Calluna vulgaris (L.) Hull: chemical characterization, evaluation of its bioactive properties and effect on the vaginal microbiota
Filipa
Mandim
a,
Lillian
Barros
 a,
Ricardo C.
Calhelha
a,
Ricardo C.
Calhelha
 a,
Rui M. V.
Abreu
a,
Rui M. V.
Abreu
 a,
José
Pinela
a,
José
Pinela
 a,
Maria José
Alves
a,
Maria José
Alves
 a,
Sandrina
Heleno
a,
Sandrina
Heleno
 a,
P. F.
Santos
a,
P. F.
Santos
 *b and
Isabel C. F. R.
Ferreira
*b and
Isabel C. F. R.
Ferreira
 *a
*a
aCentro de Investigação de Montanha (CIMO), Instituto Politécnico de Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal. E-mail: iferreira@ipb.pt; Fax: +351-273-325405; Tel: +351-273-303219
bCentro de Química – Vila Real (CQVR), Universidade de Trás-os-Montes e Alto Douro, 5001-801 Vila Real, Portugal. E-mail: psantos@utad.pt; Fax: +351-259-350480; Tel: +351-259-350276
First published on 20th November 2018
Abstract
The inflorescences of Calluna vulgaris were nutritionally and chemically characterized. Furthermore, different organic and aqueous extracts were prepared for the evaluation of their bioactive properties. From the obtained results, carbohydrates were the major compounds, followed by protein, lipid and ashes. It was possible to identify the sugars fructose and glucose, five organic acids, 26 individual fatty acids and the four tocopherol isoforms. Concerning the extract composition, 12 phenolic compounds were identified, with myricetin-3-O-glucoside and myricetin-O-rhamnoside predominating. Concerning the bioactive effects, the more polar extracts showed not only the highest amount in phenolic compounds, but also the strongest antioxidant and antibacterial activities. In contrast, for the anti-inflammatory and cytotoxic potential, the most effective extracts were the n-hexane and the ethyl acetate extracts, respectively. C. vulgaris presented a wide range of biological effects, highlighting their capacity to inhibit pathogenic bacteria without affecting beneficial microflora, corroborating their use in traditional medicine.
1. Introduction
Medicinal plants have been widely used by our ancestors as therapeutic agents. Although traditional medicine is being used less and less as the first form of treatment, medicinal plants have gained attention and have been extensively exploited for their richness in important biomolecules, able to prevent and treat different pathologies through the strengthening of the immune system or acting directly by inhibiting the mechanisms of action of these diseases.1 Phenolic compounds, vitamins, carotenoids and lipids are among the most bioactive molecules found in medicinal plants, directly correlated with their bioactive potential.1Calluna vulgaris (L.) Hull (syn. Erica vulgaris L.), known in English as Scotch heather, common heather or ling, is an acknowledged medicinal plant and is extensively used in folk medicine, being mostly consumed in the form of infusions or decoctions. This plant is also used as a condiment,2 and is usually found in foods, such as honey, providing excellent nectariferous properties.3C. vulgaris is the only representative of the genus Calluna, belonging to the order Ericales and to the Ericaceae family. This plant is native to Europe and North Africa and was further introduced in Australia, Canada and the United States of America.4 The flowering season of this plant usually starts in May and finishes in November. The inflorescences are very small, of 3 to 4 mm diameter, and have a short peduncle surrounded at the base by four ciliated bracelets. The corolla is rosy, deeply split with four lobes that persist even after drying.4 It has different ethnopharmacological uses, such as in the treatment of eczema, wounds and acne,5 rheumatic pain and arthritis problems,6 and outstanding effects on the treatment of urinary infections,4,6 disinfecting the urinary tract and mildly increasing urine production.7 Besides the mentioned biological effects, different extracts of this shrubby species have been described as having antimicrobial,8–10 antioxidant,11,12 anti-inflammatory,8,13 antinociceptive,13 antiviral,14 enzymatic inhibitory activity,8,15 and cytotoxic capacity.11,16C. vulgaris presents this wide range of biological effects due to its richness in bioactive molecules, namely, phenolic compounds, that can be present in higher or lower amounts depending on the growing conditions such as the soil, climate and altitude. The presence of these compounds is crucial to the performance of this species in exerting its bioactivity. This class of compounds is essentially provided with several OH groups with a strong ability to present antioxidant, antibacterial, anti-inflammatory and anti-proliferative properties.6 Besides phenolic compounds, also triterpenoids, namely, ursolic and oleanolic acids, were identified and related with the antitumoral properties of C. vulgaris.14,17 Lupeol and friedelin, with anti-inflammatory, antitumoral and antimicrobial properties, were identified in flower and leaf extracts of this shrub.18 The content of amino acids was also reported and related to the biological properties associated with this species. Amino acids such glutamic and aspartic acids, which are important in the neurotransmission process and also in albumin and globulin constitution, were identified.19
Considering the literature description of the bioactivity of this highly appreciated medicinal plant and taking into account its traditional application in the treatment of urinary infections, the aims of the present study were to: (i) fully characterize C. vulgaris in terms of its nutritional value and chemical composition; (ii) use different solvents to select the most bioactive extracts; (iii) evaluate the biological activities of the extracts such as antioxidant, anti-inflammatory, cytotoxic and antimicrobial against pathogenic and non-pathogenic bacteria, the latter belonging to the vaginal microbiota.
2. Materials and methods
2.1. Standards and reagents
All the solvents were of analytical grade and obtained commercially from Fisher Scientific (Lisbon, Portugal) and used as received. The FAME mixture (standard 47885-U) was purchased from Sigma-Aldrich (St Louis, MO, USA), as also sugar standards, organic acids, L-ascorbic acid, acetic and formic acid, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), trichloroacetic acid (TCA), ellipticine, sulphorhodamine B (SRB), trypan blue and Tris. Phenolic compound standards were purchased from Extra-synthèse (Genay, France). Tocol (50 mg mL−1) and individual tocopherols were obtained from Matreya (Pleasant Gap, PA, USA), 2,2-diphenyl-1-picrylhydrazyl (DPPH) from Alfa Aesar (Ward Hill, MA, USA), fetal bovine serum (FBS), L-glutamine, Hank's balanced salt solution (HBSS), trypsin–EDTA (ethylenediaminetetraacetic acid), penicillin/streptomycin solution (100 U mL−1 and 100 mg mL−1, respectively), RPMI-1640 and DMEM media were obtained from Hyclone (Logan, Utah, USA). Other reagents and solvents (analytical grade) were obtained from common commercial sources. Water was treated through a Milli-Q water purification system (TGI Pure Water Systems, Greenville, SC, USA). Tryptic Soy Broth (TSB) was obtained from Biomerieux (Marcy-l’Étoile, France). Blood agar with 7% sheep blood and MacConkey agar plates were purchased from Liofilchem, Italy. The dye p-iodonitrotetrazolium chloride (INT) was purchased from Sigma-Aldrich (Spruce Street; St Louis, MO).2.2. Plant material
The dried inflorescences of Calluna vulgaris (L.) Hull were obtained commercially through Girassol, a Portuguese herbal company. The material was reduced to a fine powder (∼20 mesh) and the homogeneous sample was stored in a refrigerator under protection from light.2.3. Chemical composition of the flowering inflorescences of C. vulgaris
2.4. Preparation of the organic and aqueous extracts
The preparation of the organic and aqueous extracts was based on the procedure previously described by the authors.24 For the preparation of the organic extracts (n-hexane, dichloromethane, ethyl acetate, acetone and methanol), 150 g of dried material were extracted through a successive extraction process, twice with each organic solvent (750 mL) for 24 h, under vigorous stirring (150 rpm), at room temperature. The extracts were filtered under reduced pressure through a Whatman No. 541 paper. The combined organic extracts were evaporated to dryness under reduced pressure at 40 °C (Büchi R-20, Flawil, Switzerland).For the decoction preparation, the dried sample (1 g) was added to 100 mL of distilled water and boiled for 5 minutes; the mixture was then left for another 5 minutes at room temperature.
