The effect of gel structure on the in vitro digestibility of wheat starch-Mesona chinensis polysaccharide gels
Anynda
Yuris
,
Kelvin Kim Tha
Goh
,
Allan Keith
Hardacre
and
Lara
Matia-Merino
 *
*
School of Food and Nutrition, Massey Institute of Food Science and Technology, Massey University, Private Bag 11222, Palmerston North 4442, New Zealand. E-mail: l.matia-merino@massey.ac.nz; a.yuris@massey.ac.nz; k.t.goh@massey.ac.nz; a.hardacre@massey.ac.nz
First published on 11th December 2018
Abstract
The digestibility of wheat starch gels in the presence of Mesona chinensis polysaccharide (MCP) was studied. MCP was found to be the most effective polysaccharide in reducing wheat starch digestion in comparison to starch gels of similar hardness containing xanthan, guar, locust bean gum (LBG) and agar. A 33% reduction in the digestibility of intact starch gels containing 5% w/w MCP (after 120 minutes of digestion) was observed and this was attributed to the strengthening of the gels in the presence of high concentration of the polysaccharide. In contrast, despite a reduction in the firmness of the gel when 2% w/w MCP was present, there was a 7% reduction in starch digestibility and hence, firmness was deduced to be not solely responsible for the digestibility of the gels. When these gels were macerated, starch digestibility was reduced regardless of the MCP concentration. Starch digestion in the macerated samples seemed to cease after 10 minutes with about 30% more starch remaining when 5% w/w MCP was present, suggesting that the amount of starch available for digestion was reduced in the presence of MCP. The reduced availability of starch for digestion was hypothesised to be due to starch-MCP interactions, which formed amylose-MCP complexes that are likely to be resistant to enzymatic digestion. Overall, this work shows the potential for MCP to be utilized as an ingredient to reduce the glycaemic index.
Introduction
Starch is the most important source of carbohydrate in the human diet and is obtained from a large variety of plant sources. Wheat starch is found in many staple food products including bread, breakfast cereals, and pasta. In the human gastrointestinal tract, wheat starch is broken down by the salivary and pancreatic amylase into glucose, which is absorbed in the small intestine into the blood stream termed as blood glucose. Under the regulation of insulin, blood glucose is metabolised or stored. However, an over consumption of easily digestible starchy foods will result in elevation of blood glucose, which can lead to Type II diabetes.1,2Digestion of starch can be divided into three types depending on their degree of digestibility: rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistant starch (RS).3 The amount of RDS corresponds to the amount of glucose released after 20 minutes, SDS between 20 and 120 minutes, and RS the remaining starch (total starch minus the amount of glucose released after 120 minutes).4 High proportions of RDS in the diet result in rapid increase in blood glucose and insulin levels. Resistant starch, on the other hand, is scarcely digested in the small intestine but is fermented in the colon. In the case of SDS, which has attracted considerable research interest in recent years, it is digested less rapidly. A small and gradual increase in blood sugars closely matches a normal glucose absorption, hence eliminating or reducing postprandial hyperglycaemia.1
The rate of starch digestion can be controlled by the addition of certain non-starch polysaccharides (NSPs) to the diet. The mechanisms by which this occurs vary depending on the type of NSP with the most common explanation being an increase in the viscosity of the gut contents.5 An increase in the viscosity of digesta slows down mixing in the lumen of the small intestine,6 reduces the mass transfer of enzyme and their substrate,7 and prolongs the time it takes for glucose to be absorbed.8 The presence of highly viscous NSPs is also known to reduce starch granular swelling by reducing water availability.9,10 Consequently, gelatinisation of these granules decreases, which leads to a lower degree of starch hydrolysis. It has been shown that completely ungelatinised starch granules were digested about 40% less than fully gelatinised granules after 60 minutes in vitro in excess of porcine pancreatic α-amylase.11 This is often associated with the presence of a more crystalline structure of the starch granules, making them less susceptible to enzymatic attack.12 Furthermore, certain NSP such as xanthan13 has been shown to encapsulate starch granules by surface association and this has been proposed as a possible mechanism to inhibit or reduce starch hydrolysis due to the presence of a physical barrier for enzyme accessibility.7,14 Alternatively, a NSP may non-competitively bind onto amylase to prevent substrate binding, which hinders digestion,15 therefore reducing postprandial glycaemia.
Mesona chinensis polysaccharide (MCP), obtained from the extract of the Mesona chinensis herb, is an anionic polysaccharide that, unlike many other hydrocolloids, has a very low viscosity in solution. It is unique in that it synergistically increases the viscosity and gel strength of certain non-waxy starches including wheat, maize and rice during the pasting process.16 However, synergistic increase in starch gel strength can only be attained at specific concentrations of starches and MCP; for instance, for a 10% w/w wheat starch suspension, at least 5% w/w MCP is required to increase the strength of the starch gel.17 Concentration dependency of starch gel properties in the presence of MCP has also been observed for corn and mung bean starches, whereby at a concentration below 0.35% w/w MCP, a decrease in the cooled paste viscosity (measured using the rapid visco analyser) was observed.16 In the former, where a synergistic increase in gel strength is observed at specific starch and MCP concentrations, the interaction between amylose and MCP creates a homogenous network as the gel sets.18,19 In the latter, heterogeneity within the gel network occurs when insufficient amylose or MCP is available for interaction. Therefore, for high starch-low MCP or low starch-high MCP gels, the decrease in the elastic moduli of the gels is probably due to the formation of a heterogeneous gel network.17 Although MCP has been traditionally used in Asia to produce a firm black coloured starch-based gel dessert known as grass jelly, the impact of its structure on starch digestibility is not known. The consumption of a solution of Mesona chinensis extract following a meal containing a high proportion of carbohydrates has been shown to reduce postprandial hyperglycaemia as a result of α-glucosidase inhibition by the polyphenols found in the extract.20 However, no study quantified the digestibility of the gels formed from starch that has been gelatinised together with MCP. The aim of this study was to compare the digestibility of MCP-wheat starch gels of various macrostructures (intact gels, fragmented gels and macerated gels) to elucidate whether different gel structures could influence the digestibility of wheat starch gels in the presence of MCP. Xanthan, guar, locust bean gum (LBG) and agar were also included in this study to compare the effectiveness of MCP relative to other polysaccharides in reducing the digestibility of wheat starch gels.
Materials and methods
Starch-polysaccharide suspension preparation
Mesona chinensis powder (Xi'an Hua Rui Bio-Engineering Co. Ltd., Xi'an, China), guar (Danisco, Copenhagen, Denmark), xanthan (Sigma-Aldrich, St Louis, MO, USA), locust bean gum (Danisco, Copenhagen, Denmark) and agar (Hawkins Watts, Auckland, New Zealand) were dissolved on a dry weight basis in MilliQ (Millipore, Billerica, MA, USA) water and left to hydrate for at least 8 hours with constant stirring. For Mesona chinensis powder, the resulting solution was centrifuged at 4000g for 30 minutes to remove insoluble materials. Wheat starch (Penford, Sydney, NSW, Australia) was suspended in water and added to the hydrated polysaccharide solution to make suspensions containing 10% w/w starch and 2–5% w/w MCP, 0.5% w/w guar, 1% w/w xanthan, 1% w/w LBG or 0.3% w/w agar. The NSP concentrations were selected to match the hardness of starch gels containing 2% w/w (16.5 ± 0.5 N) and 5% w/w (23.0 ± 1.9 N) MCP gels. The use of gels with similar hardness allow for the assumption that for all gels, the extent of which enzymes could penetrate into the gels are similar.Chemical characterisation of Mesona chinensis powder
The composition of the Mesona chinensis powder was analysed by an accredited chemical laboratory (Massey University Nutritional laboratory, Palmerston North, New Zealand). The extract was found to contain 9% protein (DUMAS combustion method – AOAC 991.36), 0.1% fat (convection oven method – AOAC 930.15), 28.2% ash (ashing at 600 °C – AOAC 942.05) containing K, Mg, P, Ca, Na, Fe and traces of Cu, I, Mn, Zn and Se as measured using the inductively coupled plasma optical emission spectrometry, 1.1% starch (amyloglucosidase-α-amylase method – AOAC 996.11) and 58.2% total carbohydrate (100% – (Moisture + Ash + Protein + Fat)). The total free sugars were determined to be 0.09% as measured using the phenol-sulphuric acid method.21 The extract was found to contain 47.9% non-starch polysaccharide (total carbohydrate % − (Starch + Free sugar) %) and this was termed as Mesona chinensis polysaccharide (MCP). The sugar composition of MCP was analysed by the Ferrier Research Institute, Wellington, New Zealand. It was found to contain 59.1% galacturonic acid, 11.6% galactose, 8.7% glucose, 6.2% rhamnose, 4.5% arabinose, 3.6% mannose, 3.0% xylose, 1.9% glucuronic acid and 1.5% fucose.Rheology
The rheological properties of the starch and polysaccharide suspensions were measured using a combination of rotational and oscillatory techniques19 as they were heated and then cooled in the starch cell (C-ETD160/ST) and spindle (ST24-2D/2 V/2V-30) geometry of a controlled-stress rheometer (MCR302, Anton Paar Physica, Graz, Austria). Briefly, the starch-polysaccharide mixture was heated from 20 °C to 95 °C to gelatinise the starch under a constant rotatory shear rate of 100 s−1. The paste was then held at 95 °C for 5 minutes before cooling to 20 °C and forming a gel under small strain oscillation (1% strain and 1 Hz frequency). The temperature was held at 20 °C for 30 minutes to allow the gels to completely set. The viscosity of the paste was recorded during heating while the elastic (G′) and viscous (G′′) moduli were recorded during cooling. Measurements were carried out in triplicate.Texture analysis
Starch-polysaccharide gels were prepared using the RVA (Rapid Visco Analyser, Perten Instruments, Massachusetts, USA). Twenty five millilitres (25 mL) of each of the various suspensions were dispersed at 960 rpm for 1 minute at 25 °C. The stirring speed was then reduced to 160 rpm and the suspensions equilibrated for 2 minutes at 25 °C. Heating then commenced at a rate of 2 °C min−1 to 95 °C and the temperature was held at 95 °C for 5 minutes to complete gelatinisation. The hot paste was then poured into a cylindrical mould (diameter = 3.2 cm, height = 2 cm) to set overnight (16 h) at 20 °C. The set gels were removed from their moulds and allowed to equilibrate to room temperature for half an hour. The gels were then subjected to a single compression cycle using the TA.XT plus texture analyser (Stable Micro Systems, England) fitted with a 61 mm plastic cylindrical probe and a load cell of 50 kg. The target deformation was set to 50% of the gel's initial height with a trigger force of 0.05 N. Measurements were carried out in triplicate.In vitro digestion
The digestibility of starch-MCP gels was evaluated under constant (intact gels) and minimal shear (intact, fragmented and macerated gels), using a rheometer and a shaking water bath, respectively. All digestions were carried out in triplicate.Constant shear digestion
