The thermal stability, structural changeability, and aggregability of glutenin and gliadin proteins induced by wheat bran dietary fiber
Sen
Ma
 *,
Wen
Han
,
Li
Li
,
Xueling
Zheng
and
Xiaoxi
Wang
*
*,
Wen
Han
,
Li
Li
,
Xueling
Zheng
and
Xiaoxi
Wang
*
College of Food science and Technology, Henan University of Technology, Zhengzhou, Henan 450001, China. E-mail: masen@haut.edu.cn; xxwanghaut@126.com; Fax: +86-67758026; Tel: +86-67758026
First published on 14th November 2018
Abstract
Wheat bran dietary fiber (WBDF) has been reported to be responsible for the low quality of whole wheat flour products due to its destructive effect on the gluten matrix. Glutenin and gliadin are the major components of gluten protein and contribute to a proper gluten structure. In this study, the thermostability, surface hydrophobicity, fluorescence characteristics, free sulfhydryl contents, and molecular weight distributions of glutenin- and gliadin-rich fractions were determined after the addition of WBDF. The addition of WBDF to glutenin resulted in an increased surface hydrophobicity and free sulfhydryl content, as well as a red-shift of the fluorescence spectrum. However, the WBDF-modified gliadin fraction changed slightly mainly due to its spherical conformation. Size exclusion chromatography profiles revealed increasing soluble gliadin aggregates and decreasing high molecular weight glutenin fractions as a result of WBDF incorporation. The results from the thermostability analyses exhibited decreased weight loss and decomposition temperatures for both glutenin and gliadin proteins at high WBDF concentration. Our results suggest that changes in the gluten matrix caused by WBDF may largely rely on glutenin structure variation.
Introduction
It is well known that the wheat flour processing quality is related to both the quantity and quality of its protein. Fractionation and reconstitution experiments revealed that gluten quality is responsible for bread-making differences among wheat cultivars.1 The gluten protein can be divided into two fractions based on its solubility in ethanol–water solution (e.g. 70% ethanol solution); the soluble fraction is gliadin, and the insoluble fraction is glutenin. Glutenin is composed of huge macropolymers of high- and low-molecular weight subunits crosslinked via interchain disulfide bonds that result in a wide range of molecular weights from 105 to 107 Da,2 while gliadin has a rather small molecular weight and can only form intramolecular disulfide bonds and noncovalent bonds.3 It has been suggested that gliadin contributes to dough viscosity and glutenin contributes to dough elasticity and, for this reason, both gliadin and glutenin are believed to be vital in providing an appropriate viscous and elastic property balance in gluten.4 In bread making, greater gas cell coalescence occurred in dough made with weak flour that had insufficient elastic gluten during processing, which was caused by low strain hardening and poor gas cell stability.5,6 Increased elasticity leads to higher loaf volumes because of more gas entrapment.7 However, when gluten is too elastic and rigid, bread cannot rise due to the impaired expansion of gas cells.8 Previous studies indicated that variations in glutenin and gliadin molecular weight distributions also greatly influenced the bread-making performance.9,10 Glutenin macropolymers are keys to wheat quality and are strongly correlated with bread loaf volume and dough extensibility.11 Moreover, numerous studies reported the vital role of glutenin in producing fine bread texture,12,13 while others contended that the gliadin fraction was more favorable and could contribute to desirable bread loaf volumes.14,15 Therefore, glutenin and gliadin should be well studied to better understand their contributions to gluten matrix formation and the quality of flour products.Wheat bran, as a good source of dietary fiber, has been used widely in wheat flour products for nutritional purposes. However, extensive studies have revealed undesirable bran-enriched food quality, which was attributed to the interaction between wheat bran dietary fiber (WBDF) and gluten fractions.16,17 Coarse bran was believed to yield an open and disaggregated gluten structure.18 Observations from standard light microscopy also revealed damage by wheat bran dietary fiber to gluten continuity and gas cell expansion.19 Moreover, bran fibers could lead to gluten conformational changes and difficulties in forming a favorable gluten network.20,21 The reduction in free thiol content and high molecular weight glutenin subunits added to the growing evidence for changes in the structure and aggregation of gluten fractions induced by the wheat bran dietary fiber.18 However, to the best of our knowledge, fewer studies have focused on the changes in glutenin and gliadin fractions as a result of WBDF addition. As demonstrated above, glutenin and gliadin are the most essential protein fractions to form a proper gluten matrix, which directly determines gluten properties. Therefore, we studied the influence of WBDF on glutenin and gliadin fractions.
This study aimed to characterize the heat, structure, and aggregation of glutenin and gliadin induced by WBDF to add growing evidence to the interactions between gluten fractions and dietary fiber. For this purpose, WBDF was added at various concentrations to glutenin and gliadin-rich fractions to study the changes in thermal decomposition characteristics, surface hydrophobicities, fluorescence characteristics, free sulfhydryl contents, and molecular weight distributions. In this study, we interpreted WBDF and gluten component interactions and deepened the understanding of the reasons behind the changes in gluten matrix formations induced by WBDF.