The infusion extract was obtained by adding the dried sample (1 g) to 100 mL of boiling distilled water and left to infuse for 5 minutes at room temperature.
For both of the aqueous extracts, the final extract was filtered under reduced pressure through a Whatman No. 541 paper, frozen at −20 °C and lyophilized (Büchi R-20, Flawil, Switzerland).
2.5. Phenolic compound analysis
For the phenolic compound analysis, the final extracts of n-hexane, dichloromethane, acetone and ethyl acetate were re-dissolved in MeOH and the methanolic extract in MeOH/H2O (80/20, v/v), to a final concentration of 15 mg mL−1. The aqueous extracts were re-dissolved in water at a final concentration of 5 mg mL−1. The extracts were further filtered through 0.22 μm nylon syringe filters. The phenolic compounds were determined by High Performance Liquid Chromatography coupled to a diode array detector and to mass spectrometry using electrospray ionization (HPLC-DAD-ESI/MS), as previously described.25 The phenolic compounds’ tentative identification was performed correlating their retention times, UV-Vis and mass spectra data with commercial standards and the available literature information. For the quantification analysis, the area of the chromatographic peaks was compared with the calibration curves of the corresponding commercial standards. The results were expressed as mg per g of dry weight.2.6. Evaluation of the biological properties
For the TBARS assay, the organic extracts were re-dissolved in MeOH and the aqueous extracts in water, at a concentration of 5 mg mL−1. The stock solutions were subjected to successive dilutions for analysis (0.078–5 mg mL−1). The antioxidant capacity was measured by the decrease of thiobarbituric acid reactive substances (TBARS) using porcine (Sus scrofa) brain homogenates. The colour intensity of malondialdehyde–thiobarbituric acid (MDA–TBA) was measured by its absorbance at 532 nm, following a procedure described previously.26
For the OxHLIA assay, a known mass of extract (100 mg) was dissolved in PBS, obtaining different solutions ranging from 12.5 to 125 μg mL−1. To determinate the inhibition capacity of oxidative hemolysis associated with each of the tested extracts, an ovine blood sample was obtained from healthy animals donated by the School of Agriculture, Bragança, Portugal, and centrifuged for 5 minutes at 1000 rpm at 10 °C. The plasma and leukocyte supernatants were discarded and the red cell suspension was washed once with a NaCl solution (150 mM) and three times with phosphate buffered saline (PBS, pH 7.4).27 The erythrocytes were re-suspended in PBS (2.8%, v/v) and an aliquot (200 μL) of this solution was placed in a 48 well microplate along with an aliquot (400 μL) of each of the samples, PBS solution (control), or water (for complete hemolysis). The mixture was then incubated with shaking at 37 °C for 10 minutes. A solution of AAPH (200 mL, 160 mM in PBS) was added and the plate was again incubated under the same conditions. The optical density was measured at 690 nm every 10 minutes28 in order to determine the percentage of erythrocytes that remained intact through the following mathematical equation: P(%) = (St −CH0/S0 − CH0) × 100, where St and S0 relate to the optical density relative to time t and time 0 (in minutes), respectively, and CH0 to the optical density of total hemolysis at time 0. The results are expressed in terms of time interval (Δt) of hemolysis delay calculated using the formula: Δt (min) = Ht50 (sample) − Ht50 (control), where Ht50 corresponds to the time, in minutes, in which there is 50% hemolysis, a value obtained graphically through the hemolysis curve of each of the extracts. Then, the Δt values were correlated to the tested extract concentrations in order to calculate the concentration required to keep 50% of the erythrocyte population (P) intact for 60 min.
Commercial strains were also tested: pathogenic Gram-negative – Neisseria gonorrhoeae ATCC 49226, and also non-pathogenic bacteria usually present in the vaginal microbiota, variable Gram – Gardnerella vaginalis ATCC 14018 (Liofilchem, Italy). Lactobacillus plantarum DSM 12028, Lactobacillus delbrueckii subs. bulgaricus LMG 6901 and Lactobacillus casei NCTC 6375, were all donated by the Catholic University of Porto.
To determine the Minimum Inhibitory Concentrations (MIC), the organic and aqueous extracts were re-dissolved in 5% DMSO and TSB culture medium, obtaining a final concentration of 20 mg mL−1.
For the evaluation of the effects on the selected vaginal strains, only the most promising extracts were tested (acetone, methanol, decoction and infusion), based on the results from the pathogenic bacteria strains. This activity was assessed by the microdilution method and the colorimetric assay using INT (p-iodonitrotetrazolium chlorite, 0.2 mg mL−1).30 Briefly, 190 μL of the stock solution (20 mg mL−1) were added to the first well of a 96-well microplate. Successive dilutions were carried out over the wells containing 90 μL of medium. Afterwards, 10 μL of inoculum (1.5 × 108 CFU mL−1) were added to all wells containing the tested concentrations in the range of 0.156–20 mg mL−1.
Three negative controls were prepared (one with TSB medium, another one with the extract, and a third one with medium and antibiotic) and a negative control with medium and inoculum. Ampicillin, imipenem and vancomycin were the antibiotics used. The plates were then incubated in an oven (Jouan, Berlin, Germany) at 37 °C for 24 h. The MICs of the samples were determined after addition of the INT (0.2 mg mL−1, 40 μL) and after incubation at 37 °C for 30 min. The viable microorganisms reduced the yellow dye to pink. MIC was defined as the lowest extract concentration that prevented this change and exhibited the complete inhibition of bacterial growth. The assays were carried out in triplicate.
2.7. Statistical analysis
The described assays were performed in triplicate and the results were expressed as the mean ± standard deviation (SD). The results were obtained by analysis of variance (ANOVA) followed by Tukey's HSD test with α = 0.05. When the results had less than three samples, they were analyzed through Student's t-test in order to determine the significant differences between two samples, with p = 0.05. These statistical treatments were performed using SPSS v. 23.0.3. Results and discussion
3.1. Nutritional composition
The results regarding the nutritional composition of the inflorescences of C. vulgaris are presented in Table 1. Carbohydrates were the most abundant macronutrients (83.1 ± 0.3 g per 100 g dw), followed by proteins (8.4 ± 0.3 g per 100 g dw), lipids (4.42 ± 0.04 g per 100 g dw), and ashes (2.3 ± 0.04 g per 100 g dw). These results are in agreement with Rodrigues et al.,31 who evaluated the nutritional composition of the wild flowers of C. vulgaris and also identified carbohydrates in higher amounts (36.2 ± 0.2 g per 100 g dw), followed by proteins (6.8 ± 0.27 g per 100 g dw), lipids (3.7 ± 0.1 g per 100 g dw), and ashes (2.3 ± 0.04 g per 100 g dw). Fructose and glucose were the two identified sugars, glucose being present in higher amounts (5.36 ± 0.08 g per 100 g dw).| Nutritional value (mg per 100 g dw) | Organic acids (g per 100 g dw) | ||
|---|---|---|---|
| Ash (mg per 100 g dw) | 4.06 ± 0.03 | Oxalic acid | 0.208 ± 0.001 |
| Proteins (mg per 100 g dw) | 8.4 ± 0.3 | Quinic acid | 1.97 ± 0.04 |
| Fat (mg per 100 g dw) | 4.42 ± 0.04 | Ascorbic acid | 0.0166 ± 0.0001 |
| Total carbohydrates (mg per 100 g dw) | 83.1 ± 0.3 | Citric acid | 0.586 ± 0.007 |
| Energy (kcal per 100 g dw) | 406.9 ± 0.1 | Total organic acids | 2.79 ± 0.04 |
| Free sugars (g per 100 g dw) | |
|---|---|
| dw – dry weight; results are expressed as the mean value ± standard deviation (SD). | |
| Fructose | 2.92 ± 0.05 |
| Glucose | 5.36 ± 0.08 |
Concerning the organic acid profile (Table 1), it was possible to identify and quantify oxalic, quinic, ascorbic and citric acids, citric acid being present in higher content (0.586 ± 0.007 g per 100 g dw), and ascorbic acid in lower amount (0.0166 ± 0.0001 g per 100 g dw). Ascorbic acid has already been identified in extracts obtained from C. vulgaris leaves in amounts of 267.6 mg per 100 g.32 Moreover, quinic acid was also found in the flowers of this species (27.07 μg mL−1).31 The differences observed between the results of these authors and the ones obtained in the present work can be due to the different origins of plant materials, since the inflorescences studied in this work were commercially obtained and the ones reported by the other authors corresponded to wild samples.
Regarding fatty acid composition (Table 2), twenty-six different compounds were identified in the inflorescences of C. vulgaris. Linolenic acid (C18:2n6) was the major compound (21%), followed by palmitic (19.6%) and linoleic (19%) acids, eicosapentarenoic acid (C20:2) being the minor compound present (0.089%). The presence of palmitic (C16:0, 21%), linolenic (C18:3n3, 35%) and linoleic (C18:2n6c, 27%) acids as the major fatty acids present in C. vulgaris flowers was also reported.31 Moreover, there was a prevalence of polyunsaturated fatty acids (PUFA) (53%), followed by saturated fatty acids (SFA) with 39.9% and lastly monounsaturated fatty acids (MUFA), with 7.1%.
| Fatty acids (relative percentage %) | |||
|---|---|---|---|
| dw – dry weight; C8:0 – caprylic acid; C10:0 – capric acid; C11:0 – undecanoic acid; C12:0 – lauric acid; C14:0 – myristic acid; C15:0 – pentadecanoic acid; C16:0 – palmitic acid; C17:0 –heptadecanoic acid; C18:0 – stearic acid; C18:1n9 – oleic acid; C18:2n6 – linoleic acid; C18:3n3 – linolenic acid; C20:0 – arachidic acid; C20:1 – gadoleic acid; C20:2 – eicosadienoic acid; C21:0 – heneicosanoic acid; C20:3n6 – eicosatrienoic acid; C20:3n3 – 11,14,17-eicosatrienoic acid; C22:0 – behenic acid; C22:1 – eicosenoic acid; C20:5n3 – eicosapentaenoic acid; C22:2 – dodecanoic acid; C23:0 – tricosanoic acid; C24:0 – lignocéric acid; C24:1 – nervonic acid. SFA – saturated fatty acids; MUFA – monounsaturated fatty acids; PUFA – polyunsaturated fatty acids. Results are expressed as the mean values ± standard deviation (SD). | |||
| C8:0 | 0.17 ± 0.01 | C20:3n3 | 11.0 ± 0.5 |
| C10:0 | 0.17 ± 0.01 | C22:0 | 2.84 ± 0.01 |
| C11:0 | 0.64 ± 0.03 | C22:1 | 0.178 ± 0.007 |
| C12:0 | 0.47 ± 0.01 | C20:5n3 | 0.307 ± 0.006 |
| C14:0 | 1.52 ± 0.06 | C22:2 | 0.669 ± 0.006 |
| C15:0 | 0.33 ± 0.02 | C23:0 | 5.6 ± 0.3 |
| C16:0 | 19.6 ± 0.6 | C24:0 | 0.108 ± 0.006 |
| C16:1 | 1.11 ± 0.03 | C24:1 | 2.40 ± 0.07 |
| C17:0 | 0.64 ± 0.03 | SFA | 39.9 ± 0.2 |
| C18:0 | 5.07 ± 0.02 | MUFA | 7.05 ± 0.05 |
| C18:1n9c | 3.097 ± 0.003 | PUFA | 53.0 ± 0.2 |
| C18:2n6c | 19.2 ± 0.1 | ||
| C18:3n3 | 21.0 ± 0.2 | Tocopherols (mg per 100 g dw) | |
| C20:0 | 2.4 ± 0.2 | α-Tocopherol | 5.84 ± 0.07 |
| C20:1 | 0.267 ± 0.005 | β-Tocopherol | 0.2500 ± 0.0001 |
| C20:2 | 0.089 ± 0.003 | γ-Tocopherol | 0.75 ± 0.03 |
| C21:0 | 0.289 ± 0.002 | δ-Tocopherol | 1.05 ± 0.08 |
| C20:3n6 | 0.73 ± 0.04 | Total tocopherols | 8.0 ± 0.2 |
With respect to tocopherols, all four isoforms of tocopherols were identified with α-tocopherol present in higher content (5.84 ± 0.07 mg per 100 g dw) and β-tocopherol in lower amounts (0.250 ± 0.001 mg per 100 g dw). The presence of the four isoforms in flowers of C. vulgaris was described by Rodrigues et al.,31 who also stated the highest content in α-tocopherol (32.5 mg per 100 g) and the lowest amount in β-tocopherol (0.39 mg per 100 g). The values of our study were found in lower content, which could be explained by the different origins of these species.