Fifteen millilitres (15 mL) of 10% w/w wheat starch suspension containing MCP (2% w/w and 5% w/w) were added into the starch cell. The sample was gelatinised as per section 2.2 and the gels were allowed to set at 20 °C overnight. The weight of the system was measured before and after gelatinisation to determine water loss due to evaporation. Appropriate quantities of water were then added to compensate for that loss during the pasting process.In vitro digestion of the gels formed in the starch cell was carried out according to22 with modifications. Three phases of digestion: oral, gastric and small intestinal were simulated in the rheometer under constant shearing (130 s−1) and a temperature of 37 °C during which the gels were broken by the imposed shear. Firstly, 4 g of water were added to the set gel sample that was left overnight in the rheometer to act as a medium for enzyme dispersion. The temperature of the gel was increased to 37 °C and allowed to equilibrate for 2 minutes at a constant shear rate of 130 s−1. These conditions were maintained throughout the digestion process. The oral phase commenced with the addition of 0.05 mL of 0.1% w/w of α-amylase (A6255, Sigma-Aldrich, St Louis, MO, USA) dispersed in milliQ water and digestion (in the oral phase) was allowed to proceed for a minute. The gastric phase was then initiated by the addition of 1.75 mL mixture of 1 M HCl and MilliQ water to reduce the pH to 2.5 ± 0.5. The ratio of water to HCl added was predetermined for each gel in order to obtain the correct pH while maintaining the same final volume. This was followed by the addition of 0.1 mL of 10% w/w pepsin (P7000, Sigma-Aldrich, St Louis, MO, USA) dissolved in 0.05 M HCl and 0.05 mL of 1% w/w lipase (L3126, Sigma-Aldrich, St Louis, MO, USA) dissolved in MilliQ water. These conditions were maintained for 30 minutes to complete the gastric phase. To begin the small intestinal phase, 0.25 mL of a mixture of water and 1 M NaHCO3 were added to increase the pH to 6.2 ± 0.5. This was followed by the addition of 0.75 mL of 10% w/w bile extract (B8631, Sigma-Aldrich, St Louis, MO, USA) suspended in 0.1 M sodium maleate buffer containing 1 mM CaCl2 and 0.02% w/w sodium azide. An aliquot (0.12 mL) was then removed and immediately dispensed into a preweighed micro-centrifuge tube containing 0.12 mL of chilled absolute ethanol (time = 0 minutes). Following this, 0.05 mL of amyloglucosidase (E-AMGDF, Megazyme, Wicklow, Ireland) and 0.05 mL of 5% w/w pancreatin (P7545, Sigma-Aldrich, St Louis, MO, USA) in 0.1 M sodium maleate buffer were added into the starch cell. Samples were removed into microcentrifuge tubes containing chilled ethanol to halt digestion at 1, 2, 5, 10, 15, 20, 30, 60 and 120 minutes after the addition of amyloglucosidase and pancreatin. Blanks were prepared by carrying out the digestion in the absence of amyloglucosidase and pancreatin to eliminate interference from the colour of MCP.
Minimal shear digestion
Sample of the starch-polysaccharide gels prepared using the RVA (Rapid Visco Analyser, Perten Instruments, Massachusetts, USA) as described in section 2.3 were each poured into a cylindrical mould (diameter = 3.2 cm and height = 0.8 cm) to allow the gel to set. The set gels were then weighed (∼7 g) and the water content determined with the oven drying method and the total starch content was calculated by subtracting the weight of the water from the weight of the gel. Three types of gels were prepared: intact cylindrical gel, fragmented gel and macerated gel. Intact cylindrical gels were prepared as described above. The intact gel was then passed twice through a woven wire mesh with a mesh diagonal aperture of 0.5 mm to create fragmented gels. Macerated gels were prepared by adding water to the fragmented gels and passing the mixture through the mesh five times to produce a dispersed gel pulp. The amount of water (10 mL) used to macerate the gels equate to the amount of water that was added for enzyme dispersion in intact and fragmented gels in order to prevent sample dilution. Digestion of the gels was carried out in a cylindrical screw top container with a 4.1 cm diameter and 5.8 cm height. The containers were placed in a 37 °C water bath (BS-11, Lab companion, Seoul, Korea) under constant linear shaking of 130 rpm. Digestion was initiated by adding 10 mL of MilliQ water (for enzyme dispersion) to all samples except for the macerated gels where 10 mL of water was already added during its preparation. Images of these gels are shown in Fig. 1. In vitro digestion was then carried out by scaling up (2.5 times) the volumes described in section 2.5.1.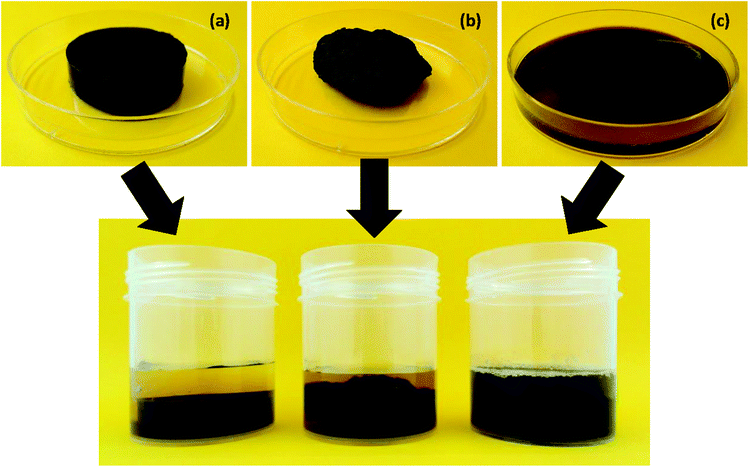 | ||
| Fig. 1 Images of prepared intact (a), fragmented (b) and macerated (c) wheat starch-MCP gels (top) and after addition of a fixed amount of water prior to the gastric phase (bottom). | ||
Sugar analysis
