Ambient air pollution and the hospital outpatient visits for eczema and dermatitis in Beijing: a time-stratified case-crossover analysis†
Qun
Guo
 a,
Fengchao
Liang
b,
Lin
Tian
a,
Fengchao
Liang
b,
Lin
Tian
 a,
Tamara
Schikowski
c,
Wei
Liu
d and
Xiaochuan
Pan
*a
a,
Tamara
Schikowski
c,
Wei
Liu
d and
Xiaochuan
Pan
*a
aDepartment of Occupational and Environmental Health, School of Public Health, Peking University, Xueyuan Road 38, Haidian District, Beijing 100191, China. E-mail: xcpan@bjmu.edu.cn; Fax: +8682802530; Tel: +8682802530
bDepartment of Epidemiology, Fuwai Hospital, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing 100037, China
cIUF-Leibniz Research Institute for Environmental Medicine, Düsseldorf, Germany
dDepartment of Dermatology, The General Hospital of Air Force of People's Liberation Army, Germany
First published on 21st December 2018
Abstract
Background: Eczema and dermatitis are a group of common skin conditions with multiple risk factors. Evidence of the effects of air pollutants on eczema and dermatitis remains limited. This study aimed to investigate the effects of short-term exposure to air pollution on eczema and dermatitis in Beijing. Methods: A time-stratified case-crossover design was used to assess the associations between short-term changes in air pollution and the hospital outpatient visits for eczema and dermatitis in Beijing. Results: A total of 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 595 outpatient visits for eczema and dermatitis were recorded from April 1, 2012 to April 30, 2014. All pollutants showed significant positive associations with the number of outpatient visits for eczema and dermatitis on lag 0 (the current day). Per IQR increase in PM2.5, PM10, NO2 and SO2 was associated with 3.81% (95% CI: 2.92–4.7%), 3.18% (95% CI: 2.39–3.97%), 5.43% (95% CI: 4.43–6.43%) and 5.57% (95% CI: 4.55–6.58%) increases in outpatient visits for eczema and dermatitis on lag 0. Associations of air pollutants with eczema and dermatitis outpatient visits varied with the seasons and were stronger among older people and females. Also, an association of consecutive days' high concentration pollution with increased outpatient visits was observed. Conclusions: Exposure to air pollution increases the exacerbation of eczema and dermatitis and stronger positive associations between air pollutants and outpatient visits for eczema and dermatitis were found among the aged, females and when high concentration air pollution occurs continuously.
595 outpatient visits for eczema and dermatitis were recorded from April 1, 2012 to April 30, 2014. All pollutants showed significant positive associations with the number of outpatient visits for eczema and dermatitis on lag 0 (the current day). Per IQR increase in PM2.5, PM10, NO2 and SO2 was associated with 3.81% (95% CI: 2.92–4.7%), 3.18% (95% CI: 2.39–3.97%), 5.43% (95% CI: 4.43–6.43%) and 5.57% (95% CI: 4.55–6.58%) increases in outpatient visits for eczema and dermatitis on lag 0. Associations of air pollutants with eczema and dermatitis outpatient visits varied with the seasons and were stronger among older people and females. Also, an association of consecutive days' high concentration pollution with increased outpatient visits was observed. Conclusions: Exposure to air pollution increases the exacerbation of eczema and dermatitis and stronger positive associations between air pollutants and outpatient visits for eczema and dermatitis were found among the aged, females and when high concentration air pollution occurs continuously.
Environmental significanceEczema and dermatitis are a group of prevalent skin diseases with multiple environmental risk factors. The prevention and the identification of harmful effects of environmental agents are important for eczema and dermatitis. We investigated the effects of air pollutants on eczema and dermatitis and how the effects changed during different seasons, among different subgroups and with different duration days. The results of this wok demonstrate that short-term exposure to air pollution could increase the exacerbation of eczema and dermatitis and the effect might be more robust among the aged, females and when high level air pollution occurs continuously. The uniqueness of this study was the use of continuous high exposure days to study the effects of continuous exposure to high concentration air pollution on eczema and dermatitis. The patients and risk groups (e.g. the aged) should avoid exposure to air pollutants and hospitals should get ready for the increasing outpatient visits on high pollution days. |
Introduction
Eczema and dermatitis are a group of chronic, inflammatory and relapsing skin diseases which place a heavy burden on patients, their families, and society.1–3 Skin and subcutaneous diseases were the cause of 41.6 million disability adjusted life years (DALYs) and 39.0 million years lived with disability (YLDs) in 2013, of which eczema and dermatitis accounted for the largest proportion.3 While the exact cause is unknown, multiple risk factors including endogenous factors (e.g. heritability) and exogenous factors (e.g. physical activity, alcohol consumption, smoking and even maternal stress) exist for eczema and dermatitis.4,5 Treatment at best achieves symptom control rather than cure. Therefore, disease prevention and the identification of harmful effects of environmental agents remain the focus in disease management.6Environmental pollution has been noted to play an important role in the development of eczema and dermatitis.5,7 A meta-analysis summarized that, for each 10 μg m−3 increase in PM10 and PM2.5 concentration, the risk of human skin diseases due to PM was determined to be 1.01% (0.08–2.05) and 1.60% (0.45–2.82), respectively.8 Birth cohort studies have suggested that pollutants such as PM and NO2 are associated with increased risks of developing eczema and dermatitis,9–11 although not all studies have shown this association.12 In addition, air pollution was found to be associated with exacerbating symptoms among previous patients. A panel study in South Korea showed that an interquartile range (IQR) increase in previous day ultrafine particle concentration (IQR: 28–140 μg m−3) was significantly associated with a 3.1% (95% confidence interval (CI), 0.2–6.1%) increase in the itch symptom score among school children with pre-existing atopic dermatitis.13 And in another study in Korea, the level of air pollutants was found to be higher on days when eczema patients were with symptoms than on days when the patients did not develop symptoms.14 Although eczema and dermatitis have been linked to air pollution, epidemiological evidence is still limited.
Preventive measures, the use of moisturizers and topical anti-inflammatory agents, such as topical corticosteroids and calcineurin inhibitors, are the main means of eczema and dermatitis management,15–17 meaning that eczema and dermatitis rarely lead to hospitalization and are more suitable for outpatient treatment. Outpatient visits were used as outcomes to estimate the effect of air pollution on skin conditions in several previous studies.18–20 One of the first publications (2010) showed that environmental ozone was positively correlated with the dermatology emergency department visits in Edmonton, Canada,20 and researchers (2016) further discovered associations of air pollutants (O3, NO2, SO2 and PM2.5) with cellulitis in Ontario, Canada.19 Similarly, two previous studies in west and southeast China showed that air pollution might increase the number of outpatient visits for eczema.21,22 However, the existing studies had several limitations. Firstly, it is still not clear that whether the effects of air pollution on eczema and dermatitis differ in different subgroups (e.g. age groups) and which subgroups might be more vulnerable to air pollution. Secondly, poor air quality could last for a long period. It is not clear if the duration days of high concentration air pollution could modify the effects of air pollution on eczema and dermatitis.
Considering the above-mentioned, we conducted this study to investigate the associations between short-term changes in air pollutants and the number of outpatient visits for eczema and dermatitis in a dermatological hospital in Beijing, China. The above associations in different seasons and different subgroups (age groups and sex groups) were further evaluated. In particular, we investigated the effects of consecutive exposure to high concentration air pollution on eczema and dermatitis. This study aimed to add evidence on the effects of air pollution on eczema and dermatitis and be useful in decision making and health policy.
Materials and methods
Data of outpatient visits
Hospital outpatient and emergency visits for eczema and dermatitis from April 1, 2012 to April 30, 2014 were collected from the Department of Dermatology, the General Hospital of Air Force of People's Liberation Army (PLA) in Beijing, China. The hospital is a full hospital with comprehensive departments. Although the hospital belongs to the military, it also provides services to the general public. For patients, military hospitals are no different from ordinary public hospitals. Moreover, the dermatology department of PLA is one of the largest and most famous dermatology departments among hospitals in Beijing. Data were collected from the HIS system of the hospital. Either a new case or a relapse of previous patients was included when counting the number of outpatient visits. Eczema and dermatitis were defined according to the International Classification of Diseases, Tenth Revision (ICD-10): L20-L30. Only patients who lived in Beijing for at least five years (recorded in the HIS system) were included in our study.Air pollutants and meteorological data
Daily data of air pollutant monitoring (including PM10, PM2.5, NO2 and SO2) during the period of April 1, 2012 to April 30, 2014 were obtained from the Beijing Municipal Environmental Monitoring Center. The data were collected from 35 monitoring stations, which were homogenously distributed all over Beijing. Hourly pollutant data were recorded at each site, which we made into daily averages at each site and then for the whole city. If there were missing data from a monitoring station on a given day, then the values from the remaining monitors were used to calculate the average concentration. Daily data of mean temperature and relative humidity during the same period in Beijing were collected from the China Meteorological Data Sharing Service System (http://data.cma.cn/).Statistical analysis
We first conducted a descriptive analysis to summarize the general distribution for hospital outpatient visits, air pollutants and meteorological factors. Spearman's correlation coefficients were used to evaluate the inter-relations between air pollutants and meteorological factors.A time-stratified case-crossover design23 was used to assess the associations between each air pollutant and outpatient visits for eczema and dermatitis. The case-crossover design has been widely used for estimating acute effects of air pollution on health outcomes.24 In this design, exposure in the case period when events occurred is compared with exposures in nearby control periods, to examine the differences in the exposure level and further to explain the differences in the daily number of cases due to the exposure factor. The influence of confounders related to individual characteristics, e.g. sex and smoking, can be controlled because each subject serves as his or her own control.25 Bias from the season and day of the week can also be successfully controlled by this design. In this study, we matched the cases and controls by day of the week within the same month and year to control for any weekly patterns. For example, for a case on the second Thursday of April 2014 (April 8, 2014), all other Thursdays within April 2014 were assigned as controls (April 1, 15, 22 and 29, 2014). This approach resulted in three or four controls.
To examine the hazard period of air pollution for eczema and dermatitis, we examined the effect of air pollutants with different lag structures: single-day lag from lag 0 to lag 4 and multi-day lag from lag 01 to lag 04. In single-day lag models, a lag of 0 day (lag 0) corresponds to the current-day pollutant concentration, while in multi-day lag models, lag 04 corresponds to 5 day moving average of pollutant concentration of the current and previous 4 days.
We fitted single pollutant and two pollutant models to assess the stability of pollutants' effect. Although individual pollutants were moderately or highly correlated, two pollutant models were widely used for sensitivity analyses in previous studies.26–28 And due to the result of two pollutant models, we did not make further sensitivity analysis, for example, using a composite air quality index or air quality health index29 or by transforming the pollutant by using cubic time.30
To assess the seasonal pattern of associations, we conducted analyses specific to the warm season (May to October) and cold season (November to next April), respectively. We introduced the interaction term of air pollutants and seasons into the model and further calculated the respective P value for interaction (Pint) in order to see whether the association between air pollutants and outpatient visits is significantly different across different seasons. We stratified the analyses by age group (≤18 years, 18–45 years, 45–65 years and >65 years) and sex (males and females). Daily data on mean temperature and relative humidity were included in all models as confounders, using the spline with 4 degrees of freedom.27. We adjusted for temperature and relative humidity with the same lag structures as pollutants. The percent increase and confidence interval (CI) in outpatient visits for eczema and dermatitis for per interquartile range (IQR) increase in air pollutant concentrations were calculated. All statistical tests were two-sided.
We further analyzed the effect of consecutive days' exposure to high concentration air pollution on outpatient visits for eczema and dermatitis. We created a categorical variable (CTM) which refers to consecutive days with high concentration air pollution. According to China's Air Quality Standards Grade II,31 we chose 75 μg m−3 for PM2.5, 150 μg m−3 for PM10, 80 μg m−3 for NO2 and 150 μg m−3 for SO2 as the reference pollution concentration, and estimated the percent increase in outpatient visits on different duration days. For example, for PM2.5, when the daily PM2.5 concentrations were lower than or equal to 75 μg m−3 on a given day, the CTM value for this day was denoted as “REF” (reference). On the first day of exceeding the reference concentration (75 μg m−3), the CTM value was denoted as “Day 1” while on the second consecutive day, it was denoted as “Day 2” and so on (an example is shown in ESI Table 1†).
The percent increase in outpatient visits was determined using the following formula. The odds ratio (OR)32 refers to the odds that an outpatient visit for eczema and dermatitis will occur given a particular exposure (per interquartile range (IQR) increase in air pollutant concentrations), compared to the odds of the visit occurring in the absence of that exposure.
| Percent increase = (OR − 1) × 100% | (1) |
The “season” package of R (version 3.4.2) was used to fit the time-stratified case-crossover33 and the “dlnm” package was used to fit the spline in the time-stratified case-crossover design.34
Results
Characteristics of subjects, air pollutants and meteorological conditions
A total of 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 595 outpatient visits for eczema and dermatitis were recorded from April 1, 2012 to April 30, 2014. The mean age of these patients was 41.5 ± 20.3 years, and most patients were males (57.8%) (Table 1).
595 outpatient visits for eczema and dermatitis were recorded from April 1, 2012 to April 30, 2014. The mean age of these patients was 41.5 ± 20.3 years, and most patients were males (57.8%) (Table 1).
| Variables |
N =157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 595 595 |
|
|---|---|---|
| a SD: standard deviation. | ||
| Age [years ± SD] | 42.5 ± 20.3 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Sex | ||
| Male (N%) | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 447 447 |
42.2% |
| Female (N%) | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144 144 |
57.8% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Season | ||
| Warm season (N%) | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 619 619 |
49.9% |
| Cold season (N%) | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 976 976 |
50.1% |
The descriptive statistics for air pollutants and meteorological conditions are shown in Table 2. The interquartile range (IQR) values of PM2.5, PM10, NO2 and SO2 were 70.8 μg m−3, 80.4 μg m−3, 27.4 μg m−3 and 28.4 μg m−3, respectively. The average temperature and relative humidity were 13.5 °C and 53.5%, respectively.
| Min | P25 | Median | P75 | Max | IQR | Mean | SD | |
|---|---|---|---|---|---|---|---|---|
| a P25 and P75 represent the percentage; Min: minimal; Max: maximal; IQR: the interquartile range; SD: standard deviation; PM2.5: particulate matter less than 2.5 μm in aerodynamic diameter; PM10: particulate matter less than 10 μm in aerodynamic diameter; SO2: sulfur dioxide; NO2: nitrogen dioxide; RH: relative humidity. | ||||||||
| PM2.5 (μg m−3) | 3.0 | 43.8 | 72.2 | 114.6 | 397.3 | 70.8 | 87.4 | 63.0 |
| PM10 (μg m−3) | 10.4 | 66.7 | 101.2 | 147.2 | 612.2 | 80.4 | 116.6 | 74.5 |
| NO2 (μg m−3) | 7.8 | 37.8 | 48.5 | 65.2 | 154.7 | 27.4 | 53.1 | 22.4 |
| SO2 (μg m−3) | 3.5 | 9.4 | 18.1 | 37.7 | 176.6 | 28.4 | 27.1 | 24.1 |
| Temperature (°C) | −9.7 | 3.5 | 15.9 | 23.4 | 31.7 | 19.9 | 13.5 | 11.1 |
| RH (%) | 9.0 | 38.0 | 54.0 | 69.0 | 97.0 | 31.0 | 53.5 | 19.6 |
ESI Fig. 1† shows the time series of air pollutants and outpatient visits for eczema and dermatitis. The concentrations of all air pollutants were highest in winter. The number of outpatient visits for eczema and dermatitis did not show a significant seasonal trend.
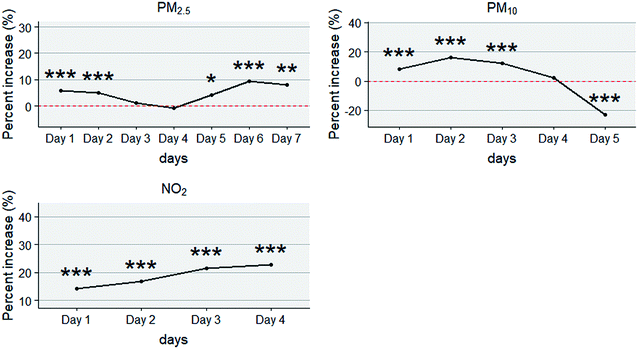
Fig. 1 presents the summary statistics for consecutive days on which residents were exposed to high concentration air pollution. The histogram showed the number of days on which air pollutants concentrations were higher than the reference concentrations. The red lines showed the average values of daily pollutant concentrations on different duration days (RER, Day 1, Day 2…). The longest period with PM2.5 over 75 μg m−3 lasted for 9 days, with PM10 over 150 μg m−3 lasted for 7 days and with NO2 over 80 μg m−3 lasted for 6 days. During the study period, SO2 over 150 μg m−3 lasted for only 1 day. Only durations (consecutive days) with frequency over 5 were calculated in the further analysis (PM2.5: Day 1 to Day 7, PM10: Day 1 to Day 5, and NO2: Day 1 to Day 4).
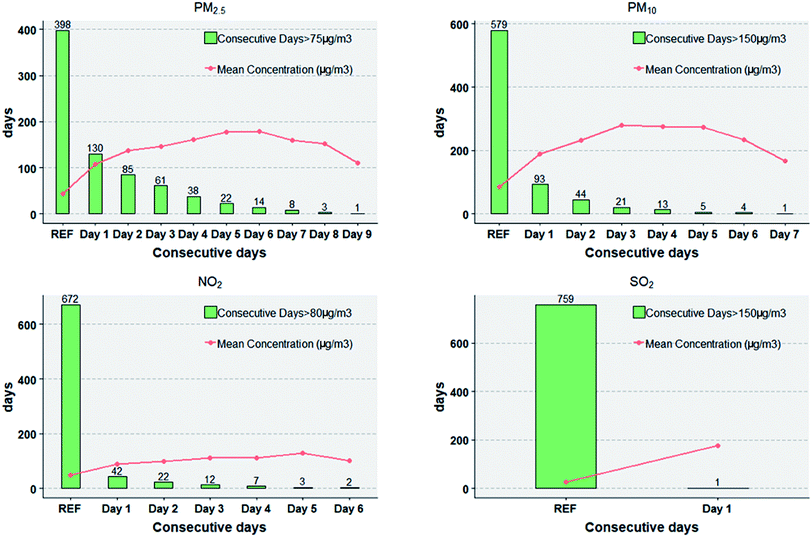 | ||
| Fig. 1 Consecutive days of exposure to high concentration air pollution and the daily mean concentrations on different duration days during the period of April 1, 2012 to April 30, 2014 in Beijing. | ||
Spearman correlation analysis for the correlation between air pollutants and meteorological factors
The correlation between air pollutants and meteorological factors is shown in ESI Table 2.† PM2.5 was positively correlated with PM10, NO2, SO2, and relative humidity (the correlation coefficients were 0.84, 0.77, 0.65, and 0.40, respectively, P < 0.05), and negatively correlated with temperature (the correlation coefficient was −0.23, P < 0.05).The association between air pollutants and outpatient visits for eczema
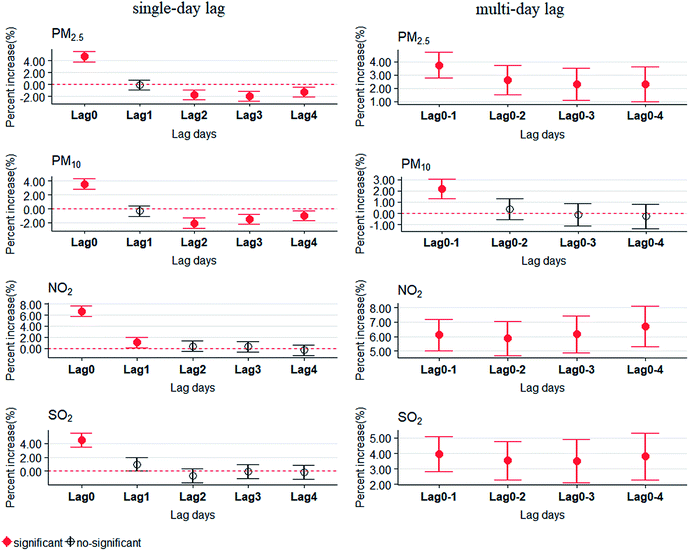
Fig. 2 shows the percentage increase in daily outpatient visits for eczema and dermatitis for per IQR increase in air pollutants at different lag structures. All pollutants showed the strongest and significant positive associations with eczema and dermatitis outpatient visits on lag 0. Thus, we chose lag 0 for all pollutants as the hazard period in the following further analysis. In addition, for PM2.5, NO2 and SO2, significant positive associations were observed from lag 0–1 to lag 0–4. For PM2.5 and PM10, significant harvesting effects were observed from lag 2 to lag 4, with the percent increase lower than 0.35Table 3 shows the percent increase in daily outpatient visits for eczema and dermatitis for per IQR increase in air pollutants in single pollutant and two pollutants models on lag 0. In single pollutant models, per IQR increases in PM2.5, PM10, NO2 and SO2 were associated with 4.72% (95% CI: 3.88–5.56%), 3.53% (95% CI: 2.78–4.28%), 6.69% (95% CI: 5.75–7.63%) and 4.50% (95% CI: 3.50–5.49%) increases in outpatient visits for eczema and dermatitis. Consistently significant positive associations were found in two pollutant models, but the effects were lower than those in the single pollutant models. For example, after controlling SO2, per IQR increase in PM2.5 was associated with a 1.65% (95% CI: 0.57–2.73%) increase in outpatient visits for eczema and dermatitis.
| Air pollutant | Percent increase (%) (95% CI) | Air pollutant | Percent increase (%) (95% CI) |
|---|---|---|---|
| a ***P < 0.001; **P < 0.01; *P < 0.05; IQR: the interquartile range; 95% CI: confidence interval. | |||
| PM2.5 | 4.72(3.88–5.56)*** | PM10 | 3.53(2.78–4.28)*** |
| +SO2 | 1.65(0.57–2.73)** | +SO2 | −0.33(−1.4–0.73) |
| +NO2 | 3.75(2.73–4.77)*** | +NO2 | 2.33(1.38–3.28)*** |
| NO2 | 6.69(5.75–7.63)*** | SO2 | 4.5(3.5–5.49)*** |
| +SO2 | 7.4(6.06–8.74)*** | +NO2 | −0.98(−2.4–0.44) |
| +PM2.5 | 5.48(4.28–6.68)*** | +PM2.5 | 1.97(0.76–3.17)** |
| +PM10 | 7(5.68–8.32)*** | +PM10 | 2.59(1.34–3.84)*** |
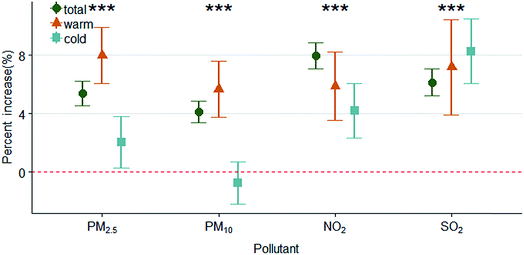
Fig. 3 and ESI Table 3† show the seasonal patterns of the associations between air pollutants and daily outpatient visits for eczema and dermatitis. The associations during the warm season period (May to October) and cold season period (November to next April) showed a significant difference (Pint < 0.001). The associations of outpatient visits for eczema and dermatitis with PM2.5, PM10 and NO2 were stronger during the warm season. Significant associations for PM10 were observed only in the warm season.
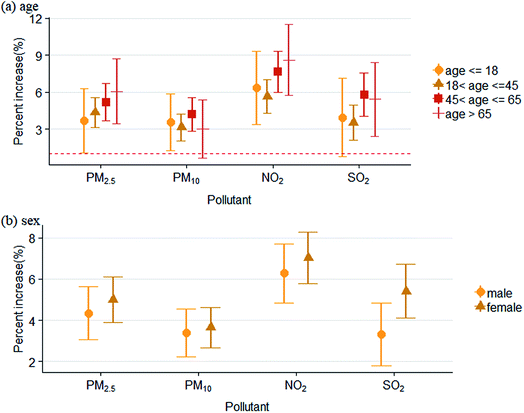
For different age groups, associations of air pollutants with outpatient visits for eczema and dermatitis were stronger among people older than 65 years old and 45 to 65 years old. The figure also shows stronger associations among females (Fig. 4).
The association between consecutive days' high concentration air pollution and outpatient visits for eczema
Fig. 5 presents the percent increase in outpatient visits for eczema and dermatitis in terms of high concentration air pollution over consecutive days. The horizontal axis stands for the number of consecutive days with pollutant concentration higher than the threshold, while the vertical axis represents the percent increase in outpatient visits caused by various durations.It can be observed that for PM2.5, a significant increase (P < 0.001) in outpatient visits for eczema and dermatitis was found on Day 1, Day 2 and from Day 5 to Day 7, peaking on Day 6 with a number of 9.29% (95% CI: 4.98–13.6%) (ESI Table 4†). For PM10, a significant increase in outpatient visits was found from Day 1 to Day 3, peaking on Day 2 with a number of 16.21% (95% CI: 13.77–18.66%). On the fifth day, there was a clear harvesting effect, with the percent increase lower than 0.35 For NO2, the increase in outpatient visits was significant and kept growing from Day 1 to Day 4, with the largest increase (22.66% (95% CI: 16.47–28.85%)) on Day 4.
Sensitivity analysis
ESI Fig. 2† shows the effects of air pollutants in two pollutant models from lag 0 to lag 4. The effects of PM (PM2.5 and PM10) from lag 1 to lag 4 did not change much in two-pollutant models compared to the effects in single-pollutant models. After adjusting PMs in the models, the effects of NO2 and SO2 from lag 1 to lag 3 were larger, but effects on lag 0 were still the largest.Discussion
In this study, a time-stratified case-crossover design was used to analyze the impact of air pollution on outpatient visits for eczema and dermatitis in Beijing, China. We analyzed 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 595 visits for eczema and dermatitis over a 2 year and 1 month period. We reported here that in both single and two pollutant models, the increase in PM2.5, NO2 and SO2 concentrations was significantly associated with an increase in the number of outpatient visits for eczema and dermatitis. The uniqueness of this study was the use of continuous high exposure days to study the effects of continuous exposure to high concentration air pollution on eczema and dermatitis. Our results contributed to limited scientific evidence that ambient air pollution may be detrimental to skin health and aggravate preexisting skin diseases.
595 visits for eczema and dermatitis over a 2 year and 1 month period. We reported here that in both single and two pollutant models, the increase in PM2.5, NO2 and SO2 concentrations was significantly associated with an increase in the number of outpatient visits for eczema and dermatitis. The uniqueness of this study was the use of continuous high exposure days to study the effects of continuous exposure to high concentration air pollution on eczema and dermatitis. Our results contributed to limited scientific evidence that ambient air pollution may be detrimental to skin health and aggravate preexisting skin diseases.
It was indicated that air pollutants were positively associated with the exacerbation of eczema and dermatitis and the results were consistent with previous studies conducted in the south east and south west of China.21,22 For example, a study in Shanghai showed that a 10 μg m−3 increase of 7 day (lag 06) average concentrations of PM10, SO2, and NO2 was associated with 0.81% (95% CI: 0.39%, 1.22%), 2.22% (95% CI: 1.27%, 3.16%) and 2.31% (95% CI: 1.17%, 3.45%) increases in outpatient visits for eczema, respectively.21 Eczema and dermatitis are chronic and easy to relapse. Either a new case, or a relapse or aggravation of previous patients can increase the number of outpatient visits for eczema and dermatitis. It's difficult to identify the initial onset time of eczema and dermatitis, and thus it is unlikely to estimate the association of air pollution with the incidence rate in this study. However, our results still indicated that increases in the concentration of air pollutants were associated with the exacerbation of eczema and dermatitis and an increased burden of medical care.
Current scientific evidence suggests that there are four mechanisms by which ambient air pollutants cause adverse effects on skin health: (a) oxidative stress, (b) alterations of microflora, (c) activation of the aryl hydrocarbon receptor (AhR), and (d) induction of the inflammatory cascade and subsequent impairment of the skin barrier.19,36–38 Air pollutants are known to enhance the production of reactive oxygen species (ROS), which reduces the content of antioxidants in the skin. This causes disturbance in the redox balance, causing oxidative stress and major damage to the skin cells.37,39–41 The negative impact of air pollutants on skin microflora can cause colonization of the stratum corneum with pathogenic strains of bacteria.19,37,42 For example, pollutant particles settle on the skin, blocking pores and therefore creating an anaerobic environment—ideal conditions for the growth of Propionibacterium acnes, the main bacteria strain responsible for acne.40 Some pollutants also tend to permeate through the stratum corneum into deeper skin layers and act as a ligand for the AhR activation, which has recently been discovered to play a key role in cutaneous mechanisms ranging from the regulation of melanogenesis to the development of inflammatory skin lesions.38,43 Air pollution induces the production of pro-inflammatory cytokines, such as interleukin 1β or interleukin 8, in the epidermis, which may alter epidermal differentiation and consequently affect the immunological barrier of the skin.38,44 For example, several studies have suggested that diesel-exhaust particles (DEPs) could induce a strong inflammatory response in human skin cells, including a significant increase in IL-8 production.44,45 In addition, pollution also causes a negative impact on other skin properties, which are necessary for proper skin function and health, such as changes of the composition of skin lipids, lactic acid content, etc.37,46 In addition to the above, there are some mechanisms that are less well known but worth considering and exploring, such as nitrosative stress,47,48 increased pH49 and reduced buffering capacities.50
Extremely short lag periods were found between exposure to air pollution and increased outpatient visits for eczema and dermatitis in this study, with the strongest associations on the current day (lag 0). The effects of short lag periods of pollutants on skin conditions were also found in a panel study in Korea, which suggested a 10 unit increase in PM10 (μg m−3) and NO2 (ppb, 18.8 μg m−3 converted at 25 °C and 1013 mb) increased the risk of eczema symptoms on the same day by 3.2% (95% CI: 1.5%, 4.9%) and 5.0% (95% CI: 1.4%, 8.8%), respectively.51 The results in the present study, combined with previous studies, suggested an acute effect of air pollution on eczema and dermatitis. Further studies on the effect of periods and lag periods are needed.
In the two pollutant models, the effects of air pollutants were attenuated compared to effects in single pollutant models. These results were consistent with previous research applying multi-pollutant models to air pollution and outpatient visits for eczema in Shanghai.28 It was noted that focusing on individual pollutants as the single risk factor is likely not to reflect the effect of combined exposure to multiple air pollutants, and it could be the mixture that represents a higher risk than the individual components.52 These results may suggest that synergistic effects among air pollutants exist and need to be further explored. In addition, co-linearity between air pollutants (r ≥ 0.5 between each other) was one factor that should not be neglected in a two-pollutant model. In order to communicate the air pollution level to the public more simply and clearly, composite measure (air quality index or air quality health index) should be considered for future studies.18,29 In addition, source apportionment analysis should also be considered because the chemical component of particle pollutants (PM2.5 and PM10) might be responsible for the adverse health effects.52
We observed that the associations of pollutants with eczema and dermatitis varied in different seasons. For most pollutants, the effects were stronger in the warm season. One explanation is that the increased cutaneous blood flow due to vasodilation in hot environments allows more pollutants to enter the systemic circulation and consequently results in increased toxicity of pollutants.53 Another explanation is that lighter clothing and more time outdoors on hot days increase skin's exposure areas to air pollutants.54,55 Few previous studies reported that the effects of air pollution on eczema and dermatitis could be modified by weather conditions but showed conflicting results. One study in the USA showed that significantly higher eczema prevalence existed in areas simultaneously with hot, humid, and rainy climate and high SO2, SO3, and PM2.5.56 A recent study in Korea showed that the risks of atopic dermatitis symptoms caused by PM2.5 and PM10 exposure were significantly increased on dry moderate days, which were characterized by mild, dry weather conditions.57,58 A study in Shanghai showed that effect estimates of pollutants in the warm season were much higher than in the cool season.23 Considering the diverse demographic, economic and climatic characteristics of the study areas, weather conditions and air pollution levels likely vary in different regions, contributing to differences in the direction and magnitude of the modification effects of seasonal factors on air pollution.
In this study, stronger associations of air pollutants with outpatient visits for eczema and dermatitis were found among older people and females. A study in the US59,60 showed that eczema prevalence in adults was significantly higher in older participants (62–85 years) compared with younger participants (18–32 years, 11.4% vs. 9.0%). Several studies in the US and Italy both showed that eczema was more highly prevalent in adult women than men.59,61 Our result suggested that elderly people and females might be more vulnerable to air pollutants. Studies on risk factors of eczema and dermatitis have been mostly performed in children, so more studies on adult populations are needed.
In the present study, we estimated the effects of consecutive exposure to high concentration air pollution on outpatients for eczema and dermatitis. A key finding was that when high level air pollution occurred continuously, the adverse effects on eczema and dermatitis were significantly larger than those on reference days. And for PM2.5 and NO2, the longer the air pollution lasted, the greater the risk was. As an international metropolis with a population of more than 20 million, Beijing's energy consumption and pollution emissions are increasing year by year. In recent years, there has been a frequent occurrence of continuous air pollution. For example, in 2014, Beijing residents suffered several serious pollution problems (daily average PM2.5 concentration ≥ 200 μg m−3) for more than three consecutive days, and one of them even lasted for 7 days.62 The problem of continuous air pollution has caused widespread concern among citizens, governments and scholars. Very few studies have specifically focused on the effect of continuous high concentration air pollution on eczema and dermatitis, so it is difficult to compare our results with previous studies. We used 75 μg m−3 for PM2.5, 150 μg m−3 for PM10, 80 μg m−3 for NO2 and 150 μg m−3 for SO2 as the reference pollution concentrations in the present study; however, the WHO 2005 guideline values are 25 μg m−3 for PM2.5, 50 μg m−3 for PM10, 40 μg m−3 for NO2 and 20 μg m−3 for SO2.63 Most days, the air quality in Beijing was categorized as “unhealthy” or worse if considering the WHO guideline values as references. We infer that when air pollution occurs continuously, the adverse effects vary at varying air pollution thresholds and different durations. So further studies about the impact of continuous air pollution under various combinations of thresholds and duration combinations are needed. Based on these results, we suggested that susceptible populations and patients of eczema and dermatitis should consider not only the concentration of air pollution but also the duration when taking precautions against air pollution.
The time-stratified case-crossover design has the ability to control many confounders by design, such as age, sex, smoking, drinking and so on. By matching on the day of the week, we avoid confounding due to generally higher pollutant levels on weekdays. The case-crossover also successfully controls seasonal patterns and long-term trends.26,27. This study was based on a large database in Beijing, China, including a period of 2 years and 1 month. The dermatology department of PLA is one of the largest and most famous dermatology departments among hospitals in Beijing. Although the hospital selection bias might exist, this hospital has good representativeness on the trend of outpatient visits of the whole city. In addition, all patients lived in a highly polluted metropolitan area with well documented air pollutant levels, which offered sufficient opportunities to explore the effects of severe and continuous air pollution on skin conditions.
This study also has some limitations. The cases were only selected from one hospital. Although the patients lived in the same city, it still cannot control their hospital selection. Outdoor average concentrations of PM2.5, PM10, NO2 and SO2 were collected from fixed sites, but the data on individual exposure were unavailable. We have no information about the severity of eczema and dermatitis and socioeconomic factors for each single outpatient visit. Despite this lack of information, we were able to observe the significant observations described above.
Conclusion
In conclusion, this study provides further evidence that short-term exposure to air pollution increases the exacerbation of eczema and dermatitis, as indicated by the increased number of outpatient visits for eczema and dermatitis. Associations of air pollutants with outpatient visits for eczema and dermatitis varied with the seasons and were stronger among older people and females. When high concentration air pollution occurs continuously, the adverse effects on eczema and dermatitis would be larger.Statement of ethics
This study was carried out by analyzing data from the HIS system of the General Hospital of Air Force of People's Liberation Army (PLA) in Beijing, China. Patients were not contacted individually for consent. The study was approved by the ethics committee of the hospital.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Department of Dermatology, the General Hospital of Air Force of People's Liberation Army (PLA) for providing the health data; we also thank the Beijing Meteorological Bureau and environmental monitoring center for providing meteorological and environmental data. This work was supported by the National Natural Science Foundation of China (grant numbers 81573030) and the Chinese Medical Association-Vichy research project (V2015110734).References
- I. A. Deckers, S. McLean, S. Linssen, M. Mommers, C. P. van Schayck and A. Sheikh, Investigating international time trends in the incidence and prevalence of atopic eczema 1990-2010: a systematic review of epidemiological studies, PLoS One, 2012, 7, e39803 CrossRef CAS PubMed.
- S. Nutten, Atopic dermatitis: global epidemiology and risk factors, Ann. Nutr. Metab., 2015, 66(suppl. 1), 8–16 CrossRef CAS PubMed.
- C. Karimkhani, R. P. Dellavalle, L. E. Coffeng, C. Flohr, R. J. Hay, S. M. Langan, E. O. Nsoesie, A. J. Ferrari, H. E. Erskine, J. I. Silverberg, T. Vos and M. Naghavi, Global Skin Disease Morbidity and Mortality: An Update From the Global Burden of Disease Study 2013, JAMA Dermatol., 2017 DOI:10.1001/jamadermatol.2016.5538.
- E. Davies, N. K. Rogers, A. Lloyd-Lavery, D. J. C. Grindlay and K. S. Thomas, What's new in atopic eczema? An analysis of systematic reviews published in 2015. Part 1: epidemiology and methodology, Clin. Exp. Dermatol., 2018 DOI:10.1111/ced.13377.
- R. Kantor and J. I. Silverberg, Environmental risk factors and their role in the management of atopic dermatitis, Expert Rev. Clin. Immunol., 2017, 13, 15–26 CrossRef CAS PubMed.
- C. Blome, M. A. Radtke, L. Eissing and M. Augustin, Quality of Life in Patients with Atopic Dermatitis: Disease Burden, Measurement, and Treatment Benefit, Am. J. Clin. Dermatol., 2016, 17, 163–169 CrossRef PubMed.
- K. Kabashima, A. Otsuka and T. Nomura, Linking air pollution to atopic dermatitis, Nat. Immunol., 2016, 18, 5–6 CrossRef PubMed.
- L. T. N. Ngoc, D. Park, Y. Lee and Y. C. Lee, Systematic Review and Meta-Analysis of Human Skin Diseases Due to Particulate Matter, Int. J. Environ. Res. Public Health, 2017, 14 Search PubMed.
- C. Lu, L. Deng, C. Ou, H. Yuan, X. Chen and Q. Deng, Preconceptional and perinatal exposure to traffic-related air pollution and eczema in preschool children, J. Dermatol. Sci., 2017, 85, 85–95 CrossRef CAS PubMed.
- W. Jedrychowski, F. Perera, U. Maugeri, D. Mrozek-Budzyn, R. L. Miller, E. Flak, E. Mroz, R. Jacek and J. D. Spengler, Effects of Prenatal and Perinatal Exposure to Fine Air Pollutants and Maternal Fish Consumption on the Occurrence of Infantile Eczema, Int. Arch. Allergy Appl. Immunol., 2011, 155, 275–281 CrossRef CAS PubMed.
- Q. Deng, C. Lu, Y. Li, J. Sundell and N. Dan, Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema, Environ. Res., 2016, 150, 119–127 CrossRef CAS PubMed.
- I. Aguilera, M. Pedersen, R. Garcia-Esteban, F. Ballester, M. Basterrechea, A. Esplugues, A. Fernandez-Somoano, A. Lertxundi, A. Tardon and J. Sunyer, Early-life exposure to outdoor air pollution and respiratory health, ear infections, and eczema in infants from the INMA study, Environ. Health Perspect., 2013, 121, 387–392 CrossRef PubMed.
- S. Song, K. Lee, Y. M. Lee, J. H. Lee, S. I. Lee, S. D. Yu and D. Paek, Acute health effects of urban fine and ultrafine particles on children with atopic dermatitis, Environ. Res., 2011, 111, 394–399 CrossRef CAS PubMed.
- J. Kim, E.-H. Kim, I. Oh, K. Jung, Y. Han, H.-K. Cheong and K. Ahn, Symptoms of atopic dermatitis are influenced by outdoor air pollution, J. Allergy Clin. Immunol., 2013, 132, 495–498 CrossRef CAS PubMed.
- G. Dhadwal, L. Albrecht, R. Gniadecki, Y. Poulin, J. Yeung, C. H. Hong and M. J. Gooderham, Approach to the Assessment and Management of Adult Patients With Atopic Dermatitis: A Consensus Document. Section IV: Treatment Options for the Management of Atopic Dermatitis, J. Cutaneous Med. Surg., 2018, 22, 21s–29s CrossRef PubMed.
- S. Chow, C. S. Seow, M. V. Dizon, K. Godse, H. Foong, V. Chan, T. H. Khang, L. Xiang, S. Hidayat, M. Y. Listiawan, D. Triwahyudi, S. P. Gondokaryono, E. Sutedja, I. A. Diana, O. Suwarsa, H. P. Dharmadji, A. S. Siswati, R. Danarti, R. Soebaryo and W. K. Budianti, A clinician's reference guide for the management of atopic dermatitis in Asians, Asia Pacific Allergy, 2018, 8, e41 CrossRef PubMed.
- F. J. de Leon, L. Berbegal and J. F. Silvestre, Management of Chronic Hand Eczema, Actas Dermo-Sifiliogr., 2015, 106, 533–544 CrossRef CAS PubMed.
- T. Kousha and G. Valacchi, The air quality health index and emergency department visits for urticaria in Windsor, Canada, J. Toxicol. Environ. Health, Part A, 2015, 78, 524–533 CrossRef CAS PubMed.
- M. Szyszkowicz, T. Kousha and G. Valacchi, Ambient air pollution and emergency department visits for skin conditions, Global Dermatology, 2016, 3, 323–329 CrossRef.
- M. Szyszkowicz, E. Porada, G. Searles and B. H. Rowe, Ambient ozone and emergency department visits for skin conditions, Air Qual., Atmos. Health, 2012, 5, 303–309 CrossRef CAS.
- Q. Li, Y. Yang, R. Chen, H. Kan, W. Song, J. Tan, F. Xu and J. Xu, Ambient Air Pollution, Meteorological Factors and Outpatient Visits for Eczema in Shanghai, China: A Time-Series Analysis, Int. J. Environ. Res. Public Health, 2016, 13(11), 1106 CrossRef PubMed.
- A. Li, L. Fan, L. Xie, Y. Ren and L. Li, Associations between air pollution, climate factors and outpatient visits for eczema in West China Hospital, Chengdu, south-western China: a time series analysis, J. Eur. Acad. Dermatol. Venereol., 2017 DOI:10.1111/jdv.14730.
- H. Janes, L. Sheppard and T. Lumley, Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias, Epidemiology, 2005, 16, 717–726 CrossRef PubMed.
- E. Carracedo-Martinez, M. Taracido, A. Tobias, M. Saez and A. Figueiras, Case-crossover analysis of air pollution health effects: a systematic review of methodology and application, Environ. Health Perspect., 2010, 118, 1173–1182 CrossRef PubMed.
- M. Maclure, The Case-Crossover Design: A Method for Studying Transient Effects on the Risk of Acute Events, Am. J. Epidemiol., 1991, 133, 144–153 CrossRef CAS PubMed.
- Y. Guo, S. Tong, Y. Zhang, A. G. Barnett, Y. Jia and X. Pan, The relationship between particulate air pollution and emergency hospital visits for hypertension in Beijing, China, Sci. Total Environ., 2010, 408, 4446–4450 CrossRef CAS PubMed.
- Y. Guo, S. Tong, S. Li, A. G. Barnett, W. Yu, Y. Zhang and X. Pan, Gaseous air pollution and emergency hospital visits for hypertension in Beijing, China: a time-stratified case-crossover study, Environ. Health, 2010, 9, 57 CrossRef PubMed.
- W. Liu, X. Pan, A. Vierkotter, Q. Guo, X. Wang, Q. Wang, S. Seite, D. Moyal, T. Schikowski and J. Krutmann, A Time-Series Study of the Effect of Air Pollution on Outpatient Visits for Acne Vulgaris in Beijing, Skin Pharmacol. Physiol., 2018, 31, 107–113 CrossRef CAS PubMed.
- T. Kousha and J. Castner, The Air Quality Health Index and Emergency Department Visits for Otitis Media, J. Nurs. Scholarship, 2016, 48, 163–171 CrossRef PubMed.
- J. Castner, L. Guo and Y. Yin, Ambient air pollution and emergency department visits for asthma in Erie County, New York 2007-2012, Int. Arch. Occup. Environ. Health, 2018, 91, 205–214 CrossRef CAS PubMed.
- China: Air Quality Standards, https://www.transportpolicy.net/standard/china-air-quality-standards/.
- M. Szumilas, Explaining odds ratios, J. Can. Acad. Child. Adolesc. Psychiatry, 2010, 19, 227–229 CrossRef PubMed.
- A. G. Barnett and A. J. Dobson, Analysing Seasonal Health Data, Springer, Berlin, Heidelberg, 2010, DOI:10.1007/978-3-642-10748-1.
- B. Armstrong, Models for the Relationship Between Ambient Temperature and Daily Mortality, 2006 Search PubMed.
- P. Kouis, M. Kakkoura, K. Ziogas, A. Paschalidou and S. I. Papatheodorou, The effect of ambient air temperature on cardiovascular and respiratory mortality in Thessaloniki, Greece, Sci. Total Environ., 2019, 647, 1351–1358 CrossRef CAS PubMed.
- K. E. Burke, Mechanisms of aging and development—A new understanding of environmental damage to the skin and prevention with topical antioxidants, Mechanisms of Ageing and Development, 2017, DOI:10.1016/j.mad.2017.12.003.
- J. Rembiesa, T. Ruzgas, J. Engblom and A. Holefors, The Impact of Pollution on Skin and Proper Efficacy Testing for Anti-Pollution Claims, Cosmetics, 2018, 5, 4 CrossRef.
- S. E. Mancebo and S. Q. Wang, Recognizing the impact of ambient air pollution on skin health, J. Eur. Acad. Dermatol. Venereol., 2015, 29, 2326–2332 CrossRef CAS PubMed.
- P. Puri, S. K. Nandar, S. Kathuria and V. Ramesh, Effects of air pollution on the skin: A review, Indian J. Dermatol. Venereol., 2017, 83, 415–423 CrossRef PubMed.
- J. Krutmann, D. Moyal, W. Liu, S. Kandahari, G.-S. Lee, N. Nopadon, L. F. Xiang and S. Seité, Pollution and acne: is there a link?, Clin., Cosmet. Invest. Dermatol., 2017, 10, 199–204 CrossRef CAS PubMed.
- K. Ahn, The role of air pollutants in atopic dermatitis, J. Allergy Clin. Immunol., 2014, 134, 993–999 CrossRef CAS PubMed.
- J. H. Jo, E. A. Kennedy and H. H. Kong, Topographical and physiological differences of the skin mycobiome in health and disease, Virulence, 2017, 8, 324–333 CrossRef PubMed.
- H. O. Kim, J. H. Kim, B. Y. Chung, M. G. Choi and C. W. Park, Increased expression of the aryl hydrocarbon receptor in patients with chronic inflammatory skin diseases, Exp. Dermatol., 2014, 23, 278–281 CrossRef CAS PubMed.
- H. Ushio, K. Nohara and H. Fujimaki, Effect of environmental pollutants on the production of pro-inflammatory cytokines by normal human dermal keratinocytes, Toxicol. Lett., 1999, 105, 17–24 CrossRef CAS PubMed.
- C. Ma, J. Wang and J. Luo, Activation of nuclear factor kappa B by diesel exhaust particles in mouse epidermal cells through phosphatidylinositol 3-kinase/Akt signaling pathway, Biochem. Pharmacol., 2004, 67, 1975–1983 CrossRef CAS PubMed.
- M. A. Lefebvre, D. M. Pham, B. Boussouira, D. Bernard, C. Camus and Q. L. Nguyen, Evaluation of the impact of urban pollution on the quality of skin: a multicentre study in Mexico, Int. J. Cosmet. Sci., 2015, 37, 329–338 CrossRef CAS PubMed.
- F. Dilek, D. Ozceker, E. Ozkaya, E. M. Guler, M. Yazici, Z. Tamay, A. Kocyigit and N. Guler, Elevated Nitrosative Stress in Children with Chronic Spontaneous Urticaria, Int. Arch. Allergy Appl. Immunol., 2017, 172, 33–39 CrossRef CAS PubMed.
- E. B. Kurutas and P. Ozturk, The evaluation of local oxidative/nitrosative stress in patients with pityriasis versicolor: a preliminary study, Mycoses, 2016, 59, 720–725 CrossRef CAS PubMed.
- S. G. Danby and M. J. Cork, pH in Atopic Dermatitis, Current problems in dermatology, 2018, vol. 54, pp. 95–107 Search PubMed.
- pH of the Skin: Issues and Challenges, Curr. Probl. Dermatol., ed. C. Surber, C. Abels, H. Maibach, Karger, Basel, 2018, vol. 54, pp. 11–18, DOI:10.1159/000489513.
- Y. M. Kim, J. Kim, Y. Han, B. H. Jeon, H. K. Cheong and K. Ahn, Short-term effects of weather and air pollution on atopic dermatitis symptoms in children: a panel study in Korea, PLoS One, 2017, 12, e0175229 CrossRef PubMed.
- T. J. Woodruff, J. D. Parker, L. A. Darrow, R. Slama, M. L. Bell, H. Choi, S. Glinianaia, K. J. Hoggatt, C. J. Karr, D. T. Lobdell and M. Wilhelm, Methodological issues in studies of air pollution and reproductive health, Environ. Res., 2009, 109, 311–320 CrossRef CAS PubMed.
- S. Ständer and M. Steinhoff, Pathophysiology of pruritus in atopic dermatitis: an overview, Exp. Dermatol., 2002, 11, 12–24 CrossRef.
- J. I. Silverberg, J. Hanifin and E. L. Simpson, Climatic Factors Are Associated with Childhood Eczema Prevalence in the United States, J. Invest. Dermatol., 2013, 133, 1752–1759 CrossRef CAS PubMed.
- E. Vocks, R. Busch, C. Fröhlich, S. Borelli, H. Mayer and J. Ring, Influence of weather and climate on subjective symptom intensity in atopic eczema, Int. J. Biometeorol., 2001, 45, 27–33 CrossRef CAS PubMed.
- P. Kathuria and J. I. Silverberg, Association of pollution and climate with atopic eczema in US children, Pediatr. Allergy Immunol., 2016, 27, 478–485 CrossRef CAS PubMed.
- Y. M. Kim, J. Kim, K. Jung, S. Eo and K. Ahn, The effects of particulate matter on atopic dermatitis symptoms are influenced by weather type: Application of spatial synoptic classification (SSC), Int. J. Hyg. Environ. Health, 2018, 221, 823–829 CrossRef CAS PubMed.
- D. G. C. Rainham, K. E. Smoyer-Tomic, S. C. Sheridan and R. T. Burnett, Synoptic weather patterns and modification of the association between air pollution and human mortality, Int. J. Environ. Health Res., 2005, 15, 347–360 CrossRef PubMed.
- J. I. Silverberg and J. M. Hanifin, Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study, J. Allergy Clin. Immunol., 2013, 132, 1132–1138 CrossRef PubMed.
- J. I. Silverberg and J. M. Hanifin, Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population–based study, J. Allergy Clin. Immunol., 2013, 132, 1132–1138 CrossRef PubMed.
- G. Pesce, A. Marcon, A. Carosso, L. Antonicelli, L. Cazzoletti, M. Ferrari, A. G. Fois, P. Marchetti, M. Olivieri, P. Pirina, G. Pocetta, R. Tassinari, G. Verlato, S. Villani and R. Marco, Adult eczema in Italy: prevalence and associations with environmental factors, J. Eur. Acad. Dermatol. Venereol., 2015, 29, 1180–1187 CrossRef CAS PubMed.
- G. Gao, As smog hangs over Beijing, Chinese cite air pollution as major concern, 2015 Search PubMed.
- WHO, Ambient (outdoor) air quality and health, http://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health, 2018.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8em00494c |
| This journal is © The Royal Society of Chemistry 2019 |