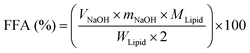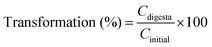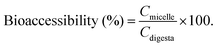Bioaccessibility and cellular uptake of β-carotene in emulsion-based delivery systems using scallop (Patinopecten yessoensis) gonad protein isolates: effects of carrier oil
Jia-Run
Han
a,
Lu-Ping
Gu
c,
Ruo-Jie
Zhang
d,
Wen-Hui
Shang
a,
Jia-Nan
Yan
a,
David Julian
McClements
 d,
Hai-Tao
Wu
d,
Hai-Tao
Wu
 *ab,
Bei-Wei
Zhu
*ab and
Hang
Xiao
*ab,
Bei-Wei
Zhu
*ab and
Hang
Xiao
 *d
*d
aSchool of Food Science and Technology, Dalian Polytechnic University, Dalian Liaoning 116034, China. E-mail: zhubeiwei@163.com; wht205@163.com; Fax: +86-411-86318655; Fax: +86-411-86318655; Tel: +86-411-86318655 Tel: +86-411-86318731
bNational Engineering Research Center of Seafood, Dalian Liaoning 116034, China
cKey Laboratory of Food Science and Technology, School of Food Science and Technology, Jiangnan University, Wuxi 214122, China
dDepartment of Food Science, University of Massachusetts, Amherst, Massachusetts 01003, USA. E-mail: hangxiao@foodsci.umass.edu; Fax: +1 (413) 545-1262; Tel: +1 (413) 545-2281
First published on 19th December 2018
Abstract
Emulsion-based delivery systems were structured by using scallop gonad protein isolates (SGPIs) as novel food-grade emulsifiers. The effects of carrier oil, including the long chain triglycerides (LCT) and medium chain triglycerides (MCT), on the bioaccessibility and cellular uptake of β-carotene (BC) were investigated. Both LCT and MCT delivery systems remained stable at pH 7–8 but aggregated at lower pH values (3–6) according to the results of light scattering and microscopy measurements. LCT droplets fabricated within SGPIs were digested and released more slowly than MCT droplets during the simulated gastrointestinal tract digestion. The LCT emulsion showed higher BC bioaccessibility (65.5%) than the MCT emulsion (23.1%) as a result of the greater solubilization of BC in mixed micelles fabricated from long-chain fatty acids. Moreover, the LCT emulsion produced higher cellular uptake of BC as compared with the MCT emulsion in intestinal epithelial cells. These results demonstrated that SGPIs could be used as novel food-grade emulsifiers to protect lipophilic bioactive compounds in emulsion-based delivery systems, in which LCT is more suitable to encapsulate and deliver BC than MCT.
1. Introduction
β-Carotene (BC) is one of the most important carotenoids existing in numerous vegetables and fruits.1,2 As the precursor of vitamin A, it is extensively used as an antioxidant and natural colorant and plays a vital role in human bodily functions.3 However, the chemical instability, low bioavailability and poor water-solubility of BC greatly restrict its application in numerous aqueous-based beverages and foods.4,5 Due to the polyunsaturated structure, BC degradation happens rapidly under certain environmental conditions.6 Nevertheless, these restrictions can be overcome by solubilising BC in lipid droplets and homogenising with a water-soluble emulsifier to form emulsion-based delivery systems.7,8Owing to the growing consumer demands for “healthy” food products, natural emulsifiers are widely considered in formulating emulsions as compared with synthetic surfactants or polymers.9 Protein is a kind of natural emulsifier with the advantages of a strong surface activity, low cell toxicity and good stability.10 Nowadays, protein-based emulsifiers applied in the food industry are derived from milk, soybean and eggs.11 Many reports have indicated that these emulsion-based delivery systems can be utilized to enhance the bioaccessibility of BC.12,13 However, due to the dietary preferences and territory restrictions, food manufacturers and consumers are looking for some alternative marine protein sources in the human diet. Based on some literature studies, several marine proteins have already been explored as food-grade emulsifiers.14,15 For instance, shrimp (Penaeus vannamei) heads with a high content of protein have been used to stabilize palm oil-in-water food emulsions.14 Silver carp (Hypophthalmichthys molitrix) protein isolate has displayed certain emulsifying capacity and foaming capacity.15 Nevertheless, the research on shellfish, especially scallop proteins, as an emulsifier to deliver BC is still relatively rare.
Scallop (Patinopecten yessoensis) is an important bivalve extensively distributed in Eastern Asia. In China, scallop production from aquatic breeding has increased to 1.86 million tons in 2016 (FAO 2017). The demand for scallop processing is increasing with the continuous expansion of scallop farming. Scallop gonads are the main edible byproducts with a high level of protein during the processing of the P. yessoensis adductor, and are regarded as good sources to develop a protein matrix. Our previous studies have shown that protein isolates from scallop gonads (SGPIs) provide high nutritional value and good emulsifying properties when compared with crude scallop gonad or soybean protein isolates. SGPIs contain a mixture of proteins, such as vitellogenin, actin, and a little myosin. These proteins have both hydrophobic and hydrophilic regions on their surfaces,16 and these regions can easily consume oil droplets forming an interfacial coating.10 As a consequence, it is necessary to study the effect of SGPIs on emulsion-based delivery systems for BC.
It has been confirmed that the bioaccessibility and bioavailability of hydrophobic bioactive compounds can be improved by combining them with digestible lipids, which is impacted by the lipid nature.17 The digested triacyglycerols form monoacylglycerols and free fatty acids (FFA), which are combined with phospholipids and bile acids forming mixed micelles to solubilise and transport the BC molecules to epithelium cells.18 After absorption, the FFA and monoacylglycerols are recombined together into triacylglycerols, which can be incorporated along with BC into lipoproteins (chylomicrons). Therefore, the type of triacylglycerols will show an important effect on the SGPI-BC emulsion delivery system.
In the current study, protein isolates were prepared from scallop gonads and further used as emulsifiers. Emulsion-based delivery systems adopting SGPIs as food-grade emulsifiers were structured by using long chain triglycerides (LCT) and medium chain triglycerides (MCT) as the carrier oils. A simulated gastrointestinal tract (GIT) and Caco-2 cells model were adopted to investigate the impact of carrier oil on the bioaccessibility and cellular uptake of BC. The knowledge gained from the present study will provide fundamental information for establishing SGPI-based delivery systems to encapsulate BC for further application.