3.2. HPLC-DAD-ESI/MS analysis of phenolic compounds
The peak characteristics and tentative identities of the compounds present in the different organic and aqueous extracts of the aerial parts of C. vulgaris are presented in Table 3. Twelve compounds were detected, two of which were phenolic acid derivatives (hydroxycinnamic acid derivatives) and ten flavonoids, particularly nine flavonols and one flavan-3-ol. Among the phenolic acids, peaks 1 ([M − H]− at m/z 353) and 2 ([M − H]− at m/z 289) were positively identified as 5-O-caffeoylquinic acid and catechin, respectively, according to their retention time, mass and UV-vis characteristics in comparison with the commercial standards. Peak 4 ([M − H]− at m/z 337) was identified as 5-p-coumaroylquinic acid taking into account the hierarchical fragmentation pattern described.33 All of the mentioned compounds have been previously reported in different plant parts of C. vulgaris.34| Peak | R t (min) | λmax (nm) | [M − H]− (m/z) | MS2 (m/z) | Tentative identification | Quantification (mg g−1 extract) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n-Hexane | Dichloromethane | Ethyl acetate | Acetone | Methanol | Decoction | Infusion | ||||||
n.d. – not detected. Calibration curves: 1 – caffeic acid (y = 388345x + 406.369, R2 = 0.994); 2 − (+)-catechin (y = 84950x − 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, R2 = 0.999); 3 – myricetin (y = 23287x − 581 200, R2 = 0.999); 3 – myricetin (y = 23287x − 581![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 708, R2 = 0.9988); 4 – p-coumaric acid (y = 301950x + 6966.7, R2 = 0.999); 5 – quercetin-3-O-glucoside (y = 34843x − 160 708, R2 = 0.9988); 4 – p-coumaric acid (y = 301950x + 6966.7, R2 = 0.999); 5 – quercetin-3-O-glucoside (y = 34843x − 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 173, R2 = 0.9988). Results are expressed as the mean values ± standard deviation (SD). Different letters correspond to significant differences (p < 0.05). 173, R2 = 0.9988). Results are expressed as the mean values ± standard deviation (SD). Different letters correspond to significant differences (p < 0.05). |
||||||||||||
| 1 | 7.07 | 325 | 353 | 191(100), 179(5), 173(2), 135(2) | 5-O-Caffeoylquinic acid1 | n.d. | n.d. | n.d. | 0.20 ± 0.02c | 3.3 ± 0.1b | 3.1 ± 0.2b | 5.0 ± 0.2q |
| 2 | 7.15 | 275 | 289 | 245(100), 203(5), 137(2) | (+)-Catechin2 | n.d. | n.d. | 0.52 ± 0.09 | n.d. | n.d. | n.d. | n.d. |
| 3 | 11.15 | 334 | 479 | 317(100) | Myricetin-O-hexoside3 | n.d. | n.d. | n.d. | 2.12 ± 0.02c | 2.09 ± 0.01c | 4.50 ± 0.03a | 4.34 ± 0.02b |
| 4 | 11.88 | 310 | 337 | 191(100), 173(16), 163(6) | 5-p-Coumaroylquinic acid4 | n.d. | n.d. | n.d. | n.d. | 0.10 ± 0.01b | 0.06 ± 0.01c | 0.150 ± 0.001a |
| 5 | 15.23 | 356 | 479 | 317(100) | Myricetin-3-O-glucoside3 | 1.672 ± 0.001e | n.d. | 1.63 ± 0.07e | 4.96 ± 0.09d | 4.30 ± 0.09c | 5.80 ± 0.01b | 8.1 ± 0.1a |
| 6 | 17.85 | 349 | 463 | 317(100) | Myricetin-O-rhamnoside3 | 1.668 ± 0.001e | n.d. | 1.66 ± 0.09e | 2.04 ± 0.06b | 2.81 ± 0.05c | 4.86 ± 0.06b | 5.8 ± 0.1a |
| 7 | 18.68 | 354 | 463 | 301(100) | Quercetin-3-O-glucoside5 | 0.310 ± 0.001d | n.d. | 0.37 ± 0.03d | 2.20 ± 0.06b | 1.680 ± 0.004c | 1.650 ± 0.001c | 3.01 ± 0.08a |
| 8 | 19.08 | 354 | 463 | 301(100) | Quercetin-O-hexoside5 | 0.3080 ± 0.0002e | n.d. | 0.3550 ± 0.0003e | 0.800 ± 0.002d | 0.97 ± 0.07c | 1.22 ± 0.03b | 2.05 ± 0.09a |
| 9 | 22.44 | 337 | 477 | 315(100) | Isorhamnetin-3-O-glucoside5 | 0.3100 ± 0.0002f | n.d. | 1.6 ± 0.3d | 1.25 ± 0.08e | 2.8 ± 0.1b | 2.33 ± 0.03c | 5.48 ± 0.08a |
| 10 | 26.42 | 343 | 463 | 301(100) | Quercetin-O-hexoside5 | n.d. | n.d. | 0.298 ± 0.005e | 0.42 ± 0.03c | 0.350 ± 0.005d | 0.810 ± 0.001a | 0.77 ± 0.01b |
| 11 | 27.30 | 341 | 431 | 285(100) | Kaempferol-O-rhamnoside5 | n.d. | n.d. | 0.36 ± 0.04b | n.d. | 0.39 ± 0.01b | 0.830 ± 0.003a | 0.81 ± 0.01a |
| 12 | 28.38 | 345 | 461 | 315(100) | Isorhamnetin-O-rhamnoside5 | 0.3070 ± 0.0001e | n.d. | 0.428 ± 0.001d | 0.41 ± 0.01d | 0.53 ± 0.01c | 0.92 ± 0.01b | 1.12 ± 0.03a |
| Total phenolic acids | n.d. | n.d. | n.d. | 0.20 ± 0.02c | 3.4 ± 0.1b | 3.2 ± 0.2b | 5.1 ± 0.2a | |||||
| Total flavonoids | 4.570 ± 0.001f | n.d. | 6.6 ± 0.5e | 13.2 ± 0.1d | 15.9 ± 0.2c | 22.92 ± 0.01b | 31.5 ± 0.5a | |||||
| Total phenolic compounds | 4.570 ± 0.001f | n.d. | 7.2 ± 0.4e | 13.39 ± 0.09d | 19.31 ± 0.02c | 26.1 ± 0.2b | 36.6 ± 0.7a | |||||
The remaining peaks corresponded to flavonol glycoside derivatives from quercetin (λmax around 350 nm and an MS2 fragment at m/z 301), kaempferol (λmax around 348 nm, MS2 fragment at m/z 285), myricetin (λmax around 354 nm, MS2 fragment at m/z 317), and isorhamnetin (λmax at 354 nm, MS2 fragment at m/z 315) (Table 3). Peaks 5 (myricetin-3-O-glucoside), 7 (quercetin-3-O-glucoside), and 9 (isorhamnetin-3-O-glucoside) were positively identified in comparison with the commercial standard. Compounds 8 and 10 ([M − H]− at m/z 463) were assigned to quercetin derivatives, while peaks 6 ([M − H]− at m/z 463), 11 ([M − H]− at m/z 431), and 12 ([M − H]− at m/z 461) were assigned to myricetin, kaempferol and isorhamnetin derivatives, respectively. They all presented one MS2 fragment, corresponding to the loss of hexosyl (−162 mu) and rhamnosyl (−146 mu) moieties, respectively. The elution order was coherent with the type of sugar substituent and according to their expected polarity, although the position and nature of the sugar moieties could not be identified, because their retention times did not correspond to any of the standards available.
Different quercetin, myricetin, kaempferol, and isorhamnetin derivatives have been found in different parts of C. vulgaris.13,31,34 In the present study myricetin-3-O-glucoside and myricetin-O-rhamnoside were the most abundant compounds present in all the different organic and aqueous extracts, followed by 5-O-caffeoylquinic acid in the methanol, infusion and decoction extracts. To the best of our knowledge, both main myricetin glycoside derivatives have not been previously identified in C. vulgaris, only the aglycone having been identified.34 The decoction revealed the highest concentration in phenolic compounds, followed by the infusion, methanol, acetone, ethyl acetate, and finally the hexane extract; therefore, the phenolic compound content is higher when solvent polarity increases. Thus, the dichloromethane extract did not present any of the identified phenolic compounds. Fig. 1 represents the phenolic profile found in the methanol extract.