Materials and methods
Materials
Commercial wheat bran (15.32% protein, 10.17% starch, 6.64% ash, 14.21% moisture) and wheat gluten (73.64% protein, 8.48% starch, 1.26% ash, 12.07% moisture) were purchased from a local market. The content of the components was measured under laboratory conditions.Wheat bran dietary fiber preparation
Wheat bran dietary fibers were prepared according to the method from our previous study.22 Briefly, wheat bran was pulverized and blended with 10-fold distilled water in a colloid mill. The resultant suspension was washed under running water, and the wheat bran was dried. Next, distilled water was blended with the wheat bran in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dilution and 1.5% (w/w) of thermostable α-amylase (40
1 dilution and 1.5% (w/w) of thermostable α-amylase (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1) and 3% (w/w) of alkaline protease (≥200
000 U g−1) and 3% (w/w) of alkaline protease (≥200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1) were added at a suitable temperature and pH. Ultimately, the solid residues were washed and dried for 24 h at 60 °C. The prepared WBDF was obtained after pulverizing the mixture to 150 μm of particle size. The purity of the WBDF was assessed using a total dietary fiber assay kit (K-TDFR, Megazyme, Bray Co., Wicklow, Ireland). The total dietary fiber content was 90.43%, including 5.76% soluble dietary fiber and 84.67% insoluble dietary fiber. The impurities consisted of primarily protein (4.72%) and starch (4.12%), measured according to the Kjeldahl method using the Kjeltec 8400 (Foss, Hoganas, Sweden) and the AACC Method 76-11.
000 U g−1) were added at a suitable temperature and pH. Ultimately, the solid residues were washed and dried for 24 h at 60 °C. The prepared WBDF was obtained after pulverizing the mixture to 150 μm of particle size. The purity of the WBDF was assessed using a total dietary fiber assay kit (K-TDFR, Megazyme, Bray Co., Wicklow, Ireland). The total dietary fiber content was 90.43%, including 5.76% soluble dietary fiber and 84.67% insoluble dietary fiber. The impurities consisted of primarily protein (4.72%) and starch (4.12%), measured according to the Kjeldahl method using the Kjeltec 8400 (Foss, Hoganas, Sweden) and the AACC Method 76-11.
Glutenin and gliadin powder preparations
The preparation of gluten-WBDF dough was conducted based on our earlier study,23 with some modifications. The WBDF contents were 0%, 3%, 6%, 9%, 12%, and 15% (w/w) in relation to gluten weights (using the same moisture basis). The gluten powder was obtained after dough-kneading, gluten-washing, freeze-drying, and grinding. The gluten and gliadin preparations were based on the report of Khatkar et al.,24 with some modifications.70% ethanol solution was used to extract the gliadin-rich fraction, and the resultant liquid–solid ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (w/v). Gluten powder was slowly added to the ethanol solution with stirring to avoid excessive protein aggregation. Extractions were conducted at 20 °C for 3 h using a magnetic stirrer and then centrifuged at 2000g for 20 min. The supernatant was diluted with a three-fold 70% ethanol solution, and then the pH was adjusted to 6.50 using 0.1 mol L−1 HCl and 0.1 mol L−1 NaOH. The solution was continued to be stirred with a glass rod, and the precipitate (gliadin-rich fraction) was collected. Because glutenin demonstrates good solubility in weak acid solutions, the glutenin-rich sediment was added to distilled water, and the pH was adjusted to 5.0. The slurry was stirred at 20 °C for 3 h and filtered using 74 μm sieves to remove insoluble WBDF particles. Next, the solution was diluted with two-fold absolute ethyl alcohol and the pH was adjusted to neutral. After three hours of stirring, the slurry was centrifuged [2000g for 20 min], and the precipitate was collected. Both the gliadin- and glutenin-rich fractions were freeze-dried and pulverized to 150 μm. Glutenin (crude protein content, 72.5%) and gliadin (crude protein content, 83.1%) were measured according to the Kjeldahl method using the Kjeltec 8400 (Foss, Hoganas, Sweden).
30 (w/v). Gluten powder was slowly added to the ethanol solution with stirring to avoid excessive protein aggregation. Extractions were conducted at 20 °C for 3 h using a magnetic stirrer and then centrifuged at 2000g for 20 min. The supernatant was diluted with a three-fold 70% ethanol solution, and then the pH was adjusted to 6.50 using 0.1 mol L−1 HCl and 0.1 mol L−1 NaOH. The solution was continued to be stirred with a glass rod, and the precipitate (gliadin-rich fraction) was collected. Because glutenin demonstrates good solubility in weak acid solutions, the glutenin-rich sediment was added to distilled water, and the pH was adjusted to 5.0. The slurry was stirred at 20 °C for 3 h and filtered using 74 μm sieves to remove insoluble WBDF particles. Next, the solution was diluted with two-fold absolute ethyl alcohol and the pH was adjusted to neutral. After three hours of stirring, the slurry was centrifuged [2000g for 20 min], and the precipitate was collected. Both the gliadin- and glutenin-rich fractions were freeze-dried and pulverized to 150 μm. Glutenin (crude protein content, 72.5%) and gliadin (crude protein content, 83.1%) were measured according to the Kjeldahl method using the Kjeltec 8400 (Foss, Hoganas, Sweden).
