Combination of curcuma zedoary and kelp inhibits growth and metastasis of liver cancer in vivo and in vitro via reducing endogenous H2S levels
Huanxiao
Han†
a,
Lupeng
Wang†
a,
Ya
Liu
a,
Xiaoyan
Shi
a,
Xiuli
Zhang
b,
Ming
Li
*a and
Tianxiao
Wang
 *a
*a
aJoint International Research Laboratory of Food & Medicine, College of Pharmacy, Henan University, Kaifeng, 475004 Henan, China. E-mail: wtx1975@126.com; 38698633@qq.com
bLiaoning Agricultural College, Yingkou, 115009 Liaoning, China
First published on 26th November 2018
Abstract
The combination of traditional Chinese medicines can improve the efficacy of cancer treatment. Furthermore, the combination of the traditional Chinese medicine curcuma zedoary and kelp was used to enhance the effect of the dissipation of blood stasis in pediatrics during the Song Dynasty. Curcumenol and laminarin, the main components of curcuma zedoary and kelp, are also reported to have a wide range of biological activities. Therefore, we hypothesize that the combination of curcuma zedoary and kelp may prevent the development of cancer. The aim of this research was to confirm whether a combination of curcuma zedoary and kelp could inhibit the proliferation and metastasis of hepatoma cells and consequently improve prognosis. In this study, we firstly found in H22-bearing mice that the combination of curcuma zedoary and kelp inhibited tumor growth and the expression of metastasis-related proteins (MMPs, VEGF, pAkt, pERK1/2). Meanwhile, the decreased cystathionine beta synthase (CBS, an endogenous hydrogen sulfide (H2S) synthetase) level was also observed in H22-bearing mice admistrated with the combination of curcuma zedoary and kelp. It was also observed that the combination of curcumenol and laminarin inhibited the proliferation, migration and invasion in human hepatoma HepG2 cells. Furthermore, we investigated the potential inhibiting mechanism of the combination of curcumenol and laminarin on HepG2 cell proliferation and metastasis. Our previous research showed that a CBS/H2S system was vital for maintaining the proliferation in hepatoma cells. Here, we found that the levels of pSTAT3 and BCL-2 were decreased in CBS knockdown HepG2 cells and the combination of curcumenol and laminarin significantly decreased the H2S level in a dose-dependent manner and down-regulated the levels of pSTAT3 and BCL-2 in HepG2 cells. Angiogenesis, positively regulated by the vascular endothelial growth factor (VEGF), is essential for human cancer metastasis. In the present study, we found that the combination of curcumenol and laminarin could significantly down-regulate the expression levels of VEGF and its downstream key genes pAkt and pERK1/2. Furthermore, previous research showed that hydrogen sulfide could stimulate angiogenesis. Here, we also observed the reduction of the VEGF, Akt, pAkt, ERK1/2 and pERK1/2 proteins levels and the inhibition of proliferation and metastasis in CBS knockdown HepG2 cells. Moreover, exogenous H2S rescued the cytological results caused by the combination of curcumenol and laminarin. Taken together, the combination of curcuma zedoary and kelp could inhibit the proliferation and metastasis of liver cancer cells in vivo and in vitro by inhibiting endogenous H2S production and down-regulating the pSTAT3/BCL-2 and VEGF pathway, which provides strong evidence for the application of curcuma zedoary and kelp in treatments of liver cancer.
1. Introduction
Liver cancer is one of the most common malignancies. High postoperative recurrence and a high metastasis rate are important factors affecting the long-term survival of patients.1 The development of drugs which prevent liver cancer from growing and metastasizing is one of the major directions for liver cancer treatments. Traditional Chinese Medicine (TCM) has been applied to cancer treatments for thousands of years in China. Many studies have shown that TCM has a certain therapeutic effect on cancer.2–4 Moreover, a drug combination can reduce toxic side effects and improve efficacy for cancer treatment.5 The combination of TCMs can treat cancer by regulating immune function, inducing the apoptosis and differentiation of cancer cells, reversing multidrug resistance of cancer, regulating cell signal transduction, inhibiting the growth of tumor blood vessels during cancer cell invasion, migration and transfer.6–8 Therefore, TCM may be vital for the development of effective anti-cancer drugs.The TCM curcuma zedoary, the dry rhizome of Curcuma phaeocaulis Val, Curcuma kwangsensis or Curcuma wenyujin, invigorates blood circulation and curcumenol is its main chemical component (according to the Chinese Pharmacopoeia). The TCM kelp is a dried thallus of Laminaria japonica Aresch or Ecklonia kurome and has the function of removing stasis. Kelp is an important medicine and food homologous plant and its main active ingredient is laminarin (according to the Chinese Pharmacopoeia). Both curcuma zedoary and kelp can pass through the liver channel. Moreover, the combination of curcuma zedoary and kelp had been used to enhance the effect of dissipation of blood stasis in the Song Dynasty (according to a medical book of pediatrics written by Liu Fang). Curcumenol and laminarin, as the main components of curcuma zedoary and kelp, respectively, possess anticancer activity and have been used to treat cancer.9–12 Curcumenol might be an antibiotic or anticancer agent when used in combination with other antitumor or antivirus drugs.13,14 Therefore, in this study, we firstly determined the effect of the combination of curcuma zedoary and kelp on tumor growth and metastasis in H22-bearing mice to evaluate the potential roles of the combination of curcuma zedoary and kelp in liver cancer treatment. Then, we further explored the effects of the combination of curcumenol and laminarin on the growth and metastasis in hepatoma cells.
