Heteroaggregation and dissolution of silver nanoparticles by iron oxide colloids under environmentally relevant conditions†
Rui
Wang
ab,
Fei
Dang
*a,
Cun
Liu
a,
Deng-jun
Wang
 c,
Pei-xin
Cui
a,
Hui-jun
Yan
ab and
Dong-mei
Zhou
c,
Pei-xin
Cui
a,
Hui-jun
Yan
ab and
Dong-mei
Zhou
 *a
*a
aKey Laboratory of Soil Environment and Pollution Remediation, Institute of Soil Science, Chinese Academy of Sciences, Nanjing 210008, China. E-mail: fdang@issas.ac.cn; dmzhou@issas.ac.cn; Fax: +86 25 86881000; Tel: +86 25 86881180
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
cNational Research Council Resident Research Associate at the U.S. Environmental Protection Agency, Ada, Oklahoma 74820, USA
First published on 16th November 2018
Abstract
The ubiquity and abundance of iron oxides in the subsurface highlight their important roles in influencing the fate and transport of engineered silver nanoparticles (AgNPs). In this study, the adsorption behaviors of AgNPs on two naturally occurring iron oxides, goethite and hematite, were investigated under environmentally relevant conditions. The maximum surface coverage of AgNPs on iron oxides ranged between 0.014 and 0.326 mg m−2 depending on the investigated ionic strength and pH. The particle interactions (AgNPs–AgNPs and AgNPs–goethite/hematite) were probed by aggregation kinetics measurements using time-resolved dynamic light scattering and Derjaguin–Landau–Verwey–Overbeek theory calculations, which confirmed the predominant role of heteroaggregation in AgNP adsorption onto iron oxides. Multiple state-of-the-art characterization studies using X-ray absorption spectroscopy, attenuated total reflection-Fourier transform infrared spectroscopy, and X-ray diffraction substantiate the dominant electrostatic attractions between AgNPs and iron oxides. Moreover, AgNP dissolution was reduced in the presence of iron oxides. Goethite was more effective than hematite in retaining AgNPs (5.1 to 16.3-fold higher) and inhibiting AgNP dissolution (1.2 to 5.7-fold lower), due to their surface charge differences. Altogether, our findings provide compelling evidence of the dominant role played by electrostatic attractions in AgNP adsorption by iron oxides and of inhibition of AgNP dissolution during the heteroaggregation process, which has important implications for better evaluating the potential environmental impacts and risks of AgNPs in the iron oxide-rich subsurface.
Environmental significanceThe released AgNPs will inevitably interact with iron oxides due to their ubiquity and abundance in the subsurface environment. However, the mechanisms of how and to what extent iron oxides interact with AgNPs are largely obscure. By identifying the aggregate structure of AgNP–iron oxides at the molecular-scale level using multiple techniques we showed that electrostatic interactions governed the adsorption processes, which highlights the importance of the surface charge properties of iron oxides and AgNPs in understanding the adsorption behavior. Both the aggregation kinetics and classical DLVO calculations showed that heteroaggregation dominated the adsorption of AgNPs onto iron oxides. Furthermore, heteroaggregation with iron oxides inhibited AgNP dissolution. These findings provide novel insights into AgNP–iron oxide interaction and improve our understanding of the fate, mobility and toxicity of AgNPs in the subsurface environment. |
Introduction
Silver nanoparticles (AgNPs) are widely used as fungicides and bactericides in diverse products of the textile, food, medical, and pharmaceutical industries (http://www.nanotechproject.org/). The widespread applications of AgNPs have resulted in their increased entry into the subsurface environment, where they pose potential risks to the environment and to human health.1 The toxicity of AgNPs to aquatic and terrestrial organisms has been extensively evaluated,2–5 but many uncertainties remain regarding the environmental impact of these particles, which is determined by their fate, persistence, and bioavailability. These properties are, in turn, a function of AgNP mobility.The mobility of AgNPs in the subsurface environment can be quantified using different strategies. Typically, the non-equilibrium adsorption coefficient (expressed as Kr) has been used to estimate the mobility of AgNPs in the natural environment.6 The positive correlations between Kr and oxalate-extractable Fe and Al contents reflected the interplay of AgNPs with Fe/Al-containing components.7 Alternatively, adsorption models can also describe the adsorption behaviors of nanoparticles in soils,8,9 and the importance of Fe and Al oxides has been demonstrated using statistical approaches.7,8,10 Strong interactions between AgNPs and natural colloids, especially Fe oxides, are confirmed by the results of packed-column experiments that used natural soils.11–13 However, despite the apparent consensus that iron oxides are key determinants controlling AgNP mobility in the natural environment, the interactions between these nanoparticles and Fe oxides have not been fully explored. Iron oxides occur ubiquitously and abundantly in sediment and soil, where their concentrations are typically orders of magnitude higher than those of AgNPs.14 Consequently, iron oxides are highly likely to affect the mobility of AgNPs in the environment. Because the collision efficiency resulting in heteroaggregation between AgNPs and iron oxides is much higher than that leading to the homoaggregation of the AgNPs themselves, heteroaggregation plays a more important role in determining the fate and mobility of AgNPs. However, the heteroaggregation of AgNPs with natural clay minerals has for the most part been ignored, as most studies have focused on homoaggregation.
AgNPs can be retained by clays via heteroaggregation,8 as previously demonstrated for TiO2-montmorillonite,15 CNT-montmorillonite,16 CNT-hematite,17 TiO2–SiO2,14 GO-montmorillonite/kaolinite/goethite,18 AuNP-hematite,19 carbon dot-goethite,20 and AgNP–SiO2/clay21 systems. In addition to shedding light on the interactions between NPs and natural clay minerals, these studies described methods for measuring the heteroaggregation of NPs14,15,17,19 in addition to providing knowledge regarding the mechanisms controlling heteroaggregation at environmentally relevant NP concentrations.15,16,18,20,21 Further, the heteroaggregation of silver nanoparticles by hematite may prevent physically the direct contact of AgNPs with the bacterial cells and thus inhibit the antimicrobial activity of AgNPs.22,23 Nonetheless, the mechanism underlying the adsorption of AgNPs by iron oxides is still poorly understood. Moreover, although iron oxides contain oxygen surface groups that react with anions and cations,24 their role in AgNP adsorption is unknown. It is important to unravel whether AgNPs and iron oxides readily interact in the subsurface environment but also to further decipher the underlying mechanisms (e.g., electrostatic or chemical reactions) governing their interactions. Such information is critical for better understanding the fate, transport, and other environmentally relevant behaviors of AgNPs in the subsurface rich in iron oxides.
In this study, we examined the adsorption behaviors (adsorption isotherms) of AgNPs under environmentally relevant solution chemistries [ionic strength (IS) and pH], using model goethite and hematite colloids differing in their surface properties and geometric dimensions. We focused on metallic AgNPs because they remain as pristine forms within two days before final transformation into Ag2S-NPs in the subsurface aerobic environment.25 The aggregation kinetics between AgNPs and iron oxide colloids were monitored using time-resolved dynamic light scattering (DLS) and related to the theoretically determined Derjaguin–Landau–Verwey–Overbeek (DLVO) energy interaction profiles of particles. The aggregate structures were characterized using X-ray absorption spectroscopy (XAS), transmission electron microscopy-energy dispersive X-ray spectroscopy (TEM-EDS), attenuated total reflectance (ATR)-Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction (XRD), which together provided information on the mechanism by which AgNPs interact with iron oxides. Although this study was conducted specifically for metallic AgNPs and naturally occurring iron oxides (i.e., goethite and hematite), our approaches of investigating their interactions and the associated conclusions can also be employed to advance mechanistic understanding of the interactions between other types of metal nanoparticles and iron oxides (e.g., ferrihydrite and siderite), and probably be extended to investigations of interactions between other metal-based nanoparticles and clays, which exist in natural environments with different coatings, size distributions and morphologies, and surface chemistries. Our findings advance current knowledge regarding the mobility, reactivity, persistence, bioavailability, and toxicity of AgNPs in subsurface environments rich in iron oxides.