The amounts of glucose resulting from the digestion of starch in the samples described above were determined using a modified DNS method.22 The tubes containing the digestate in ethanol were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 minutes to remove solids. An aliquot (0.05 mL) of the supernatant was transferred into a 10 mL Kimax tube and 0.25 mL of 1% v/v amyloglucosidase (E-AMGDF, Megazyme, Wicklow, Ireland) and 1% v/v invertase concentrate (Fisher Scientific, Hampton, NH, USA) both in 0.1 M sodium acetate buffer pH 5.2 was added to the tube to convert any remaining carbohydrates to glucose. The mixture was incubated in a 37 °C water bath for 10 minutes before the addition of 0.75 mL of DNS mixture containing, in a ratio of 1
000g for 15 minutes to remove solids. An aliquot (0.05 mL) of the supernatant was transferred into a 10 mL Kimax tube and 0.25 mL of 1% v/v amyloglucosidase (E-AMGDF, Megazyme, Wicklow, Ireland) and 1% v/v invertase concentrate (Fisher Scientific, Hampton, NH, USA) both in 0.1 M sodium acetate buffer pH 5.2 was added to the tube to convert any remaining carbohydrates to glucose. The mixture was incubated in a 37 °C water bath for 10 minutes before the addition of 0.75 mL of DNS mixture containing, in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 of the following: 0.5 mg mL−1 glucose, 4 M NaOH and DNS reagent (10 g 3,5-dinitrosalicylic acid, 16 g NaOH and 300 g Na K tartrate dissolved in 1 L water). The tubes were then capped and placed in a water bath at 95 °C for 15 minutes for colour development. The tubes were then removed from the water bath and cooled to room temperature over 15 minutes. Water (4 mL) was then added to the mixture and the tubes were inverted several times to ensure even mixing. Absorbances at 530 nm were measured using a spectrophotometer (Helios Epsilon spectrophotometer, Thermo Fisher Scientific, Waltham, MA, USA).
5 of the following: 0.5 mg mL−1 glucose, 4 M NaOH and DNS reagent (10 g 3,5-dinitrosalicylic acid, 16 g NaOH and 300 g Na K tartrate dissolved in 1 L water). The tubes were then capped and placed in a water bath at 95 °C for 15 minutes for colour development. The tubes were then removed from the water bath and cooled to room temperature over 15 minutes. Water (4 mL) was then added to the mixture and the tubes were inverted several times to ensure even mixing. Absorbances at 530 nm were measured using a spectrophotometer (Helios Epsilon spectrophotometer, Thermo Fisher Scientific, Waltham, MA, USA).
Statistical analysis
An analysis of variance (ANOVA) with Tukey's range test as the post-hoc test was performed using Minitab 16.Results and discussion
Digestibility of wheat starch gels containing MCP versus other polysaccharides
The rheological and textural properties of 10% w/w wheat starch gels with and without MCP are shown in Table 1. Increasing the concentrations of MCP increased peak viscosity (PV) of the hot paste. This is characteristic of many viscous NSPs when added to starch,23 whereby phase separation results in the localisation of the NSP in the continuous phase, hence increasing the overall viscosity.24 Due to the low viscosity of MCP (10% w/w MCP has a viscosity of <2.0 mPa s at 20 °C),19 it is likely that the rise in PV was a result of the interaction between the starch and polysaccharide, forming a network structure. Despite the formation of stronger gels when 5% w/w MCP was present (higher final G′ of 2843 ± 144 Pa and hardness of 23 N), low MCP concentration (2% w/w) resulted in a weaker gel as indicated by a lower final G′ (1489 ± 8 Pa versus 2133 Pa) and hardness (16.5 ± 0.5 N versus 18.6 ± 0.7 N). We have previously shown that the formation of strong starch-MCP gels was attributed to the interaction between MCP and amylose as it leached from the granules during pasting, hence forming a homogenous and dense network.17 However, when the concentration of MCP was lowered, the limited availability of MCP and amylose for interaction resulted in heterogeneity within the network, so a low MCP concentration disrupted/weakened the structure of the gel. Detailed explanations on the interaction and physical properties of wheat starch and MCP gels can be found in our previous publication.17 Due to the effect that MCP concentration has on the hardness of wheat starch gels (weakening and strengthening), this study investigated the digestibility of both, the weaker gel (containing 2% w/w MCP) and stronger gel (containing 5% w/w MCP) to ascertain the role of their interaction and the importance of gel strength in reducing starch-MCP digestibility.| Samples (w/w) | PV of hot paste (mPa s) | Final G′ of set gels (Pa) | Hardness of set gels (N) |
|---|---|---|---|
| 10% wheat starch | 4601 ± 75a | 2133 ± 60a | 18.6 ± 0.7a |
| 10% wheat starch + 2% MCP | 5580 ± 290b | 1489 ± 8b | 16.5 ± 0.5a |
| 10% wheat starch + 5% MCP | 7667 ± 573c | 2843 ± 144c | 23.0 ± 1.9b |
