Waste-free and efficient hydrosilylation of olefins
Valerica
Pandarus
 a,
Rosaria
Ciriminna
a,
Rosaria
Ciriminna
 b,
Geneviève
Gingras
a,
François
Béland
*a,
Serge
Kaliaguine
b,
Geneviève
Gingras
a,
François
Béland
*a,
Serge
Kaliaguine
 *c and
Mario
Pagliaro
*c and
Mario
Pagliaro
 *b
*b
aSiliCycle, 2500, Parc-Technologique Boulevard, Quebec City, Quebec, Canada G1P 4S6. E-mail: francoisbeland@silicycle.com
bIstituto per lo Studio dei Materiali Nanostrutturati, CNR, via U. La Malfa 153, 90146 Palermo, Italy. E-mail: mario.pagliaro@cnr.it
cDepartment of Chemical Engineering, Université Laval, 2325 Rue de l'Université, Quebec City, Quebec, Canada G1 V 0A6. E-mail: serge.kaliaguine@gch.ulaval.ca
First published on 21st November 2018
Abstract
High purity silicone precursors can now be synthesized by hydrosilylation of solvent-free olefins catalyzed by a highly stable and active glass hybrid catalyst consisting of mesoporous organosilica microspheres doped with Pt nanoparticles. These findings open the door to the sustainable manufacture of silicone and a way to further reduce the amount of platinum in silicones, which are ubiquitous advanced polymers with multiple uses and applications.
Introduction
Polysiloxanes (or silicones), composed of alternating silicon–oxygen backbone, are considered as polymers of unique versatility due to their high durability, chemical inertness, mechanical and thermal resistance, and waterproofing, lubricating, optical and electrical properties.1 Depending on their side group (aliphatic or aromatic) and their molecular weight and crosslinking degree, silicone fluids, elastomers, or resins are produced and commercialized either as pure silicones or as silicone-modified materials. In 2016, over 2 million tonnes of silicone polymers were produced across the world by polymerization of organosilicon compounds.2 Silicone fluids are employed in personal care, cosmetics, and medicinal/pharmaceutical products; silicone elastomers in the construction and automotive industries; and silicone resins primarily in the construction industry.Organosilicon compounds are conventionally obtained via the hydrosilylation of olefins, which are mediated by costly Pt homogeneous catalysts discovered by Speier3 in 1957 and by Karstedt4 in 1973. In order to reduce the cost and improve the safety of organosilicon compounds, which have found their way into a number of medical uses, intense research studies have been devoted to development of both new homogeneous and new efficient and recyclable solid catalysts.5 One example of new homogeneous catalysts is the platinum N-heterocyclic carbene catalysts commercialized since the early 2000s, which, being more active, stable and selective than Speier's and Karstedt's catalysts, allow lower catalyst loading and no undesired by-products during the addition of Si–H bonds to olefins to form the required Si–C bonds.6 Another one is the pyridine diimine Co(II) bis(carboxylate) complexes which were lately discovered by Chirick's team.7
Among all the new solid platinum catalysts reported so far,8 two of them shown high catalytic activity and leaching resistance for hydrosilylation reactions, which are the silica-supported Karstedt Pt-type catalyst9 and the Pt-based heterogeneous catalyst containing Pt nanoparticles (NPs) embedded into the walls of a mesostructured silica framework.10
With regard to heterogeneous catalysis, a remarkable advance was recently reported by Beller and co-workers, which is a heterogeneous single-atom catalyst (SAC) composed of Al2O3 nanorods decorated with platinum atoms. Such a catalyst can selectively catalyze the hydrosilylation of industrially relevant olefins with an unprecedented high TON (≈105).11 Specifically, 3 mmol olefins were selectively hydrosilylated in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 of olefin and silane at 100–120 °C (depending on the type of olefin) in the autoclave under 10 bar N2 and in the presence of this new single atom catalyst. Numerous olefins bearing reducible and other functional groups were converted in high yield to the corresponding silylated product, while the catalyst showed excellent stability in 6 consecutive reaction runs.
1 of olefin and silane at 100–120 °C (depending on the type of olefin) in the autoclave under 10 bar N2 and in the presence of this new single atom catalyst. Numerous olefins bearing reducible and other functional groups were converted in high yield to the corresponding silylated product, while the catalyst showed excellent stability in 6 consecutive reaction runs.
In 2013, we reported the synthesis of small Pt(0) nanoparticles (4–6 nm) encapsulated within a sol–gel porous methyl-modified ORMOSIL in irregular shape.12 A modest 0.5–1.0 mol% Pt of the hydrophobic solid catalyst selectively mediated the hydrosilylation of different olefins with triethoxysilane under an Ar atmosphere at room temperature or 65 °C, depending on the substrate. However, the catalyst failed in solvent-free alkene hydrosilylation tests. Herein, we report the discovery of a chemoselective solid catalyst (SiliaCat Pt(0)) for alkene hydrosilylation with catalytic activity similar to that of the platinum SAC catalyst11 under mild conditions, which requires no pressurized conditions, no inert gas atmosphere, and optimal reaction temperatures in the range of 65–75 °C.
Results and discussion
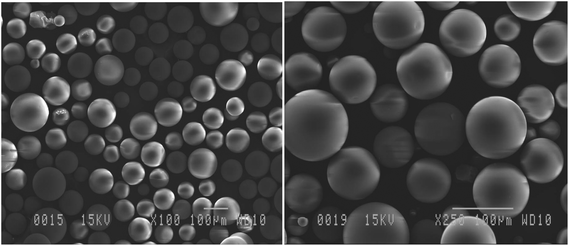
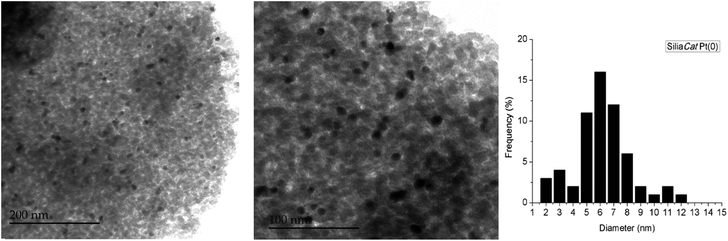
Obtained via alcohol-free sol–gel polycondensation of 100% organosilanes such as methyltriethoxysilane (MTES),13 SiliaCat Pt(0) is now synthesized in a spherical morphology using a template-based sol–gel process.14The SEM images demonstrate the uniform spherical morphology of SiliaCat Pt(0) as in Fig. 1. The TEM images reveal the highly dispersed platinum crystallites of 4–7 nm within the inner porosity of the spherical ORMOSIL matrix (Fig. 2). Our experience and previous studies have indicated that platinum crystallites with the size of 4–7 nm give the best catalytic reactivity versus crystallites stability (sintering, poisoning).15
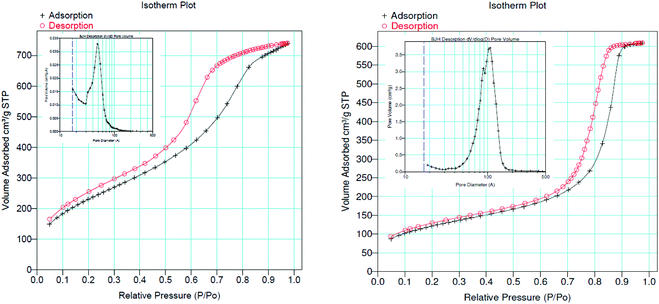
The Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods were used to evaluate the specific surface area, pore specific volume, and pore size of the SiliaCat Pt(0) and the undoped organosilica spherical support as in Table 1.
| Material | Pt load (wt%) | BET surface (m2 g−1) | Pore volume (cm3 g−1) | Pore size (nm) |
|---|---|---|---|---|
| MeSiO1.5 | — | 869 | 1.21 | 5.6 |
| Pt(0)/MeSiO1.5 (SiliaCat Pt(0)) | 5.0 | 428 | 0.94 | 8.8 |
Both types of materials demonstrate type IV N2 adsorption–desorption isotherms of mesoporous materials.16 The organosilica support presents a large BET surface area (>800 m2 g−1) and a narrow pore size distribution of mesopores (∼6.0 nm) capable of adsorbing a large volume of cryogenic nitrogen (1.21 cm3 g−1) (Fig. 3, left). On the other hand, the SiliaCat Pt(0) catalyst (Fig. 3, right) has a smaller BET surface area (average 428 m2 g−1) and a broader pore size distribution centred at 8.8 nm.
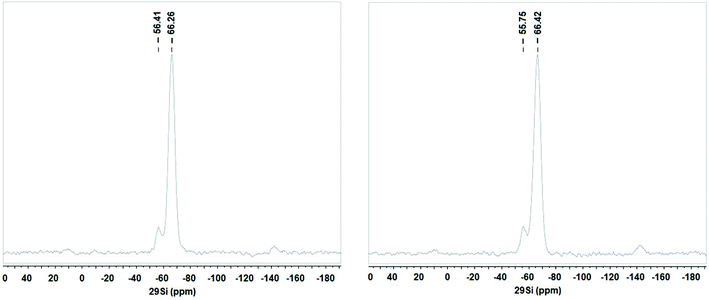
The 29Si MAS NMR spectra of both the support and SiliaCat Pt(0) show the absence of 29Si signal at −110 to −90 ppm, corresponding to (SiO)nSi(OH)4−n (Qn) sites, proving that all Si atoms are covalently linked to a carbon atom (Fig. 4).17 The spectra were further analyzed with regard to Tn band type, where n is the number of siloxane bonds on the Si atom.18 The absence of signals at −40 ppm (T1, MeSi(OSi)(OH)2) and the presence of signals at −56 ppm (T2, MeSi(OSi)2OH) and −66 ppm (T3, MeSi(OSi)3) indicate an almost complete condensation of the MTES organosilane precursor.19
Reaction condition optimization
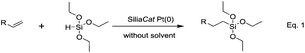
To optimize the reaction conditions, 1-octene was selected as a model olefin and triethoxysilane (TES) as a model silane. Reactions were carried out with different amounts of spherical SiliaCat Pt(0) catalyst loaded with 5 wt% Pt under solvent-free conditions at different temperatures and under air atmosphere. All reactions were carried out on a >100 mmol scale (at least 100 mmol of silane and 150 mmol of olefin).The reactions in entries 1 and 2 in Table 2 were carried out using one equivalent of 1-octene and over 0.1 mol% Pt of SiliaCat Pt(0) at 40 °C. The low yield of triethoxy(octyl)silane observed is due to the isomerisation reactions, which are detrimental to selectivity.
| SiliaCat Pt(0) loadingb | |||||||
|---|---|---|---|---|---|---|---|
| Entry | (mol% Pt) | (wt% catalyst) | 1-Octene (equiv.) | Triethoxysilane (equiv.) | T (°C) | Time (h) | Conversion/yieldd (%) |
| a Reaction conditions: 100 mmol of triethoxysilane (1 equiv.), 150 mmol of 1-octene (1.5 equiv.) (except entry 1: 100 mmol of 1-octene (1 equiv.) and 100 mmol of triethoxysilane (1 equiv.); entry 2: 100 mmol of 1-octene (1 equiv.) and 150 mmol of trietoxysilane (1.5 equiv.), SiliaCat Pt(0) (0.25 mmol g−1 Pt loading), 0.5–2 h, an atmosphere of air, 40–75 °C, mechanical stirring (700 rpm). b Molar ratio of platinum/TES calculated for the total Pt content; mass ratio of solid catalyst/TES. c Molar ratio of platinum/1-octene calculated for the total Pt content; mass ratio of solid catalyst/1-octene. d Conversion/yield evaluated by GC-MS analysis (calibration curve, internal standard used: mesitylene). | |||||||
| 1 | 0.10 | 2.44 | 1.0 | 1.0 | 40 | 0.5 | 56/55 |
| 1.0 | 70/68 | ||||||
| 2.0 | 95/85 | ||||||
| 2c | 0.10 | 2.44 | 1.0 | 1.5 | 40 | 0.5 | 73/72 |
| 1.0 | 93/84 | ||||||
| 2.0 | 100/87 | ||||||
| 3 | 0.10 | 2.44 | 1.5 | 1.0 | 40 | 0.5 | 85/84 |
| 1.0 | 100/98 | ||||||
| 4 | 0.10 | 2.44 | 1.5 | 1.0 | 55 | 0.5 | 93/92 |
| 1.0 | 100/96 | ||||||
| 5 | 0.10 | 2.44 | 1.5 | 1.0 | 65 | 0.5 | 100/97 |
| 6 | 0.05 | 1.22 | 1.5 | 1.0 | 65 | 0.5 | 68/57 |
| 1.0 | 76/74 | ||||||
| 2.0 | 100/96 | ||||||
| 7 | 0.05 | 1.22 | 1.5 | 1.0 | 75 | 0.5 | 88/87 |
| 1.0 | 100/98 | ||||||
The yield of the silyated product wasn't improved by adding excess TES (entry 2) while more secondary products have been produced. However, when 1-octene was added in excess, complete conversion of TES was obtained with an excellent yield in triethoxy(octyl)silane within 1 h (entry 3). A similar result was obtained at 55 °C (entry 4). Increasing the temperature to 65 °C, the hydrosilylation of the benchmark alkene with TES reagent was accomplished in only 30 minutes, affording once again an excellent yield in triethoxy(octyl)silane (entry 5). Entries 6 and 7 show that the catalytic activity of the catalyst decreased with the decrease of the platinum load to 0.05 mol%. However, quantitative conversion was obtained by increasing either the reaction time or the reaction temperature.
Results in Table 2 show that an increase in the conversion rate can be achieved by increasing either the amount of platinum or the temperature and the reaction time, which is in contrast with the observations lately reported by Kühn and co-workers for hydrosilylation using Karstedt's catalyst.20 These results illustrate that, in the solvent-free catalytic reaction over SiliaCat Pt(0), no inactive Pt colloids are formed.21 This hypothesis is also supported by the results obtained when the heterogeneously catalyzed reaction was conducted under inert conditions (entry 5).22 In the absence of oxygen the hydrosilylation of 1-octene with TES over 0.1 mol% of sol–gel entrapped Pt occurred with 61% yield within 1 h and 86% yield within 2 h. Upon exposure to oxygen, the 1-octene reacted vigorously with TES, making it difficult to control the reaction temperature. The reaction under inert conditions, even if it is slower, enables better temperature control.
