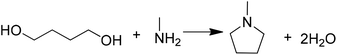
One-pot synthesis of N-methylpyrrolidine (NMPD) using Cu- and Ni-modified ZSM-5 as an efficient catalyst†
Yan
Long
ab,
Peixue
Wang
 a,
Yuqing
Fei
ab,
Dawei
Zhou
ab,
Shimin
Liu
*a and
Youquan
Deng
a,
Yuqing
Fei
ab,
Dawei
Zhou
ab,
Shimin
Liu
*a and
Youquan
Deng
 *a
*a
aCentre for Green Chemistry and Catalysis, State Key Laboratory for Oxo Synthesis and Selective Oxidation, State Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou, 730000, China. E-mail: liushm@licp.cas.cn; ydeng@licp.cas.cn
bGraduate University of Chinese Academy of Sciences, Beijing 100049, China
First published on 22nd November 2018
Abstract
In this study, a green, efficient and low-cost process for the synthesis of N-methylpyrrolidine (NMPD) from 1,4-butanediol (BDO) and methylamine (MA) via a one-pot method was developed. Under the optimized reaction conditions, more than 90% yield of NMPD was achieved over a Cu and Ni modified ZSM-5 catalyst. The catalyst could be reused for several runs retaining a satisfactory catalytic performance, and the scale-up operation showed the potential of industrial application. Characterizations of BET, XPS, XRD, TEM, SEM, H2-TPR and NH3-TPD were conducted for the developed composite catalyst, which suggested that Cu2O and NiO were the main species on the support. Meanwhile, it was found that the H2 atmosphere, the high dispersion of metal oxides and the synergic effect between Cu and Ni species on ZSM-5 contributed to the excellent catalytic performance. Furthermore, a possible mechanism based on a borrowing-hydrogen process was also proposed.
1. Introduction
N-Methylpyrrolidine (NMPD), as an important intermediate compound, can be widely used in the syntheses of pharmaceutical products and agricultural chemicals due to its high biological activities, especially for cefepime, which determines pharmacokinetic parameters and the exchange of drugs in the human body.1,2 Meanwhile, NMPD is also a key raw material for pyrrolidinium based ionic liquids. For example, 1-butyl-3-methylpyrrolidinium bis(trifluoromethyl sulfonyl)imide ([P14][NTf2]) has attracted a lot of attention as an electrolyte for electrochemical devices due to its nonvolatility, high conductivity and low viscosity.3 At present, there are several methods for preparing NMPD, mainly including (1) reductive methylation of tetrahydropyrrole (THP) with formaldehyde;4–6 (2) catalytic methylation of THP using methanol7–9 or carbon dioxide;10–15 and (3) hydrogenation of N-methylpyrrolidone (NMP).16–22 However, the above-mentioned processes often suffer from the high cost of raw materials or they involve environmental pollution. For example, using toxic formaldehyde as the methylation reagent in the first process is a hazardous threat to the environment and human health; besides, such a method needs a large amount of NaOH to neutralize inorganic acid catalysts, which generates a great deal of waste salts. For the second and third methods, both THP and NMP are relatively expensive raw materials, and sometimes noble metal catalysts are involved in those synthetic processes.Fortunately, using 1,4-butanediol (BDO) or tetrahydrofuran (THF) with methylamine (MA) as the starting materials can be considered a green and economic route to synthesize NMPD (Scheme 1), because the employed raw materials are readily available, cheap, and easy to handle. At the same time, only water is the theoretical by-product in this process. However, as far as we know, very few reports have concerned the synthesis of NMPD from BDO (or THF) and MA up to now, since it is still limited by the poor performance of the catalytic system. For example, DC Hargis23 reported cyclodialkylation between THF and MA for NMPD using titania as the catalyst, but only 63% THF conversion with 91% NMPD selectivity was obtained. Subba Rao24 employed a CrZSM-5 catalyst for the reaction between BDO and MA, but the yield of NMPD was also less than 65%. Therefore, the development of a catalyst with outstanding performance is desirable for such a green process.
In recent years, heterogeneous catalysts consisting of copper and nickel oxides have been reported for the N-alkylation of amines with alcohols by virtue of the borrowing-hydrogen method, which displayed excellent catalytic performance.25,26 Inspired by these results, we realized the synthesis of NMPD from BDO and MA via borrowing-hydrogen processes twice over Cu and Ni modified ZSM-5 catalysts in this work. Besides the influence of the components of the catalyst, reaction atmosphere, temperature, time, etc., were investigated in detail. It was found that a 3%Cu–3%Ni/ZSM-5 catalyst displayed outstanding catalytic performance, and nearly 100% conversion of BDO and >90% selectivity for NMPD could be achieved under optimum conditions. The yield of NMPD was much higher than that in previously reported results. At the same time, BET, XRD, XPS, SEM, TEM, H2-TPR studies were conducted to explore the relationship between structure and performance for the developed catalyst. Moreover, a possible mechanism was also proposed in such a catalytic system.
2. Experimental
2.1. Catalyst preparation and characterization
The Cu and Ni modified ZSM-5 catalysts were prepared by the incipient wetness method using Cu(NO3)2·3H2O and Ni(NO3)2·6H2O (Sinopharm Chemical Reagent Co., Ltd, China) as the precursors. The H-ZSM-5 support (Si/Al ratio of 50, 80, 300, supplied by the catalyst plant of NanKai University, China) was calcined at 500 °C for 3 h prior to use. After impregnation of the aqueous solutions of the precursors with a certain concentration, the sample was dried at 110 °C for 12 h, then calcined at 500 °C for 3 h. The catalysts with different weight loadings of Cu and Ni were denoted x%Cu–y%Ni/ZSM-5.The metal contents were analyzed by using graphite furnace atomic absorption spectroscopy (GF-AAS, contrAA700, Germany). X-ray diffraction (XRD) was measured on a Siemens D/max-RB powder X-ray diffractometer. Diffraction patterns were recorded with Cu Kα radiation (40 mA, 40 kV) over a 2θ range of 5° to 90° and a position-sensitive detector using a step size of 0.01° and a step time of 0.15 s.
Surface analysis of the catalysts was performed by X-ray photoelectron spectroscopy (XPS) on a VG ESCALAB210 spectrometer using Mg Kα radiation at a pass energy of 20 eV. The energy scale was calibrated and corrected for charging using the C 1s (285.0 eV) line as the binding energy (BE) reference.
Scanning electron microscopy (SEM) analysis was performed with a scanning electron microscope (JEOL-6701F) at an acceleration voltage of 5 kV. Transmission electron microscope (TEM) analysis was carried out using a TF20 field emission transmission electron microscope operating at 300 kV. Single-particle EDX mapping analysis was performed using a TF20 field emission TEM in the STEM mode.
Temperature-programmed reduction (TPR) of H2 was carried out on a TPR/TPD flow system equipped with a TCD detector. TPR analysis was conducted with 10% H2/Ar (30 ml min−1). In a typical experiment the solid sample (100 mg with particle size 160–200 μm) was pretreated at 500 °C for 1 h under air flow (30 ml min−1). The profile was recorded at a heating rate of 10 °C min−1 from room temperature to 800 °C and maintained at this temperature until the TCD signal of H2 returned to the baseline.
The surface acid properties of the catalysts were measured by temperature-programmed desorption (TPD) of NH3 and carried out on a TPD flow system equipped with a TCD detector. In a typical experiment, the solid sample (100 mg with particle size 160–200 μm) was pretreated at 500 °C for 1 h under argon gas flow (30 ml min−1) and then cooled to room temperature. The sample was subsequently exposed to an NH3 stream (30 ml min−1) at room temperature for 1 h and flushed again with argon gas for 1 h to remove any physico-adsorbed NH3. The desorption profile was recorded at a heating rate of 10 °C min−1 from room temperature to 800 °C and maintained until the TCD signal of NH3 returned to the baseline.
The BET surface areas, pore volumes and average pore radii of the catalysts were obtained with physisorption of N2 using a Micromeritics ASAP 2010 instrument.
2.2. Synthesis of NMPD
Catalytic reactions were carried out in a 100 ml stainless steel autoclave with a magnetic stirrer. In a typical process, BDO (0.05 mol), MA (0.1 mol, 40 wt% aqueous solution) and catalyst (0.45 g) were charged in the autoclave, which was then filled with H2 at 1 MPa pressure. The reactor was heated to 300 °C and magnetically stirred constantly during the reaction. After reaction, the qualitative and quantitative analyses of the resulting liquid mixture were conducted with GC-MS (Agilent 6890/5973) and GC (Agilent 7890) equipped with a SE-54 capillary column and a FID detector.3. Results and discussion
3.1. Synthesis of NMPD from 1,4-buranediol and MA under various conditions
Firstly, a series of catalysts were screened for the synthesis of NMPD from BDO and MA, and the obtained results are listed in Table 1. It was found that only 8% BDO conversion with 89% NMPD selectivity was obtained in the absence of a catalyst (entry 1, Table 1), and the catalytic performance of the support in the absence of Cu or Ni compounds was tested (entry 2, Table 1), and the conversion of BDO was merely 46% with 66% selectivity for NMPD. For 3%Cu/ZSM-5 (entry 3, Table 1), the conversion of BDO was 75%, and the selectivities for NMPD and THF were 21% and 68%, respectively; as for 3%Ni/ZSM-5, the conversion of BDO was 55%, and the selectivities for NMPD and THF were 34% and 47%, respectively (entry 4, Table 1). The selectivity for THF was higher than that for NMPD for all the single Cu or Ni on ZMS-5 catalysts. Meanwhile, it was found that with an increase in the loading of Cu or Ni, the conversion of BDO and the selectivity of THF increased, while the selectivity of NMPD declined (entries 5 and 6, Table 1). Based on the previous literature,27 the dehydration of BDO to THF only needs suitable acid sites. With an increase in the loading of metal species, the change in acid strength and amount of strong acid may be the reason for the high selectivity for THF.| Entry | Catalyst | Con. (%) | Sel. (%) | |||
|---|---|---|---|---|---|---|
| NMPD | THF | NMP | Othersb | |||
| a Reaction conditions: 0.05 mol BDO, 0.1 mol MA, 0.45 g catalyst, 300 °C, 6 h, 1 MPa H2. b Other by-products include traces of THP and N-butylpyrrolidine. c A ratio Si/Al of 50. d A ratio Si/Al of 300. | ||||||
| 1 | None | 8 | 89 | 1 | 4 | 6 |
| 2 | HZSM-5 | 46 | 66 | 32 | 2 | |
| 3 | 3%Cu/ZSM-5 | 75 | 21 | 68 | 5 | 6 |
| 4 | 3%Ni/ZSM-5 | 55 | 34 | 47 | 4 | 15 |
| 5 | 6%Cu/ZSM-5 | 100 | 5 | 94 | 1 | |
| 6 | 6%Ni/ZSM-5 | 97 | 4 | 92 | 4 | |
| 7 | 1%Cu–5%Ni/ZSM-5 | 70 | 29 | 68 | 3 | |
| 8 | 2%Cu–4%Ni/ZSM-5 | 82 | 63 | 22 | 5 | 10 |
| 9 | 3%Cu–3%Ni/ZSM-5 | 100 | 92 | 2 | 1 | 5 |
| 10 | 1.5%Cu–1.5%Ni/ZSM-5 | 73 | 56 | 27 | 5 | 12 |
| 11 | 6%Cu–6%Ni/ZSM-5 | 100 | 82 | 2 | 1 | 15 |
| 12 | 3%Cu–3%Ni/ZSM-5c | 91 | 86 | 2 | 1 | 11 |
| 13 | 3%Cu–3%Ni/ZSM-5d | 97 | 89 | 1 | 2 | 8 |
Furthermore, it could be seen that the ratio of Cu to Ni had an evident effect on the catalytic performance. As the ratio of Cu to Ni was increased, keeping the total loading amount constant at 6% (entries 7–9, Table 1), both the conversion of BDO and selectivity for NMPD were enhanced gradually, and the by-product THF declined sharply. Obviously, the 3%Cu–3%Ni/ZSM-5 catalyst expressed the highest catalytic activity for the synthesis of NMPD, and complete conversion of BDO with 92% selectivity for NMPD was achieved (entry 9, Table 1), which was much higher than the results of previous literature over CrZSM-5 (yield < 65%).24 The obtained results implied that the synergistic effect between Cu and Ni species on ZSM-5 might play a key role in the synthesis of NMPD. In addition, the impact of total loading amount of metal oxide on the performance (holding the ratio of Cu to Ni at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was also studied. As the loadings of Cu and Ni decreased to 1.5%, the BDO conversion and NMPD selectivity were only 73% and 56%, respectively (entry 10, Table 1). If both the Cu and Ni loadings were increased to 6%, the conversion of BDO could be maintained at 100%, but the product selectivity reduced to 82% (entry 11, Table 1), which might be due to the agglomeration of active sites on the surface of the ZSM-5 support.
1) was also studied. As the loadings of Cu and Ni decreased to 1.5%, the BDO conversion and NMPD selectivity were only 73% and 56%, respectively (entry 10, Table 1). If both the Cu and Ni loadings were increased to 6%, the conversion of BDO could be maintained at 100%, but the product selectivity reduced to 82% (entry 11, Table 1), which might be due to the agglomeration of active sites on the surface of the ZSM-5 support.
Meanwhile, ZSM-5 supports with different ratios of Si/Al were also tested (entries 12 and 13, Table 1). When the ratio of Si/Al was 50, the conversion of BDO was 91% with 86% selectivity for NMPD, and when the ratio of Si/Al was 300, the conversion of BDO was 97% with 87% selectivity for NMPD. The obtained yields of NMPD were lower than that of a catalyst with an Si/Al ratio of 80. As the ZSM-5 supports with different Si/Al ratios possessed similar pore sizes (Table S2†), the reason may be that the catalyst with an 80 Si/Al ratio had suitable acidity to form NMPD. According to the obtained results, it could be seen that adjusting the loading content of Cu and Ni compounds properly could allow the yield of NMPD to reach an excellent level (92%).
In order to optimize the reaction conditions, experiments focusing on the effects of reaction atmosphere, reaction temperature, time, and molar ratio of substrates (MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BDO) were investigated using 3%Cu–3%Ni/ZSM-5 as the catalyst, and the corresponding results are summarized in Table 2. Firstly, we attempted to explore the influence of reaction atmosphere on performance. Using air as the reaction atmosphere, only 53% BDO conversion with 48% NMPD selectivity were obtained, and the main by-products were THF and NMP (entry 1, Table 2). While, employing N2 as the reaction atmosphere, the conversion of BDO reached 60% and the selectivity of NMPD could increase to 64% (entry 2, Table 2). To our delight, as the reaction atmosphere was changed to H2, the BDO conversion could be further improved (81%), and the NMPD selectivity was also raised to 70% (entry 3, Table 2). It was revealed that the participation of H2 might reduce the metal oxides in situ to form active species, which could make the borrowing-hydrogen processes smoother, resulting in an improvement in the catalytic performance of the developed Cu and Ni modified ZSM-5 catalyst. On the other hand, the by-produced NMP could also be reduced to NMPD under H2 atmosphere. Therefore, the H2 atmosphere could enhance both BDO conversion and NMPD selectivity.
BDO) were investigated using 3%Cu–3%Ni/ZSM-5 as the catalyst, and the corresponding results are summarized in Table 2. Firstly, we attempted to explore the influence of reaction atmosphere on performance. Using air as the reaction atmosphere, only 53% BDO conversion with 48% NMPD selectivity were obtained, and the main by-products were THF and NMP (entry 1, Table 2). While, employing N2 as the reaction atmosphere, the conversion of BDO reached 60% and the selectivity of NMPD could increase to 64% (entry 2, Table 2). To our delight, as the reaction atmosphere was changed to H2, the BDO conversion could be further improved (81%), and the NMPD selectivity was also raised to 70% (entry 3, Table 2). It was revealed that the participation of H2 might reduce the metal oxides in situ to form active species, which could make the borrowing-hydrogen processes smoother, resulting in an improvement in the catalytic performance of the developed Cu and Ni modified ZSM-5 catalyst. On the other hand, the by-produced NMP could also be reduced to NMPD under H2 atmosphere. Therefore, the H2 atmosphere could enhance both BDO conversion and NMPD selectivity.
| Entry |
M
MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MBDO MBDO |
Atmosphere | Temperature (°C) | Time (h) | Con. (%) | Sel. (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| NMPD | THF | NMP | Others | ||||||
| a Reaction conditions: 0.05 mol BDO, 0.45 3%Cu–3%Ni/ZSM-5 catalyst. b The amount of substrates was magnified to 50 times, and the reaction was carried out in a 4L reactor. The isolated yield of NMPD was 83%. | |||||||||
| 1 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Air | 300 | 4 | 53 | 48 | 37 | 13 | 2 |
| 2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa N2 | 300 | 4 | 60 | 64 | 30 | 6 | |
| 3 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa H2 | 300 | 4 | 81 | 82 | 3 | 5 | 10 |
| 4 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa H2 | 300 | 2 | 61 | 83 | 2 | 7 | 8 |
| 5 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa H2 | 300 | 6 | 100 | 92 | 2 | 1 | 5 |
| 6 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa H2 | 300 | 8 | 100 | 91 | 2 | 1 | 6 |
| 7 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa H2 | 230 | 6 | 21 | 98 | 2 | ||
| 8 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa H2 | 250 | 6 | 35 | 97 | 3 | ||
| 9 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa H2 | 270 | 6 | 88 | 98 | 2 | ||
| 10 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa H2 | 330 | 6 | 100 | 35 | 63 | 2 | |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa H2 | 300 | 6 | 87 | 65 | 30 | 2 | 3 |
| 12 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1 Mpa H2 | 300 | 6 | 100 | 86 | 4 | 10 | |
| 13 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.5 Mpa H2 | 300 | 6 | 89 | 88 | 2 | 5 | 5 |
| 14 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 Mpa H2 | 300 | 6 | 100 | 89 | 3 | 8 | |
| 15b | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.5 Mpa H2 | 300 | 6 | 100 | 88 | 2 | 10 | |
Subsequently, the effects of reaction time and temperature on the catalytic synthesis of NMPD were examined. It was found that both the conversion of BDO and the selectivity for NMPD gradually increased with an increase in reaction time (entries 3–6, Table 2), and full conversion of BDO with 92% selectivity for NMPD could be achieved at 6 h (entry 5, Table 2). Whereas, with a further prolongation of reaction time to 8 h, no obvious improvement in the selectivity for NMPD was detected (entry 6, Table 2). Thus, the most suitable reaction time was 6 h. The reaction temperature was also a very important parameter for this process. It could be seen that the conversion of BDO could be elevated rapidly from 21% to 100% with >90% NMPD selectivity as the temperature was raised from 230 to 300 °C (entries 5, 7–10, Table 2). However, the NMPD selectivity sharply declined to 35%, as the reaction temperature went up to 330 °C (entry 10, Table 2), and the main by-product was THF, indicating that much higher temperatures promoted competitive dehydration and had an adverse effect on the formation of NMPD. Hence, to obtain more target product, the optimum reaction temperature was 300 °C.
In addition, the impact of amount of MA with the molar ratio (MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BDO) ranging from 1
BDO) ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was also studied under the same condition (entries 5, 11 and 12, Table 2). Based on the obtained results, it was necessary to have an excess of MA for this process to achieve a high yield of NMPD. But, when the molar ratio of MA
1 was also studied under the same condition (entries 5, 11 and 12, Table 2). Based on the obtained results, it was necessary to have an excess of MA for this process to achieve a high yield of NMPD. But, when the molar ratio of MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BDO increased to 3
BDO increased to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the yield of NMPD was slightly lower than that for a molar ratio of 2
1, the yield of NMPD was slightly lower than that for a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (entry 5, Table 2). The reason for this phenomenon might be that a large excess of basic MA destroyed the skeleton structure of the support, leading to a decrease in catalytic performance.
1 (entry 5, Table 2). The reason for this phenomenon might be that a large excess of basic MA destroyed the skeleton structure of the support, leading to a decrease in catalytic performance.
The pressure of H2 was also optimized in the synthesis of NMPD over the 3%Cu–3%Ni/ZSM-5 catalyst. When the pressure of H2 was adjusted from 0.5 MPa to 1 MPa (entry 5, 13 and 14, Table 2), the conversion of BDO was promoted from 89% to 100%, and the selectivity for NMPD was also improved. Nonetheless, as the pressure of H2 went up to 2Mpa, although the conversion of BDO was still maintained at 100%, the selectivity for NMPD decreased to 89%, which might be owing to excessive reduction of the metal oxides by the superfluous H2. Therefore, NMPD could be produced with high efficiency (92% yield) from MA and BDO using the composite catalyst based on Cu and Ni modified ZSM-5 at 300 °C for 6 h under a 1 MPa pressure of H2. In this work, a green, efficient and inexpensive process for NMPD was thus developed.
Moreover, a scale-up experiment (50 times) was carried out in a 4L reactor (entry 15, Table 2) under the optimum conditions. The results showed that 100% conversion of BDO with 88% selectivity for NMPD was achieved. Then, the resulting solution was separated via rectification, and the isolated yield of NMPD (99.5% purity) was 83%, which was close to the chromatographic yield. Therefore, the developed process for NMPD exhibited good prospects for industrial application.
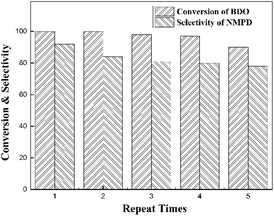
In addition, the reusability of 3%Cu–3%Ni/ZSM-5 was studied (Fig. 1). If the catalyst was used without any treatment for reusability, it showed relatively poor catalytic performance. For example, 87% BDO conversion with 86% NMPD selectivity was obtained for the 2nd run, and only 42% BDO conversion with 52% NMPD selectivity was obtained for the 5th run. The reason may be the adsorption of a small amount of heavy components and carbon deposition on the surface of catalyst. To improve the reusability, the catalyst was calcinated for 3 h at 500 °C under an ambient atmosphere after each test, and the performance could be obviously improved after the calcination treatment. The previous results showed that the BDO could be converted completely with more than 90% NMPD selectivity for the first run. In the subsequent cycles, both the conversion of BDO and NMPD selectivity declined gradually. For the 5th run, the conversion of BDO was 90% with 78% selectivity for NMPD, but the yield was still higher than that in previous reports. A possible reason for the deactivation of 3%Cu–3%Ni/ZSM-5 was the loss of metal species during the reactions.
3.2. Results of the catalyst characterization
The contents of Cu and Ni species in the catalysts were determined by atomic absorption spectroscopy (AAS, Table S1†). It could be seen that the contents of Cu and Ni in all samples were very close to their theoretical values. For example, 2.92 wt% Cu with 2.89 wt% Ni were detected for the 3%Cu–3%Ni/ZSM-5 catalyst. In addition, both the Cu and Ni contents in 3%Cu–3%Ni/ZSM-5 decreased obviously after 5 cycles, which was a possible reason for the decline in catalytic activity. The loss of Cu was more than that of Ni, which might be due to the coordination between MA and Cu.The physical properties of HZSM-5, 3%Cu–3%Ni/ZSM-5 and 6%Cu–6%Ni/ZSM-5 samples were analysed by the BET technique, including surface area (SA), pore volume, and pore size, and the data are filed in Table S2.† It can be seen that the SA, pore volume and pore radius of the HZSM-5 support were 388 m2 g−1, 0.355 cm3 g−1 and 2.549 nm, respectively. As expected, the BET surface area, pore volume and pore size of the loaded catalysts decreased with an increase in total loadings of Cu and Ni compounds. For example, 3%Cu–3%Ni/ZSM-5 displayed 285 m2 g−1 SA with a 2.348 nm radius. As the loadings of both Cu and Ni were further raised to 6%, the decline in SA to 260 m2 g−1 might be due to the agglomeration phenomenon of the oxides on the surface of the ZSM-5 support, resulting in a decline in its catalytic performance.
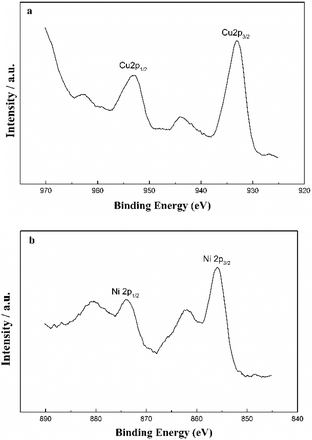
The chemical states of the metal oxides for 3%Cu–3%Ni/ZSM-5 were examined by XPS analyses, which revealed that the binding energies of Cu 2p3/2 and Ni 2p3/2 were 932.9 and 855.8 eV, respectively (Fig. 2), suggesting that Cu2O and NiO were the main species on the catalyst surface.25 In addition, the XPS results showed that the atomic ratio of Cu to Ni was 1.13 (Table S3†), which was slightly higher than the theoretical value of 1.08, indicating that Cu and Ni particles were relatively evenly distributed on the support surface.
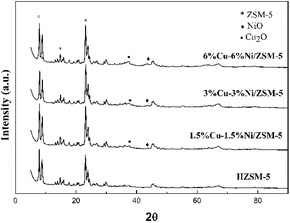
The XRD patterns for the prepared catalysts with different loadings are described in Fig. 3. As shown in Fig. 3, the corresponding peaks of ZSM-5 (appearing at 8, 8.9, 14.8 and 23°, JCPDS: 44-0002) could be observed in all samples. Except for the diffraction peaks of the support, the characteristic peaks of metal oxides could also be detected at 37° and 43°, which corresponded to the (111) facets of Cu2O and the (200) facets of NiO (JCPDS: 34-1354 and JCPDS: 47-1049), respectively. The XRD results were accordance with the XPS analyses. It was found that the peak intensity became stronger with an increase in loading content. Furthermore, the relatively wide peaks of the metal oxides indicated that the Cu2O and NiO were poorly crystallized or consisted of smaller nanoparticles.
The SEM images of HZSM-5 and 3%Cu–3%Ni/ZSM-5 are shown in Fig. S1,† and it can be seen that the morphological features of HZSM-5 and 3%Cu–3%Ni/ZSM-5 were similar, which means that the introduction of Cu and Ni species did not destroy the structure of the support. Furthermore, the high-resolution morphology of 3%Cu–3%Ni/ZSM-5 was carried out by TEM. Fig. 4a shows that no coagulation could be observed on the surface of the catalyst, meaning that both Cu2O and NiO particles were mostly well dispersed on the ZSM-5 support. Moreover, from the high resolution TEM image (HRTEM) in Fig. 4b, lattice fringes can be clearly discerned with d-spacings of 0.24 and 0.21 nm. Since the diffraction peaks of the Cu2O (111) and NiO (200) facets could be observed in the XRD patterns, and the values of the d-spacing from the TEM results were also close to the values of the PDF card (JCPDS: 34-1354, JCPDS: 47-1049), so the lattice fringes could be assigned to the Cu2O (111) and NiO (200) facets. And such speculation was also obtained from previously reported literature.28,29 EDX mapping was also conducted in order to identify the distribution of the elements (Fig. 4c). The results revealed that the Cu and Ni species were uniformly distributed in the catalyst. Additionally, the EDX pattern (Fig. S2†) of the catalyst also demonstrated the existence of Cu and Ni oxides. So, the obtained HR-TEM results agreed well with the observations from the XRD characterizations.
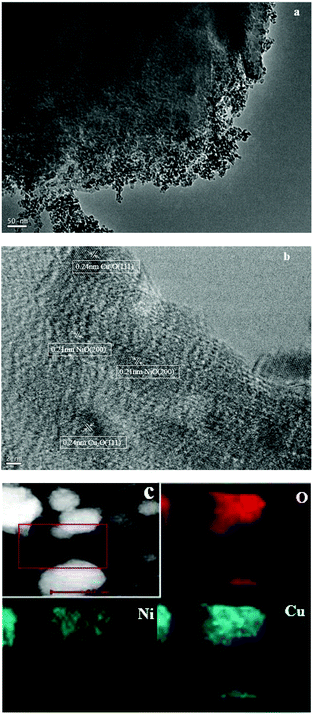 | ||
| Fig. 4 TEM images of 3%Cu–3%Ni/ZSM-5. Scale bars: (a) 50 nm, (b) 2 nm. (c) EDX mapping of 3%Cu–3%Ni/ZSM-5 for O, Cu and Ni elements. | ||
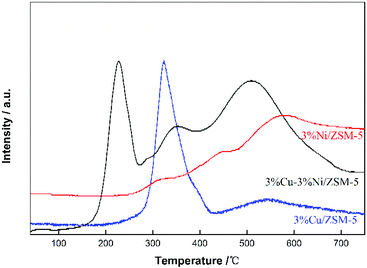
In order to explore the interaction between Cu and Ni species, H2-TPR examinations were conducted for 3%Cu–3%Ni/ZSM-5, and compared with individual loadings of 3% Cu and Ni on ZSM-5. As shown in Fig. 5, for the sample of 3%Cu/ZSM-5, the reduction temperature occurred at 323 °C; for 3%Ni/ZSM-5, there are three reduction peaks at 313, 442, 578 °C, respectively, which was in accordance with the previous report.30 The two former peaks could be assigned to the reduction of NiO of different crystal sizes located on the outer zeolite surface. For 3%Cu–3%Ni/ZSM-5, there were three peaks at 228, 348 and 508 °C, respectively. It can be seen that the reduction peak of Cu2O shifted from 323 °C to 228 °C, and the main reduction peak of NiO also shifted from 578 °C to 508 °C. So, the corresponding reduction peaks for both Cu2O and NiO declined dramatically due to the interaction between Cu and Ni. Moreover, compared to 3%Ni/ZSM-5, more NiO in 3%Cu–3%Ni/ZSM-5 was reduced in the presence of Cu species under the same conditions, which might also reflect the synergistic effect between Cu and Ni species. It should be noted that the Cu2O on the composite catalyst could be partially reduced to Cu (0) at the reaction temperature (300 °C), which was favourable for the borrowing-hydrogen process. In addition, the peak at 348 °C was possibly contributed by some Cu2O particles having a relatively weaker interaction with NiO. Therefore, the presence of the synergistic effect between metal species might be one of the reasons for the high catalytic performance of 3%Cu–3%Ni/ZSM-5 in the synthesis of NMPD from MA and BDO.
NH3-TPD was used to characterize the strength of accessible acid sites in the catalysts, and the results are shown in Fig. S3.† It can be seen that two characteristic peaks could be observed for HZSM-5, and the peak at around 100 °C indicated the weak acid sites, and the ones locating at 500 °C meant the strong acid sites. After the loading of metal, the peak at high temperature changed obviously. For 6%Cu/ZSM-5, although the peak for strong acid sites shifted to a lower temperature, the amount of strong acidity increased; while the peak of 6%Ni/ZSM-5 for the strong acid sites shifted mainly to higher temperature with a small portion to lower temperature.31 The increase in acid strength or the amount of strong acid sites may be the reason for the high selectivity for THF. As for 3%Cu–3%Ni/ZSM-5, both the amount of strong acid and the strength decreased compared with HZSM-5. Hence, the suitable acidity for the catalysts may also be an important reason for the formation of NMPD.
3.3. Possible reaction mechanism
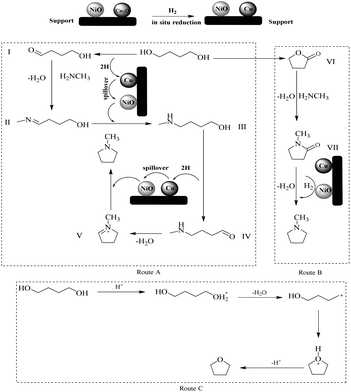
A possible reaction pathway through the borrowing-hydrogen process twice for the synthesis of NMPD was proposed in this study (Scheme 2). Firstly, with the help of Cu species being reduced in situ under the H2 atmosphere, the dehydrogenation of BDO formed the intermediate carbonyl compound (I), which underwent addition–elimination with MA to generate the imine (II). Then, the generated molecular hydrogen in the dehydrogenation step overflowed on the Ni species and reduced imine to amino alcohol (III).32 Next, the amino alcohol transformation could advance the ‘hydrogen shuttling’ process, in which the hydroxyl group dehydrogenated to the carbonyl compound (IV) followed by condensation and cyclization to form an imine (V). Finally, the imine (V) converted to NMPD via a hydrogenation reaction. For the intermediary compounds in the proposed possible mechanism, we did not find them in the present stage, and more powerful evidence requires our continued efforts. The intermediates were speculated on via the mechanism of the borrowing-hydrogen process according to the previous literature.25,33Meanwhile, a spot of NMP was detected during the synthesis of NMPD in the absence of H2, meaning that there was another route to produce NMPD. Some of the BDO could readily generate γ-butyrolactone (VI) under test conditions. The formed γ-butyrolactone easily converted to NMP (VII) in the presence of MA,34 and it was also able to yield NMPD through hydrogenation over the Ni species. So, the hydrogen atmosphere could not only promote the borrowing-hydrogen progress, but also facilitate the by-product NMP turning into the target product. Consequently, the route for NMPD from BDO and MA was likely to undergo primary Route A with a secondary Route B.
As the main product generated over the Cu/Ni separately exchanged ZSM-5 zeolites (3%Cu/ZSM-5, 3%Ni/ZSM-5, 6%Cu/ZSM-5 and 6%Ni/ZSM-5), the possible reaction pathway to THF is also given in Scheme 2 (Route C). After the ion exchange of ZSM-5 zeolites, there are still some H+ sites on the catalysts. Based on the previous literature,24,27 the BDO could undergo dehydration and cyclization with the help of H+, and such a process generally needs moderately acid sites. So, the high selectivity for THF over the Cu/Ni separately exchanged ZSM-5 zeolites maybe due to the suitable acidity. Moreover, it is difficult to induce the borrowing-hydrogen over the separately exchanged Cu or Ni on ZSM-5 in the absence of the synergistic effect.
4. Conclusions
A one-pot method to synthesise NMPD from BDO and MA over Cu-Ni/ZSM-5 was realized by virtue of the borrowing-hydrogen methodology. NMPD could be obtained with high efficiency (92% yield) over a 3%Cu–3%Ni/ZSM-5 catalyst at 300 °C for 6 h under 1 MPa pressure of H2. Meanwhile, the AAS BET, XPS, XRD, SEM and TEM characterization results indicated that the main species on the catalyst surface were Cu2O and NiO, which exhibited a high level of dispersion. H2-TPR analyses revealed that the reductive atmosphere and the synergic effect of Cu and Ni on the ZSM-5 support enhanced the catalytic activity. Moreover, a possible mechanism was also proposed, and the developed Cu–Ni/ZSM-5 could act as an excellent catalyst for the ‘borrowing-hydrogen’ reaction. This process offers a green and economic route for the synthesis of NMPD, which also has a promising prospect for industrial application.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by National Key Research and Development Program of China (No. 2017YFA0403101) and the National Natural Science Foundation of China (No. 21761132014).Notes and references
- D. G. Walker, P. R. Brodfuehrer, S. P. Brundidge, K. M. Shih and C. Sapino Jr., J. Org. Chem., 1988, 53, 983–991 CrossRef CAS.
- G. Chen, G. Liu, F. Qin and Y. Wang, J. Pharm. Biomed. Anal., 2003, 33, 797–801 CrossRef CAS.
- C. Schütter, A. R. Neale, P. Wilde, P. Goodrich, C. Hardacre, S. Passerini, J. Jacquemin and A. Balducci, Electrochim. Acta, 2016, 220, 146–155 CrossRef.
- A. J. Ward, A. F. Masters and T. Maschmeyer, RSC Adv., 2014, 4, 23327–23337 RSC.
- M. Tajbakhsh, R. Hosseinzadeh, H. Alinezhad, S. Ghahari, A. Heydari and S. Khaksar, Synthesis, 2011, 490–496, DOI:10.1055/s-0030-1258384.
- R. A. da Silva, I. H. S. Estevam and L. W. Bieber, Tetrahedron Lett., 2007, 48, 7680–7682 CrossRef CAS.
- X. Yu, Z. Yang, H. Zhang, B. Yu, Y. Zhao, Z. Liu, G. Ji and Z. Liu, Chem. Commun., 2017, 53, 5962–5965 RSC.
- L. Zhang, Y. Zhang, Y. Deng and F. Shi, RSC Adv., 2015, 5, 14514–14521 RSC.
- R. Grigg, T. R. B. Mitchell, S. Sutthivaiyakit and N. Tongpenyai, J. Chem. Soc., Chem. Commun., 1981, 611–612, 10.1039/c39810000611.
- T. Toyao, S. M. A. H. Siddiki, Y. Morita, T. Kamachi, A. S. Touchy, W. Onodera, K. Kon, S. Furukawa, H. Ariga, K. Asakura, K. Yoshizawa and K.-I. Shimizu, Chem. – Eur. J., 2017, 23, 14848–14859 CrossRef CAS PubMed.
- D. S. Morris, C. Weetman, J. T. C. Wennmacher, M. Cokoja, M. Drees, F. E. Kuhn and J. B. Love, Catal. Sci. Technol., 2017, 7, 2838–2845 RSC.
- X.-L. Du, G. Tang, H.-L. Bao, Z. Jiang, X.-H. Zhong, D. S. Su and J.-Q. Wang, ChemSusChem, 2015, 8, 3489–3496 CrossRef CAS.
- K. Kon, S. M. A. H. Siddiki, W. Onodera and K.-I. Shimizu, Chem. – Eur. J., 2014, 20, 6264–6267 CrossRef CAS PubMed.
- X. Cui, Y. Zhang, Y. Deng and F. Shi, Chem. Commun., 2014, 50, 13521–13524 RSC.
- X. Cui, X. Dai, Y. Zhang, Y. Deng and F. Shi, Chem. Sci., 2014, 5, 649–655 RSC.
- T. Mitsudome, K. Miyagawa, Z. Maeno, T. Mizugaki, K. Jitsukawa, J. Yamasaki, Y. Kitagawa and K. Kaneda, Angew. Chem., Int. Ed., 2017, 56, 9381–9385 CrossRef CAS PubMed.
- K.-I. Shimizu, W. Onodera, A. S. Touchy, S. M. A. H. Siddiki, T. Toyao and K. Kon, ChemistrySelect, 2016, 1, 736–740 CrossRef CAS.
- C. Bornschein, A. J. J. Lennox, S. Werkmeister, K. Junge and M. Beller, Eur. J. Org. Chem., 2015, 1915–1919 CrossRef CAS.
- M. Stein and B. Breit, Angew. Chem., Int. Ed., 2013, 52, 2231–2234 CrossRef CAS PubMed.
- J. A. Fernandez-Salas, S. Manzini and S. P. Nolan, Chem. Commun., 2013, 49, 9758–9760 RSC.
- J. Coetzee, H. G. Manyar, C. Hardacre and D. J. Cole-Hamilton, ChemCatChem, 2013, 5, 2843–2847 CrossRef CAS.
- R. Burch, C. Paun, X. M. Cao, P. Crawford, P. Goodrich, C. Hardacre, P. Hu, L. McLaughlin, J. Sa and J. M. Thompson, J. Catal., 2011, 283, 89–97 CrossRef CAS.
- D. C. Hargis and R. L. Shubkin, Tetrahedron Lett., 1990, 31, 2991–2994 CrossRef CAS.
- Y. V. Subba Rao, S. J. Kulkarni, M. Subrahmanyam and A. Ramo Rao, J. Org. Chem., 1994, 59, 3998–4000 CrossRef CAS.
- X. Cui, X. Dai, Y. Deng and F. Shi, Chemistry, 2013, 19, 3665–3675 CrossRef CAS.
- X. C. Dai, X. J. Cui, H. K. Yuan, Y. Q. Deng and F. Shi, RSC Adv., 2015, 5, 7970–7975 RSC.
- M. Aghaziarati, M. Kazemeini, M. Soltanieh and S. Sahebdelfar, Ind. Eng. Chem. Res., 2007, 46, 726–733 CrossRef CAS.
- Z. Xiaolong, L. Kun, S. Wenyu, W. Caihua, S. Xiaoping, Y. Sen and S. Zhanbo, Nanotechnology, 2017, 28, 045602 CrossRef.
- M. J. Siegfried and K.-S. Choi, Adv. Mater., 2004, 16, 1743–1746 CrossRef CAS.
- B. Pawelec, R. Mariscal, R. M. Navarro, J. M. Campos-Martin and J. L. G. Fierro, Appl. Catal., A, 2004, 262, 155–166 CrossRef CAS.
- A. Veses, B. Puértolas, M. S. Callén and T. García, Microporous Mesoporous Mater., 2015, 209, 189–196 CrossRef CAS.
- M. Imabeppu, K. Kiyoga, S. Okamura, H. Shoho and H. Kimura, Catal. Commun., 2009, 10, 753–757 CrossRef CAS.
- D. Pingen and D. Vogt, Catal. Sci. Technol., 2014, 4, 47–52 RSC.
- M. G. Park, Y. G. Yoo, G. J. Choi, J. H. Lee, Y. S. Yoon, G. N. Jung, S. J. Lee, S. Choi, S. H. Oh and H. S. Kim, China Pat., CN102070501A, 2011 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8gc02640h |
| This journal is © The Royal Society of Chemistry 2019 |