2. Materials and methods
2.1 Materials and chemicals
Fresh female scallop (P. yessoensis) was purchased from Dalian Changxing market (Dalian, China) in April 2017. LCT (corn oil) was purchased from Xiwang Food (Shandong, China). MCT was acquired from Coletica (Northport, NY). BC, lipase from porcine pancreas, mucin from porcine stomach (type II), porcine bile extract, pepsin from porcine gastric mucosa, fluorescein isothiocyanate (FITC), Nile red, and 3-(4,5-dime-thylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St Louis, MO, USA). Human colon carcinoma cell lines (Caco-2 cells, ATCC number: HTB-37) were acquired from the American Type Cell Collection (Manassas, VA). Cells after 25–40 passages were used in the uptake study. Fetal bovine serum (FBS), DMEM (with 4.5 g L−1 glucose, L-glutamine, and sodium pyruvate), nonessential amino acid solution, penicillin–streptomycin, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), dimethyl sulfoxide (DMSO), trypsin-EDTA and n-hexane were purchased from Fisher Scientific (Agawam, MA, USA). All the other reagents were of analytical grade.Scallop gonad protein isolates (SGPIs) were prepared from defatted scallop gonad by the isoelectric precipitation process.19 Briefly, distilled water was added to defatted scallop gonads at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v) and adjusted to pH 11.5 using NaOH solution, and then the mixture was centrifuged at 2000g for 20 min. The resulting supernatant was adjusted to pH 3.8 with 0.5 M HCl and centrifuged at 10
20 (w/v) and adjusted to pH 11.5 using NaOH solution, and then the mixture was centrifuged at 2000g for 20 min. The resulting supernatant was adjusted to pH 3.8 with 0.5 M HCl and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 30 min. Thereafter, the sediment was washed twice with distilled water, neutralised using NaOH solution, and dialyzed for 48 h. Finally, the sediment was freeze-dried and referred to as SGPIs. The proximate composition of SGPIs was found to be 86.39 ± 1.2 wt% protein, 0.17 ± 0.02 wt% lipid, 3.27 ± 0.28 wt% ash, 6.27 ± 0.31 wt% moisture and 1.9 ± 0.08 wt% carbohydrate.
000g for 30 min. Thereafter, the sediment was washed twice with distilled water, neutralised using NaOH solution, and dialyzed for 48 h. Finally, the sediment was freeze-dried and referred to as SGPIs. The proximate composition of SGPIs was found to be 86.39 ± 1.2 wt% protein, 0.17 ± 0.02 wt% lipid, 3.27 ± 0.28 wt% ash, 6.27 ± 0.31 wt% moisture and 1.9 ± 0.08 wt% carbohydrate.
2.2. Preparation of BC emulsions
An oil phase was obtained by dispersing BC (0.1%, w/w) into LCT or MCT and sonicating in an ultrasonic water bath (45 °C) for 30 min so that it dissolves completely. The aqueous phase was obtained by dispersing SGPIs at 0.25% (w/w) in 5 mM PBS (pH 7.0) and stirring overnight for complete hydration. The aqueous (98%, w/w) and oil phases (2%, w/w) were mixed at a speed of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min by using a high-speed blender (M133/128190, Biospec Products, Inc., ESGC, Switzerland). The coarse emulsion was further homogenized using a microfluidizer (M110Y, Microfluidics, Newton, MA) equipped with a 75 μm interaction chamber five times at 12
000 rpm for 2 min by using a high-speed blender (M133/128190, Biospec Products, Inc., ESGC, Switzerland). The coarse emulsion was further homogenized using a microfluidizer (M110Y, Microfluidics, Newton, MA) equipped with a 75 μm interaction chamber five times at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 psi as described previously.20 After cooling down, the final emulsions were mixed with the sodium azide (0.02%, w/w) to avoid microbial growth, and stored at 4 °C in a dark place.
000 psi as described previously.20 After cooling down, the final emulsions were mixed with the sodium azide (0.02%, w/w) to avoid microbial growth, and stored at 4 °C in a dark place.
2.3. Analysis of particle size and ζ-potential
The particle size distributions and the mean particle size of samples were determined by dilution with phosphate buffer (5 mM, pH 3.0–8.0) using laser light scattering equipment (Mastersizer 2000, Malvern Instruments Ltd, Malvern, Worcestershire, UK). The refractive index values were 1.33 for the aqueous phase and 1.47 and 1.45 for the LCT and MCT oil phase, respectively.21,22 ζ-Potential was measured using a particle electrophoresis instrument (Zetasizer Nano, Malvern Instruments, Worcestershire, UK).2.4. In vitro digestion of BC emulsions
The simulated gastrointestinal tract (GIT) model was composed of mouth, gastric and intestinal digestion as described previously with slight modification.20,23 Briefly, the simulated saliva fluid (13.64 mM NaCl, 2.05 mM NH4NO3, 2.34 mM KH2PO4, 1.35 mM KCl, 0.5 mM potassium citrate, 0.06 mM uric acid sodium salt, 1.65 mM urea and 0.65 mM lactic acid sodium salt) containing 75 U mL−1 mucin (type II) and samples were preheated at 37 °C. In order to initiate the mouth phase digestion process, the simulated saliva fluid (20 mL) including mucin (0.03 g mL−1) was added to 20 mL of the BC-emulsion (2% oil phase) sample. The mixture was agitated by adjusting to pH 6.8 and incubation at 37 °C for 2 min. Then, 20 mL of the oral digestive sample was mixed with an equal volume of simulated gastric fluid (17.11 mM NaCl and 41.91 mM HCl) including 2000 U mL−1 pepsin at pH 2.5, and the mixture sample was incubated again for 2 h to simulate the stomach phase. Finally, to the mixture 1.5 mL of simulated intestine fluid (10 mM CaCl2·2H2O and 150 mM NaCl), 3.5 mL of bile salts (10 mM) and 2.5 mL of lipase (2000 U mL−1) were added. The pH value at 7.0 was maintained using an automatic titration unit (Metrohm, USA Inc.) by titrating 0.2 M NaOH into the reaction system at 37 °C for 2 h. The volume of NaOH neutralizing the FFA was recorded. The percentage of FFA released was calculated according to the following formula:24where VNaOH is the NaOH volume (mL) titrated to neutralize the FFA generation during the lipid digestion, mNaOH is the NaOH solution molarity (M), WLipid is the weight of oil existing in the digestion system (0.15 g), and MLipid is the average molecular weight of LCT (824 g mol−1) and MCT (500 g mol−1).20 The digestion of oil-free protein micelles was used as the blank.
2.5. Microstructural analysis
Samples (50 μL) were dyed with a solution of FITC (2 μL, 10 mg mL−1) and Nile red (2 μL, 1 mg mL−1) to acquire green (protein) and red (oil) fluorescence images, respectively. Afterwards, 10 μL of the sample was added dropwise on a glass slide and covered with a coverslip, and then used to characterize the microstructure using either optical or confocal scanning laser microscopy with a 60× oil immersion objective lens and 10× eyepiece lens (Nikon D-Eclipse C1 80i, Nikon, Melville, NY, US.). The excitation and emission wavelengths were 488 nm and 515 nm for FITC, while 543 nm and 605 nm for Nile red, respectively. All images were captured and assessed using digital image analysis software (NIS-Elements, Nikon, Melville, NY, US).2.6. Transformation and bioaccessibility of BC after digestion
After in vitro digestion, the digesta samples (20 mL) were centrifuged at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (41
000 rpm (41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 657g), 4 °C for 50 min, and yellow supernatants containing the solubilized BC as the micelle fraction were collected. The concentration of BC in the supernatants was determined on the basis of a procedure described by Zhang et al. with slight modifications.25 Briefly, to the micelle fraction a mixture of n-hexane and ethanol (3
657g), 4 °C for 50 min, and yellow supernatants containing the solubilized BC as the micelle fraction were collected. The concentration of BC in the supernatants was determined on the basis of a procedure described by Zhang et al. with slight modifications.25 Briefly, to the micelle fraction a mixture of n-hexane and ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) was added at a ratio of 1
2, v/v) was added at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (v/v). After vortexing (20 s) and centrifugation (940g for 5 min), the top layer including the solubilized BC was collected. The bottom layer was extracted repeatedly with the same process until the supernatant was colorless and transparent. The collected n-hexane layers were mixed together, and the absorbance of the diluted supernatant was measured at 450 nm using a microplate reader (BioTek Instruments, Inc., Winooski, VT) with n-hexane as the control.