Glutenin and gliadin solution preparations
The preparation of the glutenin and gliadin solutions was performed based on the study of Han et al.22 An acetic acid solution was adopted to dissolve the gluten fractions using a magnetic stirrer. After 2 h of stirring, the solution was centrifuged (8000g, 5 °C, 20 min) and the supernatant was preserved at 4 °C.Surface hydrophobicity
Surface hydrophobicity (Ho) of the gluten protein was determined using 1-anilino-8-naphthalene sulfonate (ANS) as the hydrophobic probe.25 The concentration of the glutenin and gliadin protein solutions was measured using the Coomassie brilliant blue method. To make the protein concentrations within the range of 0.005–0.5 mg mL−1, the protein solution was diluted 20, 40, 80, 100, 200, and 300 times and 50, 80, 100, 200, 500, and 800 times for glutenin and gliadin, respectively, based on their original concentration. Twenty microliters of ANS (8 mol L−1) were added to 4 mL of the diluted gluten solution. The fluorescence intensity of gluten was measured after 3 min, and a diluted gluten solution without ANS was used as the zero reference. Excitation and emission wavelengths were 390 and 470 nm, respectively, and excitation and launching slit widths were set to 5 nm. Each sample was analyzed three times.The fluorescence characteristics of glutenin and gliadin
The fluorescence spectra of glutenin and gliadin were obtained using a fluorescence spectrophotometer (Cary Eclipse, EL06063220; Agilent Technologies, CA, USA). Spectra between 300–450 nm were scanned with a 290 nm excitation wavelength and a 5 nm excitation and launching slit width.Free sulfhydryl (SH) content determination
Free SH (SHfree) content was determined spectrophotometrically after reacting with [5,5′-dithiobis(2-nitrobenzoic acid)] DTNB according to Zhou et al.26 0.1 g of glutenin and gliadin powder was dissolved in 5.5 mL of an Ellmann reagent [propan-2-ol, 250 mM Tris-HCl buffer (pH 8.5) and 4 g L−1 DTNB in ethanol (5/5/1, v/v/v)] and was shaken for 10 min. The supernatant was collected after centrifugation at 8000g for 15 min and measured at 412 nm. The results are expressed in μmol per g of protein. The above analyses were performed in triplicate.Size exclusion chromatography (SEC)
The gluten solutions were separated by using an Agilent 1260 Infinity LC system (CA, USA) with a Biosep SEC-4000 column (Phenomenex, Torrance, CA, USA). The elution parameters were determined according to Zhao et al.,27 with slight modifications. The acetic acid solution (0.5 mol L−1) was passed through a 0.25 μm filter that was vacuum filtered and degassed to elute the mobile phase. The glutenin and gliadin solutions were eluted through a 0.45 μm filter before testing. The SEC experimental conditions were: 0.5 mL min−1 flow rate, 100 μL injection volume, 40 °C column temperature, and UV-detection of 220 nm. The integral area of each peak was calculated and organized using the ORIGIN software program (v. 8.5 PRO, OriginLab Corporation, USA).Thermogravimetric analyzer
Thermogravimetry (TG) measurements were performed using a thermogravimetric analyzer (Q50, Waters LLC, New Castle, Delaware USA) according to Nawrocka et al.28 Freeze-dried gluten samples within 10 mg were placed in oxide aluminum pans and heated from 40 to 600 °C at a heating rate of 20 °C min−1. The degradation temperatures (Td) and weight loss of the gliadin and glutenin samples were recorded. The TG tests were performed in replicate.Statistical analysis
Statistical analyses were performed with ANOVA (level of significance, P < 0.05) followed by Duncan's test with SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA).Results and discussion
Surface hydrophobicity
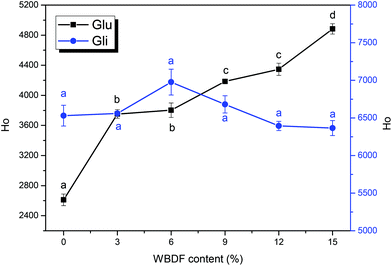
The surface hydrophobicity of glutenin and gliadin upon WBDF addition is shown in Fig. 1. ANS was used as a hydrophobic probe due to its sensitivity to polarity and its ability to amplify fluorescence emission signals to compensate for low protein quantum yields.22 Observations from the Ho intensities of the two protein fractions suggested that gliadin has higher surface hydrophobicity than glutenin and the addition of WBDF brought subtle changes to gliadin surface hydrophobicity. This could be ascribed to the spherical conformation of the gliadin proteins, which make it harder for WBDF to destroy such compact particles and reveal the hydrophobic amino acids that are buried in the protein core during the gluten matrix formation. However, with increasing WBDF content, the surface hydrophobicity of glutenin increased from 2611.2 ± 77.07 to 4883.55 ± 68.94. According to Laligant et al.,29 one of the possible reasons that contributed to the higher surface hydrophobicity might have been the dissociation of protein subunits and the extension of peptide chains. Hence, it seems that addition of WBDF leads to structural changes of the glutenin filaments.Fluorescence characteristics
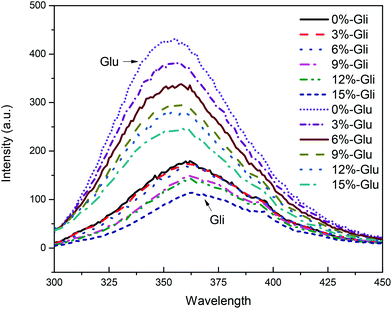
The gluten protein has fluorescence characteristics owing to the presence of aromatic amino acids including tryptophan, tyrosine, and phenylalanine. According to our earlier study,22 tryptophan fluorescence was regarded to be a useful tool to study protein structural changes not only because of its responsiveness to the microenvironment polarity but also because of its greatest fluorescence intensity. Tryptophan exhibits broad fluorescence absorption centered around 350 nm with an approximate width of 60 nm. The stretch of gluten proteins exposes tryptophan residues to the polar environment and thus leads to a red-shift, or a blue-shift in the fluorescence spectrum.30 One study assigned the fluorescence spectra of tryptophan to five spectral classes (A, S, I, II and III).22 In the current study, we show that both glutenin and gliadin exhibited fluorescence absorption peaks that centered around 350 nm, and according to the fluorescence spectra mentioned above, were assigned to spectra III (Fig. 2). The spectra III fluorescence peak corresponds to indole chromophore emissions on the protein surface that is in contact with free water molecules, which is rare in normal proteins but is common in stretched proteins. Such findings were in line with our previous work in which the gluten protein was studied and exhibited a similar fluorescence peak.22 According to these studies, it can be deduced that the gluten protein fractions are more inclined to stretch in acetic acid solution.Obviously, there are two groups of fluorescence spectra with different fluorescence intensities that were assigned to glutenin and gliadin, respectively. For the glutenin filaments, a high probability of exposure of tryptophan residues was expected, which resulted in a higher fluorescence intensity. Unlike glutenin, most of the gliadins are globular and encase tryptophan residues within their core. Therefore, gliadin protein has fewer exposed tryptophan residues compared with glutenin and thus exhibited fluorescence spectra with low intensity. Furthermore, a significant red-shift was observed in the gliadin samples, indicating a stronger gliadin tryptophan polarity compared with that of glutenin, which may be due to the fluorescence quantum yield of gliadin that mostly comes from tryptophan on the particle surfaces, rather than in the cores.
The addition of WBDF led to a gradual decrease in the fluorescence quantum yield of the glutenin and gliadin proteins. We also noticed that the tryptophan microenvironment of the glutenin samples was more sensitive to WBDF as evidenced by a significant reduction in the glutenin fluorescence intensity after WBDF was added. Such a finding corresponds well to the surface hydrophobicity changes in the two proteins induced by WBDF. Besides, a slight red-shift was observed in both protein samples, suggesting that the tryptophan residues had increased polarity. The WBDF polysaccharide is enriched with polar hydroxyl groups, which could enhance the polarity of the microenvironment around tryptophan when it interacts with proteins.
Free sulfhydryl content
Fig. 3 shows the effect of WBDF on the accessible thiol contents in glutenin and gliadin proteins during dough mixing. In agreement with the literature, we believe that changes in free sulfhydryl (SH) levels are persuasive indicators of SS bond variations and the degrees to which protein molecular chains aggregate.31,32 The results in Fig. 3 showed a significantly lower SH content of gliadin compared with that of glutenin. It is universally known that glutenin subunits develop ordered fibrous macromolecular polymers with intermolecular disulfide linkages, while gliadins only form intramolecular disulfide linkages. According to a previous study, the intermolecular disulfide linkages are more readily cleaved than intrachain bonds, which are likely to be buried in the core of the native molecule protected from chemical reduction.33 Therefore, it is possible that the low reduction reaction probability of intermolecular disulfide bonds in gliadin protein is responsible for its low SH content.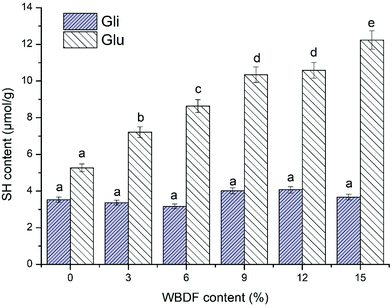 | ||
| Fig. 3 Effect of the WBDF level on the free sulfhydryl content of glutenin (Glu) and gliadin (Gli). The error bars show the corresponding standard deviation. | ||
Fig. 3 shows the significant differences between glutenin and gliadin samples in the accessible thiol content as WBDF is supplemented from 0 to 15%. For WBDF enriched glutenin, the SH glutenin content increased from 5.27 μmol g−1 to 12.24 μmol g−1, which implied that WBDF might be involved in the alteration of the glutenin tertiary structure to account for the thiol/disulfide exchange. A strong correlation between the SH content and the formation of a proper gluten network has been observed in many studies.3,31 The molecular weight distribution of glutenin has been recognized as one of the main determinants of dough quality and is governed by the state of the disulfide structure.3 Hence, the replacement of gluten with WBDF could hinder glutenin aggregation by increasing the SH content. However, it appears that intermolecular disulfide bonds in gliadin are more stable as indicated by the subtle changes in SH content as the WBDF was increased. This may be ascribed to the compact globular structure of gliadin that reduced the possibility to contact with the WBDF polysaccharide. Consequently, it is possible that the impact of WBDF on the gluten SH content was largely caused by changes in the glutenin SH content.
Molecular weight distributions
Fig. 4 shows the SEC profile of glutenin and gliadin protein fractions induced by different levels of WBDF. The elution profile shows that glutenin and gliadin are composed of two fractions, where the elution time ranges from 8 min to 13.5 min (peak 1) and 13.5 to 20 min (peak 2). Integral peak areas were calculated to represent the corresponding gluten fraction contents and are shown in Table 1. Observations from the SEC profiles demonstrated the differences in the molecular weight distributions of the gluten fractions. Gliadin exhibited a remarkably higher integral area content in fraction 1 compared with that in fraction 2, while the two glutenin fractions (peak 1 and peak 2) exhibited similar contents. The A1 and A2 values of the gliadin samples were significantly higher compared with those of the glutenin samples. There was a positive correlation between the peak area and the UV absorption intensity, which implicitly suggests a higher solubility of the gliadin fractions in acetic acid solution. A previous study observed a better solubility and dispersity of gliadin at low pH due to higher electrostatic interactions on the surface of the gliadin particles.34 The higher the gliadin nanoparticle content, the more likely the aggregate structures will be formed.35 As indicated by the peak 1 elution time of the glutenin and gliadin samples, the two eluted fractions had similar molecular weights. It is well known that gliadin has a smaller molecular weight than glutenin. However, owing to the better solubility and aggregability of the gliadin protein in solution, the protein fraction corresponding to peak 1 in the gliadin eluate profile may represent soluble aggregates.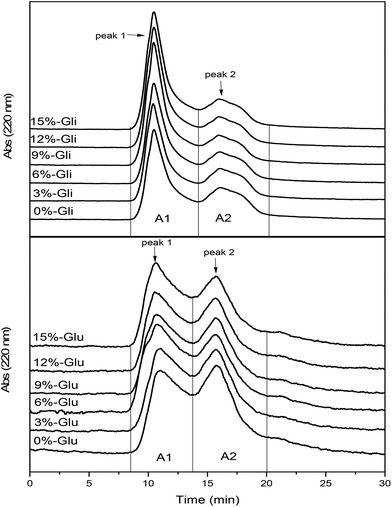 | ||
| Fig. 4 SEC profiles of changes in glutenin (Glu) and gliadin (Gli) with the addition of different amounts of WBDF. | ||
| Sample | WBDF content (%) | A1 | A2 |
|---|---|---|---|
| Mean values followed by different letters in the same column are significantly different (p < 0.05). Gli, gliadin protein; Glu, glutenin. | |||
| Gli | 0 | 1134.64 ± 3.10a | 676.91 ± 17.83a |
| 3 | 1219.93 ± 17.58b | 698.86 ± 5.32a | |
| 6 | 1246.62 ± 3.49b | 661.92 ± 11.89a | |
| 9 | 1440.99 ± 4.81c | 618.83 ± 1.95b | |
| 12 | 1438.58 ± 14.34c | 615.15 ± 19.47b | |
| 15 | 1427.90 ± 18.26c | 622.56 ± 4.78b | |
| Glu | 0 | 283.93 ± 11.32ab | 302.23 ± 13.74a |
| 3 | 286.84 ± 18.50ab | 283.03 ± 13.59ab | |
| 6 | 295.47 ± 12.62a | 264.70 ± 13.04bc | |
| 9 | 279.46 ± 0.24ab | 239.26 ± 12.92cd | |
| 12 | 265.08 ± 17.10ab | 234.94 ± 3.38d | |
| 15 | 256.94 ± 5.92b | 236.65 ± 1.22d | |
Upon the addition of WBDF, the A2 value of glutenin and gliadin decreased, which implied that this protein fraction was depolymerized. However, the A1 value showed different trends as WBDF increased from 0 to 15%. The content of soluble aggregates in gliadin samples increased from 1134.64 ± 3.10 to 1427.90 ± 18.26, suggesting enhanced aggregation induced by WBDF. As indicated from another study, the high molecular mass of gliadin (>97 kDa) was also observed in dough containing dietary fiber including highly methoxylated pectin, xanthan gum, and locust bean gum.36 These aggregates were believed to be formed by gliadins linked through noncovalent interactions.37 Therefore, it can be inferred that WBDF causes disaggregation of glutenin fractions, but yields high molecular weight gliadin aggregates.
Thermostability
Thermogravimetry (TG) measurements were applied to determine the dependence of gluten samples on temperature, and to provide information about the structural changes of the gluten fractions. Owing to the limited information obtained from the TG curves, DTG curves were combined to observe decomposition temperatures. The so-called DTG curve was the first derivative of the TG curve versus temperature. The degradation temperatures (Td) and decreases in gliadin and glutenin sample weights are presented in Table 2. The thermal degradation temperatures of gliadin and glutenin without WBDF were 319.81 ± 0.97 °C and 299.06 ± 0.82 °C, respectively, suggesting a higher thermal stability of gliadin than glutenin. This higher thermal stability could be related to the spherical construction of the gliadin proteins, which limited the ability of the protein molecular chains to stretch and the chemical bonds to break. During the thermal decomposition stage, the covalent peptide bonds, the disulfide bridges, and the O–N and O–O linkage breaks lead to the decomposition of gluten proteins.38 It was reported that gliadin intramolecular disulfide bonds tended to get buried inside the gliadin particles which made them difficult to break during heating.33 The observations of the gliadin and glutenin SH content in our study also provide indirect evidence for this. The weight loss in the gliadin and glutenin samples without WBDF was 68.07 ± 0.57 and 71.67 ± 0.31%, respectively, which further confirmed the higher thermal stability of gliadin than glutenin.| Sample | WBDF content (%) | T d (°C) | Weight loss (%) |
|---|---|---|---|
| Mean values followed by different letters in the same column are significantly different (p < 0.05). Gli, gliadin protein; Glu, glutenin. | |||
| Gli | 0 | 319.81 ± 0.97a | 68.07 ± 0.57a |
| 3 | 319.76 ± 1.32a | 72.05 ± 0.49b | |
| 6 | 319.49 ± 1.03a | 72.32 ± 0.84b | |
| 9 | 319.83 ± 1.85a | 71.35 ± 0.42b | |
| 12 | 319.87 ± 1.79a | 72.28 ± 0.63b | |
| 15 | 314.8 ± 1.12b | 72.52 ± 0.65b | |
| Glu | 0 | 299.06 ± 0.82a | 71.67 ± 0.31a |
| 3 | 299.38 ± 0.58a | 71.19 ± 0.58a | |
| 6 | 299.89 ± 0.97a | 72.82 ± 0.41ab | |
| 9 | 300.10 ± 1.69a | 72.11 ± 0.36a | |
| 12 | 296.79 ± 1.81b | 72.36 ± 0.49ab | |
| 15 | 297.57 ± 1.52b | 73.34 ± 0.29b | |
As seen from Table 2, the addition of WBDF caused similar changes in weight loss and degradation temperatures for glutenin and gliadin samples. According to Khatkar et al.,38 the weight loss changes provided information about the changes in the gluten structure with an increase in weight loss, suggesting the formation of a more open and weak gluten structure. The addition of WBDF led to a gradual increase in weight loss for both gliadin and glutenin which indicated looser gluten fractions induced by WBDF. However, the temperature at which glutenin and gliadin degrade decreased only at high WBDF content levels. The Td values remained unchanged at low WBDF content, which indicated that the gluten proteins are thermally stable in the presence of small amounts of WBDF. Similar results were obtained by Nawrocka et al.,39 who also studied the thermal properties of fiber-modified gluten. It can be concluded that high WBDF levels contribute to a looser gluten structure that impairs the thermostability of the gluten fractions.
Conclusion
The present study investigated the thermostability, structural changes, and aggregation behavior of glutenin- and gliadin-rich fractions induced by WBDF. The addition of WBDF to glutenin yielded increasing surface hydrophobicity and free sulfhydryl content, as well as a red-shift in the fluorescence spectrum. However, little changes occurred in WBDF-modified gliadin fractions, which implied that gliadin structures were more stable than glutenin structures. The SEC profiles revealed an increase in soluble gliadin aggregates, and a decrease in high molecular weight glutenin fractions after WBDF was added, indicating the disaggregation of the glutenin fraction upon WBDF addition. It can be inferred from the above findings that WBDF yields a weaker and looser glutenin structure, while the gliadin protein structure was hardly affected owing to its spherical conformation. Therefore, changes in the gluten protein network induced by WBDF may be mostly due to the interactions of WBDF with glutenin.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Fundamental Research Funds for the Henan Provincial Colleges and Universities in Henan University of Technology (no. 2018RCJH08), the Henan Province Colleges and Universities Young Backbone Teacher Plan (no. 2016GGJS-070), the Key Scientific and Technological Project of Henan Province (no. 172102110008), and the National Natural Science Foundation of China (no. 31571873).References
- M. R. Booth and M. A. Melvin, Factors responsible for the poor breadmaking quality of high yielding european wheat, J. Sci. Food Agric., 1979, 30(11), 1057–1064 CrossRef CAS.
- J. L. Carceller and T. Aussenac, Size characterisation of glutenin polymers by hpsec-malls, J. Cereal Sci., 2001, 33(2), 131–142 CrossRef CAS.
- H. Wieser, Chemistry of gluten proteins, Food Microbiol., 2007, 24(2), 115–119 CrossRef CAS.
- B. S. Khatkar and J. S. Schofield, Molecular and physicochemical basis of breadmaking-properties of wheat gluten proteins: A critical appraisal, J. Food Sci. Technol., 1997, 34, 85–102 CAS.
- B. J. Dobraszczyk, The physics of baking: rheological and polymer molecular structure–function relationships in breadmaking, J. Non-Newtonian Fluid Mech., 2004, 124(1–3), 61–69 CrossRef CAS.
- M. C. Zghal, M. G. Scanlon and H. D. Sapirstein, Effects of flour strength, baking absorption, and processing conditions on the structure and mechanical properties of bread crumb, Cereal Chem., 2001, 78(1), 178–191 CrossRef.
- S. Y. Sim, A. A. N. Aziah and L. H. Cheng, Quality and functionality of chinese steamed bread and dough added with selected non-starch polysaccharides, J. Food Sci. Technol., 2013, 52(1), 1–8 Search PubMed.
- H. Goesaert, K. Brijs, W. S. Veraverbeke, C. M. Courtin, K. Gebruers and J. A. Delcour, Wheat flour constituents: how they impact bread quality, and how to impact their functionality, Trends Food Sci. Technol., 2005, 16(1–3), 12–30 CrossRef CAS.
- F. R. Huebner, T. C. Nelsen, O. K. Chung and J. A. Bietz, Protein distributions among hard red winter wheat varieties as related to environment and baking quality, Cereal Chem., 1997, 74(2), 123–128 CrossRef CAS.
- N. K. Singh, G. R. Donovan, I. L. Batey and F. Macritchie, Use of sonication and size-exclusion high-performance liquid chromatography in the study of wheat flour proteins. I. dissolution of total proteins in the absence of reducing agents, Jew. Q. Rev., 1990, 67(2), 96–116 Search PubMed.
- C. Don, W. Lichtendonk, J. J. Plijter and R. J. Hamer, Glutenin macropolymer: a gel formed by glutenin particles, J. Cereal Sci., 2003, 37(1), 1–7 CrossRef CAS.
- R. B. Gupta, I. L. Batey and F. Macritchie, Relationships between protein composition and functional properties of wheat flours, Cereal Chem., 1992, 69(2), 125–131 CAS.
- S. Uthayakumaran, F. L. Stoddard, P. W. Gras and F. Bekes, Effects of incorporated glutenins on functional properties of wheat dough, Cereal Chem., 2000, 77(6), 737–743 CrossRef CAS.
- G. Branlard and M. Dardevet, Diversity of grain proteins and bread wheat quality. I. correlation between gliadin bands and flour quality characteristics, J. Cereal Sci., 1985, 3(4), 329–343 CrossRef CAS.
- B. S. Khatkar, Effects of high Mr glutenin subunits on dynamic rheological properties and bread making qualities of wheat gluten, J. Food Sci. Technol., 2006, 43(4), 382–387 CAS.
- M. Gómez, S. Jiménez, E. Ruiz and B. Oliete, Effect of extruded wheat bran on dough rheology and bread quality, LWT–Food Sci. Technol., 2011, 44(10), 2231–2237 CrossRef.
- A. Bakke and Z. Vickers, Consumer liking of refined and whole wheat breads, J. Food Sci., 2007, 72(7), 473–480 CrossRef PubMed.
- L. Xiong, B. Zhang, M. Niu and S. Zhao, Protein polymerization and water mobility in whole-wheat dough influenced by bran particle size distribution, LWT–Food Sci. Technol., 2017, 82(1), 396–403 CrossRef CAS.
- S. Hemdane, N. A. Langenaeken, P. J. Jacobs, J. Verspreet, J. A. Delcour and C. M. Courtin, Study of the role of bran water binding and the steric hindrance by bran in straight dough bread making, Food Chem., 2018, 253(1), 262–268 CrossRef CAS PubMed.
- J. E. Bock and S. Damodaran, Bran-induced changes in water structure and gluten conformation in model gluten dough studied by Fourier transform infrared spectroscopy, Food Hydrocolloids, 2013, 31(2), 146–155 CrossRef CAS.
- J. Li, G. G. Hou, Z. Chen, A. L. Chung and K. Gehring, Studying the effects of whole-wheat flour on the rheological properties and the quality attributes of whole-wheat saltine cracker using SRC, alveograph, rheometer, and NMR technique, LWT–Food Sci. Technol., 2014, 55(1), 43–50 CrossRef CAS.
- W. Han, S. Ma, L. Li, X. Zheng and X. Wang, Influence of wheat starch on the structural changes and size distribution of gluten induced by adding wheat bran dietary fiber, Starch/Staerke, 2018, 70, 9–10 Search PubMed.
- W. Han, S. Ma, L. Li, X. Zheng and X. Wang, Rheological properties of gluten and gluten-starch model doughs containing wheat bran dietary fiber, Int. J. Food Sci. Technol., 2018, 53(12), 2650–2656 CrossRef CAS.
- B. S. Khatkar, A. E. Bell and J. D. Schofield, The dynamic rheological properties of glutens and gluten sub-fractions from wheats of good and poor bread making quality, J. Cereal Sci., 1995, 22(1), 29–44 CrossRef CAS.
- S. Hayakawa and S. Nakai, Relationships of hydrophobicity and net charge to the solubility of milk and soy proteins, J. Food Sci., 1985, 50, 486–491 CrossRef CAS.
- Y. Zhou, D. Zhao, T. J. Foster, Y. Liu, Y. Wang and S. Nirasawa, Konjac glucomannan-induced changes in thiol/disulphide exchange and gluten conformation upon dough mixing, Food Chem., 2014, 143(2), 163–169 CrossRef CAS PubMed.
- L. Zhao, L. Li, G. Q. Liu, X. X. Liu and B. Li, Effect of frozen storage on molecular weight, size distribution and conformation of gluten by SAXS and SEC-MALLS, Molecules, 2012, 17(6), 7169–7182 CrossRef CAS.
- A. Nawrocka, M. Szymańska-Chargot, A. Miś, A. Z. Wilczewska and K. H. Markiewicz, Aggregation of gluten proteins in model dough after fibre polysaccharide addition, Food Chem., 2017, 231, 51–60 CrossRef CAS PubMed.
- A. Laligant, E. Dumay, C. Casas Valencia, J. L. Cuq and J. C. Cheftel, Surface hydrophobicity and aggregation of. beta.-lactoglobulin heated near neutral pH, J. Agric. Food Chem., 1991, 39(12), 2147–2155 CrossRef CAS.
- T. H. McCann and L. Day, Effect of sodium chloride on gluten network formation, dough microstructure and rheology in relation to breadmaking, J. Cereal Sci., 2013, 57(3), 444–452 CrossRef CAS.
- P. Wang, H. Y. Chen, B. Mohanad, L. Xu, Y. W. Ning, J. Xu, F. F. Wu, N. Yang, Z. Y. Jin and X. M. Xu, Effect of frozen storage on physico-chemistry of wheat gluten proteins: Studies on gluten-, glutenin- and gliadin-rich fractions, Food Hydrocolloids, 2014, 39, 187–194 CrossRef CAS.
- X. Y. Wang, X. N. Guo and K. X. Zhu, Polymerization of wheat gluten and the changes of glutenin macropolymer (GMP) during the production of Chinese steamed bread, Food Chem., 2016, 201(15), 275–283 CrossRef CAS.
- M. H. Morel, A. Redl and S. Guilbert, Mechanism of heat and shear mediated aggregation of wheat gluten protein upon mixing, Biomacromolecules, 2002, 3(3), 488–497 CrossRef CAS.
- W. Wu, X. Kong, C. Zhang, Y. Hua and Y. Chen, Improving the stability of wheat gliadin nanoparticles–Effect of gum arabic addition, Food Hydrocolloids, 2018, 80, 78–87 CrossRef CAS.
- R. Urade, N. Sato and M. Sugiyama, Gliadins from wheat grain: An overview, from primary structure to nanostructures of aggregates, Biophys. Rev., 2017, 10, 1–9 Search PubMed.
- N. Linlaud, E. Ferrer, M. C. Puppo and C. Ferrero, Hydrocolloid interaction with water, protein, and starch in wheat dough, J. Agric. Food Chem., 2010, 59(2), 713–719 CrossRef.
- P. R. Shewry, Y. Popineau, D. Lafiandra and P. Belton, Wheat glutenin subunits and dough elasticity: findings of the EUROWHEAT project, Trends Food Sci. Technol., 2000, 11, 433–441 CrossRef CAS.
- B. S. Khatkar, S. Barak and D. Mudgil, Effects of gliadin addition on the rheological, microscopic and thermal characteristics of wheat gluten, Int. J. Biol. Macromol., 2013, 53, 38–41 CrossRef CAS PubMed.
- A. Nawrocka, M. Szymańska-Chargot, A. Miś, A. Z. Wilczewska and K. H. Markiewicz, Dietary fiber-induced changes in the structure and thermal properties of gluten proteins studied by Fourier transform-Raman spectroscopy and thermogravimetry, J. Agric. Food Chem., 2016, 64(10), 2094–2104 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |