Retracted Article: Ginsenoside Rf alleviates dysmenorrhea and inflammation through the BDNF-TrkB-CREB pathway in a rat model of endometriosis†
Xuying
Qin
ab,
Yan
Liu
b,
Yanchong
Feng
c and
Jie
Jiang
 *a
*a
aDepartment of Obstetrics and Gynecology, Shandong University QiLu Hospital, 104 culture west Road, Jinan 250012, Shandong, China. E-mail: qljiangjie@sdu.edu.cn; Tel: +86-15653113905
bDepartment of Obstetrics and Gynecology, Liaocheng people's Hospital, No. 67 of Dongchang West Road, Liaocheng 252000, Shandong, China
cDepartment of Obstetrics and Gynecology, Heping Hospital affiliated to Changzhi Medical College, No. 110 Yanan South Road, Changzhi, 046000 Shanxi, China
First published on 14th December 2018
Abstract
To investigate the effects and the underlying mechanisms of ginsenoside Rf in a surgically induced rat endometriosis model, endometriosis was constructed through homologous transplantation and the Wistar rats were further randomly classified into the sham group, the estradiol valerate (E2V) control group, the endometriosis group, and the ginsenoside Rf groups (1.0, 2.0 and 4.0 mg kg−1, respectively). After 7 days of treatment, the implant volume and writhing responses were recorded. Vascular endothelial growth factor (VEGF), inducible nitric oxide synthase (iNOS), interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α were analyzed using enzyme-linked immunosorbent assay (ELISA) and reverse transcription polymerase chain reaction (RT-PCR) assay. Brain-derived neurotrophic factor (BDNF), tropomyosin receptor kinases (TrkB), and phosphate-c-AMP-responsive element binding protein (pCREB) were further measured. Compared with the endometriosis group, ginsenoside Rf could decrease the volume of the endometriotic implants and writhing responses. Furthermore, the expression levels of VEGF and inflammation-related iNOS, IL-6, IL-1β, and TNF-α were significantly down-regulated in the ginsenoside Rf groups in a dose-dependent manner. The results also showed that ginsenoside Rf could decrease the expression of BDNF, TrkB, and pCREB in the endometriotic implants. The alleviation of endometriosis-associated dysmenorrhea and inflammation by ginsenoside Rf may be partially mediated by the BDNF-TrkB-CREB pathway.
Introduction
Endometriosis is a pathological condition in which the functional endometrial tissue (endometrium) grows outside the uterine cavity and it can affect approximately 10% of the female population in the reproductive age.1,2 The major symptoms of endometriosis are infertility and endometriosis-related pain. Dysmenorrhea, chronic pelvic pain, dyspareunia, and dysuria are the main symptoms of endometriosis-related pain, which is really hard to endure. Surgery and hormone therapy are the predominant treatments for endometriosis. Sometimes, such treatments are insufficient and recurrence occurs. Serious treatment-associated side effects, such as hepatic injury and osteoporosis,3,4 can make it worse. So, it is urgent to develop a new therapeutic strategy for the treatment of endometriosis.Ginsenoside Rf, a glycoside of protopanaxatriol from Panax ginseng, has been traditionally used in herbal medicine in both Asian and Western countries. Ginsenoside Rf has been testified to have an antinociceptive effect in some in vivo5,6 and in vitro models,7–9 and there are still some controversial findings about the antinociceptive effect of ginsenoside Rf.10,11 Recently, ginsenoside Rf has been shown to have some anti-inflammatory effect by down-regulating the production of some proinflammatory cytokines and the activation of inflammation associated pathways,7,12 which may have a role in the development of endometriosis.
To date, no investigation has been designed to test the effects of ginsenoside Rf on rat endometriosis-associated dysmenorrhea. Thus, this study is designed to test whether ginsenoside Rf systemic treatment has antinociceptive and anti-inflammatory effects in the rat endometriosis model.
Methods and materials
Animals
Female Wistar rats aged 10 to 12 weeks (200–250 g) were obtained from Peking Vital River Laboratory Animal Ltd (Beijing, China) and fed a standard soy-free diet to avoid exposure to exogenous estrogenic compounds. The left uterine horns were exposed and ligated at the cervical end using a 4-0 silk, which were further resected longitudinally into two segments (1 cm each) and attached to the intimal surface of the right side of the abdominal wall. A further exploratory laparotomy was performed to confirm the establishment of an experimental endometriosis model after a recovery period of 28 days. Of the 70 experimental rats that underwent surgery, 12 did not develop any signs of endometriosis, and they were excluded from the study. For the measurement of ectopic endometrial tissue volume, an ellipsoid volume formula (π/6 × length × width × height) was adopted as previously reported.13Following a further recovery period of 2 weeks, the Wistar rats were assigned to 6 groups: the sham group, normal control rats (n = 16); the estradiol valerate (E2V) control group, normal rats treated with E2V (0.5 mg kg−1) daily for 14 consecutive days to induce dysmenorrhea (n = 8); the endometriosis group, rats with endometriosis treated with E2V (0.5 mg kg−1) daily for 14 consecutive days and 0.9% saline vehicle during the last 7 days (n = 13); and the three ginsenoside Rf groups (Rf 1, Rf 2, and Rf 4), rats with endometriosis treated with E2V (0.5 mg kg−1) daily for 14 consecutive days intraperitoneally injected with ginsenoside Rf (1.0 mg kg−1, 2.0 mg kg−1, and 4.0 mg kg−1, respectively) during the last 7 days (n = 15, 16, and 14 for each group). Ginsenoside Rf was obtained from Sigma-Aldrich (St Louis, MO) and the doses of ginsenoside Rf were chosen based on the previous published investigation.8
On day 14, an intraperitoneal injection of oxytocin (2 U per rat) was administered to induce writhing response one hour after the last administration of E2V. Half an hour after the observation of writhing responses, the ectopic endometrial tissues were measured and resected. All the experiments were performed according to the guidelines of Care and Use of Laboratory Animals published by the China National Institute of Health and were permitted by the Ethics Committee of Shandong University QiLu Hospital.
Quantitative real-time RT-PCR (qRT-PCR)
According to the manufacturer's instruction, TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was used to extract total RNA from ectopic endometrial tissues and a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was adopted to reverse-transcribe RNA into cDNA. SYBR Green Master Mix (Roche, Mannheim, Germany) was used to detect the amplification and the programme was set as follows: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. The relative expression levels of the targeted genes were normalized to GAPDH. The primer sequences are listed in ESI Table 1.†Western blotting
The ectopic endometrial tissues were isolated and lysed, and the soluble supernatants (30 μg) were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. Nonspecific binding was blocked with 5% non-fat dry milk, and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 diluted primary antibodies specific for brain-derived neurotrophic factor (BDNF), tropomyosin receptor kinases (TrkB), and phosphate-c-AMP-responsive element binding protein (pCREB) (Santa Cruz Biotechnology Inc., Santa Cruz, CA) were used to incubate the bands at 4 °C overnight. Peroxidase-conjugated secondary antibody (Sigma-Aldrich, MO, USA) was then used at a 1
1000 diluted primary antibodies specific for brain-derived neurotrophic factor (BDNF), tropomyosin receptor kinases (TrkB), and phosphate-c-AMP-responsive element binding protein (pCREB) (Santa Cruz Biotechnology Inc., Santa Cruz, CA) were used to incubate the bands at 4 °C overnight. Peroxidase-conjugated secondary antibody (Sigma-Aldrich, MO, USA) was then used at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution for 2 h at room temperature to develop the signal with an ECL system (GE Healthcare Life Sciences, Chalfont, UK). The relative intensity of the bands was calculated by correcting with glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology Inc., Santa Cruz, CA). Fold changes were then calculated with NIH-Image J1.51p 22 (National Institutes of Health, Bethesda, MD, USA).
1000 dilution for 2 h at room temperature to develop the signal with an ECL system (GE Healthcare Life Sciences, Chalfont, UK). The relative intensity of the bands was calculated by correcting with glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology Inc., Santa Cruz, CA). Fold changes were then calculated with NIH-Image J1.51p 22 (National Institutes of Health, Bethesda, MD, USA).
Enzyme-linked immunosorbent assay (ELISA)
According to the manufacturer's instructions, ELISA kits (eBioscience, San Diego, CA, USA) for BDNF and/or vascular endothelial growth factor (VEGF) were used to detect the secretion in the peritoneal fluid and serum. 10 mL phosphate-buffered saline buffer was injected into the rat abdominal cavity and incubated for 10 minutes; the abdominal wall was punctured with a blunt needle, and then a 10 mL syringe was utilized to extract about 8 mL of peritoneal fluid. The abdominal wall was further cut longitudinally, and 2 mL of blood was extracted from the abdominal aorta with a 5 ml syringe. The supernatant of the peritoneal fluid and serum samples were harvested and stored at −80 °C. A microplate reader (SpectraMax M5, Molecular Devices, USA) at a wavelength of 450 nm was used to measure all the standards and samples.Statistical analyses
The differences between different groups were assessed using one-way analysis of variance (ANOVA). P values less than 0.05 were considered to be statistically significant.Results
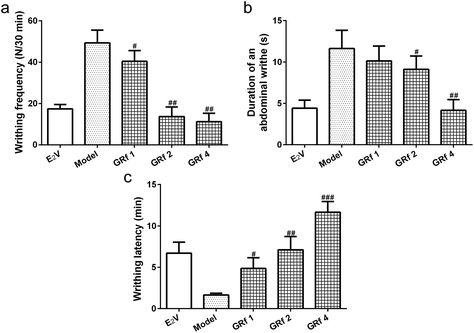
Ginsenoside Rf ameliorates dysmenorrhea in the rats with endometriosis
The endometriosis model showed a nearly 2.5-fold increase in writhing frequency (Fig. 1a) and a 2.1-fold increase in the duration of an abdominal writhe (Fig. 1b) when compared with the E2V rats, which could be reduced by ginsenoside Rf following a 7-day treatment in a dose-dependent manner (Fig. 1a and b). The endometriosis model showed decreased writhing latency from 7 minutes to 2 minutes as compared to the E2V rats and a 7-day treatment with ginsenoside Rf could lengthen the writhing latency by 2-fold at 1 mg kg−1 (p < 0.05), 3-fold at 2 mg kg−1 (p < 0.01), and 6-fold at 4 mg kg−1 (p < 0.001) (Fig. 1c). All of these data suggested that ginsenoside Rf could alleviate abdominal dysmenorrhea (constriction response) associated with endometriosis.Ginsenoside Rf alleviates endometriosis-associated inflammation in rats
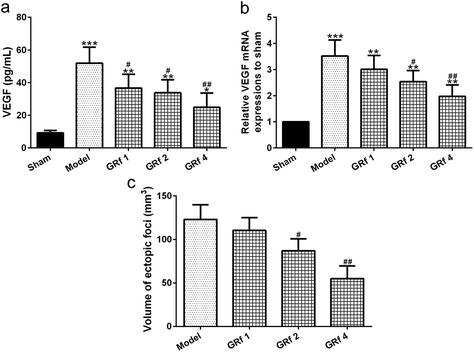
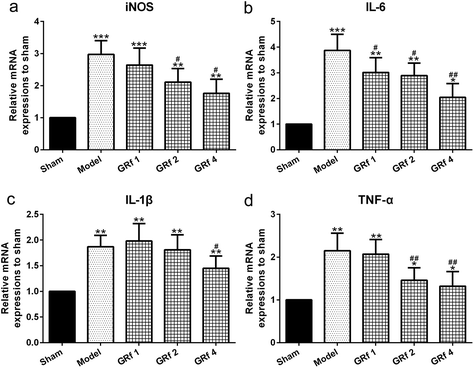
As an important neuro-angiogenic factor, the VEGF level in the peritoneal fluid increased significantly in the endometriosis group when compared with the sham group (p = 0.001), while ginsenoside Rf could attenuate the increased levels of VEGF compared with the endometriosis group in a dose-dependent manner (Fig. 2a). Such a trend could also be observed in the ectopic endometrial tissues analyzed by the RT-PCR assay (Fig. 2b). A statistically significant reduction in the implant volume was shown in the ginsenoside Rf-treated groups in a dose-dependent manner compared with the endometriosis group (Fig. 2c). At the same time, the relative expression of the inflammatory molecules iNOS (Fig. 3a), IL-6 (Fig. 3b), IL-1β (Fig. 3c) and TNF-α (Fig. 3d) was significantly down-regulated by ginsenoside Rf in the ectopic implants.Ginsenoside Rf alters the BDNF-TrkB-CREB pathway involved in endometriosis
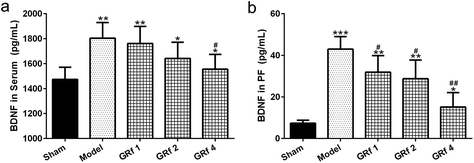
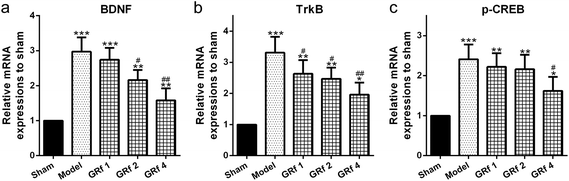
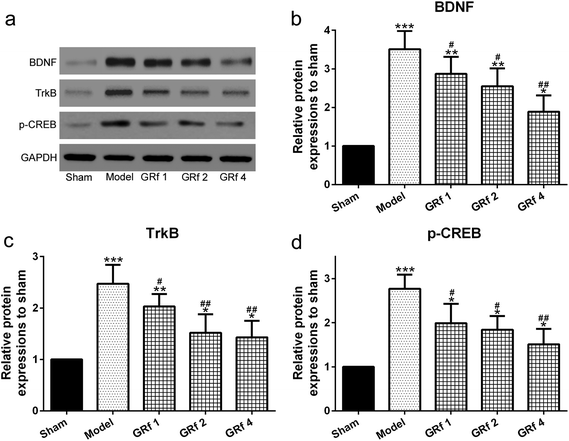
To investigate the function of BDNF, TrkB, and CREB in endometriosis, we initially detected the secretion of BDNF in the serum and peritoneal fluid. Ginsenoside Rf could decrease the circulatory level of BDNF in the serum (Fig. 4a) and peritoneal fluid (Fig. 4b) in the rats with endometriosis. At the same time, ginsenoside Rf could also down-regulate the mRNA expression levels of BDNF (Fig. 5a), TrkB (Fig. 5b), and p-CREB (Fig. 5c) in the ectopic implants of rats with endometriosis. It is worth noting that ginsenoside Rf could down-regulate the protein expressions of BDNF (Fig. 6a and b), TrkB (Fig. 6a and c), and p-CREB (Fig. 6a and d) in the ectopic implants of rats with endometriosis in a dose-dependent manner.Discussion
In this study, ginsenoside Rf can significantly reduce the volume of the endometrial explants in the endometriosis model and shows dose-dependent, incremental analgesic, and anti-inflammatory effects. The BDNF-TrkB-p-CREB pathway is altered during the treatment which indicates that such a pathway may be related to the effects of ginsenoside Rf and ginsenoside Rf could be considered as an alternative to alleviate the symptoms.Endometriosis lesions could react to hormonal stimulation during menstruation and become swollen due to insufficient clearance of blood accumulated by the immune, circulatory, and lymphatic systems, which may further trigger inflammation and pain.14,15 The anti-inflammatory effects of ginsenoside Rf, a four-ring steroidal saponin, have been testified via the inhibition of an increase in iNOS,16 IL-6,17 TNF-α,18 and IL-1β19 release through the NF-κB pathway. Such pro-inflammatory cytokines are proven to exacerbate hyperalgesia and sustain sensitization which may favor the progress to chronic pain and lead to physical, psychological, and social disability. Such a mechanism may also play a role in the endometriosis model.
It is remarkable that neoangiogenesis is common in endometriosis, that is, endometriotic lesions have their own nerve and vascular supply which is considered to be a vital step to develop endometriosis.20–22 Recent findings reveal that BDNF may be regulated by estrogen and functions as a modulator and a bridge between inflammation and neuroplasticity. BDNF and its receptor TrkB, as well as its downstream target p-CREB, mediate the transduction of the nociceptive signal.23 Such mechanisms are testified in the pathogenesis of migraine and its comorbidities.24,25 It is worth noting that BDNF signaling may regulate the primary pro-inflammatory transcription factors (NF-kB and AP-1) and restrict the magnitude of inflammatory response,26 while pro-inflammatory cytokines can provide a negative feedback to BDNF expression;27 all of these indicate the cross-talk between BDNF and pro-inflammatory cytokines. All of these lead to the prediction that BDNF/TrkB signaling may mediate endometriosis-related dysmenorrhea and inflammation.
Elevated secretion levels of VEGF have been detected in the serum and peritoneal fluid of women with endometriosis which may stimulate the proliferation and migration of endothelial cells involved in angiogenesis.28–30 In this research, the protein and mRNA levels of VEGF are significantly higher in the peritoneal fluid and in the ectopic endometrium, while ginsenoside Rf could down-regulate VEGF expression.
Our research offers a novel view of the underlying mechanisms of endometriosis and indicates that ginsenoside Rf might be a potentially promising treatment strategy for endometriosis.
In conclusion, the current study reveals that ginsenoside Rf significantly reduces the size of the endometrial explants in a rat endometriosis model and alleviates dysmenorrhea and inflammation through the BDNF-TrkB-CREB pathway. Ginsenoside Rf may be a promising therapy; however, further clinical trials are required to confirm its efficacy.
Conflicts of interest
All authors declare that they have no conflict of interest.References
- J. A. Sampson, Am. J. Pathol., 1927, 3, 93–110 CAS.
- B. RO and G. LC, Fertil. Steril., 2012, 98, 511–519 CrossRef PubMed.
- J. L. Sun, Clinical Research & Practice, 2018 Search PubMed.
- S. Kennedy, A. Bergqvist, C. Chapron, T. D'Hooghe, G. Dunselman, R. Greb, L. Hummelshoj, A. Prentice and E. Saridogan, Hum. Reprod., 2005, 20, 2698–2704 CrossRef PubMed.
- H. Lee, F. J. Gonzalez and M. Yoon, Biochem. Biophys. Res. Commun., 2006, 339, 196–203 CrossRef CAS PubMed.
- M. L. Sang, S. C. Kim, J. Oh, H. K. Jin and M. K. Na, Phytochem. Lett., 2013, 6, 620–624 CrossRef.
- P. Jongsun and C. Jaeyoul, Afr. J. Biotechnol., 2009, 8, 3682–3690 Search PubMed.
- M. K. Kim, H. Kang, C. W. Baek, Y. H. Jung, Y. C. Woo, G. J. Choi, H. Y. Shin and K. S. Kim, J. Ginseng Res., 2018, 42, 183–191 CrossRef PubMed.
- X. W. Yang, H. P. Wang, X. U. Wei, Y. P. Wang, X. U. Yong-Hua and L. X. Zhang, Chin. J. Pharm. Anal., 2017 DOI:10.16155/j.0254-1793.2017.01.04.
- S. S. Choi, E. J. Han, K. J. Han, H. K. Lee and H. W. Suh, Planta Med., 2003, 69, 1001–1004 CrossRef CAS PubMed.
- B. K. Shin, S. W. Kwon and J. H. Park, J. Ginseng Res., 2015, 39, 287–298 CrossRef CAS PubMed.
- S. Ahn, M. H. Siddiqi, V. C. Aceituno, S. Y. Simu and D. C. Yang, Immunol. Invest., 2016, 45, 1–11 CrossRef PubMed.
- S. Yavuz, N. E. Aydin, O. Celik, E. Yilmaz, E. Ozerol and K. Tanbek, J. Cancer Res. Ther., 2014, 10, 324–329 CrossRef PubMed.
- M. Morotti, K. Vincent, J. Brawn, K. T. Zondervan and C. M. Becker, Hum. Reprod. Update, 2014, 20, 717 CrossRef PubMed.
- K. L. Bruner-Tran, S. Mokshagundam, J. L. Herington, T. Ding and K. G. Osteen, Curr. Women's Health Rev., 2018, 14, 173–188 CrossRef CAS PubMed.
- S. Y. Nah, D. H. Kim and H. Rhim, CNS Drug Rev., 2010, 13, 381–404 Search PubMed.
- D. H. Kim, Y. S. Moon, T. H. Lee, J. S. Jung, H. W. Suh and D. K. Song, Neurosci. Lett., 2003, 353, 13–16 CrossRef CAS PubMed.
- D. C. W. Lee, C. L. H. Yang, S. C. C. Chik, J. C. B. Li and A. S. Y. Lau, Cytokine, 2009, 48, 101–101 CrossRef.
- S. Paul, H. S. Shin and S. C. Kang, Food Chem. Toxicol., 2012, 50, 1354–1361 CrossRef CAS PubMed.
- P. Stratton and K. J. Berkley, Hum. Reprod. Update, 2011, 17, 327–346 CrossRef PubMed.
- A. Asante and R. N. Taylor, Annu. Rev. Physiol., 2011, 73, 163–182 CrossRef CAS PubMed.
- J. Wu, H. Xie, S. Yao and Y. Liang, J. Neuroinflammation, 2017, 14, 53 CrossRef PubMed.
- J. Q. Guo, H. H. Deng, X. Bo and X. S. Yang, Biol. Open, 2017, 6, 8–16 CrossRef CAS PubMed.
- X. Cai, X. Shi, X. Zhang, A. Zhang, M. Zheng and Y. Fang, J. Headache Pain, 2017, 18, 13 CrossRef PubMed.
- C. Irkec, T. Altiparmak, D. Y. Cezayir, R. Tural and N. Altan, J. Neurol. Sci., 2017, 381, 430 CrossRef.
- D. Xu, D. Lian, J. Wu, Y. Liu, M. Zhu, J. Sun, D. He and L. Li, J. Neuroinflammation, 2017, 14, 156 CrossRef PubMed.
- Z. Guan and J. Fang, Brain, Behav., Immun., 2006, 20, 64–71 CrossRef CAS PubMed.
- V. J. Young, S. F. Ahmad, J. K. Brown, W. C. Duncan and A. W. Horne, Sci. Rep., 2015, 5, 16859 CrossRef CAS PubMed.
- A. Pupo-Nogueira, R. M. De Oliveira, C. A. Petta, S. Podgaec, J. A. Dias and M. S. Abrao, Int. J. Gynecol. Obstet., 2007, 99, 33–37 CrossRef CAS PubMed.
- E. Barcz, P. Kamiński and L. Marianowski, Ginekol. Pol., 2001, 72, 442 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8fo01839a |
| This journal is © The Royal Society of Chemistry 2019 |