2 (v/v). After vortexing (20 s) and centrifugation (940g for 5 min), the top layer including the solubilized BC was collected. The bottom layer was extracted repeatedly with the same process until the supernatant was colorless and transparent. The collected n-hexane layers were mixed together, and the absorbance of the diluted supernatant was measured at 450 nm using a microplate reader (BioTek Instruments, Inc., Winooski, VT) with n-hexane as the control.
The concentration of BC was calculated according to the standard curve: Y = 0.0723X + 0.0445. The BC concentration in the entire digesta phase was also determined using the same method. The transformation and bioaccessibility of BC were estimated using the following formulas:26
Here, Cmicelle, Cdigesta and Cinitial are the BC concentrations in the micelle fraction, entire digesta phase, and initial emulsions before digestion, respectively.
2.7. Cytotoxicity of BC-loaded SGPI emulsions after digestion
The MTT test was used to evaluate the effect of the samples on cell viability and potential cytotoxicity. Caco-2 cells were cultured in DMEM medium including 10% FBS and 100 U mL−1 penicillin–streptomycin at 37 °C under 90% humidity and 5% CO2. The cells (1.7 × 105 cells per well) were incubated in a 96-well plate for 48 h to achieve 80% post confluence. The medium was discarded, and the cells were incubated with 200 μL of mixed micelle fraction from the digesta samples diluted with DMEM (10, 20, 50, 100, and 200 times, i.e., the final concentration of BC was 0.1, 0.05, 0.02, 0.01, and 0.005 μg mL−1) for further 24 h. Simultaneously, BC dissolved in THF/DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution diluted with DMEM was used as the control. After treatment, the DMEM medium was replaced by 200 μL of MTT solution (0.5 mg mL−1) and incubated at 37 °C for 2 h. Finally, the supernatant was discarded and DMSO (100 μL) was added to dissolve the purple formazan products. Cell viability was measured in light of the absorbance at 570 nm using a microplate reader and estimated using the formula below:
1, v/v) solution diluted with DMEM was used as the control. After treatment, the DMEM medium was replaced by 200 μL of MTT solution (0.5 mg mL−1) and incubated at 37 °C for 2 h. Finally, the supernatant was discarded and DMSO (100 μL) was added to dissolve the purple formazan products. Cell viability was measured in light of the absorbance at 570 nm using a microplate reader and estimated using the formula below:Here, Acontrol is the absorbance of the cells incubated with DMEM only, Asample is the absorbance of the cells incubated with samples, and Ablank is the absorbance of cell-free wells.
2.8. Cellular uptake of BC-loaded SGPI emulsions after digestion
Caco-2 cells (3 × 104 cells per well) were seeded in 6-well plates and incubated at 37 °C under 90% humidity and 5% CO2. The DMEM medium was replaced every two days until approximately 90% confluence of the cell monolayers was observed (11–12 days). Prior to BC analysis, the mixed micelle fraction from digesta samples was diluted 20 times with DMEM to minimize the cytotoxicity of the Caco-2 cells. Meanwhile, BC in THF/DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution was also diluted with DMEM to attain the same BC concentration of 0.05 μg mL−1 as the control with the final THF concentration being less than 0.1%. After incubation for 24 h, the cell monolayer was washed twice with precooled PBS, digested using 3 mL of trypsin solution, and treated with 7 mL of DMEM to inactivate the trypsin. After centrifugation at 4000 rpm (1788.8g) for 5 min, the precipitated cells were added to 500 μL of precooled cell lysis buffer, and treated using a sonicator after incubating on ice for 30 min. Then, the cell suspension (400 μL) was extracted with a mixture of n-hexane and ethanol (3
1, v/v) solution was also diluted with DMEM to attain the same BC concentration of 0.05 μg mL−1 as the control with the final THF concentration being less than 0.1%. After incubation for 24 h, the cell monolayer was washed twice with precooled PBS, digested using 3 mL of trypsin solution, and treated with 7 mL of DMEM to inactivate the trypsin. After centrifugation at 4000 rpm (1788.8g) for 5 min, the precipitated cells were added to 500 μL of precooled cell lysis buffer, and treated using a sonicator after incubating on ice for 30 min. Then, the cell suspension (400 μL) was extracted with a mixture of n-hexane and ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v), and the BC content was analyzed using an Agilent 1100 High pressure liquid chromatography system with a DAD UV-vis absorption detector (Agilent, Santa Clara, CA) on the basis of the previous method.20 The BC quantitation range (0.005–0.05 μg mL−1) was established for HPLC analysis of BC in the mixed micelle fraction and in Caco-2 cell transport, and the standard curve was determined as Y = 1.1956X − 0.0004, R2 = 0.9992. The protein content was also determined by bicinchoninic acid (BCA) assay. All procedures avoided light exposure and were carried out on ice. The cellular uptake of BC was then calculated and expressed as pmol mg−1 protein.
2, v/v), and the BC content was analyzed using an Agilent 1100 High pressure liquid chromatography system with a DAD UV-vis absorption detector (Agilent, Santa Clara, CA) on the basis of the previous method.20 The BC quantitation range (0.005–0.05 μg mL−1) was established for HPLC analysis of BC in the mixed micelle fraction and in Caco-2 cell transport, and the standard curve was determined as Y = 1.1956X − 0.0004, R2 = 0.9992. The protein content was also determined by bicinchoninic acid (BCA) assay. All procedures avoided light exposure and were carried out on ice. The cellular uptake of BC was then calculated and expressed as pmol mg−1 protein.