The digestibility of fragmented wheat starch gels in the absence and presence of various concentrations of NSPs that had similar strength to wheat starch gel containing 2% w/w and 5% w/w MCP is shown in Fig. 2 (non-gelling NSP) and Fig. 3 (gelling NSP) respectively. Fragmented gels were chosen for this experiment since they better represent the state of gels that enter the gut (after chewing). Three viscous NSPs, 1% w/w xanthan (17.8 ± 1.8 N), 0.5% w/w guar (17.3 ± 1.4 N) and 1% w/w LBG (16.9 ± 1.9 N), were used to decrease the gels’ hardness to a level that is comparable to starch gels containing 2% w/w MCP (16.5 ± 0.5 N). Despite the hardness of these gels being lower than that of wheat starch alone, starch digestibility was reduced in the order of MCP (∼10% digested at 20 min) < xanthan < guar < LBG (∼18% digested at 20 min) in comparison to wheat starch gels alone (∼25% digested at 20 min). While no data is available on the digestibility of LBG, similar results have been previously reported for xanthan and guar, whereby xanthan was found to be more effective in reducing starch digestibility than guar.25 This was attributed to the ability of xanthan to encapsulate starch granules, which reduces enzyme accessibility.14 Since the gels were fragmented, the viscous NSPs added to the gels were leached into the digestion mixture. The increase in viscosity of the digestion mixture due to leached NSPs may be a contributing factor to the reduction in the digestibility of these gels compared to the starch gel alone (though this is not substantial for MCP). It is expected that with increased viscosity, the enzyme substrate interaction would be decreased due to reduced mobility and therefore, the rate of starch digestion will be reduced.7
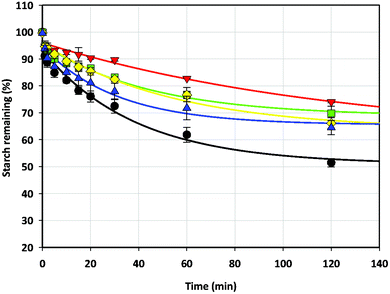 | ||
Fig. 2 The digestibility of fragmented 10% w/w wheat starch gels in the absence (●) and presence of 2% w/w MCP ( ), 1% w/w xanthan ( ), 1% w/w xanthan ( ), 0.5% w/w guar ( ), 0.5% w/w guar ( ) and 1% w/w LBG ( ) and 1% w/w LBG ( ). ). | ||
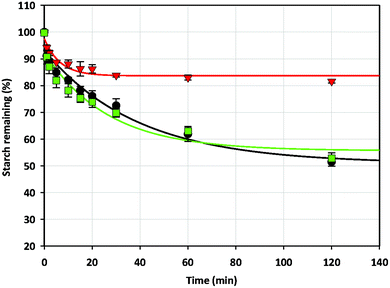 | ||
Fig. 3 The digestibility of fragmented 10% w/w wheat starch gels in the absence (●) and presence of 5% w/w MCP ( ) and 0.3% w/w agar ( ) and 0.3% w/w agar ( ). ). | ||
A large reduction in starch digestibility (80% starch remained after 120 minutes of digestion) was also observed when 5% w/w MCP was present in the starch gel (Fig. 3). However, the addition of agar, a gelling polysaccharide, did not reduce starch digestibility, despite an increase in the gel hardness to a level similar to the gel containing 5% w/w MCP. This was likely due to its gelling nature, which results in agar gels fragments being present in the digestate rather than it being leached into the digestate, therefore not contributing to a rise in viscosity. This result was consistent with the report by26 for milled rice starch-agar gels. However, they reported reduced digestibility for intact rice starch-agar gels, suggesting that gel strengthening by the addition of agar could contribute to decreased enzyme accessibility. This highlights the fact that agar would not be the ideal NSP to be used for manipulating starch digestion in starchy food since the chewing process would fragment the gels, resulting in no reduction in starch digestibility. In contrast, the fact that the digestion rates of fragmented starch gels were reduced by the addition of MCP would suggest that the polysaccharide can be used to manipulate the digestion of starchy food.
Digestion of wheat starch-MCP gels under constant and minimal shear
The digestibility of wheat starch-MCP gels was studied using both a rheometer (shear rate of 130 s−1) and a shaking water bath (linear shaking of 130 rpm) to emulate constant and minimal shear, respectively. The shear rates used ensured that the enzymes were evenly dispersed throughout the system and even mixing was achieved. The digestibility of 10% w/w wheat starch in the presence and absence of MCP under constant shear rate is shown in Fig. 4. The addition of MCP did not result in any change to the amount of RDS in wheat starch gel. However, the amount of SDS was increased in the presence of 5% w/w MCP as indicated by a decrease in the digestibility of wheat starch between 20 and 120 minutes (41 ± 0.2% versus 58 ± 2.6% starch remaining at 120 minutes in the absence and presence of 5% w/w MCP). On the other hand, the addition of 2% w/w MCP did not alter the digestibility of the starch gel. Reduced digestibility of wheat starch gel in the presence of 5% w/w MCP can be explained by in terms of the hardness of the gel itself. Due to the constant shearing in the rheometer, gels are broken up and their particle size was dependent on the gel hardness—stronger gels were less susceptible to being broken up by shear. Therefore, for a stronger gel (containing 5% w/w MCP), shearing in the rheometer would result in the formation of larger gel pieces as compared to the fine gel pieces produced from shearing a weaker gel (containing 2% w/w MCP). Starch digestibility is reduced for larger gel particles due to their smaller surface area as a result of an overall slower rate of enzyme diffusion into the gel.27 Interestingly, while the presence of 2% w/w MCP decreased the hardness of wheat starch gel, the digestibility of the gel was unaffected. Despite the reduction in starch digestibility when gel strengthening occurs at high MCP concentration (5% w/w), there is uncertainty towards the exact mechanism by which MCP reduces starch digestion due to the uneven sizes of broken gels as a result of shear in the rheometer. Hence, the effect of gel hardness on starch-MCP digestibility was further investigated by digesting gels of different macrostructure (intact cylindrical gel (Fig. 5a), fragmented gel (Fig. 5b) and macerated gel (Fig. 5c)) under minimal shear to ensure that gels were of even sizes.