Materials and methods
Silver nanoparticles and iron oxide colloids
The polyvinylpyrrolidone (PVP)-AgNP stock suspension (100 mg L−1) was purchased from XFNANO Materials Technology Co., Ltd. (Nanjing, China) and stored in the dark at 4 °C until needed. PVP coating is utilized to stabilize AgNPs sterically,26 and the PVP content is <2 wt% as specified by the manufacturer. The morphology and size of the PVP-AgNPs were determined using TEM (Tecnai G2 F30 S-Twin, USA). The intensity-weighted hydrodynamic diameter (Dh) of the particles was measured immediately after ultrasonication in a water bath for 30 min using DLS (BI-200SM, Brookhaven, USA).Goethite (98.5% on metal basis, Sigma-Aldrich, USA) and hematite (99.5% on metal basis, Aladdin, China), both in powder form, were selected as model iron oxide colloids because they are widespread in sediments and subsurface environments.27 Their sizes and morphologies were determined using field-emission scanning electron microscopy (SEM; FEI S-4800 SEM, Japan), and their specific surface areas (SSAs) were measured through a surface area and porosity analyzer (Autosorb-iQ-MP, Quantachrome Instruments Co., USA) using the BET-N2 method. The isoelectric points (IEPs) of the iron oxide colloids were obtained by determining the zeta (ζ)-potentials as a function of pH using a zeta potential analyzer (Zetasizer Nanosizer ZS, Malvern Instruments Co., UK), where electrophoretic mobility was transformed into zeta potential using Smoluchowski's approximation based on the hard sphere theory.28–30 Please note that while the content of PVP coating on AgNPs was rather low (i.e., <2 wt%), the PVP coating may cause uncertainty in zeta potential measurements, as has been demonstrated by Louie et al.31 using the soft particle electrokinetic model based on the Ohshima theory.32 The soft particle electrokinetic model allows for accurate evaluation of the polymer layer (e.g., PVP coating) parameters including the layer charge density, layer thickness, and permeability (the Brinkman screening length).28,29,31
Batch experiments
Batch experiments were conducted to examine the effects of pH and IS on AgNP adsorption onto goethite and hematite. Suspensions of each of the colloids at 2500 mg L−1 were freshly prepared using iron oxide powders containing NaNO3 concentrations of 1, 10, and 100 mM. The batch experiments were initiated by adding AgNPs to the suspensions at final concentrations of 1–20 mg Ag L−1 (nominally 1, 5, 10, 15, and 20 mg L−1). The concentrations were carefully chosen to ensure accurate detection of AgNPs by multiple analyses (see below) and were within those used in previous studies.7,8 The concentrations and particle sizes of the iron oxides and AgNPs in the suspension differed by at least one order of magnitude, mimicking the conditions found in the environment.14 The pH of the binary suspensions (AgNPs–goethite and AgNPs–hematite) was environmentally relevant33 and was maintained at 5.5 and 7.5 using 10 mM 2-(N-morpholino)ethanesulfonic acid (Biosharp Corp., China) and 3-morpholinopropanesulfonic acid (Biosharp Corp.) buffers, respectively [ESI† Fig. S1]. Introduction of either buffer solution did not change the Dh of the AgNPs (ESI† Fig. S2) or the dissolution of iron oxides (<0.01 μg L−1).34 The mixed suspension was shaken at 200 rpm on an isothermal incubator at 25 °C for 24 h in the dark.Filtration through a 0.45 μm membrane (polyethersulfone, Membrana, Germany) was effective for separating iron oxides from non-adsorbed AgNPs.6,23 The Fe concentrations were below the detection limit of inductively coupled plasma mass spectrometry (ICP-MS, Thermo Fisher iCAP QC, USA, <0.01 μg L−1) in all the filtrates, which demonstrated the negligible dissolution of the colloids under the experimental conditions of this study and the effective separation of iron oxides from non-adsorbed AgNPs. Moreover, a separate experiment showed mass recoveries of 90.5–99.3% for AgNPs when the 0.45 μm filtrate was repeatedly filtrated through the above 0.45 μm membrane (ESI† Fig. S3). The equilibrium concentration of non-adsorbed AgNPs in the filtrate was determined by the subtraction of dissolved Ag (those that passed through a 3 kDa filter, Amicon Ultra-15, Millipore, USA) from total Ag (those that passed through the 0.45 μm filter). The adsorbed mass of AgNPs was finally calculated from the difference between the initial and equilibrium concentrations. After digestion in 65% HNO3 (Merck, Darmstadt, Germany) for 24 h, the total and dissolved Ag concentrations in the filtrate were determined using ICP-MS.35,36 The retentates on the 0.45 μm membrane were collected for further characterization. The filter was pretreated with 0.1 M Cu(NO3)2 to minimize Ag+ adsorption before use.
The control treatment consisted of AgNPs without iron oxides. A mass recovery of Ag of 90.2–106.3% under the various experimental conditions indicated negligible adsorptive loss of the AgNPs onto either the walls of the vials or the filter membranes during the batch experiments (ESI† Fig. S4).
TEM-EDS, XRD, ATR-FTIR, and XAS analyses
After the batch experiments, the samples were dispersed by sonication (45 kHz and 10 min) and then loaded on a 400 mesh copper grid for TEM (Tecnai G2 F30 S-Twin, USA), performed at an accelerating voltage of 200 kV. The elemental composition of the sample was examined in selected areas using EDS (EDAX, USA) with a super-ultrathin window sapphire detector to investigate the morphology and elemental composition of the AgNP–iron oxide complexes.After the batch experiments, the 0.45 μm membrane retentates were collected, washed thoroughly with Milli-Q water, and then freeze-dried for 24 h (Alpha 1-2 LDplus, Germany). The dried samples, together with pristine AgNPs and the iron oxide colloids, were then analyzed using XRD and XAS.
The crystalline structures of the AgNP–iron oxide aggregates were analyzed using a powder X-ray diffractometer (Ultima IV, Rigaku, Japan) with Cu Kα radiation at 40 kV and 50 mA. Samples were scanned from a 2θ of 2–80° at a scanning rate of 0.5° min−1 and a scanning step of 0.02°. All experimental data were analyzed with the aid of MDI Jade 6.0 software.
Silver K-edge (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514 eV) XAS data were collected using XAS Beamline 12-ID at the Australian Synchrotron Facility (Melbourne, Australia).25 The white X-ray beam was monochromatized using a Si(311) double-crystal monochromator. Silver standards (metallic Ag, Ag2O, AgCl, Ag2S, AgNO3, Ag2CO3, and Ag3PO4) and AgNP–iron oxide samples were analyzed in fluorescence mode with a 100-element Ge fluorescence detector, using a liquid helium cryostat. All samples were measured twice. The data were processed using Athena software37 and further deconvoluted according to the wavelet transform (WT) method, using the Igor Pro script.38 WT analysis was employed due to its high resolution in both k and R spaces.39,40 This qualitative analysis primarily focuses on the nature of the backscattering atoms as well as the bond lengths, thus complementing conventional Fourier transform (FT) analysis by connecting contributions in the extended X-ray absorption fine-structure spectra to the FT peaks. The WT modules of the heteroaggregation samples were analyzed and compared with the silver standards with contributions from O/S/Cl and Ag backscattering in the first coordination shell. Furthermore, Ag foil was measured concurrently with the samples for internal energy calibration. Data processing was performed using Athena based on IFEFFIT. The χ(k) function was Fourier transformed using multiple k-weightings, and all shell-by-shell fitting was conducted in R-space. A single threshold energy value (ΔE0) was allowed to vary during fitting.41
514 eV) XAS data were collected using XAS Beamline 12-ID at the Australian Synchrotron Facility (Melbourne, Australia).25 The white X-ray beam was monochromatized using a Si(311) double-crystal monochromator. Silver standards (metallic Ag, Ag2O, AgCl, Ag2S, AgNO3, Ag2CO3, and Ag3PO4) and AgNP–iron oxide samples were analyzed in fluorescence mode with a 100-element Ge fluorescence detector, using a liquid helium cryostat. All samples were measured twice. The data were processed using Athena software37 and further deconvoluted according to the wavelet transform (WT) method, using the Igor Pro script.38 WT analysis was employed due to its high resolution in both k and R spaces.39,40 This qualitative analysis primarily focuses on the nature of the backscattering atoms as well as the bond lengths, thus complementing conventional Fourier transform (FT) analysis by connecting contributions in the extended X-ray absorption fine-structure spectra to the FT peaks. The WT modules of the heteroaggregation samples were analyzed and compared with the silver standards with contributions from O/S/Cl and Ag backscattering in the first coordination shell. Furthermore, Ag foil was measured concurrently with the samples for internal energy calibration. Data processing was performed using Athena based on IFEFFIT. The χ(k) function was Fourier transformed using multiple k-weightings, and all shell-by-shell fitting was conducted in R-space. A single threshold energy value (ΔE0) was allowed to vary during fitting.41
ATR-FTIR spectroscopy was used to analyze in situ changes in surface chemistry (spectral absorbance) during interaction.42–44 ATR-FTIR spectra of the samples measured at 25 °C were collected on a Nicolet iS10 device (Thermo Nicolet Corp., USA) and a diamond crystal as the reactor. Iron oxide films were generated by placing 30 μL of a goethite or hematite suspension directly onto the diamond crystal at 25 °C, followed by addition of 10 μL of 99.7 mg L−1 AgNPs dropwise for each measurement. Spectra were obtained at 64 scans over a wave-number range of 400–4000 cm−1, with a resolution of 2 cm−1 against an air background. All spectra data were analyzed using the OMINC 8.0 software package (Thermo Nicolet).
Aggregation kinetics of AgNPs and goethite/hematite colloids
Separate experiments were conducted to investigate the heteroaggregation kinetics of AgNPs with iron oxides, as well as the homoaggregation kinetics of AgNPs or iron oxides. The conditions of these batch experiments were similar, except that the kinetics of heteroaggregation were studied at iron oxide and AgNP concentrations of 100 mg L−1 and 1 mg L−1, respectively, and those of homoaggregation at concentrations of 100 mg L−1 and 10 mg L−1, respectively. These concentrations were chosen to ensure reliable and time-resolved measurements, as required by DLS.45 The intensity-, number-, or volume-weighted Dh values and ζ-potentials of the AgNPs and iron oxides were monitored as described above. Since all modes of particle size showed similar aggregation behaviors (Fig. S5†), we reported here the intensity-weighted particle size distribution, which were particularly sensitive to aggregation.29 The intensity-, volume-, and number-weighted average hydrodynamic diameters of AgNPs were obtained using the refractive index of the solvent (i.e., 1.33 for water) during DLS measurements.While DLS tracks the kinetics of particle size variability versus time, sedimentation might happen and affect the particle size distribution during DLS measurements.46 To test the impact of sedimentation on particle size distribution during DLS measurements, sedimentation experiments for AgNPs and iron oxide colloids were conducted under different experimental conditions over a time frame of 0–30 min, which was similar to the time frame investigated in DLS measurements. A UV-vis spectrophotometer (UV-2700, SHIMADZU Corporation, Japan) at wavelengths of 425 and 406 nm was used to monitor the concentrations of goethite/hematite and AgNPs, respectively. The results (ESI† Fig. S6) showed that sedimentation of AgNPs and iron oxides during homoaggregation and heteroaggregation over 30 min was negligible.
The initial aggregation rate was calculated as described previously, in a linear least-squares analysis of the increase in Dh (1.3-fold of the initial Dh of iron oxides) with time. Details of the calculation of the initial aggregation rate are given in the ESI† (Text S1).
The Dh values and ζ-potentials of the AgNPs and iron oxides (ESI† Table S1) were then used to calculate the interaction energies of the NPs and colloids using classical DLVO theory that considers van der Waals and electrostatic double layer interactions.47 The important role of PVP coating on the colloidal stability and aggregation of NPs due to steric repulsion has been documented well in the literature45 and is not the overarching objective of this research. Nevertheless, while the steric contribution due to PVP coating was not explicitly calculated within the framework of classical DLVO theory, the role of PVP coating on the homo- and hetero-aggregation of AgNPs and iron oxide colloids merits further investigation. Details on the DLVO interaction energy calculation, including the assumptions of this approach, can be found in the ESI† (Text S2).
Results and discussion
Characterization of the AgNPs and iron oxide colloids
The PVP-AgNPs were spherical in shape, with an average TEM diameter of 10.7 ± 3.1 nm by measuring at least 300 particles using Nano Measure software (ESI† Fig. S7A and B) and an average intensity-based DLS size (Dh) of 68.5 ± 2.1 nm (ESI† Fig. S7D). DLS measures the hydrodynamic diameters of the particles, including hydration layer and polymer shells (i.e., PVP), leading to a larger particle size.26,48 The interplanar spacing of PVP-AgNPs was 0.236 nm (ESI† Fig. S7C), corresponding to the silver (111) plane. The PVP-AgNPs were negatively charged over the solution chemistries examined (−29.3–−47.2 mV, ESI† Fig. S8), and the absolute values of their ζ-potentials decreased with increasing IS (Fig. 1), due to an enhanced charge screening effect.49SEM showed differences in the size and morphology of the goethite and hematite particles (ESI† Fig. S9). Goethite formed acicular crystals with an average width of 60 nm and an average length of 390 nm. The hematite crystals were spindle-shaped, with an average width and length of 20 nm and 80 nm, respectively. Goethite had a smaller SSA than hematite (23.1 m2 g−1vs. 105.3 m2 g−1). Its IEP was at pH 8.2 and that of hematite was at pH 7.4 (ESI† Fig. S8), consistent with the values reported in the literature (6.2–9.6 and 7.0–9.3, respectively).50–52 At pH 5.5 and 7.5, iron oxides were positively charged (except for negatively charged hematite at pH 7.5) and the AgNPs were consistently negatively charged. Electrostatic interactions were thus predicted to be the dominant mechanism between the AgNPs and iron oxides (favorable heteroaggregation) under the examined conditions.
AgNP adsorption on iron oxide colloids
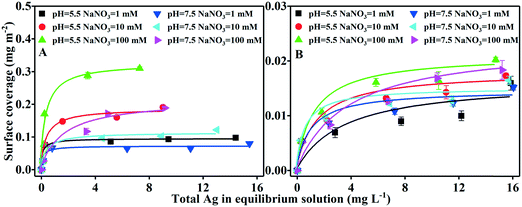
The adsorption of AgNPs on iron oxides was described using surface coverage by considering the SSA differences between goethite and hematite. The adsorption isotherm data were fitted well by the Langmuir model (ESI† Text S3 and Fig. 2). Maximum surface coverage (Γmax) by the AgNPs was consistently greater for goethite (0.072–0.326 mg m−2) than for hematite (0.014–0.022 mg m−2). No dissolved Fe was detected, consistent with its negligible role in AgNP adsorption on iron oxide colloids. Instead, the considerable increase in adsorption with increasing IS or decreasing pH provided proof that the solution chemistry (pH and electrolyte concentration) is an important determinant of the interaction between AgNPs and iron oxides.The maximum surface coverage of both iron oxides increased with increasing IS (Fig. 2 and Table S2†). At pH 5.5 and in 1 mM NaNO3, goethite and hematite had Γmax values of 0.095 and 0.016 mg m−2, respectively; at a higher IS of 100 mM NaNO3 and pH 5.5, the values were 3.4-fold (Γmax = 0.326 mg m−2) and 1.3-fold (Γmax = 0.020 mg m−2) higher. These findings were supported by the TEM images (Fig. 3), which for particles incubated at pH 5.5 showed substantially more AgNPs retained on the iron oxides at 100 mM NaNO3 than at 1 mM NaNO3. A significant increase in Γmax with increasing IS was also observed at pH 7.5. The IS-dependent adsorption of AgNPs by iron oxides was supported by DLVO theory53,54 (see ESI† Table S3 and Fig. S10). Specifically, for either homoaggregation (AgNPs–AgNPs) or heteroaggregation (AgNPs–goethite or AgNPs–hematite), the maximum repulsive interaction energy (Φmax) decreased and the attractive secondary minimum energy (Φmin2) increased with increasing IS, suggesting greater degrees of (homo- and hetero-) aggregation and thus adsorption of AgNPs in both the primary and secondary minimum wells at higher ISs.
The Γmax of the AgNPs in both iron oxides decreased with increasing pH (Fig. 2 and Table S2†). For goethite, the range was 0.095–0.326 mg m−2 at pH 5.5, but it decreased to 0.072–0.215 mg m−2 at pH 7.5. A similar trend was observed for hematite, albeit the effect of pH on Γmax was less evident. The pH-dependent adsorption of the AgNPs was unlikely to have been driven by pH-induced dissolution, since dissolved Ag at the pH values tested accounted for <7% of the total Ag in the medium (data not shown). Instead, pH more likely controlled the surface charge of both the iron oxides and the AgNPs, thereby affecting the electrostatic interactions that favored or impaired their interaction (aggregation). Specifically, at pH 5.5, both goethite and hematite were positively charged (Fig. 1), thus favoring their electrostatic attractions (heteroaggregation) with negatively charged AgNPs. By contrast, at pH 7.5, the iron oxides were less positively charged (Fig. 1 and Table S1 and Fig. S8†), resulting in less electrostatic attractions and less adsorption of the AgNPs. Again, the adsorption of AgNPs by iron oxides at varying pH values was consistent with DLVO theory (Table S3 and Fig. S10†). On the one hand, for the AgNP–AgNP system (unfavorable condition), greater Φmax and lower Φmin2 at a higher pH value (pH 7.5) cause less homoaggregation of AgNPs. On the other hand, for the AgNP–iron oxide system, greater Φmax occurred at pH 7.5, suggesting that heteroaggregation was less pronounced at pH 7.5. Therefore, both homoaggregation and heteroaggregation of AgNPs were expected to be weakened at pH 7.5, yielding a lower adsorption of AgNPs by iron oxides (Fig. 2). In a study in which the pH conditions resulted in oppositely charged TiO2 and SiO2 particles, the attachment efficiency of heteroaggregation was close to 1, indicating favorable attachment.14
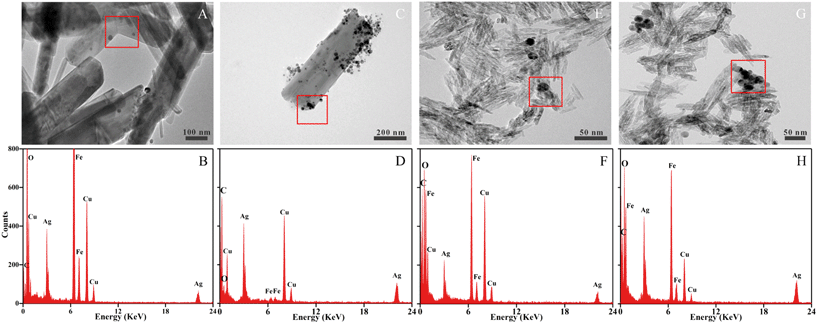
The morphology of homoaggregates and heteroaggregates were examined by TEM (Fig. 3). At 1 mM NaNO3, the size of the AgNP homoaggregates was ∼14.6 ± 3.2 nm, which was close to the initial TEM size (10.7 ± 3.1 nm) of the AgNPs (ESI† Fig. S7A and B). In the presence of iron oxides, the AgNPs occurred predominantly as dispersed nanoparticles and heteroaggregates randomly on the surfaces of the colloids at 1 mM NaNO3. At 100 mM NaNO3, however, AgNP homoaggregates up to 39.0 ± 9.3 nm (Fig. 3C and G) in size attached to the surfaces of the iron oxide particles.
Predominance of heteroaggregation of AgNPs with iron oxide colloids upon initial interaction
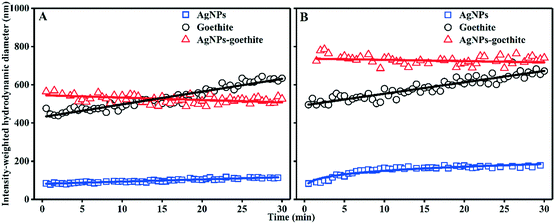
To gain further insight into the AgNP adsorption mechanism, the aggregation kinetics of AgNPs, iron oxides, and AgNPs–iron oxides were investigated. In the homoaggregation of AgNPs or iron oxides (ESI† Fig. S11 and S12), as the IS increased or pH decreased, Dh increased substantially, attributable to the increased charge-screening effect and a lower repulsive-energy barrier (ESI† Fig. S10). By contrast, the heteroaggregates were more stable and less sensitive to IS, pH, and incubation time (ESI† Fig. S13). Moreover, the measured Dh in the binary suspensions was largely contributed by the iron oxides.Representative heteroaggregation and homoaggregation profiles were extracted from ESI† Fig. S11–S13 and then plotted in Fig. 4. The addition of the AgNPs into the goethite suspension at 10 mM NaNO3 resulted in a clear, fast (0–1 min) increase in the initial Dh compared to that of the individual suspensions. In the binary system, the initial growth rate of Dh was 1.46 nm s−1, which was 14.6 times higher than the rate obtained for the original goethite suspension (0.1 nm s−1). Given that the AgNPs were resistant to homoaggregation (Fig. 4), the initially rapid growth of the particles in the binary system at the early stage reflected heteroaggregation between the colloid and the oppositely charged AgNPs. Afterwards, these particles stabilized and their size remained largely unchanged. Direct comparisons of the Dh of the binary system vs. that of the pristine goethite suspension indicated that, over the long-term, goethite homoaggregation likely played an important role in the interaction of the particles with the AgNPs. At 100 mM NaNO3, heteroaggregation of the AgNPs with goethite was more evident (initial aggregation rate of 6.07 nm s−1 in the binary system vs. 0.1 nm s−1 for goethite, Fig. 4B). A similar conclusion was drawn for the AgNP–goethite interaction at pH 7.5 and the AgNP–hematite interaction at both pH 5.5 and pH 7.5. Previous studies also showed that the heteroaggregation of nanoparticles to clays increased with increasing electrolyte concentrations.14,55
DLVO calculations showed negative energy barriers (Φmax < 0; ESI† Table S3) in most binary systems. While the repulsive energy barrier occurred for the AgNP–hematite system (Φmax = 0.1–5.9 kT), Φmax was much lower than that for the AgNPs–AgNPs or hematite–hematite system (Φmax = 0.1–66.4 kT), indicating the predominance of heteroaggregation over homoaggregation in AgNP adsorption by iron oxides. Classical DLVO theory without consideration of collision rates of particles, however, cannot completely estimate aggregation under high ionic strength conditions (100 mM NaNO3, Fig. 3), where homoaggregation of AgNPs may also occur. However, the collision between AgNPs is believed to be far weaker in comparison to the strong reaction of AgNPs with iron oxides because of their strong charge attraction and big surface area, which also confirmed that DLVO theory could simulate our experimental results well (ESI† Fig. S10).
Electrostatic attractions between AgNPs and iron oxide colloids
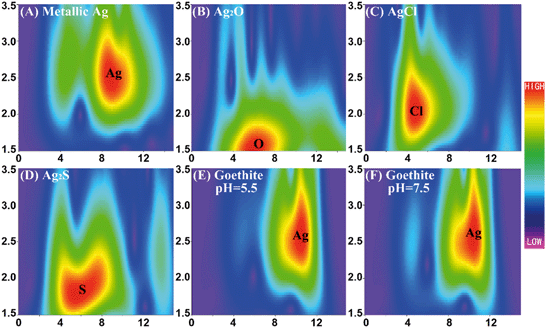
Based on the DLS measurements, TEM images, and DLVO interaction energy calculations, electrostatic attraction could be proposed as the dominant mechanism underlying the interaction between AgNPs and iron oxides. The mechanism accounting for the adsorption of AgNPs by the iron oxides was then explored using XRD, ATR-FTIR, and XAS, to search for the formation of surface complexes between AgNPs and the colloids. The XRD results showed no shift in the characteristic peaks of goethite and hematite after their interaction with the AgNPs (ESI† Fig. S14). Compared to the pristine iron oxides, two new characteristic peaks of AgNPs appeared under all experimental conditions, confirming heteroaggregation. The AgNP–goethite and AgNP–hematite aggregates were further analyzed using ATR-FTIR (ESI† Fig. S15) and the results were then compared with those obtained from the pristine iron oxides. The absorption peak at about 3100 cm−1 was ascribed to OH stretching of iron oxides.56,57 Its position and intensity had negligible changes in response to the addition of AgNPs. The absorption peak at 1620 cm−1 was due to the bending mode of the water molecule,58,59 which was closer to 1650 cm−1 with the addition of AgNPs. Strong absorption peaks at 1650 and 1295 cm−1, characteristic of the carbonyl group and –C–N bond of the PVP coating,60 respectively, were seen under all experimental conditions but no other new features of inner-sphere complexation between the iron oxides and the AgNPs were detected. Moreover, the band shifts were too small to represent possible direct surface complexation. Anyhow, ATR-FTIR did not provide solid evidence on possible direct surface complexation.XAS measurements were limited to the reaction between goethite and AgNPs, because the surface coverage (ESI† Table S2) by goethite was higher than that by hematite. Ag k-space EXAFS spectra of Ag foil and goethite after batch experiments are shown in ESI† Fig. S16A. The representative standards of metallic Ag, Ag2O, AgCl, and Ag2S were qualitatively identified in the WT contour plots by means of their features at ca. (9.5 Å−1, 2.5 Å), (6.5 Å−1, 1.6 Å), (5.0 Å−1, 2.0 Å), and (6.5 Å−1, 1.8 Å), originating from the Ag–Ag, Ag–O, Ag–Cl, and Ag–S scattering paths, respectively (Fig. 5A–D). The WT contour plots of the aggregates (Fig. 5E and F) displayed one main intensity maximum at approximately (10.0 Å−1, 2.5 Å) and a local extreme at low energy (4.5 Å−1, 2.5 Å), both of which were similar to the respective maxima of metallic Ag, suggesting Ag–Ag coordination. The WT analysis showed that almost all of the AgNPs retained on goethite maintained their pristine form, without any chemical transformation to Ag2O, AgCl, or Ag2S at any significant level. The Ag k-space EXAFS spectra of Ag foil and goethite after batch experiments were Fourier transformed to R-space (ESI† Fig. S16B) and EXAFS fitting parameters at the Ag K-edge for various samples are shown in ESI† Table S4. Only the Ag–Ag shell was fitted and the Ag–Ag distance for goethite after batch experiments was very close to that for Ag foil, which demonstrated that almost all of the AgNPs retained on goethite maintained their pristine form, consistent with the WT results. The smaller coordination number of the Ag–Ag shell for goethite after batch experiments than that for Ag foil was due to the surface effects of AgNPs. Note that we cannot completely rule out the possibility of Ag transformation on the surface, which may not be detectable by XAS analysis. Nevertheless, XRD, ATR-FTIR and XAS analysis showed that electrostatic interaction most likely dominated the adsorption of AgNPs onto iron oxides.
Greater surface coverage of AgNPs on goethite than on hematite
The Langmuir fitting results suggested that, compared to hematite, the adsorption of AgNPs onto goethite was associated with greater surface coverage and a stronger interaction of the nanoparticles with the goethite surface (ESI† Table S2). The effects of size and surface charge on the interactions between the iron oxides and AgNPs were thus assessed.Goethite and hematite differed morphologically and in size (ESI† Fig. S9), with a greater surface coverage for AgNPs on goethite (Table S2†) despite the larger SSA of hematite (105 vs. 23 m2 g−1). Hence, the SSA alone failed to explain the difference in AgNP adsorption. Rather, the faster aggregation of the smaller hematite particles may have impaired their reactivity and limited the available surface area, thereby hindering AgNP adsorption (ESI† Fig. S12B). Alternatively, the larger SSA of hematite may have implied the presence of more nano-scale pores (ESI† Fig. S17) and thus less AgNP adsorption because of the larger Dh of these particles (ESI† Fig. S11).18,61
The differences in AgNP adsorption between the two iron oxides may also have been related to differences in their surface charge. Goethite has a higher IEP than hematite (pHIEP = 8.2 vs. 7.4), which may allow its greater adsorption of AgNPs. At pH 5.5 and in 10 mM NaNO3, the ζ-potential of the goethite suspension was +29.1 mV vs. +19.0 mV for hematite. Thus, the charge attraction of goethite to the AgNPs was stronger than that of hematite, leading to greater surface coverage (Γmax) and the greater adsorption of goethite. The potential difference in surface charge therefore coincided with the differences in surface coverage and AgNP affinity, consistent with a primary role for electrostatic attraction in the adsorption of AgNPs on iron oxides.
In addition to the different adsorption patterns of goethite and hematite as demonstrated in this study, Hoppe et al.23 showed a much smaller adsorption of electrostatically stabilized than sterically stabilized AgNPs onto goethite, probably due to the degeneration or surface depletion of the stabilizer in the latter and colloidal stability in the former case. Therefore, the surface properties of the AgNPs themselves are also an important determinant of adsorption, in addition to mineralogical differences of iron oxides.
Iron oxides decrease AgNP dissolution
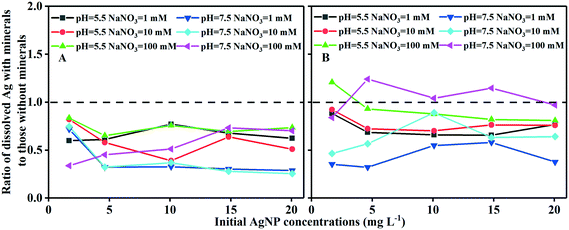
The aggregation of AgNPs and iron oxides significantly reduced the amount of dissolution of AgNPs. Since the dissolved Ag could have been re-adsorbed onto the surface of iron oxides, which would not have been experimentally detected, a separate experiment was conducted to estimate the amount of dissolved Ag (supplied as AgNO3) adsorbed at concentrations similar to those measured in the batch experiments. The amount of adsorbed dissolved Ag can then be determined via Langmuir model fitting (ESI† Fig. S18). The results ruled out potential competition for the adsorption sites between the AgNPs and dissolved Ag, in view of the low surface coverage of the iron oxides (Fig. 2). Consequently, rather than adsorption reduction as the driver, our data suggested that the decline in the dissolved Ag concentrations in the presence of iron oxides was due to a reduction of AgNP dissolution.The extent to which AgNP dissolution (defined as the ratio of dissolved Ag with vs. without iron oxides) was inhibited by goethite/hematite is presented in Fig. 6. Goethite and hematite generally inhibited AgNP dissolution by 25–75% and 3–68%, respectively. Goethite was more effective than hematite in inhibiting AgNP dissolution, but the amount of inhibition depended on the initial AgNP/goethite ratio, with a more pronounced effect at higher ratios (>0.0018, initial AgNPs > 4.62 mg L−1; Fig. 6A). This could be attributed to a loss of accessibility of active sites of AgNPs prone to oxidation, a consequence of aggregation.30 Interestingly, the IS also affected the inhibition of AgNP dissolution, with the greatest inhibition occurring at 10 mM NaNO3. This is possibly due to the fact that both AgNP dissolution45,62,63 and aggregation (Fig. S11†) increase with an increase in IS. For hematite, however, neither the initial AgNP/hematite ratio nor the IS exerted less effects on the inhibition effect (Fig. 6B), and even slight increases were shown in the dissolution of AgNPs in a few cases, such as at 100 mM NaNO3. Huynh et al.22 reported a decline in the concentration of dissolved Ag as the hematite concentration was increased, attributable to a decrease in adsorption. Our data, however, suggested that the decrease in Ag dissolution following the heteroaggregation of AgNPs with iron oxides was a key driver in lowering the dissolved Ag levels. Further studies should focus on the interplay between heteroaggregation and the dissolution kinetics of AgNPs.
Conclusions and perspectives
Our findings showed that electrostatic attractions most likely govern the adsorption behaviors of AgNPs on iron oxides under environmentally relevant solution chemistries (1–100 mM NaNO3, pH 5.5 and 7.5). These findings highlight the importance of the surface charge properties of both the iron oxides and the AgNPs in understanding the adsorption processes. Both pH and IS can modify the surface charge of the two components and thus finally determine the heteroaggregation of the AgNPs with the iron oxides. Furthermore, heteroaggregation was shown to weaken AgNP dissolution and thereby alter the fate, mobility, and potential toxicity of the nanoparticles. For example, the antimicrobial activity of AgNPs was inhibited after their heteroaggregation with hematite particles, because direct contact of the AgNPs with the bacterial cells was physically prevented22 and hematite hindered the release of more toxic ionic Ag, as shown here.The difference in the surface charge between goethite and hematite explained, at least partially, the up to 16.3-fold greater surface coverage of the former and its stronger interaction with AgNPs. In the natural environment, goethite will likely undergo chemical transformation, to form hematite, due to dehydration in arid seasons and at high temperatures.27 Transformation of goethite into hematite may release AgNPs previously retained by goethite into the aquatic environment, because hematite is less effective in sequestering AgNPs. Moreover, natural organic matter together with other mineral adsorbates often coexists with or coats on the surface of iron oxides in the natural environment, which would affect the negative charge due to the presence of carboxylic groups in NOM and complicate the adsorption behavior of AgNPs, and thus deserves further investigation to comprehensively evaluate the fate and mobilization of AgNPs in the subsurface environment.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Prof. Peng Wang of Nanjing Agricultural University for his assistance with XAS capture and analysis. We also thank the National Natural Science Foundation of China (41430752, 41671484, and 41771526) and the Natural Science Foundation of Jiangsu Province (BK20170110) for their financial support. Parts of the work were performed at the XAS (AS161/XAS/10337) Beamlines at the Australian Synchrotron Facility, Victoria, Australia (proposal number: 10337). Comments and suggestions from four anonymous reviewers and the editor helped us improve an earlier version of this manuscript.References
- S. M. Rodrigues, P. Demokritou, N. Dokoozlian, C. O. Hendren, B. Karn, M. S. Mauter, O. A. Sadik, M. Safarpour, J. M. Unrine, J. Viers, P. Welle, J. C. White, M. R. Wiesner and G. V. Lowry, Nanotechnology for sustainable food production: promising opportunities and scientific challenges, Environ. Sci.: Nano, 2017, 4, 767–781 RSC.
- J. L. Gardea-Torresdey, C. M. Rico and J. C. White, Trophic Transfer, Transformation, and Impact of Engineered Nanomaterials in Terrestrial Environments, Environ. Sci. Technol., 2014, 48, 2526–2540 CrossRef CAS PubMed.
- J. Fabrega, S. N. Luoma, C. R. Tyler, T. S. Galloway and J. R. Lead, Silver nanoparticles: Behaviour and effects in the aquatic environment, Environ. Int., 2011, 37, 517–531 CrossRef CAS PubMed.
- N. von Moos, P. Bowen and V. I. Slaveykova, Bioavailability of inorganic nanoparticles to planktonic bacteria and aquatic microalgae in freshwater, Environ. Sci.: Nano, 2014, 1, 214–232 RSC.
- A. Azimzada, N. Tufenkji and K. J. Wilkinson, Transformations of silver nanoparticles in wastewater effluents: links to Ag bioavailability, Environ. Sci.: Nano, 2017, 4, 1339–1349 RSC.
- G. Cornelis, J. K. Kirby, D. Beak, D. Chittleborough and M. J. McLaughlin, A method for determination of retention of silver and cerium oxide manufactured nanoparticles in soils, Environ. Chem., 2010, 7, 298–308 CrossRef CAS.
- G. Cornelis, C. Doolette, M. Thomas, M. J. McLaughlin, J. K. Kirby, D. G. Beak and D. Chittleborough, Retention and Dissolution of Engineered Silver Nanoparticles in Natural Soils, Soil Sci. Soc. Am. J., 2012, 76, 891–902 CrossRef CAS.
- M. Hoppe, R. Mikutta, J. Utermann, W. Duijnisveld and G. Guggenberger, Retention of Sterically and Electrosterically Stabilized Silver Nanoparticles in Soils, Environ. Sci. Technol., 2014, 48, 12628–12635 CrossRef CAS PubMed.
- G. Cornelis, B. Ryan, M. J. McLaughlin, J. K. Kirby, D. Beak and D. Chittleborough, Solubility and Batch Retention of CeO2 Nanoparticles in Soils, Environ. Sci. Technol., 2011, 45, 2777–2782 CrossRef CAS PubMed.
- R. Wang, H. Du, Y. Wang, D. Wang, Q. Sun and D. Zhou, Retention of silver nanoparticles and silver ion to natural soils: effects of soil physicochemical properties, J. Soils Sediments, 2018, 18, 2491–2499 CrossRef CAS.
- G. Cornelis, L. Pang, C. Doolette, J. K. Kirby and M. J. McLaughlin, Transport of silver nanoparticles in saturated columns of natural soils, Sci. Total Environ., 2013, 463, 120–130 CrossRef PubMed.
- Y. Liang, S. A. Bradford, J. Simunek, M. Heggen, H. Vereecken and E. Klumpp, Retention and Remobilization of Stabilized Silver Nanoparticles in an Undisturbed Loamy Sand Soil, Environ. Sci. Technol., 2013, 47, 12229–12237 CrossRef CAS PubMed.
- D. Wang, L. Ge, J. He, W. Zhang, D. P. Jaisi and D. Zhou, Hyperexponential and nonmonotonic retention of polyvinylpyrrolidone-coated silver nanoparticles in an Ultisol, J. Contam. Hydrol., 2014, 164, 35–48 CrossRef CAS PubMed.
- A. Praetorius, J. Labille, M. Scheringer, A. Thill, K. Hungerbuehler and J.-Y. Bottero, Heteroaggregation of Titanium Dioxide Nanoparticles with Model Natural Colloids under Environmentally Relevant Conditions, Environ. Sci. Technol., 2014, 48, 10690–10698 CrossRef CAS PubMed.
- D. Zhou, A. I. Abdel-Fattah and A. A. Keller, Clay Particles Destabilize Engineered Nanoparticles in Aqueous Environments, Environ. Sci. Technol., 2012, 46, 7520–7526 CrossRef CAS PubMed.
- L. Zhang, E. J. Petersen, W. Zhang, Y. Chen, M. Cabrera and Q. Huang, Interactions of C-14-labeled multi-walled carbon nanotubes with soil minerals in water, Environ. Pollut., 2012, 166, 75–81 CrossRef CAS PubMed.
- H. Khanh An, J. M. McCaffery and K. L. Chen, Heteroaggregation of Multiwalled Carbon Nanotubes and Hematite Nanoparticles: Rates and Mechanisms, Environ. Sci. Technol., 2012, 46, 5912–5920 CrossRef PubMed.
- J. Zhao, F. Liu, Z. Wang, X. Cao and B. Xing, Heteroaggregation of Graphene Oxide with Minerals in Aqueous Phase, Environ. Sci. Technol., 2015, 49, 2849–2857 CrossRef CAS PubMed.
- B. M. Smith, D. J. Pike, M. O. Kelly and J. A. Nason, Quantification of Heteroaggregation between Citrate-Stabilized Gold Nanoparticles and Hematite Colloids, Environ. Sci. Technol., 2015, 49, 12789–12797 CrossRef CAS PubMed.
- X. Liu, J. Li, Y. Huang, X. Wang, X. Zhang and X. Wang, Adsorption, Aggregation, and Deposition Behaviors of Carbon Dots on Minerals, Environ. Sci. Technol., 2017, 51, 6156–6164 CrossRef CAS PubMed.
- S. Maillette, C. Peyrot, T. Purkait, M. Iqbal, J. G. C. Veinot and K. J. Wilkinson, Heteroagglomeration of nanosilver with colloidal SiO2 and clay, Environ. Chem., 2017, 14, 1–8 CrossRef CAS.
- K. A. Huynh, J. M. McCaffery and K. L. Chen, Heteroaggregation reduces antimicrobial activity of silver nanoparticles: evidence for nanoparticle-cell proximity effects, Environ. Sci. Technol. Lett., 2014, 1, 361–366 CrossRef CAS.
- M. Hoppe, R. Mikutta, S. Kaufhold, J. Utermann, W. Duijnisveld, E. Wargenau, E. Fries and G. Guggenberger, Retention of sterically and electrosterically stabilized silver nanoparticles by soil minerals, Eur. J. Soil Sci., 2016, 67, 573–582 CrossRef CAS.
- M. Villalobos, M. A. Cheney and J. Alcaraz-Cienfuegos, Goethite surface reactivity: II. A microscopic site-density model that describes its surface area-normalized variability, J. Colloid Interface Sci., 2009, 336, 412–422 CrossRef CAS PubMed.
- M. Li, P. Wang, F. Dang and D.-M. Zhou, The transformation and fate of silver nanoparticles in paddy soil: effects of soil organic matter and redox conditions, Environ. Sci.: Nano, 2017, 4, 919–928 RSC.
- A. M. El Badawy, T. P. Luxton, R. G. Silva, K. G. Scheckel, M. T. Suidan and T. M. Tolaymat, Impact of environmental conditions (pH, ionic strength, and electrolyte type) on the surface charge and aggregation of silver nanoparticles suspensions, Environ. Sci. Technol., 2010, 44, 1260–1266 CrossRef CAS PubMed.
- R. M. Cornell and U. Schwertmann, The iron oxides : structure, properties, reactions, occurences and uses, Wiley-VCH, 2003 Search PubMed.
- S. M. Louie, T. Phenrat, M. J. Small, R. D. Tilton and G. V. Lowry, Parameter identifiability in application of soft particle electrokinetic theory to determine polymer and polyelectrolyte coating thicknesses on colloids, Langmuir, 2012, 28, 10334–10347 CrossRef CAS PubMed.
- T. Phenrat, N. Saleh, K. Sirk, H. J. Kim, R. D. Tilton and G. V. Lowry, Stabilization of aqueous nanoscale zerovalent iron dispersions by anionic polyelectrolytes: adsorbed anionic polyelectrolyte layer properties and their effect on aggregation and sedimentation, J. Nanopart. Res., 2008, 10, 795–814 CrossRef CAS.
- D. He, M. W. Bligh and T. D. Waite, Effects of aggregate structure on the dissolution kinetics of citrate-stabilized silver nanoparticles, Environ. Sci. Technol., 2013, 47, 9148–9156 CrossRef CAS PubMed.
- S. M. Louie, T. Phenrat, M. J. Small, R. D. Tilton and G. V. Lowry, Parameter Identifiability in Application of Soft Particle Electrokinetic Theory To Determine Polymer and Polyelectrolyte Coating Thicknesses on Colloids, Langmuir, 2012, 28, 10334–10347 CrossRef CAS PubMed.
- H. Ohshima, Electrophoresis of soft particles, Adv. Colloid Interface Sci., 1995, 62, 189–235 CrossRef CAS.
- E. W. Slessarev, Y. Lin, N. L. Bingham, J. E. Johnson, Y. Dai, J. P. Schimel and O. A. Chadwick, Water balance creates a threshold in soil pH at the global scale, Nature, 2016, 540, 567–569 CrossRef CAS PubMed.
- C. He, D. He, R. N. Collins, S. Garg, Y. Mu and T. D. Waite, Effects of Good's Buffers and pH on the Structural Transformation of Zero Valent Iron and the Oxidative Degradation of Contaminants, Environ. Sci. Technol., 2018, 52, 1393–1403 CrossRef CAS PubMed.
- P. M. Abraham, S. Barnikol, T. Baumann, M. Kuehn, N. P. Ivleva and G. E. Schaumann, Sorption of Silver Nanoparticles to Environmental and Model Surfaces, Environ. Sci. Technol., 2013, 47, 5083–5091 CrossRef CAS PubMed.
- H. Hagendorfer, R. Kaegi, M. Parlinska, B. Sinnet, C. Ludwig and A. Ulrich, Characterization of Silver Nanoparticle Products Using Asymmetric Flow Field Flow Fractionation with a Multidetector Approach - a Comparison to Transmission Electron Microscopy and Batch Dynamic Light Scattering, Anal. Chem., 2012, 84, 2678–2685 CrossRef CAS PubMed.
- B. Ravel and M. Newville, ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed.
- H. Funke, A. C. Scheinost and M. Chukalina, Wavelet analysis of extended x-ray absorption fine structure data, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 094110 CrossRef.
- D. E. Sayers, E. A. Stern and F. W. Lytle, New Technique for Investigating Noncrystalline Structures: Fourier Analysis of the Extended X-Ray-Absorption Fine Structure, Phys. Rev. Lett., 1971, 27, 1204–1207 CrossRef CAS.
- A. Kuzmin and J. Chaboy, EXAFS and XANES analysis of oxides at the nanoscale, IUCrJ, 2014, 1, 571–589 CrossRef CAS PubMed.
- L. Mino, G. Agostini, E. Borfecchia, D. Gianolio, A. Piovano, E. Gallo and C. Lamberti, Low-dimensional systems investigated by x-ray absorption spectroscopy: a selection of 2D, 1D and 0D cases, J. Phys. D: Appl. Phys., 2013, 46, 423001 CrossRef.
- F. M. Mirabella, Internal reflection spectroscopy : theory and applications, Marcel Dekker, 1992 Search PubMed.
- S. E. Glassford, B. Byrne and S. G. Kazarian, Recent applications of ATR FTIR spectroscopy and imaging to proteins, Biochim. Biophys. Acta, 2013, 1834, 2849–2858 CrossRef CAS PubMed.
- Q. Sun, C. Liu, M. E. Alves, S. T. Ata-Ul-Karim, D. M. Zhou, J. Z. He, P. X. Cui and Y. J. Wang, The oxidation and sorption mechanism of Sb on δ-MnO2, Chem. Eng. J., 2018, 342, 429–437 CrossRef CAS.
- K. A. Huynh and K. L. Chen, Aggregation Kinetics of Citrate and Polyvinylpyrrolidone Coated Silver Nanoparticles in Monovalent and Divalent Electrolyte Solutions, Environ. Sci. Technol., 2011, 45, 5564–5571 CrossRef CAS PubMed.
- H. J. Kim, T. Phenrat, R. D. Tilton and G. V. Lowry, Effect of kaolinite, silica fines and pH on transport of polymer-modified zero valent iron nano-particles in heterogeneous porous media, J. Colloid Interface Sci., 2012, 370, 1–10 CrossRef CAS PubMed.
- T. Phenrat, H. J. Kim, F. Fagerlund, T. Illangasekare and G. V. Lowry, Empirical correlations to estimate agglomerate size and deposition during injection of a polyelectrolyte-modified Fe0 nanoparticle at high particle concentration in saturated sand, J. Contam. Hydrol., 2010, 118, 152–164 CrossRef CAS PubMed.
- S. Kittler, C. Greulich, J. S. Gebauer, J. Diendorf, L. Treuel, L. Ruiz, J. M. Gonzalezcalbet, M. Valletregi, R. Zellner and M. Köller, The influence of proteins on the dispersability and cell-biological activity of silver nanoparticles, J. Mater. Chem., 2009, 20, 512–518 RSC.
- M. Elimelech, Particle deposition and aggregation : measurement, modelling, and simulation, Butterworth-Heinemann, 1995 Search PubMed.
- U. Schwertmann, Properties of Goethites of Varying Crystallinity, Clays Clay Miner., 1985, 33, 369–378 CrossRef CAS.
- R. M. Handler, A. J. Frierdich, C. M. Johnson, K. M. Rosso, B. L. Beard, C. Wang, D. E. Latta, A. Neumann, T. Pasakarnis, W. A. P. J. Premaratne and M. M. Scherer, Fe(II)-Catalyzed Recrystallization of Goethite Revisited, Environ. Sci. Technol., 2014, 48, 11302–11311 CrossRef CAS PubMed.
- M. Jarlbring, L. Gunneriusson, B. Hussmann and W. Forsling, Surface complex characteristics of synthetic maghemite and hematite in aqueous suspensions, J. Colloid Interface Sci., 2005, 285, 212–217 CrossRef CAS PubMed.
- B. V. Derjaguin, Theory of the stability of strongly charged lyophobic sols and the adhesion of strongly charged particles in solutions of electrolytes, Acta Physicochim. URSS, 1941, 14, 633–662 Search PubMed.
- E. Verwey, J. Overbeek and K. V. Nes, Theory of the stability of lyophobic colloids: the interaction of sol particles having an electric double layer, 1948 Search PubMed.
- N. P. Sotirelis and C. V. Chrysikopoulos, Interaction Between Graphene Oxide Nanoparticles and Quartz Sand, Environ. Sci. Technol., 2015, 49, 13413–13421 CrossRef CAS PubMed.
- E. Libowitzky and G. R. Rossman, An IR absorption calibration for water in minerals, Am. Mineral., 1997, 82, 1111–1115 CAS.
- P. Cambier, Infrared study of goethites of varying crystallinity and particle size: I. Interpretation of OH and lattice vibration frequencies, Clay Miner., 1986, 21, 191–200 CrossRef CAS.
- L. Mino, G. Spoto, S. Bordiga and A. Zecchina, Particles Morphology and Surface Properties As Investigated by HRTEM, FTIR, and Periodic DFT Calculations: From Pyrogenic TiO2 (P25) to Nanoanatase, J. Phys. Chem. C, 2012, 116, 17008–17018 CrossRef CAS.
- L. Mino, A. Zecchina, G. Martra, A. M. Rossi and G. Spoto, A surface science approach to TiO2 P25 photocatalysis: An in situ FTIR study of phenol photodegradation at controlled water coverages from sub-monolayer to multilayer, Appl. Catal., B, 2016, 196, 135–141 CrossRef CAS.
- R. Bryaskova, D. Pencheva, S. Nikolov and T. Kantardjiev, Synthesis and comparative study on the antimicrobial activity of hybrid materials based on silver nanoparticles (AgNps) stabilized by polyvinylpyrrolidone (PVP), J. Chem. Biol., 2011, 4, 185–191 CrossRef PubMed.
- O. Sagee, I. Dror and B. Berkowitz, Transport of silver nanoparticles (AgNPs) in soil, Chemosphere, 2012, 88, 670–675 CrossRef CAS PubMed.
- B. Molleman and T. Hiemstra, Time, pH, and size dependency of silver nanoparticle dissolution: the road to equilibrium, Environ. Sci.: Nano, 2017, 4, 1314–1327 RSC.
- B. A. Chambers, A. R. M. N. Afrooz, S. Bae, N. Aich, L. Katz, N. B. Saleh and M. J. Kirisits, Effects of Chloride and Ionic Strength on Physical Morphology, Dissolution, and Bacterial Toxicity of Silver Nanoparticles, Environ. Sci. Technol., 2014, 48, 761–769 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed information on determination of hydrodynamic diameters and zeta-potentials of AgNPs and iron oxides (Text S1), DLVO interaction energy calculation (Text S2), Langmuir model (Text S3), average hydrodynamic diameters and zeta-potentials of AgNPs and iron oxides used in the DLVO calculations (Table S1), Langmuir model-fitted parameters (Table S2), calculated DLVO interaction energies (Table S3), EXAFS fitting parameters at the Ag K-edge for various samples (Table S4), variation in the suspension pH (Fig. S1), average hydrodynamic diameter of AgNPs with 10 mM MES or 10 mM MOPS or without buffer (Fig. S2), mass recoveries of Ag during the filtering process in 100 mM NaNO3 at pH 7.5 (Fig. S3), mass recoveries of Ag during the 24 h batch experiment in the absence of goethite and hematite (Fig. S4), the volume- (A and B) and number- (C and D) average hydrodynamic diameters of AgNPs and goethite (Fig. S5), sedimentation history of AgNPs (A), goethite (B), hematite (C), AgNPs–goethite (D), and AgNPs–hematite (E) over the time frame of 0–30 min under different experimental conditions (Fig. S6), AgNP characterization (Fig. S7), zeta-potentials of AgNP, goethite, and hematite suspensions (Fig. S8), characterization of goethite and hematite (Fig. S9), calculated DLVO interaction energy as a function of the separation distance (Fig. S10), hydrodynamic diameter of AgNPs (Fig. S11), hydrodynamic diameter of goethite and hematite (Fig. S12), hydrodynamic diameter (Dh) of AgNPs–goethite and AgNPs–hematite (Fig. S13), XRD patterns of AgNPs–goethite and AgNPs–hematite (Fig. S14), ATR-FTIR spectra of AgNPs with goethite and hematite (Fig. S15), Ag k-space EXAFS spectra (A) and the Fourier transforms (B) for Ag foil and goethite after batch experiments (Fig. S16), cumulative pore volume calculated from the BET-N2 method (Fig. S17), and adsorption isotherms of Ag+ on goethite and hematite (Fig. S18). See DOI: 10.1039/c8en00543e |
| This journal is © The Royal Society of Chemistry 2019 |