2.9. Statistical analysis
All values were expressed as means ± SD. One-way analysis of variance was computed using the SPSS software (version 7.5). The differences in the experimental results were analyzed by Duncan's multiple range test with p < 0.05 as significant.3. Results and discussion
3.1. Effect of pH on particle stability of different carrier oil-based BC emulsions stabilized by SGPIs
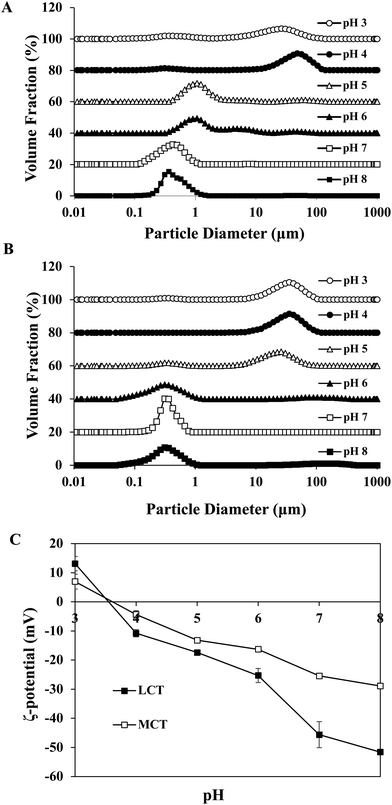
Certain protein isolates extracted from different sources, such as eggs, whey, and soybean, possess strong surface activity and can form a thin oil–water interface cohesive layer during the emulsification process.25,27 Our preliminary studies have shown that SGPIs have a higher surface activity than soybean protein isolate as confirmed by their emulsion activity and stability (Data not shown). Thus, they can be utilized as a favourable emulsifier for embedding BC emulsion. The aqueous phase pH value during microfluidisation was a critical factor in determining the emulsion stability. Therefore, a variety of emulsions were obtained by microfluidisation of 2% LCT or MCT (0.1% BC) and 98% aqueous phase (0.25% SGPIs) at pH 3.0–8.0 since this range comprises the majority of food applications.The particle size distributions and mean particle diameters of both LCT and MCT-based BC emulsions are shown in Fig. 1A, B and Table 1, respectively. In the range of pH 3.0–4.0, both emulsions exhibited physical instability. The emulsions had obviously broad particle size distribution coinciding with visible phase separation after microfluidisation (Fig. 1A and B). Mean particle diameters higher than 10 μm were observed (Table 1). Furthermore, with the increase in pH value, the mean particle diameters of both emulsions significantly decreased (p < 0.05) and tended to be flat when the pH reached 6.0, and the particle size distribution at pH 7.0–8.0 showed narrow single peaks within the mean particle size less than 0.35 μm. As shown in Fig. 1C, both emulsions exhibited fairly similar ζ potential-pH profiles altering from moderately positive changes to notably negative charges at pH 3.0–8.0. The zero-charge point was determined to be approximately pH 3.8. Meanwhile, the ζ-potential magnitude of LCT emulsions was always higher than that of MCT when the pH reached above 4.0, and the ζ-potential of emulsions for LCT and MCT was less than −20 mV at pH 7.0–8.0. Furthermore, the oil droplets seemed to be evenly spread throughout the BC emulsions at pH 7.0–8.0 as revealed from the optical and confocal microscopy images, while an extensive droplet flocculation occurred in both systems at pH 3.0–6.0 (Fig. 2). These results suggest that both LCT and MCT emulsions stabilized by SGPIs exhibited good stability under pH 7.0–8.0, and LCT emulsions have a relatively large particle diameter and ζ-potential magnitude than MCT emulsions.
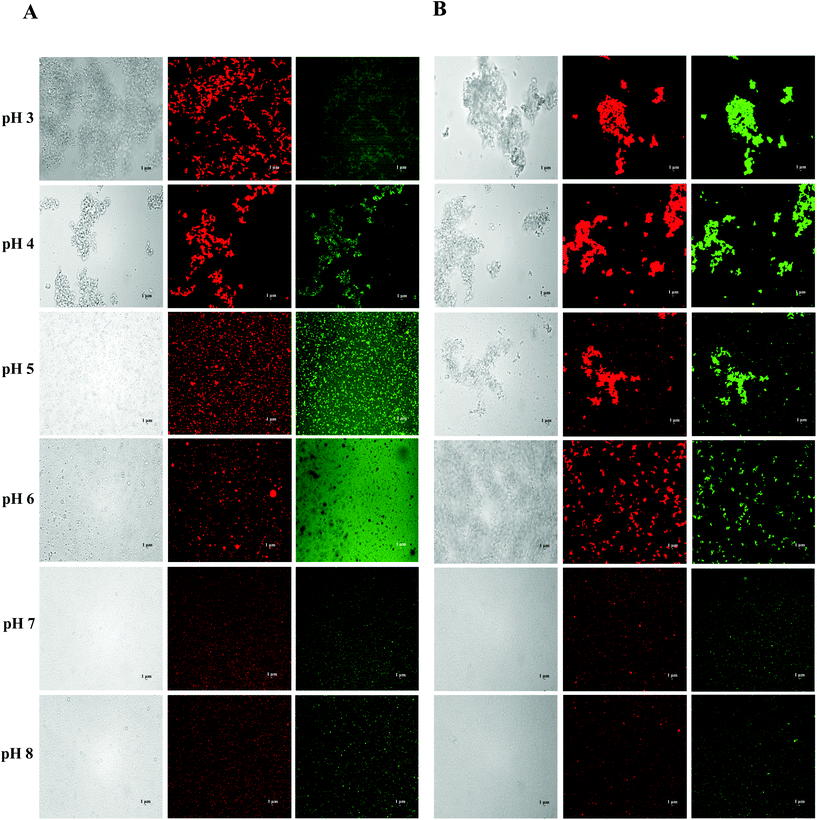 | ||
| Fig. 2 Effect of carrier oil on the microstructures of BC emulsions stabilized by SGPIs under different pH conditions: (A) LCT and (B) MCT (scale bar is 1 μm). | ||
| Samples | Mean particle diameter (μm) | |||||
|---|---|---|---|---|---|---|
| pH 3 | pH 4 | pH 5 | pH 6 | pH 7 | pH 8 | |
| Data with different letters in the same row are significantly different (p < 0.05). | ||||||
| LCT-emulsion | 19.8 ± 1.28a | 25.42 ± 5.79a | 2.58 ± 0.06b | 0.45 ± 0.03c | 0.34 ± 0.03c | 0.31 ± 0.01c |
| MCT-emulsion | 10.99 ± 0.18a | 23.94 ± 2.92b | 1.72 ± 0.05c | 0.39 ± 0.02d | 0.30 ± 0.06d | 0.26 ± 0.02d |
The stability of the emulsion at different pH values is attributed to the electrostatic repulsion between the lipid droplets coated with protein.13 Similar consequences were found in whey protein isolate (WPI) stabilized emulsion. It has been suggested that the WPI-soybean oil emulsion exhibited the greatest phase separation resistance and the emulsions led to more stability at pH 7 than at pH 3 as the WPI possessed a pI at 4.6.28 In contrast, the egg white protein (EWP)-stabilized BC emulsion showed good stability under acidic conditions as the EWP had pI at approximately 6.1 and 10.7 for ovotransferrin and lysozyme, respectively.29 The SGPIs had pI at approximately 3.8, and the oil droplets’ surface potential was supposed to be rather low at pH 3.0–4.0. The charge on the droplet surface was reduced by partial protonation of the fatty acids, which led to the gradual replacement of combined repulsive interactions by the attractive colloidal interaction resulting in the physical destabilization of emulsions.30,31 However, when the pH was far from the pI, relatively stable emulsions could be observed with mean particle diameters less than 0.35 μm at pH 7.0–8.0. Moreover, the LCT emulsions have a relatively large particle diameter and ζ-potential magnitude than MCT emulsions. The reason for this phenomenon may be due to the changes in the dispersed phase viscosity of the emulsions.32 Generally, the efficiency of droplet crushing in a microfluidizer increases as the viscosity of the dispersed phase decreases. LCT has a distinctly higher viscosity and contains more anionic impurities than MCT.33 Therefore, the droplet breakup of the LCT emulsion becomes less efficient during microfluidisation, which results in the formation of larger droplets and with more negative charge.
3.2. Effect of carrier oil on particle characteristics of BC emulsion stabilized using SGPIs during in vitro digestion
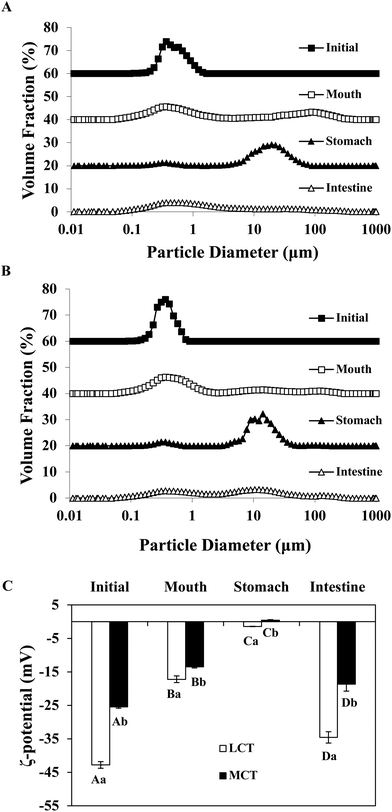
In order to adapt to the in vitro digestion and cell uptake, the emulsions were prepared at pH 7.0 for further study. The behavior of BC emulsions by using two different oils (LCT and MCT) was determined as they were subjected to the simulated GIT. The particle characteristics of both emulsions were evaluated at different GIT stages to offer some information on the changes in interfacial characterization. The mean particle sizes and particle size distributions of both LCT and MCT-based BC emulsions stabilized by SGPIs during digestion are shown in Fig. 3A, B and Table 2, respectively. Initially, the BC emulsions containing both triacylglycerol oils had a good aggregation ability and comparatively small particle diameter (0.35 and 0.31 μm, for LCT and MCT, respectively) with a monomodal particle size distribution. Nevertheless, there was a visible difference between the particle electrical properties depending on the carrier oil type: −43 and −26 mV for LCT and MCT, respectively (Fig. 3C), the reason for the difference in electric charges may be due to the anionic or cationic impurities (e.g. phospholipids, mineral ions, or FFA) present within the lipid phase.34 The oil droplets were evenly distributed throughout both BC emulsions without any significant droplet aggregation as confirmed by confocal scanning laser microscopy (Fig. 4).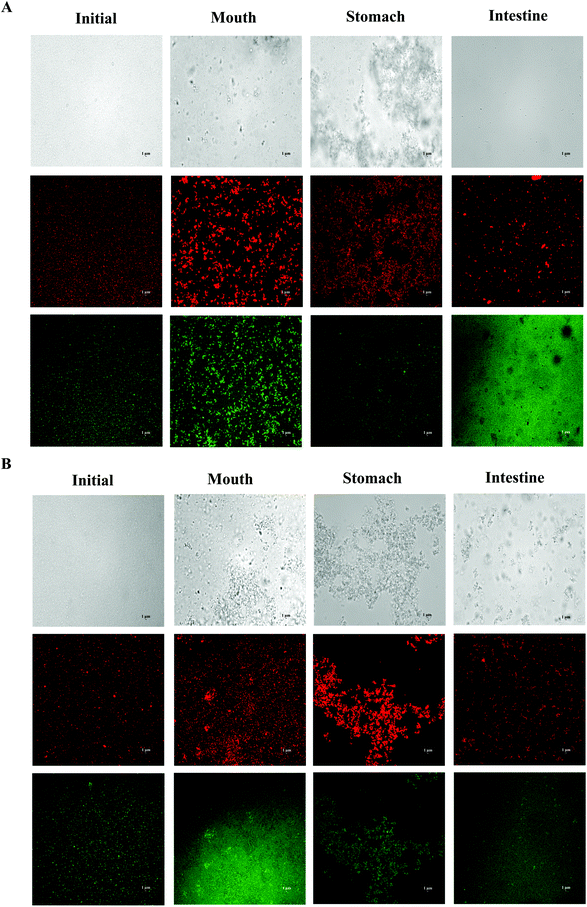 | ||
| Fig. 4 Microstructure of SGPI-stabilized BC emulsions with different carrier lipids: (A) LCT; (B) MCT after they were exposed to different stages of a GIT. | ||
| Samples | Mean particle diameter (μm) | |||
|---|---|---|---|---|
| Initial | Mouth | Stomach | Intestine | |
| Data with different letters in the same column are significantly different (p < 0.05). | ||||
| LCT-emulsion | 0.35 ± 0.01a | 1.92 ± 0.51a | 15.69 ± 2.26a | 3.36 ± 0.25a |
| MCT-emulsion | 0.31 ± 0.05a | 0.84 ± 0.48b | 12.77 ± 1.63a | 10.81 ± 0.72b |
After getting through the mouth and stomach stage, the particle diameter distributions of both BC emulsions became bimodal, accompanied by a comparative rise in mean particle size (Fig. 3A, B and Table 2). Extensive oil droplet accumulation was also observed after exposure to the oral and stomach stages (Fig. 4), which indicated that some flocculation had happened, especially in the LCT emulsions. Meanwhile, there was a palpable decline in the magnitude of negative charge on both the oil droplets after the exposure to the simulated oral phase, with the surface potential of −17.20 and −13.47 mV for LCT and MCT, respectively. Subsequently, a further decrease in ζ-potential was observed when the emulsions were passed through the simulated stomach phase with the surface potential of −1.4 and 0.5 mV for LCT and MCT, respectively (Fig. 3C).
The MCT emulsions exhibited relatively larger particles than LCT emulsions after simulating the small intestinal stage, and the particle size distribution was broad bimodal in MCT emulsions (Fig. 3A, B and Table 2). Moreover, the LCT emulsion had more negative charges than the MCT emulsion (Fig. 3C). During the small intestinal phase, although the aggregated oil droplet breakup has occurred, the MCT emulsions also contained some relatively large particles (Fig. 4), which is in accordance with the light scattering measurements (Fig. 3B). Overall, all these results indicate that both LCT and MCT-based BC emulsions stabilized by SGPIs showed exactly similar behavior when they were subjected to the simulated digestion model while the systems containing LCT may have more anionic fatty acids at the surface of the particle than those containing MCT.
It has been reported that emulsions stabilized by proteins are likely to accumulate during the stomach phase on account of impaired electrostatic repulsion, hydrolysis of adsorbed proteins, and bridging flocculation or depletion induced by mucin.35,36 Our results draw a similar conclusion as well. Meanwhile, a significant reduction in the negative charge after the exposure to the gastric phase is noted, and the ζ-potential is nearly close to zero. This phenomenon is probably due to the fact that some of the proteins may have been displaced and digested, and at the same time, some of the anionic substances (for example mucin) are also adsorbed on the surface of the protein-coated lipid droplet.37 Furthermore, our results coincided with those of the previous studies which have interpreted that the MCT emulsion results in the generation of larger particles than the LCT emulsion after small intestine digestion by using Tween 20 and WPI as the emulsifier.38,39 The high surface negative charge of the particles in both emulsions was observed in the small intestine stage, which can be ascribed to the chemisorption of anionic colloidal particles on their surface, for instance phospholipids, bile salts, free fatty acids, and peptides.35 Interestingly, LCT emulsions had pronouncedly higher negative charges than MCT after digestion, which indicated that the fatty acids formed by digestion of LCT were accumulated at the surface of the particles, whereas the fatty acids produced by digestion of MCT were still retained in the aqueous phase.38 Actually, it is hard to confirm the accurate nature of these particles after digestion because of the particle diversity including micelles, undigested protein aggregates, undigested lipid droplets, and insoluble calcium salts. The entire particles conduce to the integral signal for calculating the ζ-potential.
3.3. Effect of carrier oil on lipid digestion of BC emulsion stabilized by SGPIs
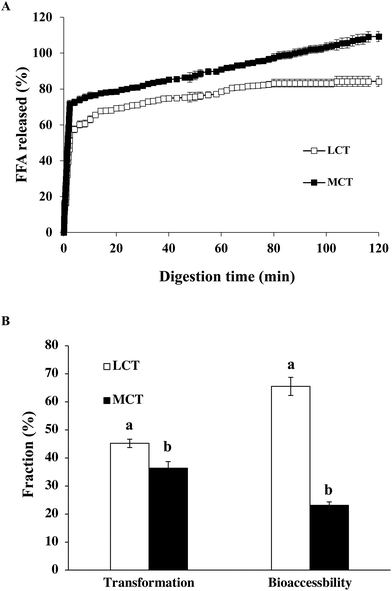
After the GIT model, the impact of oil type on the extent and rate of lipid digestion was assessed by an automatic titration (pH stat) assay. The amount of FFA liberated from the lipid phase was determined during the small intestine stage (Fig. 5A). The profiles of lipid digestion in both LCT and MCT emulsions followed quite similar trends. For LCT emulsions, FFA was swiftly released throughout the first 5 minutes and afterwards more smoothly until a comparatively stable value of around 85% was reached after digestion for 2 h, whereas the MCT emulsion showed a noticeable increase during the first 5 minutes of reaction, and subsequently a more progressive release at a longer digestion time until 110% digestion was observed (Fig. 5A). These results suggest that the lipid digestion extent and the rate of MCT emulsion were obviously higher than those of the LCT emulsion stabilized with SGPIs.It has been illuminated that long-chain fatty acid lipids are digested more slowly than medium-chain fatty acid lipids.40 For instance, LCT (i.e., corn oil) includes appreciably long-chain fatty acids (i.e., C16, C18 and C20), while MCT includes comparatively shorter chain ones (i.e., C8 and C10). The relatively low amount of digested LCT is possibly on account of the fact that long chain fatty acids are prone to aggregation at the surface of oil–water, and consequently lipase was restricted access to the surface of the droplets.41 In contrast, the digestive products of MCT-emulsion have high water affinity making the lipase more liable to close to lipid surfaces.42 Interestingly, the release of the total amount of FFA reached 110% at the end of 2 h of simulated GIT digestion in the MCT-emulsions. The final amount of FFA released was greater than 100% in the MCT emulsion which is attributed to some other components, such as proteins (SGPIs) in the digestion models, which were hydrolyzed and dedicated to the pH-stat process. Moreover, some monoacylglycerols might have been transformed into FFA and glycerol,43 which was not considered in the calculations. Our results are also in accordance with other latest reports, which have demonstrated a faster digestion of MCT when they are merged into Tween 20, β-lactoglobulin and modified starch-stabilized BC emulsions in comparison with LCT, reaching 123%, 113% and 120% after 2 h, respectively.24,33,43 As a result, MCT possessing a high specific surface area are easily released from the small intestine leading to quick lipid digestion.
3.4. Effect of carrier oil on the transformation and bioaccessibility of BC emulsion stabilized by SGPIs
The transformation refers to the amount of BC that remains in the bioactive form, whereas the bioaccessibility provides information on the BC fraction available for absorption in mixed micelles arriving at the small intestine phase. The middle layer of the digesta samples which contained the solubilized BC was collected as the micelle fractions after small intestine digestion.Our results clearly showed that the transformation, i.e., the fraction of BC retaining in its original form after passing through the GIT, was distinctly higher in the LCT-emulsion (45.2%) than in the MCT-emulsions (36.3%) stabilized by SGPIs (Fig. 5B). These results suggest that a greater fraction of the BC in the LCT emulsion was not transformed at the small intestine stage, which may be due to the fact that BC is not subject to degradation by trapping into the interior of the micelle fractions. Thus, encapsulation of BC in the LCT emulsion stabilized by SGPIs gave better protection against chemical degradation. In spite of the fact that the triacylglycerols in both LCT and MCT emulsions were nearly completely digested, the bioaccessibility of BC was significantly distinct. The determined bioaccessibility of BC was approximately 23.1% in the MCT-emulsion, while it was about 65.5% in the LCT-emulsion (Fig. 5B). BC is a highly hydrophobic linear rod-like structure molecule, which favorably passed through the entire micelle core to successfully combine with the surfactant micelles.24 The long-chain fatty acids are more likely to form micelles with a larger solubilisation ability on account of the larger sizes of their hydrophobic core in comparison with medium-chain fatty acids. Our studies are consistent with some research studies showing that BC shows a higher bioaccessibility in Tween 80 stabilized emulsion systems when LCT is utilized as a carrier oil rather than MCT.44 Furthermore, the unsaturation degree of fatty acids can decide the GIT fate of BC and its bioaccessibility.45 Corn oil contains huge amounts of monounsaturated and polyunsaturated long chain fatty acids, which are presumed to be beneficial for BC bioaccessibility. In short, LCT is a more effective carrier oil for ensuring a comparatively high BC bioaccessibility than MCT in the SGPI stabilized BC emulsion system since MCT does not form large enough mixed micelle fractions to dissolve BC.
3.5. Cell toxicity and uptake of BC emulsion stabilized by SGPIs after digestion in Caco-2 cells
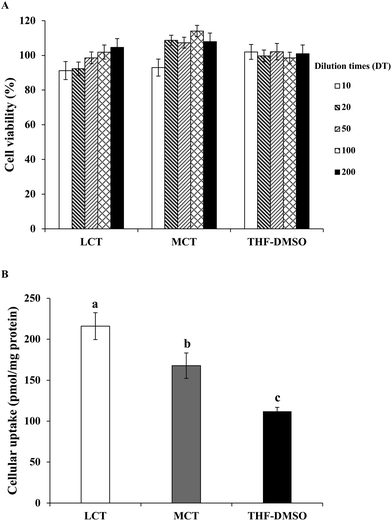
After digestion, the BC solubilized micelle fractions are likely to be absorbed by the epithelial cells by different mechanisms such as active or passive transport.46 Thus, the latent cytotoxicity of the different digested samples on the Caco-2 cells was evaluated. The cells were pretreated with BC-loaded micelles stabilized by either LCT or MCT of different dilution times (DT) for 24 h. As shown in Fig. 6A, no cytotoxicity was found in the Caco-2 cells treated with pure BC (dispersed in THF/DMSO) at a concentration of 0.1–0.005 μg mL−1. The micelle samples included lipid digestion products, protein, and GIT components (for instance bile salts). The cell viability was greater than 92% after treatment with both LCT and MCT mixed micelles at any diluted times (Fig. 6A), which indicates that the micelles from SGPI stabilized emulsion and GIT components did not damage the cells within the incubation period, and also did not adversely affect the integrity of the Caco-2 cell monolayer. These results were consistent with those of the preceding study,17 which showed that the micelles from quillaja saponins stabilized VE emulsion consisted of LCT and MCT which were both nontoxic and biocompatible with Caco-2 cells. Hence, this model of cell culture was appropriate for evaluating the BC absorption in the native SGPI-based emulsion delivery systems.It is supposed that the mixed micelle fractions solubilize the BC and subsequently transport it to the surface of the Caco-2 cells.47 Therefore, a Caco-2 cell model was utilized to simulate the uptake of the dissolved BC by epithelial cells. After incubation with the samples (DT = 20, the final BC concentration was 0.05 μg mL−1) for 24 h, the content of BC that had accumulated in cells was measured. The cellular uptake of BC in THF/DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) suspension was 112.0 pmol mg−1 protein, whereas the cellular uptake of BC in the LCT and MCT emulsions stabilized by SGPIs showed a 1.9- and 1.5-fold increase, respectively (Fig. 6B). These results are consistent with those of previous studies which indicated that the cellular uptake values of whey protein isolates, sodium caseinate, and soybean protein isolate loaded BC emulsions were 687, 891, and 452 pmol mg−1 protein, respectively, which were significantly higher than that of the THF/DMSO-BC control (246 pmol mg−1 protein).48 The potential reason for the difference between our results and previously published results is that previous studies mainly focused on the emulsions that were directly applied to the Caco-2 cell model without GIT digestion, while the emulsion of our study was applied to the Caco-2 cell model after in vitro GIT digestion. The LCT resulted in a higher absorption of the BC than MCT in the SGPI-stabilized emulsion, which is similar to the effect of oil type on VE bioaccessibility as reported previously.17 Actually, there are several reasons for explaining this phenomenon. In the first place, the property of the mixed micelle fractions formed is known to lie in the type of fatty acid, which may have affected the BC transport to the cell surface. The mixed micelle fractions include liquid crystalline phases, vesicles, and micelles which may change in their dimensions and structure, consequently altering their absorption characteristics. Secondly, some fatty acids have the ability to enhance the permeability of the cell membrane, which would change the absorption of the hydrophobic bioactive component. As a result, for disparate kinds of lipid digestion products generated by LCT and MCT, Caco-2 cells may have absorbed different amounts of BC. Thirdly, the intracellular processing and absorption of hydrophobic substances like BC were also influenced by the chain length of fatty acids. Medium chain fatty acids are generally diffused through the epithelial cells rather than being shipped to the endoplasmic reticulum like long chain fatty acids, which are then transformed into triacylglycerols for secreting into the lymphatic system.17,49 As a result, more BC in the LCT emulsion was absorbed by the epithelial cells than that in the MCT emulsion.
1, v/v) suspension was 112.0 pmol mg−1 protein, whereas the cellular uptake of BC in the LCT and MCT emulsions stabilized by SGPIs showed a 1.9- and 1.5-fold increase, respectively (Fig. 6B). These results are consistent with those of previous studies which indicated that the cellular uptake values of whey protein isolates, sodium caseinate, and soybean protein isolate loaded BC emulsions were 687, 891, and 452 pmol mg−1 protein, respectively, which were significantly higher than that of the THF/DMSO-BC control (246 pmol mg−1 protein).48 The potential reason for the difference between our results and previously published results is that previous studies mainly focused on the emulsions that were directly applied to the Caco-2 cell model without GIT digestion, while the emulsion of our study was applied to the Caco-2 cell model after in vitro GIT digestion. The LCT resulted in a higher absorption of the BC than MCT in the SGPI-stabilized emulsion, which is similar to the effect of oil type on VE bioaccessibility as reported previously.17 Actually, there are several reasons for explaining this phenomenon. In the first place, the property of the mixed micelle fractions formed is known to lie in the type of fatty acid, which may have affected the BC transport to the cell surface. The mixed micelle fractions include liquid crystalline phases, vesicles, and micelles which may change in their dimensions and structure, consequently altering their absorption characteristics. Secondly, some fatty acids have the ability to enhance the permeability of the cell membrane, which would change the absorption of the hydrophobic bioactive component. As a result, for disparate kinds of lipid digestion products generated by LCT and MCT, Caco-2 cells may have absorbed different amounts of BC. Thirdly, the intracellular processing and absorption of hydrophobic substances like BC were also influenced by the chain length of fatty acids. Medium chain fatty acids are generally diffused through the epithelial cells rather than being shipped to the endoplasmic reticulum like long chain fatty acids, which are then transformed into triacylglycerols for secreting into the lymphatic system.17,49 As a result, more BC in the LCT emulsion was absorbed by the epithelial cells than that in the MCT emulsion.
In our study, a comparatively simple HPLC technique was utilized to measure the amount of BC. In our future research, it would be beneficial to utilize more comprehensive analytical techniques, for instance HPLC-MS/MS, to offer more detailed information with regard to the changes in the BC chemical structure throughout the GIT and Caco-2 cells.
4. Conclusion
To sum up, our study reveals that BC-loaded emulsions were produced and stabilized using SGPIs with either LCT or MCT as a carrier oil. Both the LCT and MCT delivery systems exhibited favorable stability at pH 7–8. The particle size of both emulsions increased considerably after passing through the oral and gastric stages, which was ascribed to the aggregation of droplets, while the relatively small particle diameters with lower FFA released, but higher transformation and bioaccessibility of BC after exposure to the small intestine phase was observed in the LCT emulsion than that in in MCT emulsion. Moreover, the micelles from both SGPI-stabilized emulsions were nontoxic. LCT was more valid for increasing the absorption of BC in the Caco-2 cells than MCT, and they were both significantly higher than that of pure BC. In general, our results indicate that SGPI-based emulsions fabricated using LCT (i.e. corn oil) as a carrier oil are more appropriate for delivering BC than MCT, which provide significant instructions for designing efficient natural emulsion-based delivery systems for these hydrophobic bioactive compounds.Abbreviations
| BC | β-Carotene |
| LCT | Long chain triglyceride |
| MCT | Medium chain triglyceride |
| SGPIs | Scallop gonad protein isolate |
| GIT | Gastrointestinal tract |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| FITC | Fluorescein isothiocyanate |
| DMSO | Dimethyl sulfoxide |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported financially by the National Key R&D Program of China (No. 2018YFC0311200), the National Natural Science Foundation of China (No. 31671808), the Innovative Talent Support Program for Colleges and Universities of Liaoning Province (No. LR2017031), and the U.S. Department of Agriculture (MAS00450, MAS00492).References
- J. Berman, U. Zorrilla-Lopez, G. Farre, C. F. Zhu, G. Sandmann, R. M. Twyman and P. Christou, Phytochem. Rev., 2015, 14, 727–743 CrossRef CAS.
- A. V. Rao and L. G. Rao, Pharmacol. Res., 2007, 55, 207–216 CrossRef CAS PubMed.
- Z. Hou, Y. Liu, F. Lei and Y. Gao, LWT – Food Sci. Technol., 2014, 59, 867–873 CrossRef CAS.