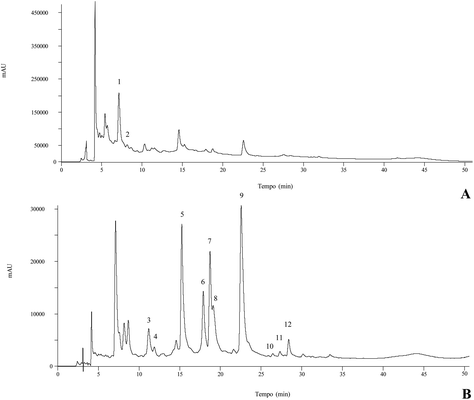 | ||
| Fig. 1 Phenolic compound profile of the methanolic extract of C. vulgaris at 280 nm (A) and 370 nm (B). Numbers correspond to peak identification in Table 3. | ||
3.3. Bioactive properties
Two methods were used to evaluate the antioxidant activity of organic and aqueous extracts of C. vulgaris (Table 4). In both assays, acetone was the most active extract with an EC50 of 8 μg mL−1 for the TBARS and 14 μg mL−1 for the OxHLIA assay. These EC50 values are higher than the ones exhibited by Trolox, a recognized antioxidant agent (EC50 of 9.1 μg mL−1 and 21.2 μg mL−1 for the TBARS and the OxHLIA assay, respectively), highlighting these extracts as a promising source of compounds with antioxidant potential. In turn, the dichloromethane extract revealed the weakest potential, presenting no activity in the TBARS assay and an EC50 value of 384 μg mL−1 in the OxHLIA assay. The antioxidant potential is widely described in the literature, especially the scavenging capacity through the DPPH assay. To the best of our knowledge, the TBARS assay in C. vulgaris was only evaluated in the petroleum ether extract of the roots of this species; at a concentration of 25 μg mL−1 the TBARS inhibition was about 20%.8| n-Hexane | Dichloromethane | Ethyl acetate | Acetone | Methanol | Decoction | Infusion | |
|---|---|---|---|---|---|---|---|
| EC50 values corresponded to the extract concentration that inhibits in 50% the oxidation and inflammatory process. Trolox (EC50 values): TBARS: 9.1 ± 0.3; OxHLIA: 21.2 ± 0.7. Dexametasona (IC50 values): 16 ± 1. GI50 values correspond to the concentration that causes 50% inhibition of cell proliferation; n.d. – not detected; AGS – human gastric adenocarcinoma; MCF-7 – human breast adenocarcinoma; NCI-H460 – human lung carcinoma; HeLa – human cervix adenocarcinoma; HepG2 – hepatocellular carcinoma; PLP2 – primary culture of non-tumoral pig liver cells. Ellipticine (GI50 values). AGS: 2.59 ± 0.05; MCF-7: 1.21 ± 0.02; NCI-H460: 0.91 ± 0.11; HeLa: 1.03 ± 0.09; HepG2: 1.1 ± 0.09; PLP2: 2.29 ± 0.18. Results are expressed as the mean values ± standard deviation (SD). Different letters represent significant differences (p < 0.05). | |||||||
| Antioxidant activity (EC 50 , μg mL −1 ) | |||||||
| TBARS | 95 ± 4a | n.d. | 33 ± 3e | 8 ± 0.2f | 53 ± 1c | 79 ± 2b | 61 ± 1d |
| OxHLIA (Δt = 60 min) | 320 ± 10b | 384 ± 10a | 80 ± 2c | 14 ± 0.4e | 34 ± 0.6d | 38 ± 1d | 66 ± 0.3c |
| Anti-inflammatory activity (EC 50 , μg mL −1 ) | |||||||
| RAW 246.7 | 66 ± 3e | 81 ± 3de | 81 ± 6de | 90 ± 4c | 189 ± 3b | 280 ± 13a | 297 ± 17a |
| Cytotoxic activity (GI 50 , μg mL −1 ) | |||||||
| AGS | 58 ± 5e | 106 ± 9c | 49 ± 4e | 100 ± 6c | 78 ± 4d | 281 ± 12b | 305 ± 10a |
| MCF-7 | 41 ± 4d | 101 ± 5b | 40 ± 3d | 89 ± 5bc | 65 ± 4cd | 353 ± 32a | 351 ± 6a |
| NCI-H460 | 61 ± 3d | 133 ± 6a | 50 ± 1e | 121 ± 4b | 101 ± 9c | n.d. | n.d. |
| HeLa | 33 ± 2d | 83 ± 3c | 31± 3d | 69 ± 5c | 76 ± 6c | 224 ± 22b | 258 ± 14a |
| HepG2 | 43 ± 4e | 120 ± 12c | 44 ± 1e | 145 ± 12b | 97 ± 6d | 368 ± 23a | 352 ± 7a |
| PLP2 | 61 ± 3d | 240 ± 8a | 41 ± 2e | 198 ± 5b | 163 ± 8c | >400 | >400 |
The highest levels of phenolic compounds found in this species have been correlated to the antioxidant activity.6,35 Nevertheless, in the present study the extracts with the highest phenolic compound content (decoction and infusion extracts) were not the most active regarding the antioxidant properties, whereas the acetone extract revealed the highest potential. This fact can be due to the antioxidant activity of other less polar compounds extracted with acetone.
All the extracts were also evaluated regarding LPS-induced NO production in the murine macrophage (RAW 264.7) cell line and the results are presented in Table 4. Nitric oxide (NO) has an important role in the inflammatory process; thus, obtaining compounds with a high capacity to inhibit NO production is a promising advance in the development of active formulations. All the tested extracts showed anti-inflammatory capacity with EC50 values between 66 and 297 μg mL−1. In general, an increase in the polarity solvent decreased the anti-inflammatory potential, suggesting that the phytochemical constituents responsible for this biological potential are less polar. Accordingly, the n-hexane extract was the one exhibiting the highest activity (EC50 = 66 ± 3 μg mL−1). The decoction and infusion extracts presented the highest EC50 values (280 ± 13 and 297 ± 17 μg mL−1, respectively), presenting the weakest potential. The anti-inflammatory activity of the different constituents of C. vulgaris has already been demonstrated through different biological assays, such as the capacity to stabilize the HRBC membrane and to scavenge NO and in vivo assays with the induction of inflammation in mice,13 and the ability to suppress the signaling of a nuclear factor (NF-KB).36 Nevertheless, an evaluation on the RAW 264.7 cell line was not reported until now.
The results obtained for the five human tumor cell lines and for the primary culture cell line are presented in Table 4. All the tested extracts demonstrated cytotoxic activity for almost all the analyzed cell lines, except for the aqueous extracts that presented no activity against the lung tumor cell line (NCI-H460). HeLa was the most susceptible cell line, presenting GI50 ranging from 33 to 83 μg mL−1 for the organic extracts, and 224 to 258 μg mL−1 for the aqueous extracts. The n-hexane and the ethyl acetate extracts were the most active against all the tested cell lines, presenting GI50 values in the range of 33–61 μg mL−1 and 31–50 μg mL−1, respectively. Nevertheless, regarding the cytotoxic effects on normal cells, these were also the extracts presenting the strongest activity, in some cases presenting the same GI50 values as the ones needed to inhibit the tumor cell growth. In this perspective, among the most active extracts, it can be considered that the acetone and the methanol extracts are the most promising candidates to be applied in this field, since the GI50 values needed to inhibit the tumor cells are significantly lower than the ones needed to inhibit the normal cells (Table 4).
The cytotoxic activity of different extracts from the aerial parts of C. vulgaris obtained by sequential percolation and with solvents of different polarities (n-hexane, chloroform, ethyl acetate, methanol, water) was evaluated in a rat melanoma (B16) cell line.16 These authors described that the extracts with lower polarity presented better results, whereas the aqueous extracts were less active, which is in agreement with the results obtained in the present work. In another study,14 which studied C. vulgaris extracted under moderate pressure conditions (30 MPa), triterpenoids, namely ursolic and oleanolic acids, were identified as the major compounds. These compounds have also been described as being responsible for the cytotoxic effects of C. vulgaris not only against breast cancer (MCF-7), mouse melanoma (B16F10), glioma (U87-MG), hepatic cancer (Hep-G2), and leukemic cells (HL-60),17 but also against normal kidney cells of monkeys.37
One of the ethnopharmacological applications of C. vulgaris most frequently described in the literature is in the treatment of infections related to the urinary tract. As a result of this important activity, the extracts obtained were tested against twelve bacterial strains, which could be responsible for infections of the urogenital tract. The results for the different tested extracts are presented in Table 5, and the results are expressed as MIC values (mg mL−1). In general, all the extracts showed inhibiting potential against the pathogenic bacteria. The Gram-positive bacteria presented lower MIC values, being the most susceptible strains when compared with the Gram-negative strains. The most polar extracts revealed the highest antibacterial activity, such as decoction and infusion, and the opposite effect was observed for the extracts such as the n-hexane, dichloromethane and ethyl acetate extracts, which revealed the weakest potential. The n-hexane extract was the weakest extract, presenting the highest MIC values, while the methanol, acetone and aqueous extracts exhibited the strongest antibacterial capacity with lower MIC values. The decoction was the one presenting the most interesting potential, with lower MIC and MBC values for almost all the bacterial strains tested, with the exception of Pseudomonas aeroginosa and Enterococcus faecalis, for which the most active extract was acetone. This fact corroborates the knowledge applied in traditional medicine, since the infusions and decoctions of this species are traditionally used for the treatment of different infections, as previously mentioned.
| n-Hexane | Dichloromethane | Ethyl acetate | Acetone | Methanol | Decoction | Infusion | Ampicillin (20 mg mL−1) | Imipenem (1 mg mL−1) | Vancomicin (1 mg mL−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| MIC – minimal inhibitory concentration; MBC – minimal bactericidal concentration; n.t. – not tested; MRSA – methicillin resistant Staphylococcus aureus; MSSA – methicillin sensitive Staphylococcus aureus. | ||||||||||||||||||||
| Pathogenic | ||||||||||||||||||||
| Gram-negative | ||||||||||||||||||||
| Escherichia coli | 20 | >20 | 20 | >20 | 20 | >20 | 5 | >20 | 2.5 | >20 | 2.5 | >20 | 2.5 | >20 | <0.15 | <0.15 | <0.0078 | <0.0078 | n.t. | n.t. |
| Klebsiella pneumoniae | 20 | >20 | 20 | >20 | 20 | >20 | 5 | >20 | 2.5 | >20 | 2.5 | >20 | 2.5 | >20 | 10 | 20 | <0.0078 | <0.0078 | n.t. | n.t. |
| Morganella morganii | 20 | >20 | 5 | >20 | 5 | >20 | 5 | >20 | 2.5 | >20 | 2.5 | >20 | 2.5 | >20 | 20 | >20 | <0.0078 | <0.0078 | n.t. | n.t. |
| Proteus mirabilis | >20 | >20 | 10 | >20 | 20 | >20 | 10 | >20 | 20 | >20 | 10 | >20 | 20 | >20 | <0.15 | <0.15 | <0.0078 | <0.0078 | n.t. | n.t. |
| Pseudomonas aeruginosa | 20 | >20 | 20 | >20 | 20 | >20 | 5 | 20 | 10 | >20 | 10 | >20 | 10 | >20 | >20 | >20 | 0.5 | 1 | n.t. | n.t. |
| Neisseria gonorrohoeae | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | 2.5 | >20 | 5 | >20 | 2.5 | >20 | 2.5 | >20 | <0.15 | <0.15 | n.t. | n.t. | n.t. | n.t. |
| Gram-positive | ||||||||||||||||||||
| Enterococcus faecalis | >20 | >20 | 5 | >20 | 20 | >20 | 2.5 | >20 | 10 | >20 | 20 | >20 | 10 | >20 | <0.15 | <0.15 | n.t. | n.t. | <0.0078 | <0.0078 |
| Listeria monocytogenes | >20 | >20 | 20 | >20 | 10 | 20 | 2.5 | >20 | 2.5 | >20 | 2.5 | >20 | 2.5 | >20 | <0.15 | <0.15 | n.t. | n.t. | n.t. | n.t. |
| MRSA | 20 | 20 | 10 | 20 | 20 | 20 | 1.25 | >20 | 1.25 | 20 | 1.25 | 20 | 1.25 | 20 | <0.15 | <0.15 | n.t. | n.t. | <0.0078 | <0.0078 |
| MSSA | 10 | 20 | 0.6 | 20 | 0.15 | 20 | 0.6 | 20 | 0.15 | 20 | <0.15 | 20 | 0.15 | 20 | <0.15 | <0.15 | n.t. | n.t. | 0.25 | 0.5 |
| Variable gram | ||||||||||||||||||||
| Gardnerella vaginallis | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | 5 | n.t. | 5 | n.t. | 5 | n.t. | 5 | n.t. | <0.15 | <0.15 | n.t. | n.t. | n.t. | n.t. |
| Non-pathogenic | ||||||||||||||||||||
| L delbrueckii | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | 10 | n.t. | 20 | n.t. | 10 | n.t. | 10 | n.t. | <0.15 | <0.15 | n.t. | n.t. | n.t. | n.t. |
| L. casei | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | 10 | n.t. | >20 | n.t. | 10 | n.t. | 10 | n.t. | <0.15 | <0.15 | n.t. | n.t. | n.t. | n.t. |
| L. plantarum | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | 20 | n.t. | >20 | n.t. | 10 | n.t. | 10 | n.t. | <0.15 | <0.15 | n.t. | n.t. | n.t. | n.t. |
Different authors described that the extracts of C. vulgaris presented no activity against some of the bacterial strains tested in the present work, such as Escherichia coli,8,9,31,35Klebsiella pneumoniae,9,31Pseudomonas aeruginosa,9,31,35 methicillin-resistant Staphylococcus aureus (MRSA) and Proteus mirabilis.35 This fact can be justified by the use of different parts of C. vulgaris, since these authors used the seeds and roots.
Most of the results described in the literature evaluate the activity of organic extracts.10,31 The antibacterial activity associated with aqueous, ethanolic and hydroethanolic extracts of wild C. vulgaris flowers were studied against seven bacterial strains: Staphylococcus epidermidis, Staphylococcus aureus, MRSA, Pseudomonas aeroginosa, Escherichia coli, Klebsiella pneumoniae and Candida albicans.31 In contrast to the present work, the extract that showed the best activity was the hydroethanolic (MIC 2.0–8.5 mg mL−1), and the extract with the lowest potential was the ethanolic (MIC 10.8–43.2 mg mL−1). Infusions obtained with water, ethanol and ethyl acetate were tested against three different bacteria: Escherichia coli, Enterococcus faecalis and Proteus vulgaris, describing that the aqueous extract was the most active and the ethyl acetate was the weakest.10 These results are in agreement with the ones obtained in the present work.
The effects on the vaginal microbiota were studied using bacteria belonging to the vaginal microbiota that have a direct effect on the host, since they have the ability to protect the organism from possible infections as a result of the antimicrobial effect they exert against pathogenic organisms. This ability derives from the production of substances such as lactic acid, bacteriocins and hydrogen peroxide, mainly by Lactobacillus.38 Each of these compounds effectively has the ability to kill pathogenic substances:39 lactic acid allows the maintenance of a low vaginal pH, between 3.5 and 4.5, limiting the growth of potentially dangerous microorganisms; bacteriocins inhibit the growth of several bacteria including Gardnerella vaginalis; and finally, hydrogen peroxide has the ability to inhibit some Gram-negative bacteria, such as Pseudomonas aeruginosa, and Gram-positive bacteria, such as Staphylococcus aureus.40,41
As a result of the promising antibacterial activity associated with some of the tested extracts (acetone, methanol, decoction and infusion), and based on the knowledge of traditional medicine, the antibacterial activity of these extracts was also evaluated in the vaginal microbiota (Table 5). Gardnerella vaginalis and Neisseria gonorrhoeae are the strains usually associated with bacterial vaginosis, a vaginal infection that occurs when the vaginal microbiota suffers an imbalance, namely with the decrease of Lactobacillus.38 In this perspective, the main objective was to demonstrate that the extracts present the ability to inhibit these bacteria without affecting Lactobacillus strains. Through the comparison of the results for pathogenic and non-pathogenic strain bacteria, it is possible to state that MIC values presented by the extracts are higher for the Lactobacillus strains compared to the ones needed to inhibit the pathogenic microorganisms, with the exception of Proteus mirabilis, Pseudomonas aeruginosa and Enterococcus faecalis.
From Table 5 it is possible to visualize that for a MIC of 5 mg mL−1 the majority of the pathogenic strains are inhibited, without affecting Lactobacillus strains that showed a higher MIC value (≥10 mg mL−1). Regarding the microorganisms belonging to the vaginal microbiota, MIC values for Gardnerella vaginalis and Neisseria gonorrhoeae are lower than those exhibited for Lactobacillus, which allows the inhibition of the pathogens without affecting beneficial bacterial strains. It can be highlighted that the methanolic and acetone extracts revealed the best potential, by inhibiting Gardnerella vaginalis and Neisseria gonorrhoeae MIC values of 5 and 2.5 mg mL−1, respectively, and presenting a much higher MIC value for all the Lactobacillus strains (20 mg mL−1).
To the best of our knowledge, there is no report in the literature regarding the antibacterial activity of C. vulgaris extracts relative to the vaginal microbiota. However, a study recently published,42 also using a natural product, has shown that it is possible to inhibit vaginal Gardnerella without interfering with the vaginal microbiota.
4. Conclusions
C. vulgaris was evaluated regarding its nutritional value and chemical composition. Moreover, five organic and two aqueous extracts were obtained from inflorescences of C. vulgaris by extraction with solvents of different polarities, which were fully characterized regarding their phenolic composition and their biological effects evaluated. From a nutritional point of view, carbohydrates were the major compounds found in C. vulgaris, followed by proteins, lipids and ashes. Fructose and glucose were the only sugars identified with glucose predominating. Five organic acids were also quantified, citric acid being present in the highest amount. Regarding fatty acids, twenty-six compounds were identified, PUFA being the most predominant fatty acid. The four isoforms of tocopherol were identified, α-tocopherol being the most abundant. Furthermore, twelve phenolic compounds were identified in the extracts, myricetin-3-O-glucoside and myricetin-O-rhamnoside being the main molecules present. The obtained extracts revealed different biological potentials according to the studied biological properties. Most polar extracts, such as the methanolic, acetone and aqueous extracts, showed the strongest potential for the antioxidant and antibacterial activities and the highest content of phenolic compounds. In contrast, for the anti-inflammatory and cytotoxic effects, the n-hexane extract was the most effective. Overall, it should be stated that the inflorescences of C. vulgaris presented huge potential in terms of bioactivity, especially against a range of pathogenic bacteria strains, and most importantly, without affecting normal vaginal microbiota, highlighting their potential for application in the pharmaceutical sector. Moreover, the obtained results corroborate the use of the aqueous extracts in traditional medicine.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) and FEDER under Programme PT2020 for financial support through CIMO (UID/AGR/00690/2013), CQ-VR (UID/QUI/00616/2013), and S. Heleno (SFRH/BPD/101413/2014) grants, and L. Barros and R. C. Calhelha contracts. The authors are also grateful to the FEDER-Interreg España-Portugal programme for financial support through the project 0377_Iberphenol_6_E. The authors are thankful to Dr Ana Maria Carvalho from the Instituto Politécnico de Bragança for her botanical expertise.References
- N. Martins and I. C. F. R. Ferreira, Mountain food products: A broad spectrum of market potential to be exploited, Trends Food Sci. Technol., 2017, 67, 12–18 CrossRef CAS.
- Plants for a Future, https://pfaf.org/user/Plant.aspx?LatinName=Calluna+vulgaris, (accessed October 2018).
- O. Escuredo, M. Míguez, M. Fernández-González and M. C. Seijo, Nutritional value and antioxidant activity of honeys produced in a European Atlantic area, Food Chem., 2013, 138, 851–856 CrossRef CAS PubMed.
- K. Ghedira and P. Goetz, Bruyère commune: Calluna vulgaris (L.) Hull ou Calluna vulgáris Salisb. (Ericaceae), Phytotherapie, 2013, 11, 52–55 CrossRef.
- J. M. Neves, C. Matos, C. Moutinho, G. Queiroz and L. R. Gomes, Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal), J. Ethnopharmacol., 2009, 124, 270–283 CrossRef PubMed.
- M. Monschein, J. I. Neira, O. Kumert and F. Bucar, Phytochemistry of heather (Calluna vulgaris (L.) Hull) and its altitudinal alteration, Phytochem. Rev., 2010, 9, 205–215 CrossRef CAS.
- A. Chevallier, The Encyclopedia of Medicinal Plants, Dorling Kindersley, London, 1996 Search PubMed.
- D. A. Ghareeb, A. M. D. ElAhwany, S. M. El-mallawany and A. A. Saif, In vitro screening for anti-acetylcholiesterase, anti-oxidant, anti-glucosidase, anti-inflammatory and anti-bacterial effect of three tradicional medicinal plants, Biotechnol. Biotechnol. Equip., 2014, 28, 1155–1164 CrossRef PubMed.
- D. Pavlović, B. Lakušić, D. Kitić, M. Milutinović, M. Kostić, B. Miladinović and N. Kovačević, Antimicrobial activity of selected plant species of genera Arbutus L., Bruckenthalia, Rchb., Calluna Salisb. and Erica L. (Ericaceae), Acta Fac. Med. Naissensis, 2014, 31, 81–85 Search PubMed.
- D. M. Vučić, M. R. Petković, B. B. Rodić-Grabovac, O. D. Stefanović, S. M. Vasić and L. R. Čomić, In vitro activity of heather [Calluna vulgaris (L.) Hull] extracts on selected urinary tract pathogens, Bosn. J. Basic. Med. Sci., 2014, 14, 234–238 CrossRef.
- G. A. Filip, I. D. Postescu, C. Tatomir, A. Muresan and S. Clichici, Calluna vulgaris extract modulates NF-kB/ERK signaling pathway and matrix metalloproteinase expression on SKH-1 hairless mice skin exposed to ultraviolet B irradiation, J. Physiol. Pharmacol., 2012, 63, 423–432 CAS.
- G. Rieger, M. Müller, H. Guttenberger and F. Bucar, Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus, J. Agric. Food Chem., 2008, 56, 9080–9086 CrossRef CAS.
- I. Orhan, E. Küpeli, S. Terzioglu and E. Yesilada, Bioassay-guided isolation of kaempferol-3-O-β-D-galactoside with anti-inflammatory and antinociceptive activity from the aerial part of Calluna vulgaris L, J. Ethnopharmacol., 2007, 114, 32–37 CrossRef CAS.
- M. R. García-Risco, E. Vázquez, J. Sheldon, E. Steinmann, N. Riebesehl, T. Fornari and G. Reglero, Supercritical fluid extraction of heather (Calluna vulgaris) and evaluation of anti-hepatitis C virus activity of the extracts, Virus Res., 2014, 198, 9–14 CrossRef PubMed.
- L. Saaby, H. B. Rasmussen and A. K. Jäger, MAO-A innhibitory activity of quercetin from Calluna vulgaris (L.) Hull, J. Ethnopharmacol., 2009, 121, 178–181 CrossRef CAS PubMed.
- C.-A. Calliste, P. Trouillas, D.-P. Allais, A. Simon and J.-L. Duroux, Free radical scavenging activities measured by electron spin resonance spectroscopy and B16 cell antiproliferative behaviors of seven plants, J. Agric. Food Chem., 2001, 49, 3321–3327 CrossRef CAS PubMed.
- W. Hai, H. Chen, M. Zhao, Y. Wang, L. Hong, H. Tang and X. Tian, Two new cytotoxic triterpenoid saponins from the roots of Clematis argentilucida, Fitoterapia, 2012, 83, 759–764 CrossRef CAS PubMed.
- M. Chudzik, I. Korzonek-Szlacheta and W. Król, Triterpenes as potentially cytotoxic compounds, Molecules, 2015, 20, 1610–1625 CrossRef PubMed.
- G. Y. Starchenko and A. R. Grytsyk, Analysis of amino acid composition of Calluna vulgaris, L. (Hull), Pharm. Innov. J., 2017, 6, 88–89 CrossRef CAS.
- AOAC, Official methods of analysis of AOAC international, ed. W. George and G. W. Latimer Jr., AOAC International, Gaithersburg, MD, 20th edn, 2016 Search PubMed.
- H. P. Souza, R. C. G. Corrêa, L. Barros, R. C. Calhelha, C. Santos-Buelga, R. M. Peralta, A. Bracht, M. Matsushita and I. C. F. R. Ferreira, Phytochemicals and bioactive properties of Ilex paraguariensis: An in vitro comparative study between the whole plant, leaves and stems, Food Res. Int., 2015, 78, 286–294 CrossRef PubMed.
- L. Barros, C. Pereira and I. C. F. R. Ferreira, Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L, Food Anal. Methods, 2013a, 6, 309–316 Search PubMed.
- L. Barros, E. Pereira, R. C. Calhelha, M. Dueñas, A. M. Carvalho, C. Santos-Buelga and I. C. F. R. Ferreira, Optimized analysis of organic acids in edible mushrooms from Portugal by ultra fast liquid chromatography and photodiode array detection, J. Funct. Foods, 2013b, 5, 1732–1740 Search PubMed.
- V. C. Graça, L. Barros, R. C. Calhelha, M. I. Dias, A. M. Carvalho, C. Santos-Buelga, I. C. F. R. Ferreira and P. F. Santos, Chemical characterization and bioactive properties of Geranium molle L.: from the plant to the most active extract and its phytochemicals, Food Funct., 2016, 7, 2204–2212 RSC.
- S. M. F. Bessada, J. C. M. Barreira, L. Barros, I. C. F. R. Ferreira and B. P. P. Oliveira, Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species, Ind. Crops Prod., 2016, 89, 45–51 CrossRef CAS.
- F. Souilem, Â. Fernandes, R. C. Calhelha, J. C. Barreira, L. Barros, F. Skhiri, A. Martins and I. C. F. R. Ferreira, Wild mushrooms and their mycelia as sources of bioactive compounds: Antioxidant, anti-inflammatory and cytotoxic properties, Food Chem., 2017, 230, 40–48 CrossRef CAS.
- B. C. Evans, C. E. Nelson, S. S. Yu, K. R. Beavers, A. J. Kim, H. M. Nelson, T. D. Giorgio and C. L. Duvall, Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs, J. Visualized Exp., 2013, 73, e50166 Search PubMed.
- J. Takebayashi, N. Iwahashi, Y. Ishimi and A. Tai, Development of a simple 96-well plate method for evaluation of antioxidant activity based on the oxidative haemolysis inhibition assay (OxHLIA), Food Chem., 2012, 134, 606–610 CrossRef CAS.
- F. Sobral, A. Sampaio, S. Falcão, M. J. Queiroz, R. C. Calhelha, M. Vilas-Boas and I. C. F. R. Ferreira, Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal, Food Chem. Toxicol., 2016, 94, 172–177 CrossRef CAS PubMed.
- J. Glamočlija, D. Stojković, M. Nikolić, A. Ćirić, F. S. Reis, L. Barros, I. C. F. R. Ferreira and M. Soković, A comparative study on edible Agaricus mushrooms as functional foods, Food Funct., 2015, 6, 1900–1910 RSC.
- F. Rodrigues, T. Moreira, D. Pinto, F. B. Pimentel, A. Costa, M. A. Nunes, T. G. Albuquerque, H. S. Costa, A. Palmeira-de-Oliveira, A. I. Oliveira, S. Sut, S. Dall'Acqua and M. B. Oliveira, The phytochemical and bioactivity profiles of wild Calluna vulgaris L. flowers, Food Res. Int., 2018, 111, 724–731 CrossRef CAS PubMed.
- E. Jones and R. E. Hughes, Foliar ascorbic acid in some Angiosperms, Phytochemistry, 1983, 22, 2493–2499 CrossRef CAS.
- M. N. Clifford, K. L. Johnston, S. Knight and N. Kuhnert, Hierarchical scheme for LC-MSn identification of chlorogenic acids, J. Agric. Food Chem., 2003, 51, 2900–2911 CrossRef CAS PubMed.
- M. A. F. Jalal, D. J. Read and E. Haslam, Phenolic composition and its seasonal variation in Calluna vulgaris, Phytochemistry, 1982, 21, 1397–1401 CrossRef CAS.
- Y. Kumarasamy, P. J. Cox, M. Jaspars, L. Nahar and S. D. Sarker, Screening seeds of Scottish plants for antibacterial activity, J. Ethnopharmacol., 2002, 83, 73–77 CrossRef.
- A. Salminen, M. Lehtonen, T. Suuronen, K. Kaarninanta and J. Huuskonen, Terpenoids: natural inhibitors of NF-kB signaling with anti-inflammatory and anticancer potencial, Cell. Mol. Life Sci., 2008, 65, 2979–2999 CrossRef CAS PubMed.
- T. A. Mokota, L. J. McGraw, L. K. Mdee, V. P. Bagla, E. O. Iwalewa and J. N. Eloff, Antimicrobial activity and cytotoxic of triterpenes isolated from leaves of Maytenus undata (Celastraceae), BMC Complementary Altern. Med., 2013, 13, 111 CrossRef PubMed.
- B. Ma, L. J. Forney and J. Ravel, Vaginal microbiome: rethinking health and disease, Annu. Rev. Microbiol., 2012, 66, 371–389 CrossRef CAS PubMed.
- C. Mishra and J. Lambert, Production of anti-microbial substances by probiotics, Asia Pac. J. Clin. Nutr., 1996, 5, 20–24 CAS.
- R. Brunelli, M. Papi, G. Arcovito, A. Bompiani, M. Castagnola, T. Parasassi, B. Sampaolese, F. Vincenzoni and M. De Spirito, Globular structure of human ovulatory cervical mucus, FASEB J., 2007, 21, 3872–3876 CrossRef CAS.
- B. Huang, J. M. Fettweis, J. P. Brooks, K. K. Jefferson and G. A. Buck, The changing landscape of the vaginal microbiome, Clin. Lab. Med., 2014, 34, 747–761 CrossRef.
- D. Machado, C. Gaspar, A. Palmeira-de-Oliveira, C. Cavaleiro, L. Salgueiro, J. Martinez-de-Oliveira and N. Cerca, Thymbra capitata essential oil as potential therapeutic agent against Gardnerella vaginalis biofilm-related infections, Future Microbiol., 2017, 12, 407–416 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |

