process was very simple (Fig. 3): after the completion of the reaction, the reaction mixture (solid) was transferred to a Buchner funnel (or a short chromatography column with cotton plug) for filtration washed with ethyl acetate; the filter cake containing Al2O3 and inorganic salts (K2SO4, KBr, and an excess of oxone) could be reused directly without further activation or purification. This easy recycling operation largely benefited from the use of oxone and KBr, which did not produce organic byproducts. Additionally, these solvent-free conditions did not require specialized equipment such as a ball mill/mixer used in mechanochemistry.57–59
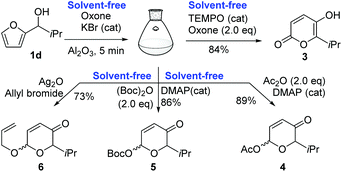
To further exploit the synthetic advantages of our protocol over solvent-assisted methods, we were attracted to explore the possibility of solvent-free, one-pot, sequential reactions. We selected four important transformations of the AchR products, i.e., oxidation, O-acetylation, O-Boc protection, and O-allylation, to examine such a possibility (Scheme 1). Oxidation of the AchR product 2d to 5-hydroxy-2-pyrone 3 was successfully achieved in a one-pot, solvent-free manner by using oxone/TEMPO (cat).60 Of note, KBr was used as the dual-role catalyst for both the AchR and subsequent oxidation. The sequential reactions were performed as follows: treatment of furfuryl alcohol 1d (0.2 mmol) suspended in Al2O3 (120 mg) with oxone (123 mg, 1.0 eq.), KBr (7.1 mg, 0.3 eq.), H2O (7.2 μL, 2.0 eq.), and NaHCO3 (16.8 mg, 1.0 eq.) and the reaction mixture was stirred vigorously at room temperature for 2 min, then to this reaction mixture were added oxone (246 mg, 2.0 eq.) and catalytic TEMPO (6.25 mg, 0.2 eq.), the solid reaction mixture was stirred for 10 min, and then filtered through a pad of silica gel with ethyl acetate to remove the solid byproduct and Al2O3, and concentrated in a vacuum, to provide 3 in 84% overall yield. This is remarkable because we have realized two solvent-free catalytic reactions in a one pot. Next, we derivatized 2d as acetate 4 and tert-butyl carbonate 5 in excellent yields (89% and 86% for two steps) by adding Ac2O/DMAP and (Boc)2O/DMAP, respectively, to the reaction vessel of the AchR. O-Acetylation and O-Boc protection are among the most used direct transformations of the AchR products since they afford substrates for O-glycosylation,61,62 [5 + 2]-cycloaddition, and C-arylation. Surprisingly, these transformations have been conventionally performed with the purified AchR products in a separate reaction vessel because the stoichiometric byproduct from the oxidant (m-CPBA, NBS, tert-BuOOH, Br2/MeOH, etc.) or aqueous reaction medium of the AchR did not permit a one-pot anhydrous acetylation. The final example of our sequential reactions was O-alkylation of 2d in 73% yield by adding Ag2O (92.7 mg, 2.0 eq.) and allyl bromide (69 μL, 4.0 eq.) to the solvent-free AchR mixture. Importantly, no aqueous workup was needed for all these two-step, one-pot, solvent-free reactions and the products (3–6) could be purified easily by transferring the solid reaction mixture to the top of the silica gel in a chromatography column eluting with hexane/ethyl acetate.
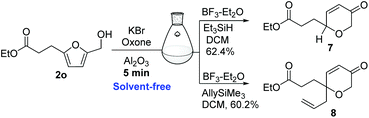
Nevertheless, we recognized that the one-pot, solvent-free conditions could not be extended to the AchR–Kishi reduction or AchR–Ferrier allylation. Since the Kishi reduction and Ferrier-type allylation have been widely used for the synthesis of tetrahydropyrans from the AchR products, we aimed to achieve the one-pot reaction goal (Scheme 2). Fortunately, we found that an excess of BF3–Et2O (8.0 eq.) could effect the Kishi reduction and Ferrier-type allylation of the AchR products in the same reaction vessel by adding solvent dichloromethane, Et3SiH (12.0 eq.) and allyltrimethylsilane (12.0 eq.), respectively. The presence of neutral alumina might reduce the BF3–Et2O acidity and thus an excess of BF3–Et2O was required for both the Kishi reduction and Ferrier allylation. It was also noted that without the addition of dichloromethane, the AchR product was rapidly decomposed by BF3–Et2O. Nevertheless, the one-pot process of two reactions still presented sufficient greenness, cost, and time advantages over the two-pot operation.
Conclusions
In summary, we have established a solvent-free catalytic protocol for the AchR, representing the greenest conditions to date. The efficiency and utility of this new protocol was demonstrated with (1) 29 examples, (2) six-times recycling of Al2O3 on a gram scale, (3) the successful integration of oxidation, O-acylation or O-allylation as a one-pot solvent-free reaction, and (4) one-pot AchR/Kishi reduction and AchR–Ferrier allylation. The most striking advantages of this protocol over all previous methods include no solvent, no stoichiometric organic byproduct generated from the oxidant, no liquid–liquid extraction in the workup, and no column chromatography for purification. Furthermore, this new protocol is operationally simple and does not require specialized equipment (ball mill). It is expected that this new green protocol will aid the application of the AchR in organic synthesis and drug discovery.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was financially supported by the Research Grant Council of Hong Kong (GRF 605113, 16311716, and 16303617) and the National Natural Science Foundation of China (NSFC 21472160 and 21772167).Notes and references
- O. Achmatowicz, P. Bukowski, B. Szechner, Z. Zwierzchowska and A. Zamojski, Tetrahedron, 1971, 27, 1973–1996 CrossRef CAS.
- A. K. Ghosh and M. Brindisi, RSC Adv., 2016, 6, 111564–111598 RSC.
- P. F. Koh and T. P. Loh, Green Chem., 2015, 17, 3746–3750 RSC.
- D. Noutsias, I. Alexopoulou, T. Montagnon and G. Vassilikogiannakis, Green Chem., 2012, 14, 601–604 RSC.
- J. Liu, J. Wu, J.-H. Fan, X. Yan, G. Mei and C.-C. Li, J. Am. Chem. Soc., 2018, 140, 5365–5369 CrossRef CAS PubMed.
- Y. Sridhar and P. Srihari, Eur. J. Org. Chem., 2013, 578–587 CrossRef CAS.
- L. Zhu, L. Song and R. Tong, Org. Lett., 2012, 14, 5892–5895 CrossRef CAS PubMed.
- A. Orue, U. Uria, E. Reyes, L. Carrillo and J. L. Vicario, Angew. Chem., Int. Ed., 2015, 54, 3043–3046 CrossRef CAS PubMed.
- H. Suga, T. Iwai, M. Shimizu, K. Takahashi and Y. Toda, Chem. Commun., 2018, 54, 1109–1112 RSC.
- R. H. Kaufman, C. M. Law, J. A. Simanis, E. L. Woodall, C. R. Zwick, H. B. Wedler, P. Wendelboe, C. G. Hamaker, J. R. Goodell, D. J. Tantillo and T. A. Mitchell, J. Org. Chem., 2018, 83, 9818–9838 CrossRef CAS PubMed.
- M. I. Thomson, G. S. Nichol and A. L. Lawrence, Org. Lett., 2017, 19, 2199–2201 CrossRef CAS PubMed.
- H.-Y. Wang, K. Yang, S. R. Bennett, S.-r. Guo and W. Tang, Angew. Chem., Int. Ed., 2015, 54, 8756–8759 CrossRef CAS PubMed.
- X. Liang, L. Zhou, L. Min, W. Ye, W. Bao, W. Ma, Q. Yang, F. Qiao, X. Zhang and C.-S. Lee, J. Org. Chem., 2017, 82, 3463–3481 CrossRef CAS PubMed.
- C. Zhao, F. Li and J. Wang, Angew. Chem., Int. Ed., 2015, 55, 1820–1824 CrossRef PubMed.
- R. S. Babu, Q. Chen, S.-W. Kang, M. Zhou and G. A. O'Doherty, J. Am. Chem. Soc., 2012, 134, 11952–11955 CrossRef CAS PubMed.
- Z. Li, F. C. Ip, N. Y. Ip and R. Tong, Chem. – Eur. J., 2015, 21, 11152–11157 CrossRef CAS PubMed.
- Z. Li and R. Tong, J. Org. Chem., 2017, 82, 1127–1135 CrossRef CAS PubMed.
- J. Ren, Y. Liu, L. Song and R. Tong, Org. Lett., 2014, 16, 2986–2989 CrossRef CAS PubMed.
- J. Ren and R. Tong, J. Org. Chem., 2014, 79, 6987–6995 CrossRef CAS PubMed.
- J. Ren, J. Wang and R. Tong, Org. Lett., 2015, 17, 744–747 CrossRef CAS PubMed.
- Z. Li and R. Tong, Synthesis, 2016, 48, 1630–1636 CrossRef CAS.
- L. Zhu and R. Tong, Synlett, 2015, 26, 1643–1648 CrossRef CAS.
- L. Zhu, Y. Liu, R. Ma and R. Tong, Angew. Chem., Int. Ed., 2015, 54, 627–632 CAS.
- L. Zhu and R. Tong, Org. Lett., 2015, 17, 1966–1969 CrossRef CAS PubMed.
- Z. Li, T.-F. Leung and R. Tong, Chem. Commun., 2014, 50, 10990–10993 RSC.
- B. M. Adger, C. Barrett, J. Brennan, M. A. McKervey and R. W. Murray, J. Chem. Soc., Chem. Commun., 1991, 1553–1554 RSC.
- C. Dominguez, A. G. Csáky and J. Plumet, Tetrahedron Lett., 1990, 31, 7669–7670 CrossRef CAS.
- G. Piancatelli, A. Scettri and M. D'Auria, Tetrahedron Lett., 1977, 18, 2199–2200 CrossRef.
- T.-L. Ho and S. G. Sapp, Synth. Commun., 1983, 13, 207–211 CrossRef CAS.
- J. Wahlen, B. Moens, D. E. De Vos, P. L. Alsters and P. A. Jacobs, Adv. Synth. Catal., 2004, 346, 333–338 CrossRef CAS.
- A. De Mico, R. Margarita and G. Piancatelli, Tetrahedron Lett., 1995, 36, 3553–3556 CrossRef CAS.
- D. Noutsias, A. Kouridaki and G. Vassilikogiannakis, Org. Lett., 2011, 13, 1166–1169 CrossRef CAS PubMed.
- T. Shono and Y. Matsumura, Tetrahedron Lett., 1976, 17, 1363–1364 CrossRef.
- D. Thiel, D. Doknić and J. Deska, Nat. Commun., 2014, 5, 5278 CrossRef CAS PubMed.
- M. B. Plutschack, P. H. Seeberger and K. Gilmore, Org. Lett., 2017, 19, 30–33 CrossRef CAS PubMed.
- C. Wei, R. Zhao, Z. Shen, D. Chang and L. Shi, Org. Biomol. Chem., 2018, 16, 5566–5569 RSC.
- J. Finlay, M. A. McKervey and H. Q. N. Gunaratne, Tetrahedron Lett., 1998, 39, 5651–5654 CrossRef CAS.
- T. Oishi, M. Suzuki, K. Watanabe and M. Murata, Tetrahedron Lett., 2006, 47, 3975–3978 CrossRef CAS.
- F. Blume, P. Sprengart and J. Deska, Synlett, 2018, 29, 1293–1296 CrossRef CAS.
- E. Fernández-Fueyo, S. H. H. Younes, S. V. Rootselaar, R. W. M. Aben, R. Renirie, R. Wever, D. Holtmann, F. P. J. T. Rutjes and F. Hollmann, ACS Catal., 2016, 6, 5904–5907 CrossRef.
- It was noted that an oxidant-free method has been reported for the synthesis of pyranone acetals from 2,5-bis-hydroxymethylfuran, see: A. Gelmini, S. Albonetti, F. Cavani, C. Cesari, A. Lolli, V. Zanotti and R. Mazzoni, Appl. Catal., B, 2016, 180, 38–43 CrossRef CAS.
- E. A. Couladouros and M. P. Georgiadis, J. Org. Chem., 1986, 51, 2725–2727 CrossRef CAS.
- R. Laliberte, G. Medawar and Y. Lefebvre, J. Med. Chem., 1973, 16, 1084–1089 CrossRef CAS PubMed.
- Z. Li and R. Tong, J. Org. Chem., 2016, 81, 4847–4855 CrossRef CAS PubMed.
- M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machado, Chem. Rev., 2009, 109, 4140–4182 CrossRef CAS PubMed.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- K. Tanaka and F. Toda, Chem. Rev., 2000, 100, 1025–1074 CrossRef CAS PubMed.
- J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC.
- A. Nasser and U. Mingelgrin, Appl. Clay Sci., 2012, 67–68, 141–150 CrossRef CAS.
- P. J. Walsh, H. Li and C. A. de Parrodi, Chem. Rev., 2007, 107, 2503–2545 CrossRef CAS PubMed.
- P. J. Kropp, G. W. Breton, J. D. Fields, J. C. Tung and B. R. Loomis, J. Am. Chem. Soc., 2000, 122, 4280–4285 CrossRef CAS.
- J. D. Fields and P. J. Kropp, J. Org. Chem., 2000, 65, 5937–5941 CrossRef CAS PubMed.
- N. Narender, P. Srinivasu, M. Ramakrishna Prasad, S. J. Kulkarni and K. V. Raghavan, Synth. Commun., 2002, 32, 2313–2318 CrossRef CAS.
- B. V. Tamhankar, U. V. Desai, R. B. Mane, P. P. Wadgaonkar and A. V. Bedekar, Synth. Commun., 2001, 31, 2021–2027 CrossRef CAS.
- G.-W. Wang and J. Gao, Green Chem., 2012, 14, 1125–1131 RSC.
- B.-S. Koo, C. K. Lee and K.-J. Lee, Synth. Commun., 2002, 32, 2115–2123 CrossRef CAS.
- J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC.
- G. D. Bo, Chem. Sci., 2018, 9, 15–21 RSC.
- J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19 CrossRef CAS PubMed.
- C. Bolm, A. S. Magnus and J. P. Hildebrand, Org. Lett., 2000, 2, 1173–1175 CrossRef CAS PubMed.
- R. S. Babu and G. A. O'Doherty, J. Am. Chem. Soc., 2003, 125, 12406–12407 CrossRef CAS PubMed.
- R. S. Babu, M. Zhou and G. A. O'Doherty, J. Am. Chem. Soc., 2004, 126, 3428–3429 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8gc03030h |
| This journal is © The Royal Society of Chemistry 2019 |