- H. O. Akinosho and L. Wicker, LWT – Food Sci. Technol., 2015, 63, 582–589 CrossRef CAS.
- D. J. McClements and H. Xiao, Food Funct., 2012, 3, 202–220 RSC.
- L. Gu, Y. Su, M. Zhang, C. Chang, J. Li and D. J. Mcclements, Food Res. Int., 2017, 96, 84–93 CrossRef CAS PubMed.
- L. K. Mao and S. Miao, Food Eng. Rev., 2015, 7, 439–451 CrossRef CAS.
- D. J. McClements and Y. Li, Adv. Colloid Interface Sci., 2010, 159, 213–228 CrossRef CAS PubMed.
- D. J. McClements and C. E. Gumus, Adv. Colloid Interface Sci., 2016, 234, 3–26 CrossRef CAS PubMed.
- R. S. H. Lam and M. T. Nickerson, Food Chem., 2013, 141, 975–984 CrossRef CAS PubMed.
- M. Hu, D. J. McClements and E. A. Decker, J. Agric. Food Chem., 2003, 51, 1696–1700 CrossRef CAS PubMed.
- Y. Yang and D. J. Mcclements, Food Chem., 2013, 141, 473–481 CrossRef CAS PubMed.
- R. Zhang, Z. Zhang, H. Zhang, E. A. Decker and D. J. Mcclements, Food Res. Int., 2015, 75, 71–78 CrossRef CAS PubMed.
- Y. Cano, L. A. G. Zapateiro and Y. Zárate, Ing. Invest., 2017, 37, 17–22 CrossRef.
- M. Azadian, M. Moosavi-Nasab and E. Abedi, Eur. Food Res. Technol., 2012, 235, 83–90 CrossRef CAS.
- L. Day, J. L. Zhai, M. Xu, N. C. Jones, S. V. Hoffmann and T. J. Wooster, Food Hydrocolloids, 2014, 34, 78–87 CrossRef CAS.
- Y. Yang, H. Xiao and D. J. Mcclements, J. Agric. Food Chem., 2017, 65, 3946–3955 CrossRef CAS PubMed.
- V. Tyssandier, B. Lyan and P. Borel, Biochim. Biophys. Acta, 2001, 1533, 285–292 CrossRef CAS.
- S. K. Marmon and I. Undeland, J. Agric. Food Chem., 2010, 58, 10480–10486 CrossRef CAS.
- R. Adjonu, P. Torley and S. Agboola, Food Hydrocolloids, 2014, 41, 169–177 CrossRef CAS.
- B. R. Shah, Y. Li, W. Jin, Y. An, L. He and Z. Li, Food Hydrocolloids, 2016, 52, 369–377 CrossRef CAS.
- L. Bai, S. Huan, J. Gu and D. J. Mcclements, Food Hydrocolloids, 2016, 61, 703–711 CrossRef CAS.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn and C. Bourlieu, Food Funct., 2014, 5, 1113–1124 RSC.
- Y. Li and D. J. Mcclements, J. Agric. Food Chem., 2010, 58, 8085–8092 CrossRef CAS PubMed.
- R. Zhang, Z. Zhang, L. Zou, H. Xiao, G. Zhang, E. A. Decker and D. J. McClements, Food Funct., 2016, 7, 93–103 RSC.
- W. Liu, J. Wang, D. J. Mcclements and L. Zou, J. Funct. Foods, 2018, 40, 527–535 CrossRef CAS.
- L. Gu, N. Peng, C. Chang, D. J. Mcclements, Y. Su and Y. Yang, Food Biophys., 2017, 12, 1–13 CrossRef.
- M. Sobhaninia, A. Nasirpour, M. Shahedi and A. Golkar, J. Dispersion Sci. Technol., 2016, 38, 1366–1373 CrossRef.
- L. Gu, Y. Su, Z. Zhang, B. Zheng, R. Zhang and D. J. Mcclements, J. Agric. Food Chem., 2017, 65, 6919 CrossRef CAS PubMed.
- J. Rao and D. J. Mcclements, Food Hydrocolloids, 2012, 29, 326–334 CrossRef CAS.
- T. K. Dey, P. Banerjee, R. Chatterjee and P. Dhar, Colloids Surf., A, 2018, 538, 36–44 CrossRef CAS.
- D. J. Mcclements, Langmuir, 2005, 21, 9777–9785 CrossRef CAS.
- L. Salvia-Trujillo, C. Qian, O. Martín-Belloso and D. J. Mcclements, Food Chem., 2013, 141, 1472–1480 CrossRef CAS PubMed.
- Z. Zhang, R. Zhang and D. J. Mcclements, Food Hydrocolloids, 2016, 61, 1–10 CrossRef CAS.
- H. Singh, A. Ye and D. Horne, Prog. Lipid Res., 2009, 48, 92–100 CrossRef CAS PubMed.
- R. Zhang, Z. Zhang, H. Zhang, E. A. Decker and D. J. Mcclements, Food Hydrocolloids, 2015, 45, 175–185 CrossRef CAS.
- M. H. Vingerhoeds, B. Tbj, F. D. Zoet and A. Gavan, Food Hydrocolloids, 2005, 19, 915–922 CrossRef CAS.
- C. Qian, E. A. Decker, H. Xiao and D. J. Mcclements, Food Chem., 2012, 135, 1440–1447 CrossRef CAS PubMed.
- L. Cornacchia and Y. H. Roos, J. Food Sci., 2011, 76, 1211–1218 CrossRef PubMed.
- C. J. H. Porter, N. L. Trevaskis and W. N. Charman, Nat. Rev. Drug Discovery, 2007, 6, 231–248 CrossRef CAS PubMed.
- L. Sek, C. J. H. Porter, A. M. Kaukonen and W. N. Charman, J. Pharm. Pharmacol., 2002, 54, 29–41 CrossRef CAS PubMed.
- J. L. Periago, M. D. Suarez and M. L. Pita, J. Nutri., 1990, 120, 986 CrossRef CAS PubMed.
- K. Ahmed, Y. Li, D. J. McClenents and H. Xiao, Food Chem., 2012, 132, 799–807 CrossRef CAS.
- L. Salvia-Trujillo and D. J. Mcclements, J. Agric. Food Chem., 2016, 64, 4639–4647 CrossRef CAS PubMed.
- J. Corte-Real, E. Richling, L. Hoffmann and T. Bohn, Nutr. Res., 2014, 34, 1101–1110 CrossRef CAS PubMed.
- D. J. McClements, L. Saliva-Trujillo, R. Zhang, Z. Zhang, L. Zou, M. Yao and H. Xiao, Food Res. Int., 2016, 88, 140–152 CrossRef CAS PubMed.
- M. L. Failla, C. Chitchumronchokchai, M. G. Ferruzzi, S. R. Goltz and W. W. Campbell, Food Funct., 2014, 5, 1101–1112 RSC.
- J. Yi, T. I. Lam, W. Yokoyama, L. W. Cheng and F. Zhong, J. Agric. Food Chem., 2014, 62, 1096–1104 CrossRef CAS PubMed.
- D. J. Brayden, J. Gleeson and E. G. Walsh, Eur. J. Pharm. Biopharm., 2014, 88, 830–839 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |