A one-pot catalyst/external oxidant/solvent-free cascade approach to pyrimidines via a 1,5-hydride transfer†
Mohit L.
Deb
 *,
Paran J.
Borpatra
*,
Paran J.
Borpatra
 and
Pranjal K.
Baruah
and
Pranjal K.
Baruah
 *
*
Department of Applied Sciences, GUIST, Gauhati University, Guwahati-781014, Assam, India. E-mail: mohitdd.deb@gmail.com; baruah.pranjal@gmail.com; Fax: +91-8876998905; Tel: +91-8876998905
First published on 5th December 2018
Abstract
A cascade 3-component reaction of 6-aminouracil, aldehyde and tetrahydroisoquinolines under heating in the absence of catalyst, external oxidant and solvent is performed. The reaction constructs a new pyrimidine ring by the functionalization of the C–H bond adjacent to nitrogen through a 1,5-hydride shift. No chromatographic techniques were required to purify the products.
C–H functionalization is a growing field that has continued to draw the attention of enthusiastic organic chemists for the last two decades due to it not having the prefunctionalization requirements of substrates, being step economical, being directive, and having environment-friendly features.1 The functionalization of the α-sp3 C–H bonds of tertiary amines is very common and much more studied because of its massive importance. A lot of progress has been made by various research groups to extend the scope and to develop new synthetic strategies for this particular reaction.2 Salts of many transition metals such as Cu, Fe, Ru, Rh, V, and Co are usually used as catalysts along with various oxidants for this purpose.3 Molecular iodine or its salts has also been used to catalyze the reaction.4 Brønsted acids were recently reported to catalyze the reaction in the presence of an oxidant.5 Very recently, our group reported one such C–H functionalization in the presence of only molecular oxygen in H2O.6
Redox-neutral processes which operate via hydride transfer mechanisms have recently attracted the attention of researchers due to their simple mode of operation, low cost, and step and atom economy.7 This technique was also utilized for the functionalization of the α-sp3 C–H bonds of tertiary amines.8 However, most of these synthetic methods require pre-structurally arranged amine substrates in the presence of a catalyst, in which various chiral nitrogen heterocycles have been synthesized.8a,c,f–j
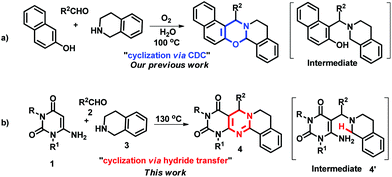
Inspired by our recently published work on the synthesis of 1,3-oxazines (Scheme 1a), we planned to construct a pyrimidine nucleus under identical reaction conditions to our previous report, but failed. Pyrimidines and pyrimido[4,5-d]pyrimidines are highly useful bio-active ingredients.9 Many procedures are available in the literature for their synthesis, however long synthetic steps, toxic catalysts/solvents and low yields make them less attractive.10 Multi-component one-pot processes have gained much attention in synthetic chemistry due to their advantages of reduced reaction steps/waste/cost, inherent atom economy, simpler procedures, and energy savings.11 Many of the solvents used in the laboratory and for industrial purposes are volatile organic compounds (VOCs), which are unsafe for human beings and the surroundings.12 Therefore, a solvent-free synthesis is now in high demand. Moreover, the use of external oxidants sometimes generates unwanted oxidation products, which can be avoided by using aerial oxidation.13 Nowadays, catalyst-free synthetic methods have attracted much attention because of their lower costs, mild conditions, reduced pollution and ease of purification.14 On top of that, avoiding all types of chromatographic purification adds additional advantages in terms of cost and time savings.15 Keeping these advantages in mind, here we disclose a one-pot cascade reaction of 6-aminouracil, aldehyde and tetrahydroisoquinolines (THIQs).