Hydrogen sulfide (H2S), as a member of the three molecule gases, plays an important role in many biological processes, and its metabolic and functional abnormalities are also associated with many diseases.15 Studies have shown that H2S can promote the proliferation of various tumor cells.16,17 Our previous study has also shown that a cystathionine beta synthase (CBS)/hydrogen sulfide (H2S) system is vital for maintaining the proliferation and progression in hepatoma cells.18 Meanwhile, H2S possesses the function of stimulating angiogenesis,19–21 which is essential for human cancer metastasis. The process of liver cancer metastasis includes basement membrane degradation, epithelial mesenchymal transition (EMT), tumor migration and invasion. Matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) can degrade the extracellular matrix and promote cell migration. The growth of solid tumors is dependent on angiogenesis and neovascularization.22 So, endogenous H2S, MMP-2, MMP-9 and the angiogenesis factor VEGF could be applied together for the assessment of liver cancer as tumor metastatic markers. Here we hypothesize that the combination of curcumenol and laminarin can inhibit the proliferation and metastasis of hepatoma cells by inhibiting endogenous H2S production and down-regulating MMPs and the VEGF pathway.
To demonstrate the hypothesis, in the present study we further investigated the effect of the combination of curcumenol and laminarin on H2S production and MMPs, as well as the VEGF pathway, in human hepatoma HepG2 cells to further appraise the potency of the combination of curcuma zedoary and kelp against the recurrence and metastasis of human liver cancer.
2. Experimental section
2.1 Traditional Chinese medicines and their active monomers
The traditional Chinese medicine curcuma zedoary (No. 161021, place of origin: Guangxi, China) and kelp (No. 160101CP0349, place of origin: Fujian, China) were purchased from the Zhang Zhongjing Pharmacy (Kaifeng, Henan, China). Curcumenol (HPLC ≥98%, No. B20342) and laminarin (BR98%, No. S11059) were obtained from the Shanghai Source Leaf Biotechnology Co., Ltd (Shanghai China).2.2 Animals and cell lines
KM mice (male and female in half) weighing 20 ± 2 g were supplied by the Henan Experimental Animal Center (Zhengzhou, Henan, China, Certificate No. SCXK [Yu] 2017-0001). The mice were allowed to access food and water freely, and housed in a constant temperature (22 ± 1 °C) and humidity (65 ± 5%) environment under a 12 h light/dark cycle.Human hepatoma HepG2 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), and cultivated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Zeta-life, USA) in a 37 °C incubator with 5% CO2.
2.3 Establishment and grouping of a mouse liver cancer model
To establish a mouse liver cancer model, eighty-four KM mice were injected with H22 cells through a subaxillary injection. Firstly, 0.2 mL of mouse hepatoma H22 cells (1 × 107 ml−1) were intraperitoneally injected into the mice. One week later, H22 well growing ascites were sucked out with syringes, cleaned with physiological saline and centrifuged at 1500 rpm for 5 min. Then 0.2 ml of the H22 cells (1 × 107 mL−1) were subcutaneously injected into the mice. The next day, the mice were randomly divided into groups and administered for 7 days (0.2 mL/10 g d−1) in a row. On the eighth day, the mice were sacrificed by cervical dislocations and the tumors were stripped and weighed. Meanwhile, the proteins related to growth and metastasis were detected in the tumor tissues.The mice were randomly divided into 7 groups (n = 12, male and female in half): a negative control group; a liver cancer model group with a positive control Cisplatin; model groups treated with a 4000 mg kg−1curcuma zedoary water decoction, a 4000 mg kg−1kelp water decoction, a 1000 mg kg−1curcuma zedoary water decoction combined with a 1000 mg kg−1kelp water decoction, a 2000 mg kg−1curcuma zedoary water decoction combined with a 2000 mg kg−1kelp water decoction or a 4000 mg kg−1curcuma zedoary water decoction combined with a 4000 mg kg−1kelp water decoction. From the second day of establishing the liver cancer model, the negative control group was injected with saline once a day, and the treated groups of mice were given 0.2 mL per 10 grams of a weight intragastric administration of a curcuma zedoary water decoction and/or a kelp water decoction once a day. The mice in the Cisplatin group were given 0.2 mL per 10 grams of weight intraperitoneal injections of 1 mg kg−1 cisplatin (H37021358, Qilu Pharmaceutical Co., Ltd, Shandong, China) every day. All of the experiment procedures involving animal maintenance and treatments were carried out according to the guidelines in the Principles of Laboratory Animal Care established by the National Institutes of Health, China. The experimental protocol was approved by the ethics committee of the Medical School, Henan University, China (the approval number is HUSOM-2016-134).
2.4 Cell viability and proliferation assays
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and 5-ethynyl-2′-deoxyuridine (EdU) assays were used to assess cell viability and cell proliferation. HepG2 cells (1 × 104) were plated into 96-well plates, and treated with curcumenol alone, laminarin alone and curcumenol combined with laminarin for 24 h, respectively. The cell viability was evaluated by determining the number of cells with MTS (Sigma-Aldrich). The assay was carried out in triplicate for three independent experiments. Cisplatin (DDP) served as positive controls.The effects of curcumenol alone, laminarin alone and curcumenol combined with laminarin on cell proliferation were tested by the EdU assay kit (Ruibobio, Guangzhou, China). Cells were cultured in 96-well plates and exposed to curcumenol or laminarin or curcumenol combined with laminarin for 24 h. Then, the cells were incubated with 50 μm of EdU for 2 h at 37 °C. The cells were fixed with 4% formaldehyde for 30 min, incubated with glycine (2 mg mL−1) for 5 min and treated with 0.5% Triton X-100 for 10 min to permeabilize the cells. After being washed with phosphate-buffered saline (PBS) for 5 min, the cells were incubated with Apollo for 30 min and treated twice with 0.5% Triton X-100. DNA was stained with Hoechst 33342 stain for 30 min and visualized with fluorescence microscopy. Five groups of cells in the images were randomly selected.