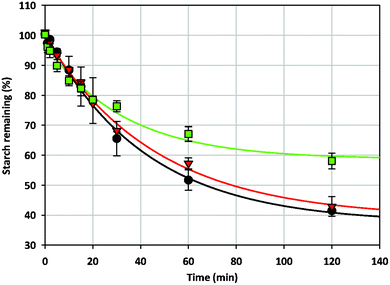 | ||
Fig. 4 The digestibility of 10% w/w wheat starch gels in the absence (●) and presence of 2% w/w ( ) and 5% w/w ( ) and 5% w/w ( ) MCP under constant shear rate. ) MCP under constant shear rate. | ||
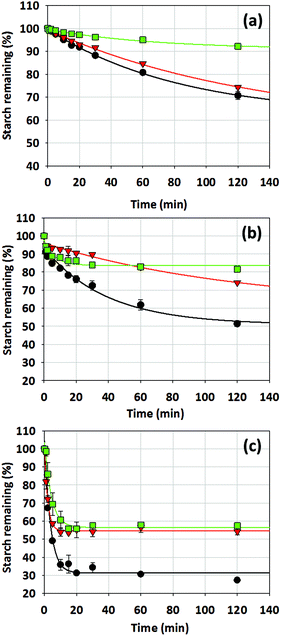 | ||
Fig. 5 The digestibility of 10% w/w wheat starch (a) intact cylindrical gels, (b) fragmented gels and (c) macerated gels in the absence (●) and presence of 2% w/w ( ) and 5% w/w ( ) and 5% w/w ( ) MCP. ) MCP. | ||
For intact gels, the rate of hydrolysis was much lower than that for the fragmented and macerated gels. This was expected due to a lower surface area for enzymes to hydrolyse the starch in the gel. Higher rates of digestion were observed, particularly in the first 20 minutes when macerated gels were used (31 ± 1.0% starch remaining versus 76 ± 2.1% and 92 ± 0.3% starch remaining for fragmented and intact gels, respectively). The same observation was reported by26 for intact and milled rice starch whereby more starch hydrolysis was observed for the milled sample.
The digestibility of intact wheat starch gel in the presence or absence of MCP is shown in Fig. 5a, whereby a decrease in starch digestibility was observed for wheat starch gel containing 5% w/w MCP. For an intact gel, the digestibility of the gel is dependent primarily on the ability of the enzymes to penetrate into the gel to access the substrate. Hence, the structure and network of the gels influences the rate and extent of digestion. Therefore, in this instance, the digestibility of wheat starch gel containing 5% w/w MCP may reflect the higher strength of the gel due to the presence of a stronger network, which limits enzyme diffusion into the gel to access its substrate. However, despite a decrease in the gel hardness when 2% w/w MCP is present, its digestibility was slightly lower or comparable to 10% w/w wheat starch on its own, a similar finding to the starch-MCP gels being digested in the rheometer. These findings indicate that gel hardness was not the sole factor in determining the digestibility of starch-MCP gels. Our previous studies17,19 have shown that granule swelling was reduced in the presence of MCP, with intact granules being present at 5% w/w starch in the presence of high MCP concentrations following gelatinisation. Nevertheless, at high starch concentration, such as that used in this study (10% w/w), the close packing of gelatinised starch granules would result in many granules being broken up by shear during gel preparation. However, we cannot discount the fact that starch-MCP composite gel is likely to contain a higher proportion of granules that are not fully gelatinised, meaning that they are more rigid and resistant to shear. In the presence of these less gelatinised granules, starch digestion would consequently be reduced due to the tight packing of the crystalline structure that reduces enzyme accessibility.
The digestibility of fragmented gels is shown in Fig. 5b. The total amount of starch remaining after 120 minutes of digesting these gels were lower than that of the intact gels and this was due to an increase in the surface area of the gels, which allowed enzymes to access their substrate more easily. For a fragmented gel, the addition of 5% w/w MCP reduced starch digestibility by 60% after 120 minutes as compared to a 30% reduction for intact gels (Fig. 5a and b), indicating that the ability of MCP to reduce starch digestibility was more prominent in a fragmented gel. The digestibility of fragmented gels is affected not only by the size of the gel fragments but more so by the release of NSPs from the matrix into the digestate, resulting in an increase in viscosity as in the case of xanthan and konjac glucomannan.26 In such instance, starch digestibility can be hindered as a result of restricted enzyme mobility due to the high viscosity of the digestate in the presence of the NSP.5 This is unlikely for MCP due to its low viscosity and, hence, its release from the gel matrix into the digestate would not greatly impact the digestate viscosity. However, leaching of MCP into the digestate may have had other effects that influenced starch digestion.
In the absence of MCP, the total amount of starch digested (after 120 minutes) was 30% higher when the intact gels were fragmented. In contrast, this remained at a similar level when MCP was present despite gel fragmentation. This suggests that in the presence of MCP, the degree of starch hydrolysis by enzyme remained limited despite the disruption of the gel structure. Such result may reflect a situation in which MCP encapsulate the starch granules, thereby limiting enzyme access, as hypothesised by28 for other NSPs. The ability of MCP to encapsulate starch granules is not known but its interaction with amylose has been hypothesised to occur as the polymer leaches out, creating an amylose-MCP coat around the granule.19 The formation of barriers around starch granules, together with NSP's hydration capacity,10 could limit water mobility, which would consequently result in reduced granular swelling. This effect is often accompanied by reduced starch hydrolysis as a result of not only limited access to the granule, but also the unavailability of substrate due to the tight packing of starch polymer within the partially gelatinised granules.12
Interestingly, the initial rate of digestion (first 30 minutes) of fragmented gels was lowest when a low concentration of MCP (2% w/w) was present, despite being a weaker gel. We hypothesised that the presence of MCP affected the stickiness of the fragmented gels so that those containing low concentration of MCP (2% w/w) tended to stick more and form large gel clumps that had lower surface area, resulting in an initial reduction in digestibility. The hypothesis was proposed based on the fact that the stickiness of a starch gel is primarily dependent on the cohesive effect of amylopectin. The formation of an amylose network is known to interfere with amylopectin–amylopectin interactions, therefore creating a less sticky gel.29 At higher concentrations of MCP, there was an increase in the extent of network formation as indicated by the higher G′ of the gel. This may have interfered with amylopectin-amylopectin interaction, therefore reducing the stickiness of the gel. The opposite was observed for starch gels containing 2% w/w MCP, whereby a decrease in its G′ indicates a less extensive network formation. In this instance, interference to the amylopectin–amylopectin interaction would be reduced, and hence gels would become stickier. With time, these aggregates were loosened up and the gel containing 2% w/w MCP was digested more readily than that containing 5% w/w MCP, suggesting that multiple factors were involved which affected the overall digestibility of wheat starch-MCP gels. This result may have positive implications on the digestibility of a starchy food product that is produced using low MCP concentration, since fragmented gels resulting from mastication may reassociate to form larger clumps that are digested with less ease. This showed that gel adhesiveness was, in part, contributing to the digestion rate of the fragmented starch gel and should be further investigated.
In order to eliminate the effect of stickiness on the gel digestibility, the fragmented gels were macerated in water to disperse them completely (consistency of a paste). In this case, the rate of starch digestion was then not limited by the gel hardness as the macrostructure was disrupted. The rate and total amount of starch digested was increased greatly as a result of an increased enzyme accessibility due to a larger surface area. For the macerated gel (Fig. 5c), it was observed that the presence of MCP resulted in a reduction in the rate of starch digestion and, more importantly, the amount of starch digested. Starch digestion ceased after 10 minutes, with 27 ± 0.6% starch remaining in the absence of MCP and this was increased to 57 ± 1.0% when 5% w/w MCP was present, suggesting that there was a reduction in the amount of starch available for digestion. The results thus far suggested that the association between amylose and MCP forming complexes, which have previously been proposed, could account for the 30% of undigested starch in the presence of MCP implying that these MCP-amylose complexes are somewhat resistant to the enzymes. Resistance of amylose complexes to enzymatic hydrolysis is not unreported and has been shown for amylose-lipid complexes.30 While other studies have suggested that the decrease in the rate of starch digestion in the presence of a non-starch polysaccharide was due to the ability of certain NSPs such as guar gum to inhibit enzyme activity,15 the ability of MCP to do so is still unknown. There is also the added possibility for other compounds present in the extract of Mesona chinensis such as polyphenols that may possess inhibitory activities against digestive enzymes. Hence, these possibilities cannot be excluded and should be studied in future work.
Conclusion
The digestibility of 10% w/w wheat starch gels containing xanthan, guar, LBG, agar and MCP were compared. Despite their similar gel hardness, it was found that MCP was the most effective polysaccharide in reducing wheat starch digestibility, making it a potential ingredient to formulate starchy products with low glycaemic load. The mechanism by which MCP reduces starch digestibility was found to be complex, possibly consisting of several different factors. The hardness of the gel, in part, contributed to reducing enzyme accessibility to starch trapped within the gel. Consequently, reduced digestibility was observed for the intact strong gels (high MCP concentration) but not for the weak gels (low MCP concentration). When the gel structure was fragmented, starch digestibility was reduced in the presence of both low and high MCP concentration. The stickiness of the fragmented gel pieces contributed to reducing starch digestibility, whereby at low MCP concentration, fragmented gels pieces aggregated to form larger clumps, leading to more reduction in digestibility for the first 30 minutes. Finally, macerated gels dispersed in water showed that ∼30% of the starch in gels containing MCP was not available for digestion. This is likely to be largely attributed to the formation of MCP-amylose that survived enzymatic hydrolysis.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the scholarship from Massey University's College of Health for Anynda Yuris.References
- U. Lehmann and F. Robin, Slowly digestible starch – its structure and health implications: a review, Trends Food Sci. Technol., 2007, 18, 346–355 CrossRef CAS.
- M. Miao, B. Jiang, S. W. Cui, T. Zhang and Z. Jin, Slowly digestible starch-a review, Crit. Rev. Food Sci. Nutr., 2015, 55, 1642–1657 CrossRef CAS PubMed.
- H. N. Englyst, S. Kingman and J. H. Cummings, Classifications and measurements of nutritionally important starch fractions, Eur. J. Clin. Nutr., 1992, 46, S33–S50 Search PubMed.
- J. Singh, A. Dartois and L. Kaur, Starch digestibility in food matrix: a review, Trends Food Sci. Technol., 2010, 21, 168–180 CrossRef CAS.
- C. S. Brennan, M. Suter, T. Luethi, L. Matia-Merino and J. Qvortrup, The Relationship Between Wheat Flour and Starch Pasting Properties and Starch Hydrolysis: Effect of Non-starch Polysaccharides in a Starch Gel System, Starch/Staerke, 2008, 60, 23–33 CrossRef CAS.
- R. G. Lentle and P. W. Janssen, Physical characteristics of digesta and their influence on flow and mixing in the mammalian intestine: a review, J. Comp. Physiol., B, 2008, 178, 673–690 CrossRef CAS PubMed.
- A. Dartois, J. Singh, L. Kaur and H. Singh, Influence of guar gum on the in vitro starch digestibility-Rheological and microstructural characteristics, Food Biophys., 2010, 5, 149–160 CrossRef.
- P. Vaugelade, C. Hoebler, F. Bernard, F. Guillon, M. Lahaye, P. Duee and B. Darcy-Vrillon, Non-starch polysaccharides extracted from seaweed can modulate intestinal absoprtion of glucose and insulin response in the pig, Reprod., Nutr., Dev., 2000, 40, 33–47 CrossRef CAS.
- A. Krüger, C. Ferrero and N. E. Zaritzky, Modelling corn starch swelling in batch systems: effect of sucrose and hydrocolloids, J. Food Eng., 2003, 58, 125–133 CrossRef.
- R. F. Tester and M. D. Sommerville, The effects of non-starch polysaccharides on the extent of gelatinisation, swelling and α-amylase hydrolysis of maize and wheat starches, Food Hydrocolloids, 2003, 17, 41–54 CrossRef CAS.
- J. Holm, I. Lundquist, I. Bjorck, A. C. Eliasson and N. G. Asp, Degree of starch gelatinization, digestion rate of starch in vitro, and metabolic response in rats, Am. J. Clin. Nutr., 1988, 47, 1010–1016 CrossRef CAS PubMed.
- H.-J. Chung, H. S. Lim and S.-T. Lim, Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch, J. Cereal Sci., 2006, 43, 353–359 CrossRef CAS.
- A. Gonera and P. Cornillon, Gelatinization of starch gum sugar systems studied by using DSC, NMR, and CSLM, Starch/Staerke, 2002, 54, 508–516 CrossRef CAS.
- C. S. Brennan, V. Kuri and C. M. Tudorica, Inulin-enriched pasta: effects on textural properties and starch degradation, Food Chem., 2004, 86, 189–193 CrossRef CAS.
- S. L. Slaughter, P. R. Ellis, E. C. Jackson and P. J. Butterworth, The effect of guar galactomannan and water availability during hydrothermal processing on the hydrolysis of starch catalysed by pancreatic α−amylase, Biochim. Biophys. Acta, 2002, 1571, 55–63 CrossRef CAS.
- T. Feng, Q. Su, H. N. Zhuang, R. Ye, Z. B. Gu and Z. Y. Jin, Ghost structures, pasting, rheological and textural properties between mesona blumes gum and various starches, J. Food Qual., 2014, 37, 73–82 CrossRef CAS.
- A. Yuris, L. Matia-Merino, A. K. Hardacre, J. Hindmarsh and K. K. T. Goh, Molecular interactions in composite wheat starch-Mesona chinensis polysaccharide gels: Rheological, textural, microstructural and retrogradation properties, Food Hydrocolloids, 2018, 79, 1–12 CrossRef CAS.
- L. S. Lai and S. J. Chao, A DSC study on the gel-sol transition of a starch and hsian-tsao leaf gum mixed system, J. Agric. Food Chem., 2000, 48, 3267–3274 CrossRef CAS PubMed.
- A. Yuris, K. K. T. Goh, A. K. Hardacre and L. Matia-Merino, Understanding the interaction between wheat starch and Mesona chinensis polysaccharide, LWT – Food Sci. Technol., 2017, 84, 212–221 CrossRef CAS.
- C. Chusak, T. Thilavech and S. Adisakwattana, Consumption of Mesona chinensis attenuates postprandial glucose and improves antioxidant status induced by a high carbohydrate meal in overweight subjects, Am. J. Chin. Med., 2014, 42, 315–336 CrossRef CAS PubMed.
- M. B. Hall, W. H. Hoover, J. P. Jennings and T. K. M. Webster, A method for partitioning neutral detergent-soluble carbohydrates, J. Sci. Food Agric., 1999, 79, 2079–2086 CrossRef CAS.
- S. Mishra, J. Monro and D. Hedderley, Effect of Processing on Slowly Digestible Starch and Resistant Starch in Potato, Starch/Staerke, 2008, 60, 500–507 CrossRef CAS.
- T. Funami, Y. Kataoka, T. Omoto, Y. Goto, I. Asai and K. Nishinari, Effects of non-ionic polysaccharides on the gelatinization and retrogradation behavior of wheat starch, Food Hydrocolloids, 2005, 19, 1–13 CrossRef CAS.
- M. Alloncle and J. L. Doublier, Viscoelastic properties of maize starch/hydrocolloid pastes and gels, Food Hydrocolloids, 1991, 5, 455–467 CrossRef CAS.
- T. Sasaki and K. Kohyama, Influence of non-starch polysaccharides on the in vitro digestibility and viscosity of starch suspensions, Food Chem., 2012, 133, 1420–1426 CrossRef CAS.
- T. Sasaki and K. Kohyama, Effect of non-starch polysaccharides on the in vitro digestibility and rheological properties of rice starch gel, Food Chem., 2011, 127, 541–546 CrossRef CAS PubMed.
- K. Mahasukhonthachat, P. A. Sopade and M. J. Gidley, Kinetics of starch digestion in sorghum as affected by particle size, J. Food Eng., 2010, 96, 18–28 CrossRef.
- C. S. Brennan and C. M. Tudorica, Evaluation of potential mechanisms by which dietary fibre additions reduce the predicted glycaemic index of fresh pastas, Int. J. Food Sci. Technol., 2008, 43, 2151–2162 CrossRef CAS.
- L. B. Iturriaga, B. L. de Mishima and M. C. Añon, Effect of amylose on starch pastes viscoelasticity and cooked grains stickiness in rice from seven argentine genotypes, Food Res. Int., 2006, 39, 660–666 CrossRef CAS.
- Y. Ai, J. Hasjim and J. L. Jane, Effects of lipids on enzymatic hydrolysis and physical properties of starch, Carbohydr. Polym., 2013, 92, 120–127 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |