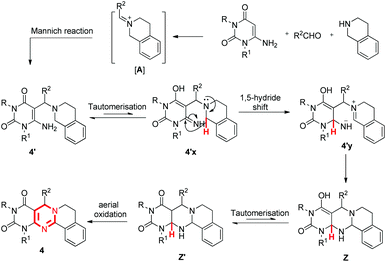
The reaction constructs a new pyrimidine ring in the absence of any catalyst, external oxidant and solvent under heating which proceeds through the cyclization of intermediate 4′ by a 1,5-hydride shift (Scheme 1b). The cyclization of this type of intermediate usually requires a catalyst and/or oxidant and therefore, this method has advantages over previously known ones.16 The products of the reactions were isolated in pure form only by trituration with ethanol and no chromatographic techniques were required. To the best of our knowledge, this is the first example of C–H functionalization adjacent to a tertiary nitrogen atom in which no external agents are required.
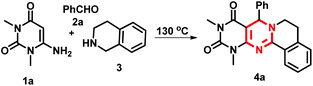
We started our study with the 3-component reaction of 1,3-dimethyl-6-aminouracil (1a), benzaldehyde (2a) and THIQ (3) in the presence of an oxygen balloon in water under reflux conditions (entry 1, Table 1). However, the reaction completely failed to form the product. We then performed the reaction in different solvents like ethanol, toluene, p-xylene, and DMF in the presence of molecular oxygen. However, only DMF produced an average yield after 4 h of heating at 120 °C (entry 6, Table 1). Very interestingly, when we performed the reaction in open air in DMF, we obtained the same yield as in the presence of molecular oxygen (entry 7, Table 1). This result tells us that external oxygen is not required for the reaction. We further attempted the reaction in DMSO and acetonitrile in open air under heating, but afforded a poor yield (entries 8–9, Table 1). However, the reaction under neat conditions gave us an excellent yield only after 2 h of stirring at 130 °C, which we considered as the optimal conditions for the reaction (entry 11, Table 1). At room temperature the reaction only formed the intermediate 4′a (27%) and we recovered the unreacted 6-aminouracil 1a (entry 13, Table 1).
| Entry | Oxidant | Solvent | Temp. (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| a Unless otherwise mentioned, all of the reactions were performed using 1a (1 mmol, 155 mg), 2a (1 mmol, 106 mg) and 3a (1 mmol, 133 mg) under neat conditions under heating. The products were purified by trituration with ethanol and the yields are for the isolated products. b At room temperature the reaction gave 27% of the intermediate 4′. | |||||
| 1 | O2 (1 atm) | H2O | 100 °C | 12 h | — |
| 2 | O2 (1 atm) | EtOH | 90 °C | 12 h | 10 |
| 3 | O2 (1 atm) | Toluene | 110 °C | 12 h | — |
| 4 | O2 (1 atm) | p-Xylene | 130 °C | 12 h | — |
| 5 | O2 (1 atm) | DMF | 120 °C | 12 h | 58 |
| 6 | O2 (1 atm) | DMF | 120 °C | 4 h | 65 |
| 7 | Open air | DMF | 120 °C | 4 h | 65 |
| 8 | Open air | DMSO | 120 °C | 4 h | 15 |
| 9 | Open air | CH3CN | 90 °C | 4 h | Trace |
| 10 | Open air | Neat | 120 °C | 4 h | 80 |
| 11 | Open air | Neat | 130 °C | 2 h | 92 |
| 12 | Open air | Neat | 140 °C | 2 h | 84 |
| 13b | Open air | Neat | RT | 12 h | — |
To examine the substrate scope we used a variety of aldehydes in the reaction. We observed that aromatic aldehydes and aromatic heterocyclic aldehydes both gave excellent yields of the product. However, aliphatic aldehydes such as isobutyraldehyde and formaldehyde gave a slightly lower yield (4o–p, Scheme 2). We did not notice much difference in the yield of 4, obtained by electron-donating and -withdrawing aldehydes. When we used -o, -m and -p substituted aldehydes for the reaction, an excellent yield of 4 was obtained in each case (4b–d, Scheme 2). We then treated various N-substituted/unsubstituted 6-aminouracils and observed relatively lower yields in the case of 1,3-dipropyl-6-aminouracil (4q–t, Scheme 2). On the other hand, 1-methyl-6-aminouracils and unsubstituted-6-aminouracils produced a higher yield of the products (4u–z, Scheme 2). When we replaced THIQ with tryptoline, an indole fused derivative of THIQ, we obtained a comparatively lower yield of 4 (4z1 & 4z2, Scheme 2).
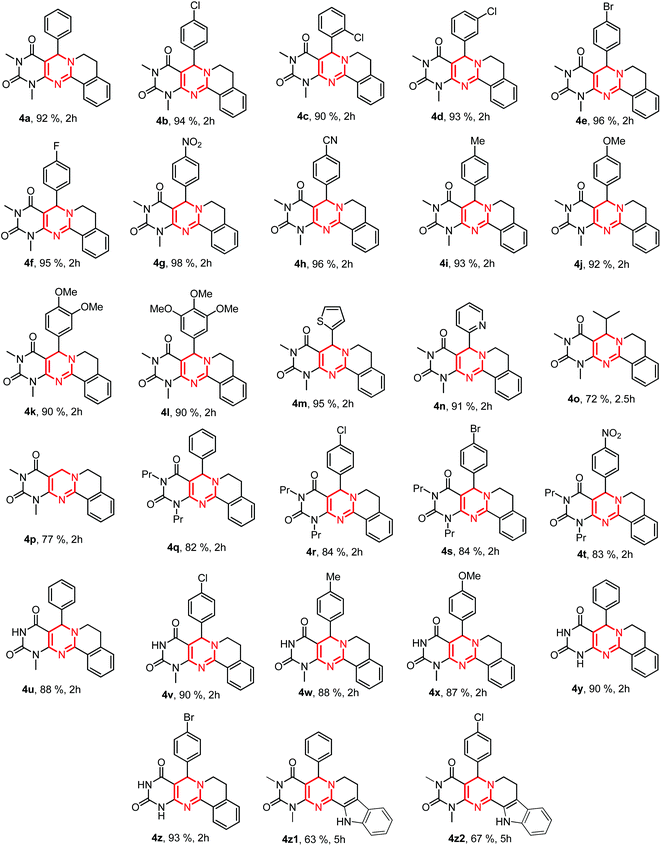 | ||
| Scheme 2 Synthesis of pyrimido[4,5-d]pyrimidines. The products were purified by trituration with ethanol except 4q–t in which column chromatography was required for purification. | ||
In our previous report, we isolated both the regioisomers of the intermediate Mannich product,6 which we did not observe here and therefore, the reaction was clean. The structures of all of the products were established through spectroscopic analysis as well as single crystal X-ray studies of a few compounds (Fig. 1). To scale-up the reaction process, we carried out the first reaction in a 10 g scale under the optimized conditions and obtained a 97% yield of 4a only after trituration with ethanol.
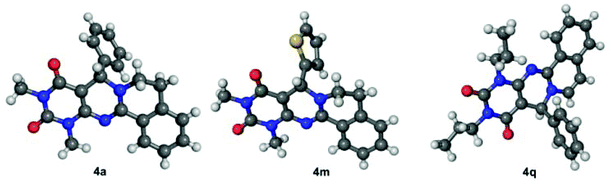 | ||
| Fig. 1 X-ray structures of 4a, 4m and 4q. CCDC no. for 4a is 1850632, 4m is 1850625 and 4q is 1850631.† | ||
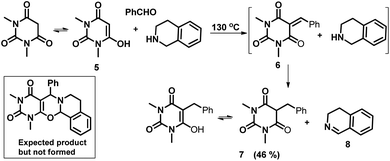
To extend the scope of the reaction further, we performed a reaction using 1,3-dimethylbarbituric acid assuming that it would produce compounds like pyrimido-1,3-oxazines. However, we didn't obtain the expected one and instead, the reduced compound of the Knoevenagel product was isolated (Scheme 3). THIQ reduced the unsaturated double bond to a single bond and consequently isolated DHIQ (8) from the reaction mixture. The reducing properties of THIQ were reported earlier by Seidel et al., in which they observed the reduction of iminium ions by THIQ.7d One of the possible explanations they proposed was an intermolecular hydride transfer from THIQ to the iminium ions with the concurrent oxidation of the former to DHIQ. Here, we for the first time observed that THIQ can even reduce the activated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond. To understand the route of the reaction, we first synthesized compound 6
C double bond. To understand the route of the reaction, we first synthesized compound 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 which was then reacted with THIQ under the same reaction conditions. In both of the reactions, we obtained the product 7.
17 which was then reacted with THIQ under the same reaction conditions. In both of the reactions, we obtained the product 7.
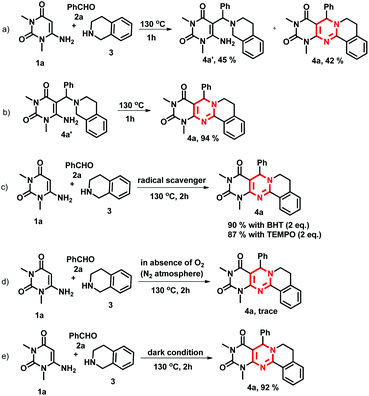
To study the mechanism of the formation of 4, we performed some control experiments (Scheme 4). We isolated compound 4a′ along with 4a when the reaction was stopped after 1 h (Scheme 4a). When 4a′ was heated at 130 °C, it produced 4a in excellent yield (Scheme 4b). Therefore, we believe that the reaction proceeded through 4′ which was formed from the 3-component Mannich reaction. Since, in many instances, the α-C–H functionalization/cyclization of tertiary nitrogen proceeds via a free radical route,18 we carried out the reaction by adding radical scavengers. In the presence of BHT and TEMPO the reaction gave 90% and 87% of 4a, respectively, under the optimized reaction conditions (Scheme 4c). These reactions ruled out the possibility of a radical pathway for the cyclization step. As our reactions were performed in open air, we thought aerial oxygen might play some crucial role in the cyclization. When the reaction was carried out under the careful exclusion of air/oxygen (in a N2 atmosphere), the reaction gave a trace amount of 4a (Scheme 4d). This reaction inferred that oxygen is essential for the formation of the product 4, however an external supply of oxygen is not required. Next, we attempted a reaction under dark conditions bearing in mind that light may promote the reaction, but did not observe any decrease in the yield of 4a (Scheme 4e). Therefore, based on our control experiments and relevant reports8 a tentative mechanism for the formation of 4 is proposed (Scheme 5).
The 3-component reaction of 6-aminouracil, aldehyde and THIQ gave the Mannich product 4′via an iminium ion [A]. Under the reaction conditions, 4′ may tautomerise to 4′x, which undergoes a 1,5-hydride shift to form a zwitterion 4′y. This molecule then cyclizes to the intermediate Z′ which finally oxidises to the desired product 4. The final oxidation probably takes place by aerial oxygen. Further work is under way to develop a better understanding of the mechanism.
Conclusions
In conclusion, here we have developed a 3-component one-pot coupling of 6-aminouracil, aldehyde and tetrahydroisoquinolines in which a new pyrimidine ring is constructed. The reaction proceeds through the cyclization of the Mannich intermediate. The reaction does not necessitate any catalyst, external oxidant and solvent. No inert atmosphere is required for the reaction and it could be scaled up with an excellent yield. Furthermore, the reaction avoids chromatographic techniques for the purification of the products. Further studies to evaluate the bioactivity of the synthesized compounds are in progress in our collaborating laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MLD is thankful to the Science and Engineering Research Board (SERB), India, (Grant No. SB/FT/CS-073/2014) for the financial support. PKB is also thankful to SERB, India (Grant No. SB/FT/CS-100/2012) for the financial support. PJB acknowledges MHRD, Govt of India, for the research fellowship under the TEQIP-III Project. We acknowledge the Sophisticated Analytical Instrumentation Facility (SAIF), GU, for the use of the single-crystal X-ray diffractometer. The authors acknowledge Dr Ranjit Thakuria, Dept. of Chemistry, Gauhati University for the X-ray structure analysis.Notes and references
- (a) M. Klussmann and D. Sureshkumar, Synthesis, 2011, 353 CrossRef CAS; (b) W.-J. Yoo and C.-J. Li, Top. Curr. Chem., 2010, 292, 281 CrossRef; (c) C. S. Yeung and V. M. Dong, Chem. Rev., 2011, 111, 1215 CrossRef CAS PubMed; (d) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC; (e) C.-J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (f) Z. Shi and F. Glorius, Angew. Chem., Int. Ed., 2012, 124, 9354 CrossRef; (g) C. Zhang, C. Tang and N. Jiao, Chem. Soc. Rev., 2012, 41, 3464 RSC; (h) Z. Li, D. S. Bohle and C.-J. Li, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8928 CrossRef CAS.
- (a) K. Alagiri and K. R. Prabhu, Org. Biomol. Chem., 2012, 10, 835 RSC; (b) K. R. Campos, Chem. Soc. Rev., 2007, 36, 1069 RSC; (c) X. Z. Shu, X. F. Xia, Y. F. Yang, K. G. Ji, X. Y. Liu and Y. M. Liang, J. Org. Chem., 2009, 74, 7464 CrossRef CAS PubMed; (d) G. Jiang, J. Chen, J. S. Huang and C. M. Che, Org. Lett., 2009, 11, 4568 CrossRef CAS PubMed; (e) K. M. Jones and M. Klussmann, Synlett, 2012, 23, 159 CAS; (f) F. Kienzle, Tetrahedron Lett., 1983, 24, 2213 CrossRef CAS; (g) C. L. Mathis, B. M. Gist, C. K. Frederickson, K. M. Midkiff and C. C. Marvin, Tetrahedron Lett., 2013, 54, 2101 CrossRef CAS; (h) C.-K. Chen, A. G. Hortmann and M. R. Marzabadi, J. Am. Chem. Soc., 1988, 110, 4829 CrossRef CAS; (i) J. Xuan, Z.-J. Feng, S.-W. Duan and W.-J. Xiao, RSC Adv., 2012, 2, 4065 RSC; (j) G. Pandey, G. Kumaraswamy and P. Y. Reddy, Tetrahedron, 1992, 48, 8295 CrossRef CAS; (k) M. Okimoto, K. Ohashi, H. Yamamori, S. Nishikawa, M. Hoshi and T. Yoshida, Synthesis, 2012, 44, 1315 CrossRef CAS; (l) M. L. Deb, C. D. Pegu, P. J. Borpatra and P. K. Baruah, RSC Adv., 2016, 6, 40552 RSC.
- (a) O. Basle, N. Borduas, P. Dubois, J. M. Chapuzet, T.-H. Chan, J. Lessard and C.-J. Li, Chem. – Eur. J., 2010, 16, 8162 CrossRef CAS PubMed; (b) W. Han and A. R. Ofial, Chem. Commun., 2009, 45, 5024 RSC; (c) W. Han, P. Mayer and A. R. Ofial, Adv. Synth. Catal., 2010, 352, 1667 CrossRef CAS; (d) S.-I. Murahashi and D. Zhang, Chem. Soc. Rev., 2008, 37, 1490 RSC; (e) M. Rueping, C. Vila, R. M. Koenigs, K. Poscharny and D. C. Fabry, Chem. Commun., 2011, 47, 2360 RSC; (f) A. J. Catino, J. M. Nichols, B. J. Nettles and M. P. Doyle, J. Am. Chem. Soc., 2006, 128, 5648 CrossRef CAS PubMed; (g) S. Singhal, S. L. Jain and B. Sain, Chem. Commun., 2009, 45, 2371 RSC; (h) M. Ghobrial, K. Harhammer, M. D. Mihovilovic and M. Schnurch, Chem. Commun., 2010, 46, 8836 RSC; (i) A. Sud, D. Sureshkumar and M. Klussmann, Chem. Commun., 2009, 45, 3169 RSC; (j) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 3672 CrossRef CAS PubMed; (k) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 6968 CrossRef CAS PubMed.
- (a) U. Kloeckner, N. M. Weckenmann and B. J. Nachtsheim, Synlett, 2012, 97 CAS; (b) Z.-Q. Lao, W.-H. Zhong, Q.-H. Lou, Z.-J. Li and X.-B. Meng, Org. Biomol. Chem., 2012, 10, 7869 RSC; (c) Y. Yan, Y. Zhang, Z. Zha and Z. Wang, Org. Lett., 2013, 15, 2274 CrossRef CAS PubMed; (d) J. Zhao, P. Li, C. Xia and F. Li, Chem. Commun., 2014, 50, 4751 RSC; (e) I. Andivelu and S. Gandhesiri, J. Org. Chem., 2014, 79, 4984 CrossRef PubMed; (f) L.-T. Li, H.-Y. Li, L.-J. Xing, L.-J. Wen, P. Wang and B. Wang, Org. Biomol. Chem., 2012, 10, 9519 RSC; (g) Z. Ningning, C. Ran, Z.-N. Daisy, D. Yunfei and Z. Kang, J. Org. Chem., 2014, 79, 10581 CrossRef; (h) L.-T. Li, J. Huang, H.-Y. Li, L.-J. Wen, P. Wang and B. Wang, Chem. Commun., 2012, 48, 5187 RSC; (i) L. Le, D. Liang, Z.-N. Daisy and D. Yunfei, RSC Adv., 2015, 5, 29774 RSC; (j) T. Froehr, C. P. Sindlinger, U. Kloeckner, P. Finkbeiner and B. J. Nachtsheim, Org. Lett., 2011, 13, 3754 CrossRef CAS PubMed; (k) M. L. Deb, C. D. Pegu, P. J. Borpatra and P. K. Baruah, Tetrahedron Lett., 2016, 57, 5479 CrossRef CAS.
- (a) B. Schweitzer-Chaput, J. Demaerel, H. Engler and M. Klussmann, Angew. Chem., Int. Ed., 2014, 53, 8737 CrossRef CAS PubMed; (b) A. Pinter, A. Sud, D. Sureshkumar and M. Klussmann, Angew. Chem., Int. Ed., 2010, 49, 5004 CrossRef CAS PubMed; (c) W. Chen and D. Seidel, Org. Lett., 2016, 18, 1024 CrossRef CAS; (d) S. Lai, X. Ren, J. Zhao, Z. Tang and G. Li, Tetrahedron Lett., 2016, 57, 2957 CrossRef CAS; (e) B. Schweitzer-Chaput and M. Klussmann, Eur. J. Org. Chem., 2013, 666 CrossRef CAS.
- M. L. Deb, C. D. Pegu, P. J. Borpatra, P. J. Saikia and P. K. Baruah, Green Chem., 2017, 19, 4036 RSC.
- (a) S. Mahato, M. A. Haque, S. Dwaria and C. K. Jana, RSC Adv., 2014, 4, 46214 RSC; (b) B. M. Trost and R. J. Kulaweic, J. Am. Chem. Soc., 1993, 115, 2027 CrossRef CAS; (c) M. T. Richers, M. Breugst, A. Y. Platonova, A. Ullrich, A. Dieckmann, K. N. Houk and D. Seidel, J. Am. Chem. Soc., 2014, 136, 6123 CrossRef CAS PubMed; (d) D. A. Evans and A. H. Hoveyda, J. Am. Chem. Soc., 1990, 112, 6447 CrossRef CAS; (e) L. Ma, A. Paul, M. Breugst and D. Seidel, Chem. – Eur. J., 2016, 22, 18179 CrossRef CAS PubMed.
- (a) Y. K. Kang, S. M. Kim and D. Y. Kim, J. Am. Chem. Soc., 2010, 132, 11847 CrossRef CAS PubMed; (b) A. Dieckmann, M. T. Richers, A. Y. Platonova, C. Zhang, D. Seidel and K. N. Houk, J. Org. Chem., 2013, 78, 4132 CrossRef CAS PubMed; (c) Y.-P. He, Y.-L. Du, S.-W. Luo and L.-Z. Gong, Tetrahedron Lett., 2011, 52, 7064 CrossRef CAS; (d) C. Zhang, C. K. De, R. Mal and D. Seidel, J. Am. Chem. Soc., 2008, 130, 416 CrossRef CAS PubMed; (e) C. Zhang, S. Murarka and D. Seidel, J. Org. Chem., 2009, 74, 419 CrossRef CAS PubMed; (f) Y.-Y. Han, W.-Y. Han, X. Hou, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2012, 14, 4054 CrossRef CAS PubMed; (g) K. Mori, K. Ehara, K. Kurihara and T. Akiyama, J. Am. Chem. Soc., 2011, 133, 6166 CrossRef CAS; (h) W. Cao, X. Liu, W. Wang, L. Lin and X. Feng, Org. Lett., 2011, 13, 600 CrossRef CAS PubMed; (i) L. Chen, L. Zhang, J. Lv, J.-P. Cheng and S. Luo, Chem. – Eur. J., 2012, 18, 8891 CrossRef CAS PubMed; (j) S. Murarka, I. Deb, C. Zhang and D. Seidel, J. Am. Chem. Soc., 2009, 131, 13226 CrossRef CAS PubMed; (k) M. Asikainen, O. Jauhiainen, O. Aaltonen and A. Harlin, Green Chem., 2013, 15, 3230 RSC.
- (a) A. S. Jones, J. R. Sayers, R. T. Walker and E. D. Clercq, J. Med. Chem., 1988, 31, 268 CrossRef CAS PubMed; (b) H. Mitsuya, R. Yarchoan and S. Broder, Science, 1990, 249, 1533 CrossRef CAS; (c) R. Pontikis and C. Monneret, Tetrahedron Lett., 1994, 35, 4351 CrossRef CAS; (d) N. J. Curtin, H. C. Barlow, K. J. Bowman, A. H. Calvert, R. Davison, B. T. Golding, B. Huang, P. J. Loughlin, D. R. Newell, P. G. Smith and R. J. Griffin, J. Med. Chem., 2004, 47, 4905 CrossRef CAS PubMed.
- (a) S. Badvel, R. R. Gopireddy, T. B. Shaik, S. Hasti, V. R. Tummaluru and N. R. Chamarthi, Chem. Heterocycl. Compd., 2015, 51, 749 CrossRef CAS; (b) F. M. Moghaddam, M. R. Khodabakhshi and A. Mariyeh, Tetrahedron Lett., 2014, 55, 4720 CrossRef CAS; (c) H. Wang, C. Wang and T. D. Bannister, Tetrahedron Lett., 2015, 56, 1949 CrossRef CAS PubMed; (d) L. Suresh, P. S. V. Kumar, Y. Poornachandra, C. G. Kumar and G. V. P. Chandramouli, Bioorg. Med. Chem. Lett., 2017, 27, 1451 CrossRef CAS PubMed; (e) M. Kidwai, K. Singhal and S. Kukreja, Z. Naturforsch., B: J. Chem. Sci., 2007, 62, 732 CAS; (f) W. S. Hamama, M. A. Ismail, H. A. Al-Saman and H. H. Zoorob, J. Adv. Res., 2013, 4, 115 CrossRef CAS.
- (a) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef; (b) M. Shiri, Chem. Rev., 2012, 112, 3508 CrossRef CAS PubMed; (c) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (d) J.-C. Xiang, Z.-X. Wang, Y. Cheng, S.-Q. Xia, M. Wang, B.-C. Tang, Y.-D. Wu and A.-X. Wu, J. Org. Chem., 2017, 82, 9210 CrossRef CAS PubMed; (e) H. Wang, Q. Xu, S. Shen and S. Yu, J. Org. Chem., 2017, 82, 770 CrossRef CAS PubMed; (f) L.-J. Cheng and N. P. Mankad, J. Am. Chem. Soc., 2017, 139, 10200 CrossRef CAS PubMed; (g) M. L. Deb, P. J. Borpatra, P. J. Saikia and P. K. Baruah, Synthesis, 2017, 49, 1401 CrossRef CAS.
- (a) J.-Y. Chin, C. Godwin, E. Parker, T. Robins, T. Lewis, P. Harbin and S. Batterman, Indoor Air, 2014, 24, 403 CrossRef CAS PubMed; (b) W.-T. Tsai, Environments, 2016, 3, 23 CrossRef.
- (a) A. N. Campbell and S. S. Stahl, Acc. Chem. Res., 2012, 45, 851 CrossRef CAS PubMed; (b) D. Munz and T. Strassner, Inorg. Chem., 2015, 54, 5043 CrossRef CAS PubMed; (c) M. L. Deb, B.-S. Saikia, G. K. Rastogi and P. K. Baruah, ChemistrySelect, 2018, 3, 1693 CrossRef CAS.
- (a) J. Zhang, Z. Cui, F. Wang, Y. Wang, Z. Miao and R. Chen, Green Chem., 2007, 9, 1341 RSC; (b) G. Choudhary and R. K. Peddinti, Green Chem., 2011, 13, 276 RSC; (c) J. Liu, M. Lei and L. Hu, Green Chem., 2012, 14, 2534 RSC.
- (a) A. M. Wawro, T. Muraoka, M. Kato and K. Kinbara, Org. Chem. Front., 2016, 3, 1524 RSC; (b) X. Lei, A. Jalla, M. A. A. Shama, J. M. Stafford and B. Cao, Synthesis, 2015, 47, 2578 CrossRef CAS; (c) P. A. Byrne, K. V. Rajendran, J. Muldoon and D. G. Gilheany, Org. Biomol. Chem., 2012, 10, 3531 RSC.
- (a) K. S. V. Gupta, D. V. Ramana, B. Vinayak, B. Sridhar and M. Chandrasekharam, New J. Chem., 2016, 40, 6389 RSC; (b) S. Mahato, S. Haldar and C. K. Jana, Chem. Commun., 2014, 50, 332 RSC; (c) X.-J. Shang and Z.-Q. Liu, Tetrahedron Lett., 2015, 56, 482 CrossRef CAS; (d) V. Satheesh, M. Sengoden and T. Punniyamurthy, J. Org. Chem., 2016, 81, 9792 CrossRef CAS PubMed.
- M. L. Deb and P. J. Bhuyan, Tetrahedron Lett., 2005, 46, 6453 CrossRef CAS.
- (a) M. L. Deb, S. S. Dey, I. Bento, M. T. Barros and C. D. Maycock, Angew. Chem., Int. Ed., 2013, 52, 9791 CrossRef CAS PubMed; (b) P. J. Borpatra, M. L. Deb and P. K. Baruah, Synlett, 2018, 29, 1171 CrossRef CAS; (c) K. Chen, B. Gao, Y. Shang, J. Du, Q. Gu and J. Wang, Org. Biomol. Chem., 2017, 15, 8770 RSC; (d) A. V. A. Gholap, S. Maity, C. Schulzke, D. Maiti and A. R. Kapdi, Org. Biomol. Chem., 2017, 15, 7140 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1850632, 1850625 and 1850631. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8gc03507e |
| This journal is © The Royal Society of Chemistry 2019 |