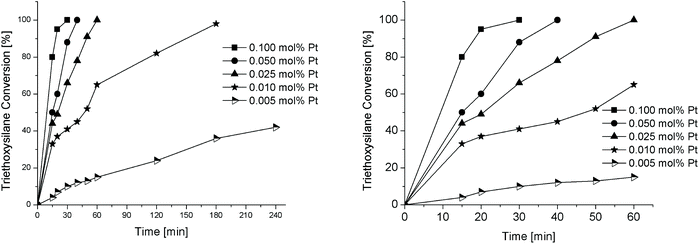
Effect of catalyst loading
All the reactions were carried out at 75 °C with respect to the TES regent. Fig. 5 shows the time evolution of TES conversion using the conditions developed in entry 7 of Table 2. The Pt reaction load was varied from 0.1 mol% to 0.005 mol%. Entry 1 in Table 3 shows that the hydrosilylation of 1-octene with TES over 0.1 mol% Pt proceeds with full conversion to triethoxy(octyl)silane in 30 minutes only, whereas complete conversion of TES to triethoxy(octyl)silane was obtained within 1 h at lower platinum loadings: 0.05 mol% (entry 2) and 0.025 mol% (entry 3).| Entry | SiliaCat Pt(0) loadingb | |||||
|---|---|---|---|---|---|---|
| Entry | (mol% Pt) | (wt% catalyst) | Time (h) | Conversion/yieldc (%) | TONd | TOFd (h−1) |
| a Reaction conditions: 100 mmol of triethoxysilane (1 equiv.), 150 mmol of 1-octene (1.5 equiv.), SiliaCat Pt(0) (0.25 mmol g−1 Pt loading), an atmosphere of air, 75 °C, mechanical stirring (700 rpm). b Molar ratio of platinum/TES calculated for the total Pt content; mass ratio of solid catalyst/TES. c Conversion of TES/triethoxy(octyl)silane yield evaluated by GC-MS analysis (calibration curve, internal standard used: mesitylene). d TON = mol product/mol Pt. TOF = TON/reaction time. e 200 mmol of triethoxysilane (TES), 300 mmol of 1-octene were used. | ||||||
| 1 | 0.100 | 2.44 | 0.5 | 100/98 | 980 | 1960 |
| 2 | 0.050 | 1.22 | 0.5 | 88/87 | 1980 | 1980 |
| 1.0 | 100/98 | |||||
| 3 | 0.025 | 0.61 | 0.5 | 62/61 | 3920 | 3920 |
| 1.0 | 100/98 | |||||
| 4 | 0.010 | 0.24 | 1.0 | 65/64 | 9500 | 3920 |
| 2.0 | 82/80 | |||||
| 3.0 | 98/95 | |||||
| 5e | 0.005 | 0.12 | 2.0 | 25/25 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
2650 |
| 4.0 | 42/42 | |||||
Furthermore, reducing the catalyst load to 0.01 mol% Pt lengthened the reaction time for almost full conversion to triethoxy(octyl)silane from 1 h to 3 h (entry 4), while over the ultralow 0.005 mol% Pt catalyst the conversion to triethoxy(octyl)silane was not complete (42% yield) even after 4 h (entry 5).
The drastic decrease in catalytic activity of the SiliaCat Pt(0) catalyst in the hydrosilylation of 1-octene with TES over 0.005 mol% Pt (corresponding to 3 × 10–4 mmol Pt per gram of triethoxysilane calculated from the total Pt content; entry 5 in Table 3) requires to study the influence of mechanical stirring and reaction temperature to evaluate their impact on the mass transfer resistance. Different experiments were carried out at different stirring rates and temperatures to determine whether the low conversion was due to the deactivation of the Pt nanoparticles or to limitations in the diffusion of the reactants (starting from the bulk liquid phase to the outer surface of the sol–gel catalyst and from the external surface into and through the inner porosity of the solid catalyst to access the active sites on the Pt nanoparticles dispersed throughout the internal pore structure).
Entries 1–3 in Table 4 show that on increasing the stirring rate from 700 rpm to 1000 rpm, the conversion of TES to triethoxy(octyl)silane increased from 42% to 54%, which indicates the mass transfer limitation of reactants from the bulk fluid to the external surface of the solid catalyst. However, increasing the reaction temperature from 75 °C to 85 °C at 1000 rpm significantly increased the yield in triethoxy(octyl)silane after 4 h from 54% to 86%, suggesting the same internal mass transfer limitation (entry 4). Any further increase in the reaction temperature beyond 85 °C resulted in a decrease of the yield (entries 5 and 6 in Table 4), suggesting that the overall reaction rate in the solid catalyst has become higher relative to the rate of mass transfer to the organosilica inner surface.
| Entry | T (°C) | Stirringb (rpm) | Time (h) | Conversion/yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: 200 mmol of triethoxysilane (1 equiv.), 300 mmol of 1-octene (1.5 equiv.), 40 mg SiliaCatPt(0) (0.25 mmol g−1 Pt loading), an atmosphere of air, mechanical stirring. b rpm: revolutions per minute. c Triethoxy(octyl)silane yield evaluated by GC-MS analysis (calibration curve, internal standard used: mesitylene). | ||||
| 1 | 75 | 700 | 4 | 42/42 |
| 2 | 75 | 850 | 4 | 49/48 |
| 3 | 75 | 1000 | 4 | 54/54 |
| 4 | 85 | 1000 | 4 | 89/86 |
| 5 | 95 | 1000 | 4 | 55/53 |
| 6 | 105 | 1000 | 4 | 53/51 |
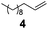
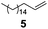
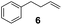
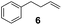
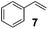
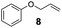
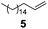
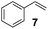
Showing evidence of the broad scope of the new catalytic method, Table 5 reports the outcomes of the reaction in a 100 mL reaction flask charged with 0.025 mol% Pt of SiliaCat Pt(0) spherical catalyst and 150 mmol of different alkenes. The catalyst/olefin mixture was heated at 75 °C under air stirring at 700 rpm and adding 100 mmol of TES dropwise over 15 min. The hydrosilylation reaction proceeded smoothly with various terminal alkenes (entries 1–5). Phenyl-1-butene (6), styrene (7) and allyl benzyl ether (8) were hydrosilylated using 1.25 equiv. of triethoxysilane due to the high olefin cost. The olefin conversions varied between 80% for styrene (entry 8) and 100% for allyl benzyl ether (entry 9). However, isomerisation reactions which are detrimental to selectivity directly affected the overall yield in silylated products, which varied between 73% for styrene within 4 h (entry 8) and 85% for the 4-phenyl-1-butene within 3 h (entry 6).