2.5 Migration and invasion assays
The scratch wound assay was used to determine the cell migration. Cells were seeded into a 6-well plate and scraped with a 10 μL pipette tip at approximately 90% confluency to generate a scratch wound and rinsed twice with PBS. Then cells were cultivated in the medium with 5% FBS and the distance was measured at the beginning and after 24 h.The transwell invasion assay proceeded as follows: 24-well transwell chambers (8 μm pores; Corning, NY, USA) were coated with a matrigel matrix (BD Biosciences, CA, USA). 2.5 × 104 HepG2 cells, suspended in 200 μL DMEM (with 1% FBS), were seeded into each top chamber. A DMEM medium with 15% FBS was placed in the 24-well's bottom wells. After a treatment of curcumenol or laminarin and incubation at 37 °C for 48 h in the incubator, the cells on the upper surface of the membrane were wiped off with a cotton swab. The invasive cells attaching to the outside bottom surface of the membrane were fixed with 4% polyoxymethylene and stained with 0.1% crystal violet for 15–20 min. The staining cells were dissolved by 10% acetic acid and counted under a microscope and the OD570 was read using a microplate spectrofluorometer.
2.6 siRNA transfection
HepG2 cells in 6-well plates (30–40% cell confluence) were transfected with Scramble siRNA (Sc siRNA) or specific siRNA against human CBS (Invitrogen, Shanghai, China) using Lipofectamine 2000. The medium was replaced at 6 h post-transfection, and the silencing efficiency was determined by WB 48 h after transfection. CBS-specific siRNA (sense, 5′-CCAAGUGUGAGUUCUUCAAdTdT-3′ and antisense, 5′-UUGAAGAACUCACACUUGG dTdT-3′) was designed to target the open reading frame (ORF) region of the CBS mRNA.2.7 WB analysis
The mice tumor tissues or HepG2 cells (transfected with CBS siRNA for 48 h or treated with the combination of curcumenol and laminarin for 24 h) were lysed by a RIPA buffer (50 mM Tris-HCl, pH 8.0; 150 mM sodium chloride; 1.0% NP-40; 0.5% sodium deoxycholate; and 0.1% SDS) with 10 μg mL−1 of the protease inhibitor, PMSF (Sigma-Aldrich) and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. This was followed by the determination of the protein concentration with a BCA protein quantitative kit (Solarbio, Beijing, China). Then, 40 μg of protein was separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, MA, USA) at 70 mA for 2 h. The membrane was then blocked in 5% fat-free milk, and probed with specific primary antibodies against CBS, STAT3, pSTAT3, BCL-2, MMP-2, MMP-9, VEGF, Akt, pAkt, ERK1/2 and pERK1/2 at 4 °C overnight. After incubation with the secondary antibody, the proteins were visualized using an EasyBlot Enhanced Chemiluminescence kit (Sangon Biotech Co., Ltd, Shanghai, China) and detected using a FluorChem Q Multifluor System (ProteinSimple, San Jose, CA, USA). GAPDH was used as a loading control. Primary antibodies including a CBS rabbit polyclonal antibody (1
000g for 10 min. This was followed by the determination of the protein concentration with a BCA protein quantitative kit (Solarbio, Beijing, China). Then, 40 μg of protein was separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, MA, USA) at 70 mA for 2 h. The membrane was then blocked in 5% fat-free milk, and probed with specific primary antibodies against CBS, STAT3, pSTAT3, BCL-2, MMP-2, MMP-9, VEGF, Akt, pAkt, ERK1/2 and pERK1/2 at 4 °C overnight. After incubation with the secondary antibody, the proteins were visualized using an EasyBlot Enhanced Chemiluminescence kit (Sangon Biotech Co., Ltd, Shanghai, China) and detected using a FluorChem Q Multifluor System (ProteinSimple, San Jose, CA, USA). GAPDH was used as a loading control. Primary antibodies including a CBS rabbit polyclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), STAT3 rabbit monoclonal antibody (1
1000), STAT3 rabbit monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), pSTAT3 rabbit monoclonal antibody (1
1000), pSTAT3 rabbit monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), MMP-2 rabbit monoclonal antibody (1
1000), MMP-2 rabbit monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), MMP-9 rabbit monoclonal antibody (1
1000), MMP-9 rabbit monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), VEGF-C rabbit recombinant antibody (1
1000), VEGF-C rabbit recombinant antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), Akt mouse monoclonal antibody (1
1000), Akt mouse monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), pAkt rabbit monoclonal antibody (1
1000), pAkt rabbit monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), ERK1/2 rabbit monoclonal antibody (1
1000), ERK1/2 rabbit monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000), pERK1/2 rabbit monoclonal antibody (1
1000), pERK1/2 rabbit monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) and a GAPDH mouse monoclonal antibody (1
1000) and a GAPDH mouse monoclonal antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) were purchased from Cell Signaling Technology (Danvers, MA, USA). Secondary antibodies were horseradish peroxidase-conjugated goat anti-rabbit and horseradish peroxidase-conjugated goat anti-mouse (1
1000) were purchased from Cell Signaling Technology (Danvers, MA, USA). Secondary antibodies were horseradish peroxidase-conjugated goat anti-rabbit and horseradish peroxidase-conjugated goat anti-mouse (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Proteintech Group, Inc., Chicago, IL, USA).
000, Proteintech Group, Inc., Chicago, IL, USA).
2.8 Quantification of H2S production
H2S determination was performed using the methylene blue method. HepG2 cells (transfected with CBS siRNA for 48 h or treated with the combination of curcumenol and laminarin for 24 h) were treated with 2 mM L-cysteine and 0.5 mM pyridoxal phosphate. Meanwhile, 1% (w/v) zinc acetate (500 μL) was added to the filter papers that adhered to the tissue culture plate cover to absorb H2S. After 24 h, the filter papers were put in the tubes containing 0.2% (w/v) N,N-dimethyl-p-phenylenediamine-dihydrochloride dye (500 μL), 10% (w/v) ammonium ferric sulfate (50 μL) and 3 ml H2O and incubated for 20 min at room temperature. The absorbance at 670 nm was subsequently monitored. The production of H2S was determined using a standard curve of NaHS (0–1 mM; R2 = 0.9997) and presented as nmol min−1 per 1 × 106 cells.2.9 Statistical analysis
Statistical analyses were performed with SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). The data are expressed as mean ± s.d. The differences between the two groups were analyzed using one-way ANOVA, followed by Student's t-tests. p < 0.05 was considered statistically significant.3. Results
3.1 The combination of the traditional Chinese medicine curcuma zedoary and kelp inhibits tumor growth and the expression of metastasis-related proteins in H22-bearing mice
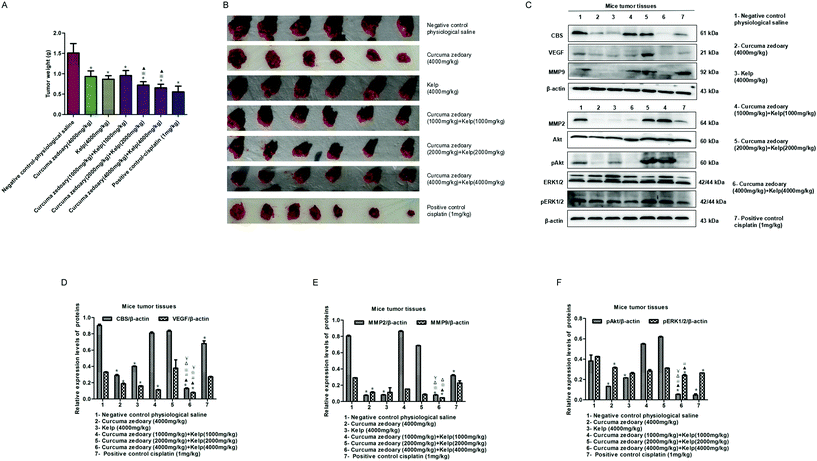
According to the ancient medical records, the combination of curcuma zedoary and kelp is used to enhance the effect of the dissipation. Therefore, we here investigated the inhibitory effect of the combination of curcuma zedoary and kelp on tumor growth. As shown in Fig. 1A and B, the combination of curcuma zedoary and kelp distinctly inhibited the tumor growth in H22-bearing mice and the inhibitory effect was stronger than the curcuma zedoary alone group and kelp alone group. Meanwhile, we found that the high concentration combination (4000 mg kg−1 + 4000 mg kg−1) of curcuma zedoary and kelp distinctly decreased the levels of MMP2/9 and the VEGF pathway proteins including pAkt and pERK1/2, as well as the CBS protein. Moreover, the inhibitory effects of the combination of curcuma zedoary and kelp on metastasis-related proteins, except for MMP2, were stronger than the curcuma zedoary alone group and the kelp alone group (Fig. 1C–F). We also detected the levels of apoptosis-related proteins in mice tumor tissues and found that the combination of curcuma zedoary and kelp hardly affected the expression of Bcl-2 and Bax (data not shown). The data indicated that the combination of curcuma zedoary and kelp possessed potential significance in the treatment of liver cancer and the CBS/H2S system and VEGF pathway could be responsible for the diminution of tumor growth. So in the next study, we will further explore the effect and mechanism of the combination of curcumenol and laminarin, the main active component of curcuma zedoary and kelp respectively, on human liver cancer cells in vitro.3.2 The combination of curcumenol and laminarin inhibits the proliferation of human hepatoma cells
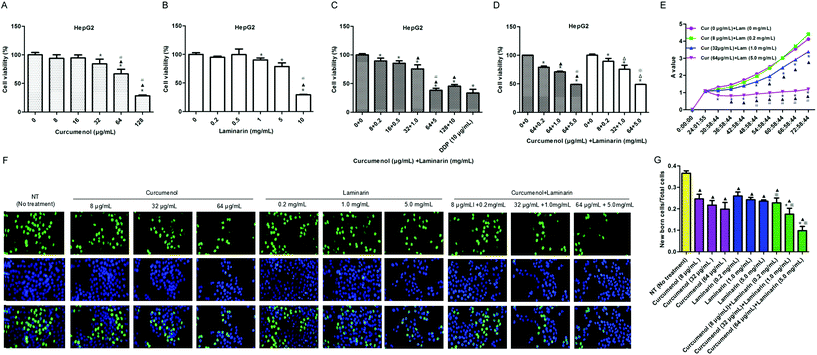
Based on the above animal experiment, we further explored the roles of the combination of curcumenol and laminarin, as the main components of the traditional Chinese medicine curcuma zedoary and kelp respectively, in human hepatoma cells. As shown in Fig. 2A–C, the HepG2 cell viability was significantly reduced in a dose-dependent manner under treatment with the combination of curcumenol and laminarin and the inhibitory effect was stronger than the treatment with curcumenol or laminarin alone, except for the 128 μg mL−1 curcumenol and 10 mg mL−1 laminarin group. This phenomenon could be due to the different enhancement magnitudes of the pharmacological effects caused