| Entry | Olefin | Olefin/H–Si (molar ratio) | SiliaCat Pt(0) (mol% Pt) | T (°C) | t (h) | Conversionb (%) | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: Entries 1–5: 100 mmol of triethoxysilane (1 equiv.), 150 mmol of olefin (1.5 equiv.), 0.025 mol% Pt (100 mg SiliaCat Pt(0), 0. 25 mmol g−1 Pt loading), an atmosphere of air, mechanical stirring. Entries 6–9: 100 mmol of olefin, 125 mmol of triethoxysilane (TES), 0.025 mol% Pt (100 mg SiliaCat Pt(0), 0.25 mmol g−1 Pt loading), an atmosphere of air, mechanical stirring. b Conversion evaluated by GC-MS analysis. Yields evaluated by 1H NMR and by GC-MS (internal standard used: mesytylene). c 0.05 mol% Pt (200 mg SiliaCat Pt(0)); β- and α-silylated products in 73% and in 5% yields, respectively. | |||||||
| 1 |
|
1.50/1.00 | 0.025 | 75 | 1 | 100 | 95 |
| 2 |
|
1.50/1.00 | 0.025 | 75 | 2 | 100 | 96 |
| 3 |
|
1.50/1.00 | 0.025 | 75 | 1 | 100 | 98 |
| 4 |
|
1.50/1.00 | 0.025 | 75 | 2 | 100 | 96 |
| 5 |
|
1.50/1.00 | 0.025 | 75 | 2 | 100 | 98 |
| 6 |
|
1.00/1.25 | 0.025 | 75 | 2 | 88 | 83 |
| 3 | 92 | 85 | |||||
| 7 |
|
1.00/1.25 | 0.050 | 75 | 2 | 100 | 81 |
| 8c |
|
1.00/1.25 | 0.050 | 85 | 4 | 80 | 73 |
| 9 |
|
1.00/1.25 | 0.025 | 75 | 2 | 100 | 75 |
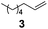
We then studied the commercially important hydrosilylation of various olefins by chlorodimethylsilane (DMCS). Preliminary experiments with 1-octene over 0.025 mol% Pt of spherical catalyst at 75 °C were conducted to determine the best olefin/silane molar ratio. Entries 1 and 2 in Table 6 show that when 1-octene was added in excess, the conversion of DMCS to chloro(dimethyl)octylsilane was smoothly achieved within 2 h. Again, with one equivalent of 1-octene only (entry 3), the isomerisation reactions detrimental to selectivity directly affected the reaction yield in the silylated product. However, by adding the silane in a slight excess (1.25 equiv.), chloro(dimethyl)octylsilane was obtained in 91% yield at 75 °C (entry 4) and in 93% yield at 60–65 °C (entry 5) within 1 h. The hydrosilylation reaction occurred with high anti-Markovnikov selectivity and low alkene isomerisation. Once more, the hydrosilylation of linear olefins involves a certain degree of isomerization, namely either a shift of the double bond or even skeletal modification of the starting molecules. Hence, to slow down isomerization and obtain higher yields in silylated products, the hydrosilylation with DMCS was optimally carried out at 60–65 °C.
| Entry | Olefin | Olefin /HSi (molar ratio) | Catalyst (mol% Pt) | T (°C) | t (h) | Conversionc (%) | Yieldc (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 100 mmol of olefin (1 equiv.), 125 mmol of chlorodimethylsilane (1.25 equiv.), 0.025 mol% Pt (100 mg SiliaCat Pt(0), 0.25 mmol g−1 Pt loading), an atmosphere of air, mechanical stirring. b 100 mmol of chlorodimethylsilane, from 100 mmol to 150 mmol of 1-octene, 0.025 mol% Pt (100 mg SiliaCat Pt(0), 0.25 mmol g−1 Pt loading), an atmosphere of air, mechanical stirring. c Conversion/Yields evaluated by GC-MS and by 1H NMR. d TES conversion. e 1-Octene conversion. | |||||||
| 1b |
|
1.50/1.00 | 0.025 | 75 | 2 | 100d | 93 |
| 2b | 1.25/1.00 | 0.025 | 75 | 2 | 100d | 92 | |
| 3b | 1.00/1.00 | 0.025 | 75 | 2 | 92d | 88 | |
| 4 | 1.00/1.25 | 0.025 | 75 | 1 | 100e | 91 | |
| 5 | 1.00/1.25 | 0.025 | 65 | 1 | 100e | 93 | |
| 6 |
|
1.00/1.25 | 0.025 | 65 | 1 | 99 | 89 |
| 7 |
|
1.00/1.25 | 0.025 | 65 | 1 | 100 | 91 |
| 8 |
|
1.00/1.25 | 0.05 | 65 | 1 | 100 | 95 (β/α = 70/25) |
| 9 |
|
1.00/1.25 | 0.025 | 65 | 1 | 100 | 85 |
| 10 |
|
1.00/1.25 | 0.025 | 75 | 1 | 100 | 82 |
| 11 |
|
1.00/1.25 | 0.025 | 65 | 1 | 99 | 89 |
| 12 |
|
1.00/1.25 | 0.050 | 75 | 1 | 92 | 77 |
| 13 |
|
1.00/1.25 | 0.025 | 65 | 1 | 100 | 90 (β-(E)/α = 75/15) |
| 14 |
|
1.00/1.25 | 0.025 | 65 | 1 | 100 | 95 (β-(E)/α = 75/20) |
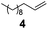
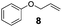
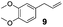
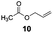
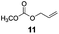
The hydrosilylation of 1-dodecene (entry 6) and of 1-octadecene (entry 7) afforded the corresponding silylated products in very good yields of 89% and 91%, respectively, showing evidence of the smooth access of these long chain olefins to the lipophilic inner porosity of the mesoporous catalyst. Hydrosilylation of styrene (7) also occurred with complete conversion and afforded the β- and α-silylated products in 70% and 25% yields, respectively (entry 8). Allylic compounds such as allyl phenyl ether (8), 4-allyl-1,2-dimethoxybenzene (9), allyl acetate (10), and allyl carbonate (11) were also smoothly hydrosilylated by chlorodimethylsilane over the SiliaCat Pt(0) at either 65 °C or 75 °C to form the corresponding silylated products in more than 75% yields (entries 9–11).
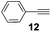
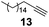
Other, more reactive allyl derivatives including allyl chloride, allyl bromide, allyl alcohol, α-vinylbenzyl alcohol, and 3-butene-2-one were also tested in solvent-free hydrosilylation reaction with DMCS. The use of SiliaCat Pt(0) resulted in a rapid consumption of these compounds but gave a complex mixture of excessive by-products formed via allylic rearrangement.23 Hence, optimized conditions were applied to the catalytic hydrosilylation of 1-alkynes with DMCS. When phenylacetylene (12) and 1-octadecyne (13) were hydrosilylated in the presence of SiliaCat Pt(0) only β-(E) and α isomers were produced, with the first being predominant, similar to other platinum catalysts.24 The reaction of phenylacetylene with DMCS gave 90% yield with an isomeric ratio of β-(E)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α of around 5
α of around 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The reaction of 1-octadecyne with DMCS gave 95% yield with an isomeric ratio of β-(E)
1. The reaction of 1-octadecyne with DMCS gave 95% yield with an isomeric ratio of β-(E)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) α of around 3.75
α of around 3.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
The optimized conditions were also applied to the catalytic hydrosilylation of internal alkenes with DMCS including cyclohexene and trans-2-octene. Unfortunately, the cyclohexene did not undergo hydrosilylation and the trans-2-octene reacted to a little extent, leading to 13% yield in chloro(dimethyl)octylsilane, the same product obtained in the hydrosilylation of 1-octene. These results are in good agreement with the results obtained by Kühn and co-workers20 that the internal octenes, reluctant to undergo hydrosilylation, isomerise to terminal octene, which then undergoes hydrosilylation.
SiliaCat Pt(0) catalysed hydrosilylation of 1-octadecene with other related hydrosilanes
The high reactivity of chlorodimethylsilane (Cl(Me)2SiH, DMCS) in the olefin hydrosilylation reaction over the SiliaCat Pt0 has motivated us to test other electronically and structurally related hydrosilanes to determine the reactivity value of the catalyst. The general protocol established for the hydrosilylation of olefins using DMCS in Table 6, entry 5, was used to examine the hydrosilylation of 1-octadecene with triethoxysilane ((EtO)3SiH, TES), dimethylphenylsilane (Ph(Me)2SiH, DMPS), and trichlorosilane (Cl3SiH, TCS) in the presence of 0.025 mol% Pt of SiliaCat Pt0 and without solvent. The reaction mixtures were stirred under air atmosphere at 65 °C from 1 h to 2 h (Table 7). Whereas the hydrosilylations with DMCS, TCS, and DMPS proceeded smoothly within 1 h to afford the hydrosilylated product in greater than 85% yield (entries 1–3), the hydrosilylation with TES was much slower (entry 4). A possible explanation for the reactivity difference between DMCS, TCS, DMPS and TES in the platinum catalysed hydrosilylation reaction could be the presence of electron-donating groups on the silicon atom.| Entry | Olefins | Silane | Olefin /HSi (molar ratio) | Catalyst (mol% Pt) | T (°C) | t (h) | Conversionb (%) | Yieldb (%) |
|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: 100 mmol of olefin (1 equiv.), 125 mmol of silane (1.25 equiv.), 0.025 mol% Pt (100 mg SiliaCat Pt(0), 0.25 mmol g−1 Pt loading), an atmosphere of air, mechanical stirring. b Conversion/yields evaluated by GC-MS and by 1H NMR. | ||||||||
| 1 |
|
Cl(Me)2SiH | 1.00/1.25 | 0.025 | 65 | 1 | 100 | 91 |
| 2 |
|
Cl3SiH | 1.00/1.25 | 0.025 | 65 | 1 | 100 | 87 |
| 3 |
|
Ph(Me)2SiH | 1.00/1.25 | 0.025 | 65 | 1 | 98 | 86 |
| 4 |
|
(EtO)3SiH | 1.00/1.25 | 0.025 | 65 | 1 | 85 | 82 |
| 2 | 98 | |||||||
Catalyst stability
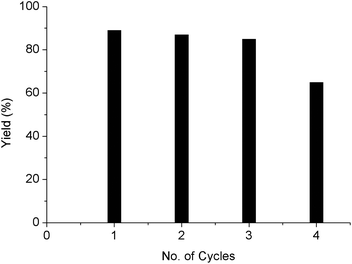
In order to evaluate the stability of the new SiliaCat Pt(0) spherical catalyst, we attempted the hydrosilylation reaction in four consecutive multigram experiments starting from 660 mmol of 1-octadecene (166.64 g) with 1.25 equiv. of chlorodimethylsilane over 0.025 mol% Pt of SiliaCat Pt(0) (0.66 g) under the optimized reaction conditions (Table 6, entry 7).In each consecutive reaction, once the maximum conversion of the 1-octadecene was achieved, the catalyst was collected by filtration, washed with 20 mL of anhydrous THF (3× for 10 minutes), dried at room temperature, and reused in a subsequent cycle. As shown in Table 8 and Fig. 6, no significant decrease in yield was observed for three consecutive reaction runs. However, the fourth reaction run proceeded with a significant decrease in yield (65%). The deactivation observed in the fourth reaction runs could be mainly related to the sintering of the platinum nanoparticles. This observation is not consistent with the TEM image of the catalyst after the fourth reaction run, which reveals that the size of Pt nanoparticles is almost unchanged (from 5–7 nm to 6–8 nm, Fig. 8A). Possibly,the deactivation of the catalyst is more likely due to catalyst poisoning by HCl produced in the reaction mixture by the decomposition of excess DMCS.25
| Entry | Time (h) | Yieldb (%) | Pt leaching (mg kg−1) |
|---|---|---|---|
| a Reaction conditions: 1 equiv. of 1-octadecene (660 mmol, 166.64 g), 1.25 equiv. of chlorodimethylsilane (825 mmol, 78.06 g), 0.660 g of SiliaCat Pt(0) (0.25 mmol g−1 Pt loading), an atmosphere of air, 65 °C, mechanical stirring (800 rpm). b Chloro(dimethyl)octadecylsilane yield evaluated by GC-MS analysis. | |||
| Run 1 | 1 | 89 | 2.62 |
| Run 2 | 1 | 87 | 3.63 |
| Run 3 | 2 | 85 | 3.51 |
| Run 4 | 2 | 65 | 3.94 |
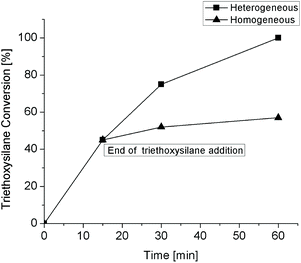
The analysis of the metal content via ICP-OES in the isolated crude products, obtained during the reusability tests after hot filtration of the catalyst,26 shows very low values of Pt leached, which never exceeded 5 ppm. To assess whether this small Pt leaching could impact catalysis, we performed the hot filtration test in the hydrosilylation reaction of 1-octene with triethoxysilane over 0.025 mol% Pt of SiliaCat Pt(0) under the optimized reaction conditions of entry 3 in Table 3, namely under an atmosphere of air at 75 °C. As a reminder, it is important to perform the test at a conversion which is neither too low (to ensure that enough Pt has leached) nor too high (to avoid the redeposition of the leached Pt on the catalyst, as observed in the literature for other supported metal catalysts).27 After adding triethoxysilane for 15 min, an aliquot of the reaction mixture was recovered, hot-filtered using a 0.45 μm syringe filter to remove the solid catalyst, and placed in another flask to continue the experiment at 75 °C.
Fig. 7 shows a modest residual activity, which is most likely due to platinum nanoparticles in suspension, known to be as effective as Karsted's catalyst for alkene hydrosilylation.10,28
The catalytic activity of the organically modified spherical 5 wt% Pt(0)/MeSiO1.5 was compared with the catalytic activity of the irregular 5 wt% Pt(0)/MeSiO1.5,12 as well as with the organically modified aluminosilicate 5 wt% Pt(0)/MeSiO1.5-Al2O3 catalyst made by our group (unpublished results).
Under the optimised conditions developed in entry 3 of Table 3, the spherical organically modified 5 wt% Pt(0)/MeSiO1.5 catalyst showed high activity and selectivity (98% yield within 1 h), whereas the organically modified 5 wt% Pt(0)/MeSiO1.5 xerogel with irregular particle morphologies showed a modest activity (21% yield within 2 h and 26% yield after 4 h). This low catalytic activity could be explained by the weak mechanical robustness of the support under the applied reaction conditions, which favor sintering of the Pt nanoparticles. This observation is consistent with the TEM image of the catalyst after only one reaction run, which revealed that the size of the NPs increased from 4–6 nm to almost 25–40 nm (Fig. 8B).
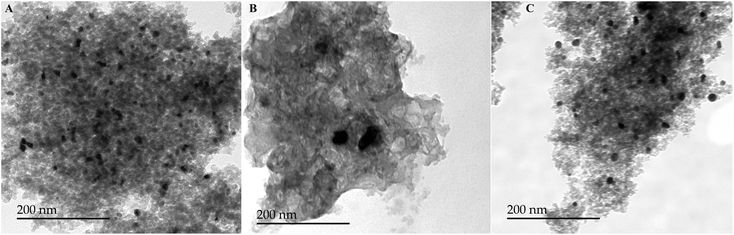 | ||
| Fig. 8 TEM images after the reusability test: (A) spherical Pt/MeSiO1.5 (O/W emulsion synthesis), (B) Pt/MeSiO1.5 and (C) Pt/SiO1.5-Al2O3 in irregular shape. | ||
To improve the robustness of the support, an organically modified aluminosilicate Pt(0)/MeSiO1.5-Al2O3 catalyst with irregular morphologies was prepared which indeed showed enhanced catalytic activity in solvent-free hydrosilylation reaction with 79% yield within 1 h and 92% yield after 2 h. However, this Pt sol–gel composite catalyst failed in the reusability test. Again, the TEM analysis of the catalyst after a single reaction run revealed that the size of the Pt nanoparticles increased from 4–5 nm to almost 15–20 nm (Fig. 8C). These results clearly show that the catalytic activity critically depends on the mechanical resistance of the support to nanoparticle sintering and to catalyst poisoning.
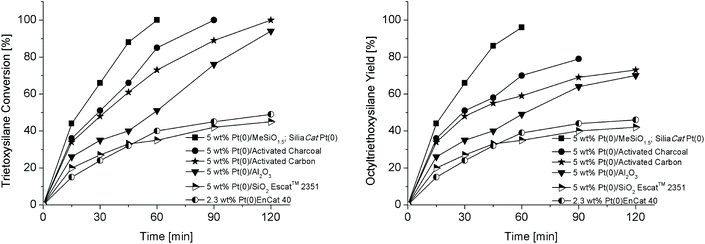
Finally, the catalytic activity of the spherical SiliaCat Pt(0) was compared to that of several different commercial solid catalysts, including 5 wt% Pt/charcoal, 5 wt% Pt/C, 5 wt% Pt/Al2O3, 5 wt% Pt/SiO2 (Escat 2351), and 2.3 wt% Pt EnCat 40, under the optimised reaction conditions (Table 3, entry 3).
The graphs in Fig. 9 show that, invariably, the spherical organically modified 5 wt% Pt(0)/MeSiO1.5 showed considerably higher catalytic activity, achieving complete conversion of the olefin in the silylated product in 98% yield within 1 h, whereas the 5 wt% Pt/charcoal and the 5 wt% Pt/C showed complete conversion of the silane within 1.5 h and 2 h, respectively, though with significantly lower yield in functionalised silane (Fig. 9, right). The 5 wt% Pt/Al2O3 showed 94% conversion of the silane within 2 h, but again with a much lower yield (70%) in octyltriethoxysilane. Under the same reaction conditions, both 5 wt% Pt/SiO2 (Escat 2351) and 2.3 wt% Pt EnCat 40 showed modest activity with 30% yield and 40% yield, respectively, in 2 h reaction time.
We ascribed the pronounced catalytic activity and the selectivity of Pt(0)/MeSiO1.5 organosilica catalysts to the ideal hydrophilic–lipophilic balance of the organosilica hybrid matrix, and hypothesized that the spherical geometry of the catalyst could lead to further enhanced activity.29
In 1965 Chalk and Harrod proposed the catalytic mechanism of the olefin hydrosilylation reaction over a homogeneous Pt catalyst consisting of conventional oxidative addition of the trisubstituted silane HSiR3 to Pt, coordination of the olefin, migratory insertion of the olefin into the Pt–H bond, followed by reductive elimination of the hydrosilylation product by the Si–C bond formation as the rate determining step.30 The latter model was improved by the fundamental mechanistic work of Lewis and Stein,21 Roy,31 and others.32,33 Their work supports and improves the basic steps of the Chalk–Harrod mechanism for Pt-catalysed hydrosilylation, instead of the modified version involving the insertion of the olefins into the Pt–Si bond.34 In brief, it has been established that the hydrosilylation reaction proceeds homogeneously and the catalytic active species contain Pt–C and Pt–Si bonds,21 with the insertion of the olefin into the Pt–Si bond being not favoured and competing isomerisation and hydrogenation reactions taking place if no silane is present in excess.31 It was also established that O2 has a beneficial effect on the hydrosilylation reaction.20,21
However, the variable induction periods, the formation of color bodies, and the requirement of oxygen in the same hydrosilylation reactions are not explained by the Chalk–Harrod mechanism. In the late 1980s and early 1990s, the induction period was attributed to the formation of colloidal active species stabilized by oxygen and responsible for the color bodies.35 In contrast, the extensive studies carried out by Lewis and Stein and co-workers allow to conclude that the catalytic hydrosilylation reaction is a molecular process, with the active platinum catalyst existing as a mononuclear species which contains Pt–C and Pt–Si bonds regardless of the nature of the reagents or stoichiometry, and the formation of colloids during the hydrosilylation reaction being responsible for the deactivation of the catalyst.21
Furthermore, the platinum complex proposed as the active species in the Chalk–Harrod mechanism has not been observed with highly active catalysts.21 While a multitude of mechanistic studies have been presented in detail since 1965 for hydrosilylation processes catalysed by less active transition metals, reports on kinetic and mechanistic studies of the reaction catalysed by the platinum-based systems and especially by supported platinum systems remain relatively scarce, likely due to the high activity level and extremely sensitive nature of the corresponding intermediates.23,36
Our experimental results indicate that in the presence of the spherical Pt/MeSiO1.5 heterogeneous catalyst the Chalk–Harrod mechanism is most favoured and responsible for the olefin hydrosilylation. However, the high catalytic activity of this catalyst, either at low or high platinum concentrations, suggests that no inactive Pt colloids are formed. Moreover, the hot filtration experiment (Fig. 7) clearly demonstrates the heterogeneous nature of the main catalytic process taking place in the inner porosity of SiliaCat Pt(0). In brief, it seems that the spherical organosilica support protects the platinum surface from colloid formation. The beneficial role of O2 could be to introduce a new oxygen-containing ligand into the coordination sphere of the surface Pt atom,37 which facilitates either the reductive elimination of the hydrosilylation product or the migratory insertion of the olefin.20 A detailed mechanistic study will be conducted to demonstrate this hypothesis.
Conclusions
We have discovered that an organically modified silica (ORMOSIL) xerogel in a spherical morphology functionalized with Pt nanoparticles is a highly active and selective hydrosilylation catalyst of broad scope. The catalyst is recyclable, retaining its spherical morphology with minor sintering of the Pt nanoparticles in three consecutive reaction runs even when reactions were carried out on the 660 mmol scale (660 mmol of olefin), with ultralow amounts (<5 ppm) of Pt leached in the silylated reaction product. Comparative tests using several commercial supported platinum catalysts invariably showed a superior performance for the SiliaCat Pt(0) catalyst. Partial deactivation of the catalyst in the fourth reaction run when using the low cost chlorodimethylsilane as a silylating reagent is due to catalyst poisoning by HCl produced in the reaction mixture by the decomposition of excess DMCS.In 2003, the determination of platinum (and siloxanes) in tissues of women with silicone gel-filled implants demonstrated that platinum and siloxanes leak from prostheses and accumulate in their surrounding tissues.38 The amounts of platinum leached from implants were extremely small (25–90 ng g−1 in the fibrin layer and fat tissue).29 Furthermore, as an element in the zero oxidation state, platinum poses the lowest risk, leading regulatory authorities across the world to generally conclude that platinum leaching does not represent a significant risk for people with silicone implants.39 A reduction in the amount of Pt leached by using silicones with ever lower Pt amounts is nonetheless highly desirable, which explains the relevance of findings such as those lately reported by Chirik,7 involving a new cobalt-based catalysis, and by Beller,11 involving a new single-atom platinum catalysis. Now, a new stable and highly active solid platinum catalyst, the sol–gel SiliaCat Pt(0) organosilica in a spherical morphology, is made available to carry out hydrosilyation under solvent-free (no longer requiring a solvent as in the case of the catalyst in irregular shape) and mild reaction conditions. This provides a method to make silicone precursors of high purity at low cost using a green reaction process. The highly porous glassy catalyst is stable and robust, being ideally suited for conversions in batch such as those presented here, and under flow, as will be reported shortly.
Experimental section
All reactions were performed on a multi-gram scale without solvent. The catalytic tests were run under an atmosphere of air in classic 100–1000 mL glass reactors equipped with a condenser and a mechanical stirring and temperature control systems. Unless otherwise noted, olefin samples with different purities (90–98%, from Sigma Aldrich), triethoxysilane, 95% chlorodimethylsilane, 98% dimethylphenylsilane, and 95% trichlorosilane (from Gelest) were used without purification. The commercial platinum catalysts 5 wt% Pt/charcoal, 5 wt% Pt/C, and 5 wt% Pt/Al2O3 (from Sigma Aldrich) and 5 wt% Pt/SiO2 (Escat 2351) (from Strem Chemicals) were used as received from the suppliers. The commercial catalyst 2.3 wt% Pt0 EnCat 40, supplied as a water-wet solid, was washed with ethanol and dried before use.SiliaCat Pt(0) was characterized using transmission electron microscopy (TEM), N2-isotherms, 29Si MAS NMR (magic angle spinning nuclear magnetic resonance) spectroscopy, and ICP-OES (inductively coupled plasma-optical emission spectroscopy). The TEM pictures were taken using a JEOL-2010 microscope equipped with a LaB6 electron gun source operated at 200 kV. Nitrogen adsorption and desorption isotherms were measured at 77 K using a Micrometrics TriStar II 3020 system. The resulting data were analysed with the TriStar II 3020 version 3.02 software. The Barrett–Joyner–Halenda (BJH) pore size and volume analysis was applied to the desorption branches of the isotherms. Solid-state 29Si NMR spectra were recorded on a Bruker Avance spectrometer (Milton, ON, Canada) at a Si frequency of 79.5 MHz. The sample was spun at 8 kHz at a magic angle and at room temperature in a 4 mm ZrO2 rotor. A Hahn echo sequence synchronized with the spinning speed was used while applying a TPPM15 composite pulse decoupling during acquisition. 2400 acquisitions were recorded with a recycling delay of 30 seconds. The leaching of Pt was assessed by ICP-OES analysis of the crude product in DMF or in 1,1,2-trichloroethane (concentration 100 mg mL−1) using a PerkinElmer Optima 2100 DV system. The values of Pt leached in ppm are mg of Pt per kg of crude product.
The batch reactor was charged with the desired amount of 5 wt% Pt/MeSiO1.5 catalyst (SiliaCat Pt(0) in a spherical morphology) and with the olefin. The olefin/SiliaCat Pt(0) catalyst mixture was heated under an atmosphere of air to desired temperature. Hydrosilylations are highly exothermic reactions in which the reaction temperature must be closely controlled. The Si–H silane precursor was therefore added at 1 mL min−1 addition rate with an additional ampoule under mechanical stirring at a controlled temperature ranging from 75 °C to 85 °C. The reaction mixture was kept under mechanical stirring at 700 rpm (rotation per minute) until maximum conversion was observed.
The heterogeneous catalyst was found to be very stable at the degree of olefin used. When using 1-octadecene of industrial grade (90% purity) or 1-octene in 95% purity, conversions were not affected. At the end of the reaction, samples were regularly collected, diluted in CDCl3 and analysed by 1H NMR or in anhydrous CH2Cl2 and analysed by GC-MS (internal standard used: mesitylene) to obtain the Si–H or olefin conversion. Once the reaction was complete, the catalyst was recovered by filtration at 50 °C (to avoid the hydrolysis of functionalized Si–H) through a Buchner funnel using a glass filter (fiber grade 691). The catalyst (between 0.1 and0.5 g) was washed with anhydrous toluene (3 × 10 mL) and with anhydrous THF (3 × 10 mL), after which it was dried under air at room temperature and stored in a closed vessel prior to reuse. The isolated yields were assessed by fractionary distillation under vacuum of the crude product, except for the functionalized chlorosilanes, which were isolated by rotavap distillation at 20 °C to remove the excess unreacted silane. The GC-MS analyses were performed using a 7890B GC System (Agilent Technologies) equipped with a HP-5MS 30 m capillary column (composed of (5%-phenyl)-methylpolysiloxane, 0.25 mm inner diameter and 0.25 μm film thickness) equipped with a mass spectrometer of 5977B Series with a selective detector operated in the electron impact ionization mode (70 eV). The analyses were carried out in the split mode, using helium as a carrier gas (1 mL min−1 flow rate). The injection temperature was of 250 °C, the interface was set at 325 °C, and the ion source was adjusted to 230 °C. The column was maintained at an initial temperature of 50 °C for 4.5 min, then ramped to 325 °C at 100 °C min−1 heating rate and maintained at 325 °C for 5 min. Mass spectra were recorded at 5.5 scans per s (m/z 50–550). The identification of the compounds was based on the comparison of their retention times with those of authentic samples and on the comparison of their EI-mass spectra with the NIST/NBS, Wiley library spectra and the literature.
Conflicts of interest
The authors declare no conflict of interest.References
- S. J. Clarson, J. J. Fitzgerald, M. J. Owen, S. D. Smith and M. E. Van Dyke, Science and Technology of Silicones and Silicone-Modified Materials, American Chemical Society, Washington DC, USA and Oxford University Press, Oxford, 2007 Search PubMed.
- The global market is estimated to reach a volume of around 3 million tonnes by 2022: Imarc Services Private Limited, Silicones Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2017-2022, Delhi, 2017 Search PubMed.
- J. L. Speier, J. A. Webster and G. H. Barnes, J. Am. Chem. Soc., 1957, 79, 974–979 CrossRef CAS.
- B. D. Karstedt, U.S. Patent, 3775452, 1973 Search PubMed.
- M. Pagliaro, R. Ciriminna, F. Béland and V. Pandarus, Eur. J. Org. Chem., 2013, 6227–6235 CrossRef CAS.
- Umicore, New Catalysts for Hydrosilylation Reactions, http://pmc.umicore.com/storage/pmc/umicore-pmc-new-hs-catalysts.pdf.
- C. H. Schuster, T. N. Diao, I. Pappas and P. J. Chirik, ACS Catal., 2016, 6, 2632–2636 CrossRef CAS.
- H. Ba, Ind. Eng. Chem. Res., 2014, 53, 1588–1597 CrossRef.
- Q. J. Miao, Z. P. Fang and G. P. Cai, Catal. Commun., 2003, 4, 637–639 CrossRef CAS.
- T. Galeandro-Diamant, R. Sayah, M. Zanota, S. Marrot, L. Veyre, C. Thieuleux and V. Meille, Chem. Commun., 2017, 53, 2962–2965 RSC.
- X. Cui, K. Junge, X. Dai, C. Kreyenschulte, M.-M. Pohl, S. Wohlrab, F. Shi, A. Brückner and M. Beller, ACS Cent. Sci., 2017, 3, 580–585 CrossRef CAS PubMed.
- R. Ciriminna, V. Pandarus, G. Gingras, F. Béland and M. Pagliaro, ACS Sustainable Chem. Eng., 2013, 1, 249–253 CrossRef CAS.
- M. Pagliaro, V. Pandarus, F. Béland, R. Ciriminna, G. Palmisano and P. Demma Carà, Catal. Sci. Technol., 2011, 1, 736–739 RSC.
- For the template-synthesis of spherical silica microparticles with tunable pore size, see for example: A. B. D. Nandiyanto, S.-G. Kim, F. Iskandar and K. Okuyama, Microporous Mesoporous Mater., 2009, 120, 447–453 CrossRef CAS.
- M. Boudart and G. Djega-Mariadassou, Kinetics of Heterogeneous Catalytic Reactions, Princeton University Press, Princeton, New Jerseym, 1984 Search PubMed.
- G. Leonfanti, M. Padovan, G. Tozzola and B. Venturelli, Catal. Today, 1998, 41, 207–219 CrossRef.
- A. S. Lilly Thankamony, O. Lafon, X. Lu, F. Aussenac, M. Rosay, J. Trébosc, H. Vezin and J.-P. Amoureux, Appl. Magn. Reson., 2012, 43, 237–250 CrossRef CAS.
- R. H. Glaser, G. L. Wilkes and C. E. Bronnimann, J. Non-Cryst. Solids, 1989, 113, 73–87 CrossRef CAS.
- A. Grunenwald, A. Ayral, P.-A. Albouy, C. Licitra, P. Gergaud, D. Quemener, A. Deratani, V. Rouessac, A. Zenasni and V. Jousseaume, Microporous Mesoporous Mater., 2012, 150, 64–75 CrossRef CAS.
- T. K. Meister, K. Riener, P. Gigler, J. Stohrer, W. A. Herrmann and F. E. Kühn, ACS Catal., 2016, 6, 1274–1284 CrossRef CAS.
- J. Stein, L. N. Lewis, Y. Gao and R. A. Scott, J. Am. Chem. Soc., 1999, 121, 3693–3703 CrossRef CAS.
- L. N. Lewis and R. J. Uriarte, Organometallics, 1990, 9, 621–625 CrossRef CAS.
- A. K. Roy, Adv. Organomet. Chem., 2008, 55, 1–59 CAS.
- (a) G. De Bo, G. Berthon-Gelloz, B. Tinant and I. E. Markó, Organometallics, 2006, 25, 1881–1890 CrossRef CAS; (b) R. Cano, M. Yus and D. J. Ramon, ACS Catal., 2012, 2, 1070–1078 CrossRef CAS.
- T. J. Geldbach, D. Zhao, N. C. Castillo, G. Laurenczy, B. Weyershausen and P. J. Dyson, J. Am. Chem. Soc., 2006, 128, 9773–9780 CrossRef CAS PubMed.
- Leaching values are given in mg kg−1 (ppm). Limit of detection: LODPt = 0.05 ppm in solution (100 mg mL−1 concentration) or 0.50 mg kg−1 in the crude product.
- R. G. Heidenreich, J. G. E. Krauter, J. Pietsch and K. Köhler, J. Mol. Catal. A: Chem., 2002, 182–183, 499–509 CrossRef CAS.
- T. Galeandro-Diamant, M.-L. Zanota, R. Sayah, L. Veyre, C. de Bellefon, V. Meille and C. Thieuleux, Chem. Commun., 2015, 51, 16194–16196 RSC.
- R. Ciriminna, A. Fidalgo, F. Béland, V. Pandarus, L. M. Ilharco and M. Pagliaro, Chem. Rev., 2013, 113, 6592–6620 CrossRef CAS PubMed.
- A. J. Chalk and J. F. Harrod, J. Am. Chem. Soc., 1965, 87, 16–21 CrossRef CAS.
- A. K. Roy and R. B. Taylor, J. Am. Chem. Soc., 2002, 124, 9510–9524 CrossRef CAS PubMed.
- W. Caseri and P. S. Pregosin, J. Organomet. Chem., 1988, 356, 259–269 CrossRef CAS; W. Caseri and P. S. Pregosin, Organometallics, 1988, 7, 1373–1380 CrossRef.
- X. Coqueret and G. Wegner, Organometallics, 1991, 10, 3139–3145 CrossRef CAS.
- J. C. Mitchener and M. S. Wrighton, J. Am. Chem. Soc., 1981, 103, 975–977 CrossRef CAS.
- (a) L. N. Lewis and N. Lewis, J. Am. Chem. Soc., 1986, 108, 7228–7231 CrossRef CAS; L. N. Lewis, J. Am. Chem. Soc., 1990, 112, 5998–6004 Search PubMed; (b) L. N. Lewis, R. J. Uriarte and N. Lewis, J. Mol. Catal., 1991, 66, 105–113 CrossRef CAS; (c) L. N. Lewis, R. J. Uriarte and N. Lewis, J. Catal., 1991, 127, 67–74 CrossRef CAS; L. N. Lewis and R. J. Uriarte, Organometallics, 1990, 9, 621–625 Search PubMed.
- Comprehensive Hendbook on Hydrosilylation, ed. B. Marciniec, Pergamon, Oxford, 1992 Search PubMed.
- B. Marciniec, H. Maciejewski, W. Duczmal, R. Fiedorow and D. Kityńki, Appl. Organomet. Chem., 2003, 17, 127–134 CrossRef CAS.
- D. Flassbeck, B. Pfleiderer, P. Klemens, K. G. Heumann, E. Eltze and A. V. Hirner, Anal. Bioanal. Chem., 2003, 375, 356–362 CrossRef CAS PubMed.
- See, for example: U.S. Food and Drug Administration, FDA Backgrounder on Platinum in Silicone Breast Implants, fda.gov, 18 January 2018. See the URL: https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/UCM064040.
| This journal is © The Royal Society of Chemistry 2019 |