by curcumenol or laminarin, so the bidirectional effects of low concentration promotion and high concentration inhibition occurred when they were combined at different doses. Then we detected the effects of the combination of an optimal amount of curcumenol and different ratios of laminarin on HepG2 cell growth. The results showed that the effects of the combination of 64 μg mL−1 curcumenol and 0.2, 1, 5 mg mL−1 laminarin on cell viability were not significantly different with the combination of curcumenol and laminarin (8 + 0.2, 32 + 1, 64 + 5) (Fig. 2D). According to the results, we chose three concentrations of low (Cur 8 μg mL−1 + Lam 0.2 mg mL−1), middle (Cur 32 μg mL−1 + Lam 1.0 mg mL−1) and high (Cur 64 μg mL−1 + Lam 5.0 mg mL−1) levels for follow-up experiments. In terms of cell growth, we also observed that the combination of curcumenol and laminarin yielded the strongest inhibition to HepG2 cells in a time-dependent manner (Fig. 2E). Meanwhile, an EdU assay was performed to further evaluate the inhibitory effect of the combination of curcumenol and laminarin on cell proliferation. As shown in Fig. 2F and G, the combination of curcumenol and laminarin distinctly decreased the number of EdU positive cells compared with the curcumenol or laminarin alone groups in the HepG2 cell lines. Taken together, these data demonstrated that the combination of curcumenol and laminarin inhibited the proliferation in human hepatoma cells.3.3 The combination of curcumenol and laminarin inhibits the metastasis in human hepatoma cells
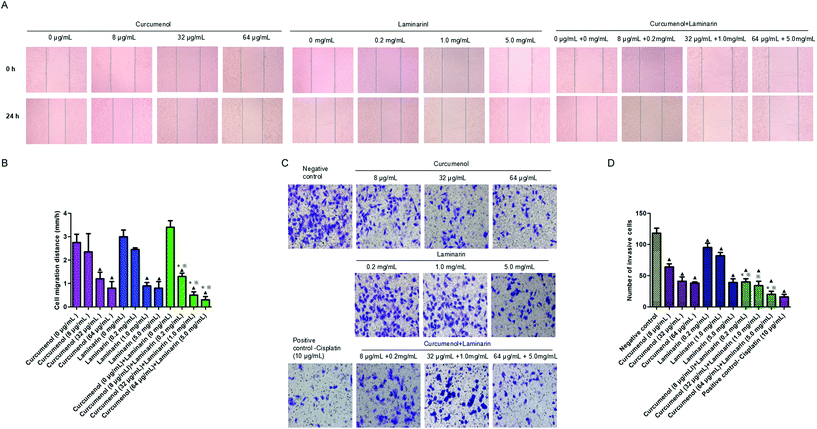
To explore the potential role of the combination of curcumenol and laminarin in human hepatoma metastasis, the scratch test and the transwell migration assay were performed in HepG2 cells. The scratch test data showed that the combination of curcumenol and laminarin significantly inhibited HepG2 cell migration in a dose-dependent manner (Fig. 3A and B). We also observed a decreased cell invasion rate in HepG2 cells treated with the combination of curcumenol and laminarin in a dose-dependent manner (Fig. 3C and D). Moreover, the inhibitory effects of migration and invasion were stronger than the treatment of curcumenol or laminarin alone at the same concentrations (Fig. 3A and D).3.4 The combination of curcumenol and laminarin inhibits endogenous H2S production and STAT3 activation in human hepatoma cells
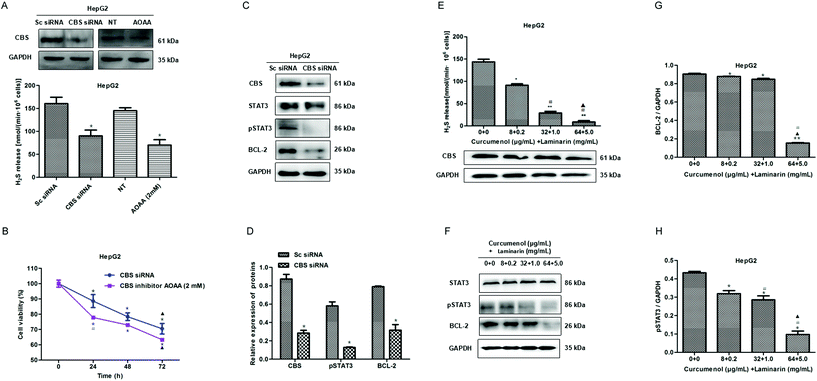
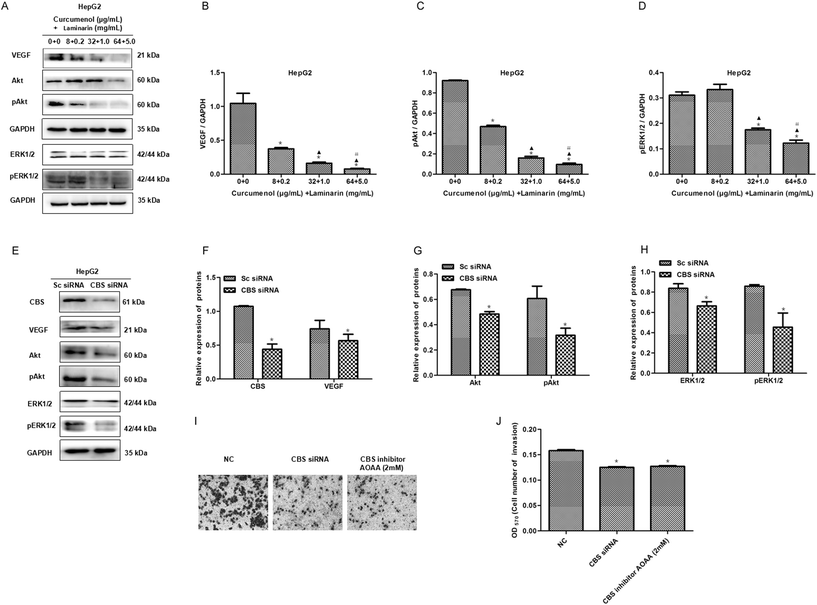
Our previous study found that a cystathionine beta synthase (CBS)/H2S system is vital for maintaining the proliferation of hepatoma cells.17 In this study, we further confirmed the inhibitory effect of a CBS knockdown or inactivation on proliferation in HepG2 cells (Fig. 4A and B). Then, we investigated the possible downstream pathway in a CBS/H2S system and observed the reduced levels of pSTAT3 and BCL-2 proteins in CBS knockdown HepG2 cells (Fig. 4C and D), which indicated that the CBS/H2S system might regulate proliferation via a STAT3/BCL-2 pathway in HepG2 cells.To explore the mechanism of the combination of curcumenol and laminarin inhibiting proliferation in hepatoma, we detected the effect of the combination of curcumenol and laminarin on a CBS/H2S system. The data showed that the combination of curcumenol and laminarin significantly decreased the H2S level in a dose-dependent manner although there is no obvious change in the expression of CBS (Fig. 4E). Moreover, we also observed the down-regulation of pSTAT3 and BCL-2 in the HepG2 cells treated with the combination of curcumenol and laminarin (Fig. 4F–H). The results suggested that endogenous H2S mediated the inhibition of the proliferation of hepatoma cells caused by the combination of curcumenol and laminarin via the STAT3/BCL-2 pathway.
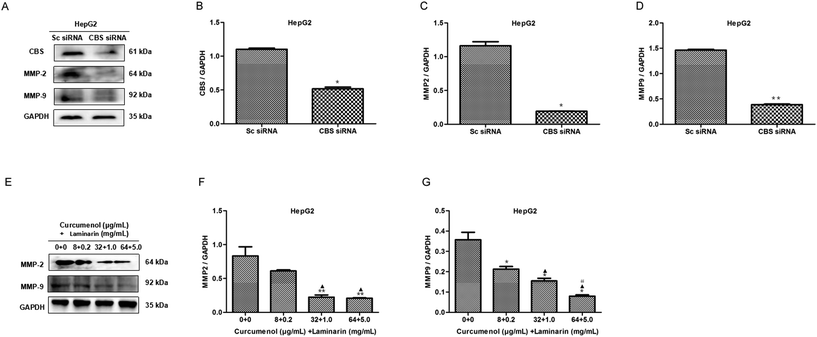
3.5 The combination of curcumenol and laminarin reduces the levels of MMP2 and MMP9
The expression of the matrix metalloproteinases MMP2 and MMP9 is vital to the migration of cancer cells. To explore the mechanism of the combination of curcumenol and laminarin inhibiting migration and invasion in hepatoma, we firstly investigated the effect of CBS siRNA on the levels of MMP2 and MMP9 in HepG2 cells. As shown in Fig. 5A–D, CBS siRNA distinctly decreased the levels of the MMP2 and MMP9 proteins, which suggested that the CBS/H2S system could regulate the expression of MMP2 and MMP9. We further found that the combination of curcumenol and laminarin at the concentration of 32 μg mL−1 + 1.0 mg mL−1 and 64 μg mL−1 + 5.0 mg mL−1 distinctly decreased the levels of MMP2, while the combination of curcumenol and laminarin, in a dose-dependent manner, inhibited the expression of MMP9 (Fig. 5E–G). These data suggested that the combination of curcumenol and laminarin weakened the degradation of the basement membrane and consequently inhibited the migration of human hepatoma cells via the CBS/H2S system.3.6 The combination of curcumenol and laminarin depresses the VEGF signaling pathway via a CBS/H2S system
To further investigate the mechanism of the combination of curcumenol and laminarin inhibiting metastasis in hepatoma, we tested whether angiogenesis was influenced by the combination of curcumenol and laminarin. The expression of VEGF and its downstream signaling molecules ERK1/2/pERK1/2 and Akt/pAkt were assayed in HepG2 cells. western blot results showed that the combination of curcumenol and laminarin significantly decreased the levels of VEGF, pERK1/2 and pAkt proteins (Fig. 6A–D). Here we also observed that the inhibition of the CBS/H2S system depressed the expression of VEGF and the phosphorylation of ERK1/2 and Akt in HepG2 cells (Fig. 6E–H). Moreover, the reduced invasion of HepG2 cells was observed in the case of the CBS knockdown or inactivation (Fig. 6I and J). Taken together with the above results of the combination of curcumenol and laminarin inhibiting H2S production, the combination of curcumenol and laminarin inhibited angiogenesis of hepatoma by H2S and the VEGF pathway and consequently inhibited the metastasis of hepatoma cells.3.7 Exogenous H2S relieves the cytological results caused by the combination of curcumenol and laminarin
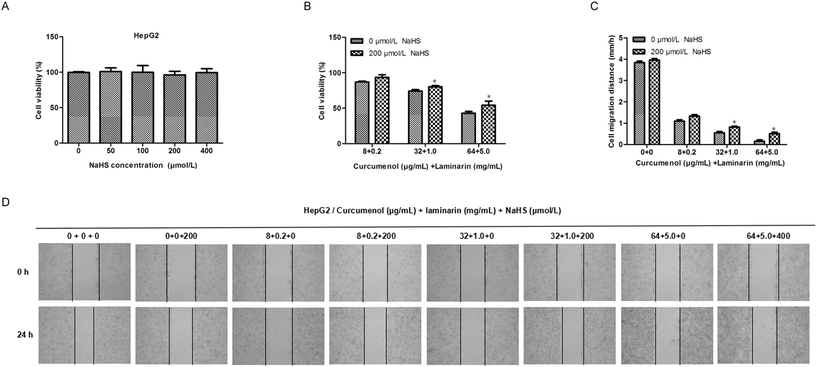
To further confirm the roles of H2S mediating the antihepatic activity of the combination of curcumenol and laminarin, we investigated the effects of exogenous H2S on the cytological results caused by the combination of curcumenol and laminarin. The MTS assay and scratch test assay showed that the addition of exogenous H2S relieved the inhibition of cell viability and migration caused by the combination of curcumenol and laminarin (Fig. 7). The data further confirmed the potential function of H2S mediating the antihepatic activity of the combination of curcumenol and laminarin.4. Discussion
Based on the Chinese Medicine (CM) theory, liver cancer belongs to the accumulation syndrome caused by the stasis of blood. So, increasing blood circulation and dissipating blood stasis are important therapies for treating primary liver cancer in the clinic. Traditional Chinese medicine (TCM) has been used for preventing and treating liver diseases in China for centuries23,24 and the combination of TCM has also been widely explored for liver cancer treatment in recent years.25,26 However, exploring the scientific and rational combination for the treatment of liver cancer is still necessary.Curcuma zedoary and kelp had been used to enhance the effect of dissipation in the Song Dynasty. Moreover, both curcuma zedoary and kelp enter the liver. So, in this study, we explored the potential role of the combination of curcuma zedoary and kelp in the treatment of liver cancer. Firstly, we established a mouse liver cancer model and set up three concentrations of high (4000 mg kg−1), medium (2000 mg kg−1) and low (1000 mg kg−1) concentrations of curcuma zedoary and kelp in mice based on the traditional Chinese medicine prescription kunbusan (both curcuma zedoary and kelp are half in human), citing Youyou Xin Shu, a New Book of Pediatrics in the Song Dynasty. Here, Cisplatin was a positive control. The dosage of the Chinese medicine in animals was calculated according to the dosage of Chinese medicine in humans. The mice were given 0.2 mL per 10 grams of weight intraperitoneal injections of 1 mg kg−1 Cisplatin every day, referring to relevant reports. In the present study, we, for the first time, demonstrated that the combination of curcuma zedoary and kelp could inhibit tumor growth in H22-bearing mice. Meanwhile, we also observed the inhibition of metastatic proteins in H22-bearing mice administered with a combination of curcuma zedoary and kelp. It can be seen that the combination of curcuma zedoary and kelp may play a potential role in the treatment of liver cancer.
Based on the results of the experiment in vivo, we further explored the roles of the combination of curcumenol and laminarin, the effective components extracted from curcuma zedoary and kelp respectively, in human hepatoma cells. The concentrations of the pure compounds in cells were taken from the repeated screening of the preliminary experiment because the concentration using in vivo had no correlation with the concentration using in vitro. Here we found that the combination of curcumenol and laminarin inhibited the growth and proliferation in HepG2 cells in a dose-dependent manner at the concentration of 8 μg mL−1 curcumenol combined with 0.2 mg mL−1 laminarin, 32 μg mL−1 curcumenol combined with 1.0 mg mL−1 laminarin and 64 μg mL−1 curcumenol combined with 5.0 mg mL−1 laminarin. Meanwhile, HepG2 cells treated with combination of curcumenol and laminarin at the same concentrations for a short duration of time already exhibited less migration and invasion. Moreover, studies have shown that curcumenol can protect the liver from damage27 and kelp is both a traditional Chinese medicine and a vegetable, which can be used for daily consumption and the concentrations can be obtained through diet in humans. So, the combination of curcumenol and laminarin set in this study does not damage the hepatocytes. The findings indicated that the combination of curcumenol and laminarin might be beneficial for the treatment of liver cancer.
We further investigated the mechanism of the combination of curcumenol and laminarin inhibiting growth and metastasis of hepatocellular carcinoma. A CBS/H2S system is vital for maintaining the proliferation of hepatoma cells.18 In this study, we confirmed the inhibitory effect of a CBS knockdown or inactivation on the proliferation of HepG2 cells and found that a CBS/H2S system may regulate proliferation via a STAT3/BCL-2 pathway in HepG2 cells. Therefore, we explored the effect of the combination of curcumenol and laminarin on a CBS/H2S system and STAT3/BCL-2 expression. The decreased H2S level and the inhibition of the pSTAT3 and BCL-2 expression were observed in the HepG2 cells treated with combination of curcumenol and laminarin, which suggested that endogenous H2S mediated the inhibition of the combination of curcumenol and laminarin on the proliferation of hepatoma cells via a STAT3/BCL-2 pathway.
Metastasis is responsible for the rapid recurrence and poor survival of malignancies. We firstly found that the combination of curcumenol and laminarin inhibited the expression of MMP-2 and MMP-9 in HepG2 cells, ultimately leading to a reduction of migration. Angiogenesis is essential for human cancer metastasis and the angiogenic process is positively regulated by the vascular endothelial growth factor (VEGF).28–32 In the present study, we found that the combination of curcumenol and laminarin distinctly downregulated the levels of VEGF and its downstream pAkt and pERK1/2 proteins in HepG2 cells. Moreover, the addition of exogenous H2S rescued the inhibition of cell viability and migration caused by the combination of curcumenol and laminarin. Therefore, the combination of curcumenol and laminarin may inhibit angiogenesis of hepatoma via H2S and the VEGF pathway and consequently inhibit the metastasis of hepatoma cells.
5. Conclusion
The present study demonstrates the inhibitory effect of the combination of curcuma zedoary and kelp on the proliferation and metastasis of hepatoma cells in vivo and in vitro. Its anti-proliferation and anti-metastasis effect is probably related to the inhibition of CBS activity and restraint of angiogenesis (Fig. 8). The data provide a foundation for further research on the combination of curcumenol and laminarin in tumors.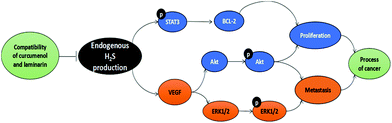 | ||
| Fig. 8 The schematic diagram of the mechanism of the combination of curcumenol and laminarin inhibiting proliferation and metastasis of hepatoma cells. | ||
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to acknowledge the financial assistance provided by a grant from the Key Science and Technology Fund of Henan Province in China (grant no. 162300410035) and Henan province university science and technology innovation team (grant no. 16IRTSTHN019) to support the present project.References
- J. He, D. Gu and X. Wu, N. Engl. J. Med., 2005, 353, 1124–1134 CrossRef CAS PubMed.
- W. L. Hsiao and L. Liu, Planta Med., 2010, 76, 1118–1131 CrossRef CAS PubMed.
- M. Li, C. Qiao, L. Qin, J. Zhang and C. Ling, J. Tradit. Chin. Med., 2012, 32, 299–307 CrossRef PubMed.
- L. Ma, L. Liu, Y. C. Ma, H. Xie, X. Yu, X. Wang, A. G. Fan, D. Y. Ge, Y. Y. Xu, Q. Zhang and C. Song, Biol. Pharm. Bull., 2017, 40, 1706–1715 CrossRef CAS PubMed.
- M. Zhou, Y. Hong, X. Lin, L. Shen and Y. Feng, J. Ethnopharmacol., 2017, 206, 363–375 CrossRef PubMed.
- J. Zhang, H. F. Li, W. Fan, Z. Liu, S. L. Man, S. Y. Si and W. Y. Gao, Zhongguo Zhongyao Zazhi, 2014, 39, 3870–3875 Search PubMed.
- J. Zhou, T. Zhou, M. Jiang, X. Wang, Q. Liu, Z. Zhan and X. Zhang, J. Tradit. Chin. Med., 2014, 34, 100–105 CrossRef PubMed.
- Y. He, X. Zheng, C. Sit, W. T. Loo, Z. Wang, T. Xie, B. Jia, Q. Ye, K. Tsui, L. W. Chow and J. Chen, J. Transl. Med., 2012, 10(Suppl. 1), S12 CrossRef PubMed.
- E. Abdel-Lateef, F. Mahmoud, O. Hammam, E. El-Ahwany, E. El-Wakil, S. Kandil, H. Abu Taleb, M. El-Sayed and H. Hassenein, Acta Pharm., 2016, 66, 387–398 CAS.
- J. Y. Lo, M. N. Kamarudin, O. A. Hamdi, K. Awang and H. A. Kadir, Food Funct., 2015, 6, 3550–3559 RSC.
- K. Song, L. Xu, W. Zhang, Y. Cai, B. Jang, J. Oh and J. O. Jin, Oncotarget., 2017, 8, 38554–38567 Search PubMed.
- H. K. Park, I. H. Kim, J. Kim and T. J. Nam, Int. J. Mol. Med., 2013, 32, 291–295 CrossRef CAS PubMed.
- X. H. Han, Y. Y. Ye, B. F. Guo and S. Liu, Zhongxiyi Jiehe Xuebao, 2012, 10, 67–75 CAS.
- J. H. Beijnen and J. H. Schellens, Lancet Oncol., 2004, 5, 489–496 CrossRef CAS PubMed.
- M. Leslie, Science, 2008, 320, 1155–1115 CrossRef CAS PubMed.
- P. Yin, C. Zhao, Z. Li, C. Mei, W. Yao, Y. Liu, N. Li, J. Qi, L. Wang, Y. Shi, S. Qiu, J. Fan and X. Zha, Cell. Signalling, 2012, 24, 1229–1240 CrossRef CAS PubMed.
- W. J. Cai, M. J. Wang, L. H. Ju, C. Wang and Y. C. Zhu, Cell Biol. Int., 2010, 34, 565–572 CrossRef CAS PubMed.
- H. A. Jia, J. Ye, J. You, X. Y. Shi, W. Y. Kang and T. X. Wang, Oncol. Rep., 2017, 37, 3001–3009 CrossRef CAS PubMed.
- C. Szabó and A. Papapetropoulos, Br. J. Pharmacol., 2011, 164, 853–865 CrossRef PubMed.
- A. Papapetropoulos, A. Pyriochou and Z. Altaany, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21972–21977 CrossRef CAS PubMed.
- W. J. Cai, M. J. Wang and P. K. Moore, Cardiovasc. Res., 2007, 76, 29–40 CrossRef CAS PubMed.
- N. S. Vasudev and A. R. Reynolds, Angiogenesis, 2014, 17, 471–494 CrossRef CAS PubMed.
- Y. H. Lin and J. H. Chiu, J. Altern. Complement. Med., 2010, 16, 527–528 CrossRef PubMed.
- B. Carmady and C. A. Smith, China's Med., 2011, 6, 22 CrossRef PubMed.
- S. Xi, Y. Peng, G. Y. Minuk, M. Shi, B. Fu, J. Yang, Q. Li, Y. Gong, L. Yue, L. Li, J. Guo, Y. Peng and Y. Wang, J. Ethnopharmacol., 2016, 190, 1–12 CrossRef PubMed.
- X. Wang, N. Wang, F. Cheung, L. Lao, C. Li and Y. Feng, J. Integr. Med., 2015, 13, 142–164 CrossRef PubMed.
- T. Morikawa, H. Matsuda, K. Ninomiya and M. Yoshikawa, Biol. Pharm. Bull., 2002, 25, 627–631 CrossRef CAS.
- V. Baeriswyl and G. Christofori, Semin. Cancer Biol., 2009, 19, 329–337 CrossRef CAS PubMed.
- S. P. Herbert and D. Y. Stainier, Nat. Rev. Mol. Cell Biol., 2011, 12, 551–564 CrossRef CAS PubMed.
- H. E. Tzeng, P. C. Chen, K. W. Lin, C. Y. Lin, C. H. Tsai, S. M. Han, C. L. Teng, W. L. Hwang, S. W. Wang and C. H. Tang, Clin. Sci., 2015, 129, 147–158 CrossRef CAS PubMed.
- B. K. McColl, S. A. Stacker and M. G. Achen, APMIS, 2004, 112, 463–480 CrossRef CAS PubMed.
- C. Y. Lin, S. Y. Hung, H. T. Chen, H. K. Tsou, Y. C. Fong, S. W. Wang and C. H. Tang, Biochem. Pharmacol., 2014, 91, 522–533 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |