Hazard assessment of quinaldine-, alkylcarbazole-, benzene- and toluene-based liquid organic hydrogen carrier (LOHCs) systems†
Marta
Markiewicz
 *ab,
Ya-Qi
Zhang
*ab,
Ya-Qi
Zhang
 ab,
Michael T.
Empl
c,
Marianna
Lykaki
ab,
Michael T.
Empl
c,
Marianna
Lykaki
 ab,
Jorg
Thöming
ab,
Jorg
Thöming
 a,
Pablo
Steinberg
c and
Stefan
Stolte
ab
a,
Pablo
Steinberg
c and
Stefan
Stolte
ab
aCentre for Environmental Science and Sustainable Technologies (UFT), University of Bremen, Bremen, Germany
bInstitute of Water Chemistry, Faculty of Environmental Sciences, Technische Universität Dresden, 01062 Dresden, Germany. E-mail: marta.markiewicz@tu-dresden.de
cInstitute for Food Toxicology, University of Veterinary Medicine Hannover, Hannover, Germany
First published on 6th December 2018
Abstract
Due to the finite nature and scarcity of crude oil-based fuels, increasing attention is being directed towards various forms of renewable energy. To obtain a fully functional renewable energy system, some compensation for the spatiotemporal fluctuations in renewable energy sources is necessary. In this context, the so-called liquid organic hydrogen carrier (LOHC) systems are promising from a technological perspective, although their impact on the environment and on animal/human health has not been considered in great detail. Hence, we present a proactive, comparative environmental hazard assessment of LOHC systems based on three alkylcarbazoles and quinaldine, including H2-rich, H2-lean and intermediate (i.e., partially hydrogenated) forms as well as on benzene and toluene, also including H2-rich forms (cyclohexane and methylcyclohexane, respectively). Specifically, the present study reports on the enzyme inhibitory (acetylcholinesterase), mutagenic (Ames test) and cytotoxic (IPC-81 cell line) activities of the above-mentioned compounds. Furthermore, the aquatic toxicity of the test compounds to marine bacteria (Vibrio fischeri), green algae (Raphidocelis subcapitata), aquatic plants (Lemna minor) and water fleas (Daphnia magna) was assessed in addition to their biodegradability, using inocula from a wastewater treatment plant. To complete the picture, we compared the effects of the LOHCs investigated herein with those of diesel oil. In this context, our results suggest that the quinaldine-based LOHC system is comparable with diesel oil in terms of ecotoxicity but is less biodegradable. The alkylcarbazoles appear to be more toxic and poorly biodegradable. Lastly, the benzene and toluene-based LOHC systems give serious reasons for concern in terms of human and aquatic toxicity. Nevertheless, due to their less complex composition, the assessment of the LOHC systems carries much lower levels of uncertainty regarding adverse effects. Additionally, because of more favourable physicochemical properties (e.g., higher boiling points), some LOHCs are safer for handling and transportation. Finally, the possibility of storing and using renewable energy offers a significant environmental and economic benefit.
Broader contextLiquid organic hydrogen carrier (LOHC) systems offer a very attractive way to store and transport hydrogen as an energy vector greatly facilitating the step-wise transition from today's fossil system to a CO2 emission-free energy supply for both stationary and mobile applications. To satisfy current world energy demand 2.1–2.9 × 1013 kg of LOHC would need to be handled, processed, stored and transported worldwide with consumers having access to rather large quantities. Consequently, the carrier might be released into the environment and the consequences of such a release are currently unknown. The LOHC-based energy storage is still a relatively new technology, requiring research and development efforts to optimise its performance to commercially attractive levels. This opens the possibility of proactively designing carriers with increased operational and environmental safety. This work presents a proactive comparative environmental hazard assessment of six LOHC systems based on quinaldine, ethylcarbazole, butylcarbazole, propylcarbazole, benzene and toluene using diesel oil as a reference. The assessment is based on mutagenicity, cytotoxicity, acute aquatic toxicity and biodegradability of each LOHC system including H2-lean, intermediate and H2-rich forms of the carriers. The results are presented in regulatory context by applying REACH and GHS screening criteria. |
Introduction
Global use of fossil fuels such as coal, oil, and natural gas has tremendously increased in recent decades to meet energy needs caused by rapid economic development. However, growing demand for energy from fossil fuels raises concerns not only regarding their limited availability but also with respect to their impact on the environment and human health. The combustion of fossil fuels is one of the biggest sources of air pollution, and the production of fossil fuels has undeniable negative environmental consequences.1 Therefore, the world's largest economies increasingly direct their attention towards renewable energy sources to reduce their dependence on fossil fuels.2–4 In theory, the solar energy available on Earth could fully cover the energy needs of our civilisation.1 In practice, even all renewable energy sources taken together cannot yet form a stable and adequate energy system due to the intermittent nature of such energy sources, i.e., spatial and temporal limitations in energy production or the inability to interconnect them into one system that would provide a stable, continuous output. Because the sun and wind cannot be controlled at will, a way to store solar or wind-derived energy, when it is present in excess, must be found for this energy to be available when it is needed. In current concepts of the future energy economy, a special place is taken by hydrogen – a clean, high energy density resource (the gravimetric energy density of H2 equals 120 MJ kg−1).5,6 Considerable efforts are being invested into optimising the process of H2 generation by water splitting using renewable energy.7 However, developing economical H2 production is only half the task because the storage and distribution of H2 are not trivial. Due to the low density of H2 (0.091 g L−1),5 its volumetric energy density is also low (10.9 kJ L−1). Therefore, any form of storage essentially includes lowering the energy content-to-volume ratio. Several methods can be applied to achieve this, including compression, liquefaction, physisorption on high surface area materials or chemisorption in the form of various hydrides.8,9 Unfortunately, all of these methods suffer from drawbacks hampering their direct implementation, including low storage capacity, high mass of the storage system, slow loading/unloading and H2 losses.10 Thus, another system based on storage of H2 chemically bound in so-called liquid organic hydrogen carriers (LOHCs) was proposed.The LOHC system consists of a tandem of organic compounds in which one H2-lean compound (usually aromatic or heteroaromatic) is hydrogenated to yield another H2-rich cyclic or heterocyclic equivalent. Hydrogen is drawn from the loaded H2-rich form in a catalytic reaction. In this process, the liquid carrier is reverted to its H2-lean form and can be rehydrogenated to close the cycle (Fig. 1). Two main advantages of this system over conventional fuels become immediately clear. First, the energy stored in LOHCs can be obtained from renewable sources. Second, the carrier is not spent in the process but is fed back into the cycle after H2 is released, where it can undergo multiple hydrogenation–dehydrogenation rounds. Because the carriers are generally similar to diesel fuel regarding the properties defining handling, they could be stored, transported and distributed using the existing infrastructure, which would reduce the implementation costs of LOHC technology. In some respects, the LOHCs (see Table 1) are superior to presently used fuels. For example, they usually have higher boiling points, which means lower evaporation (less atmospheric pollution and greater safety during handling), and their composition is much better defined than that of fossil fuels. This facilitates all types of evaluation, standardisation and quality control. Demonstration units of LOHC-based energy storage were recently launched in Stuttgart‡ and Arzberg,§ Germany, as well as in Japan¶ and China.|| Additionally, commercially available solutions using LOHC technology include off-grid storage/generator units (66 to 792 kW) for buildings, renewable energy farms, and the shipping industry as well as water desalination units, air-conditioning units and a CO2-free energy autonomous megayacht.**
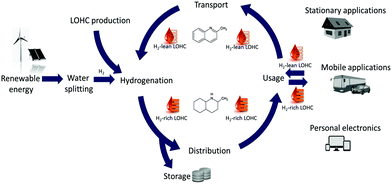 | ||
| Fig. 1 Schematic representation of the LOHC cycle using the quinaldine-based LOHC system as an example. | ||
| LOHC system | Ethylcarbazole | Benzyltoluene | Dibenzyltoluene | Quinaldine | Diesel |
|---|---|---|---|---|---|
| a European Chemical Agency (ECHA) for automotive diesel oil CAS68334-30-5 (https://echa.europa.eu/). b Assuming a density of 0.85 kg L−1. c Assuming the consumption of 1 kg H2 per 100 km and zero losses. | |||||
| Structure |
|
|
|
|
Mixture of paraffins, olefins, naphthenes and aromaticsa |
| Melting point [°C] | 7017 | −3021 | −39 to −3421 | −9 to −222 | −40 to 6a |
| Boiling point [°C] | 27021 | 28021 | 39021 | 24822 | 141 to 462a |
| kg fuel/100 km driving range | 17.3b | 15.2b | 16.1b | 15.3b | 7.35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
| H2-Carrying capacity [wt%] | 5.8 | 6.2 | 6.2 | 6.6 | n/a |
| Dehydrogenation temperature [°C] | 200–23023 | 250–27021 | 270–29021 | 13812 | n/a |
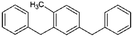
In principle, any molecule having an unsaturated bond could serve as an LOHC. Technological feasibility and safety concerns limit the choice, however, and to date, only a few LOHC systems have been investigated (Table 1). Initially, LOHC systems like benzene–cyclohexane (Benz–Chex) and toluene–methylcyclohexane (Tol–MChex) were suggested.11 However, due to the restriction of use of benzene and toluene under Regulation (EC) No. 1907/2006, i.e. REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals—the list of substances restricted under REACH is available from the European Chemical Agency [ECHA]), LOHC systems based on these compounds can currently not be placed on the EU market. Additionally, the H2-lean and H2-rich forms of both of these LOHC systems have very low flash points (<4 °C), which are well below the typical temperature of dehydrogenation.12 Nevertheless, they are being considered as LOHC outside of the EU.13 In Europe, the most established LOHC system is based on ethylcarbazole.14–16 However, the high melting point of ethylcarbazole and the limited thermal stability of that LOHC system has stimulated further research for more suitable carriers, e.g., chemical homologues substituted with longer alkyl chains (e.g., propyl- and butylcarbazole). Nonetheless, the melting points of the H2-lean forms of propyl- and butylcarbazole are still not sufficiently low (49 °C and 59 °C, respectively17). The hydrogenated equivalents remain liquid at much lower temperatures (e.g., perhydro-ethylcarbazole solidifies at −84 °C17), and the melting points of the partially hydrogenated species are between these values, as was shown for ethylcarbazole.17 In this context, intentionally preventing the full dehydrogenation of dodecahydro-alkylcarbazoles, although counterproductive in terms of the amount of H2 obtained, might be technologically favourable because these mixtures do not solidify as readily as pure H2-lean alkylcarbazoles. As a result, a mixture of the molecules with different levels of hydrogenation (e.g., see Table 2 for the structures of partially hydrogenated alkylcarbazoles) might be used. Both hydrogenation and dehydrogenation are catalytic processes occurring at high temperatures known to result in a certain level of carrier degradation (e.g., transalkylation or dealkylation), especially in the case of heteroaromatic carriers.18 All three carbazole derivatives are prone to dealkylation. Additionally, each methyl group added to the molecule acts as a ballast, decreasing the H2 storage capacity.18 Despite all these drawbacks, most research efforts are presently directed towards improving this LOHC system. Another LOHC system based on quinaldine (2-methylquinoline) has gained significant interest, due to a low dehydrogenation temperature (Table 1).19 The temperature of dehydrogenation of decahydroquinaldine is low enough that it could be supplied by the waste heat of a polymer electrolyte membrane fuel cell, unlike any of the other LOHC systems mentioned herein.20 Recently, Brückner et al. examined the applicability of a heat transfer fluid composed of benzyl- or dibenzyltoluene isomers in H2 storage.21 These LOHC systems have a greater H2 storage capacity than carbazoles, with all of the components remaining liquid down to −40 °C, but require higher temperatures to achieve complete dehydrogenation (see Table 1 for details on the properties of the different LOHC systems). The H2-lean/H2-rich forms of both carriers are complex mixtures of isomers, and the complexity increases even further for the partially hydrogenated forms.6 Benzyl- or dibenzyltoluene isomers are additionally characterised by a significantly lower aqueous solubility than the other systems. Because of their extremely low solubility and because the REACH registration of these systems is underway, they were not included in the test set.
| Sample acronym | Name | Structure | Formula/MW [gmol−1] | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D (pH 7.4) D (pH 7.4) |
pKb |
|---|---|---|---|---|---|
a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D and pKa were obtained using Chemaxon. log D and pKa were obtained using Chemaxon. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D was calculated using the atom contribution method as a ratio of the sum of concentration of neutral and charged species in octanol to the same ratio in water (log D was calculated using the atom contribution method as a ratio of the sum of concentration of neutral and charged species in octanol to the same ratio in water (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D = [B]octanol + [HB+]octanol/[B]water + [HB+]water). In some cases the pKb value is outside of the environmentally relevant scale. D = [B]octanol + [HB+]octanol/[B]water + [HB+]water). In some cases the pKb value is outside of the environmentally relevant scale.
|
|||||
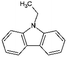
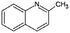
| Quin-2Me | Quinaldine |
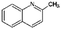
|
C10H9N | 2.5946 | 4.9447 |
| 143.2 | 2.26a | 5.15a | |||
| Quin-2Me-ph | Tetrahydro-quinaldine |
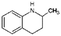
|
C10H13N | 2.35a | 4.88a |
| 147.2 | |||||
| Quin-2Me-10H | Decahydro-quinaldine |
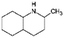
|
C10H19N | −0.84a | 10.75a |
| 153.3 | |||||
| Car2 | Ethylcarbazole |
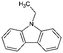
|
C14H13N | 3.67a | —a |
| 195.3 | |||||
| Car2-ph | Tetrahydro-ethylcarbazole (Car2-4H) |
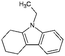
|
C14H17N | 3.87a | —a |
| 199.3 | |||||
| Octahydro-ethylcarbazole (Car2-8H) |
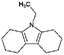
|
C14H21N | 4.07a | —a | |
| 203.3 | |||||
| Car2-12H | Dodecahydro-ethylcarbazole |
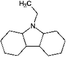
|
C14H25N | 0.09a | 11.69a |
| 207.4 | |||||
| Car3 | Propylcarbazole |
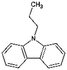
|
C15H15N | 4.19a | —a |
| 209.3 | |||||
| Car3-ph | Tetrahydro-propylcarbazole (Car3-4H) |
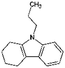
|
C15H19N | 4.39a | —a |
| 213.3 | |||||
| Octahydro-propylcarbazole (Car3-8H) |
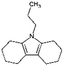
|
C15H23N | 4.59a | —a | |
| 217.3 | |||||
| Car3-12H | Dodecahydro-propylcarbazole |
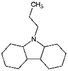
|
C15H27N | 0.58a | 11.97a |
| 221.4 | |||||
| Car4 | Butylcarbazole |
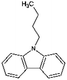
|
C16H17N | 4.64a | —a |
| 223.3 | |||||
| Car4-ph | Tetrahydro-butylcarbazole (Car4-4H) |
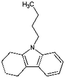
|
C16H21N | 4.84a | —a |
| 227.3 | |||||
| Octahydro-butylcarbazole (Car4-8H) |
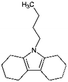
|
C16H25N | 1.02a | 4.90a | |
| 231.3 | |||||
| Car4-12H | Dodecahydro-butylcarbazole |
|
C16H29N | 1.02a | 12.09a |
| 235.4 | |||||
The annual global fuel oil demand was estimated to be 5.5 × 1012 L in 2016.24 If all of that demand were to be replaced by any of the above-mentioned LOHC systems, 2.1–2.9 × 1013 kg of the carrier would be necessary. Even if LOHC technology was only implemented as a niche application, the carrier chemicals would be handled, processed, stored and transported on a multi-ton scale, with consumers having access to rather large quantities. Consequently, the carrier might be released into the environment by leakage or an accidental spill, as has often been the case for fossil fuels.6 The LOHC-based energy storage is still a relatively new technology, requiring research and development efforts to optimise its performance to commercially attractive levels. This opens the possibility of proactively designing carriers with increased operational and environmental safety according to the rules of green chemistry.25 To achieve this, a hazard assessment regarding their possible impact on human health and the environment must be performed first.
Regarding human toxicity of LOHC chemicals, benzene (Benz) is classified as a known human carcinogen (Cat.1A) and presumed to cause germ cell mutagenicity (Cat.1B), while toluene (Tol) is classified as presumed to cause reproductive toxicity (Cat.2; details available from the ECHA). None of them was classified as acutely toxic to humans based on animal tests, although Benz, cyclohexene (Chex), Tol and methylcyclohexene (MChex) are hazardous when aspirated (Cat.1). The LD50 (lethal dose 50%) of orally administered Quin-2Me in rats is 1230 mg kg−1 and is higher than the 5000 mg kg−1 for Car2,26 which allows a classification of these compounds in the acute oral toxicity categories 4 and 5 (the second lowest and lowest category), respectively, according to the GHS (globally harmonised system).27,28
Concerning environmental hazards, both Chex and MChex are classified as acutely and chronically toxic to aquatic life (acute and chronic Cat.1). Knowledge regarding the effects and behaviour of the components of the LOHC systems mentioned in Table 1 in the environment is scarce. In the absence of actual experimental data, some insight into environmental and health hazards can be gained from the analysis of a compound's physicochemical properties or a read-across with structural analogues. The hydrophobicity, expressed as the octanol–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow) for neutral compounds or the octanol–water distribution coefficient (pH-dependent log
Kow) for neutral compounds or the octanol–water distribution coefficient (pH-dependent log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D) for ionisable compounds, is used to estimate the affinity of chemicals for biological membranes.29,30 From such values, the minimum toxicity of chemicals deprived of specific modes of toxic action (i.e., the baseline toxicity) can be derived. Table 2 summarises the most important parameters potentially influencing the toxicity of LOHC systems under investigation.
D) for ionisable compounds, is used to estimate the affinity of chemicals for biological membranes.29,30 From such values, the minimum toxicity of chemicals deprived of specific modes of toxic action (i.e., the baseline toxicity) can be derived. Table 2 summarises the most important parameters potentially influencing the toxicity of LOHC systems under investigation.
Generally, the hydrophobicity of azaarenes such as quinolines and carbazoles plays an important role in their toxic and mutagenic activity.31,32 In addition, the mutagenicity of such compounds can be modulated by introducing certain substituents at different positions of the aromatic rings or at the nitrogen atom. For example, the addition of a methyl or ethyl group to the nitrogen atom in the carbazole structure profoundly enhances mutagenicity in the Salmonella bacterial reverse mutation assay (the so-called Ames test), whereas the attachment of such groups to the aromatic rings at positions 1–4 does not significantly influence mutagenicity.33 Similarly, quinolines with different substituents vary greatly in their mutagenic activity.34–36 Although quinolines and carbazoles can potentially induce mutations, mostly through intercalation between DNA bases due to their planar structure,31,37,38 most azaarenes need to undergo a metabolic transformation (activation) prior to acting as mutagens.33,34,39–42 On the other hand, this mutagenicity seems to be due to the formation of reactive epoxides, which can ultimately lead to the formation of damaging DNA adducts (reviewed in ref. 31 and 43). Although this holds true for quinoline, in which case the mutagenic metabolite has been proposed to be the quinoline-2,3-epoxide,44 the mutagenic activity of N- and non-N-substituted carbazoles seems to emanate from hydroxylated metabolites.33,45
Members of Benz–Chex or Tol–MChex LOHC systems do not exert mutagenic effects detectable in the Ames test using similar strains as well as a metabolizing fraction.48–53 Although not mutagenic in the Ames test, it is well-known that Benz is a carcinogen causing leukaemia, which has been consequently classified as belonging to IARC group 1 since 1979.53,54
We have previously performed a preliminary assessment of the two LOHC systems (based on quinaldine and ethylcarbazole) discussed above based on QSAR predictions to highlight the necessity and the difficulty of hazard assessment.6 We have shown that the ecotoxicity of the components of a quinaldine-based LOHC system cannot be accurately predicted using presently available models (e.g., ECOSAR) based on log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values.6 In this work, we present extensive experimental results for two previously mentioned and two new LOHC systems, allowing for hazard assessments with lower levels of uncertainty. We have used a test battery composed of six test systems, encompassing (eco)toxicity, biodegradability and bacterial mutagenicity tests. We have also added two other LOHC systems for which a minimum of data is available to the comparative assessment, namely benzene–cyclohexane and toluene–methylcyclohexane.
Kow values.6 In this work, we present extensive experimental results for two previously mentioned and two new LOHC systems, allowing for hazard assessments with lower levels of uncertainty. We have used a test battery composed of six test systems, encompassing (eco)toxicity, biodegradability and bacterial mutagenicity tests. We have also added two other LOHC systems for which a minimum of data is available to the comparative assessment, namely benzene–cyclohexane and toluene–methylcyclohexane.
Should energy systems based on H2 storage in LOHCs be implemented on a broad scale, a hazard assessment considering at the very least the physicochemical properties, stability, (eco-)toxicity, biodegradability and bioaccumulation potential would need to be conducted. Four potential LOHC systems based on ethyl-, propyl- and butylcarbazole as well as quinaldine were experimentally assessed in this work. For each LOHC system, three forms of the carrier were assessed: the H2-lean (aromatic), the H2-rich (perhydrogenated) and the partially hydrogenated form (i.e., the hydrogenation reaction was stopped before full hydrogenation could occur). The rationale underlying the inclusion of the partially hydrogenated samples was to test the molecules that can be formed as unavoidable impurities during multiple hydrogenation–dehydrogenation cycles of LOHC chemicals. Moreover, as mentioned above, the prevention of complete dehydrogenation is sometimes technologically advantageous to avoid the solidification of the H2-lean carrier. We have therefore investigated H2-rich and H2-lean molecules as well as mixtures containing technical-grade partially hydrogenated carriers (after one cycle of hydrogenation). We thereby focused on the aquatic environment because it is likely to be the most affected compartment. We based the evaluation on acute and sub-chronic (eco)toxicity in six biological test models with increasing complexity of the test subjects: enzymes (acetylcholine esterase, AChE), mammalian cell lines (IPC-81 promyelocytic leukaemia rat cell line), luminescent bacteria (Vibrio fischeri), unicellular limnic algae (Raphidocelis subcapitata), freshwater vascular plants (Lemna minor) and freshwater invertebrates (Daphnia magna). We additionally investigated biodegradability since the persistence in the environment often leads to a concentration build-up, so that even relatively non-toxic compounds can have a detrimental impact on the environment. Finally, we performed a bacterial mutagenicity assay using Salmonella typhimurium strains TA98 and TA100, with and without metabolic activation (S9 mix) since many heterocyclic aromatic hydrocarbons are known to be mutagenic.43,55 Using several test systems allowed us to generate an ecotoxicological profile of the different LOHC systems investigated in the present manuscript based on different modes of action and on different levels of biological organisation. We also added LOHC systems based on Benz and Tol to the comparative hazard assessment, for which we gathered the data from the literature.
Materials and methods
Chemicals
2-Methylquinoline (quinaldine, Quin-2Me, CAS number 91-63-4), N-ethylcarbazole (Car2, CAS number 86-28-2), N-propylcarbazole (Car3, CAS number 1484-10-2) and N-butylcarbazole (Car4, CAS number 1484-08-8) were kindly supplied by the research group of Professor Peter Wasserscheid (Institute of Chemical Reaction Engineering, University of Erlangen, Germany). Tetrahydro-2-methylquinoline (Quin-2Me-ph) and decahydro-2-methylquinoline (Quin-2Me-10H) were prepared by catalytic hydrogenation using Ru on Al2O3 as the catalyst at 140 °C and 50 bar H2-pressure, as described in detail in ref. 22. Dodecahydro-N-ethylcarbazole (Car2-12H, CAS number 146900-30-3), dodecahydro-N-propylcarbazole (Car3-12H, CAS number 1612790-27-8), dodecahydro-N-butylcarbazole (Car4-12H, CAS number 1612790-29-0) and mixtures of partially hydrogenated carbazoles (abbreviated in the text as Car2-ph, Car3-ph and Car4-ph) were prepared by hydrogenation using Ru on Al2O3 as the catalyst at 150 °C and 40 bar H2-pressure.17 Cetyldimethylbenzylammonium chloride (C16-benzalkonium chloride, CAS number 122-18-9; Sigma-Aldrich), carbendazim (CAS number 10605-21-7; Sigma-Aldrich), 1-octyl-3-methylimidazolium chloride (CAS number 64697-40-1; Merck), sodium chloride (Sigma-Aldrich), 3,5-dichlorophenol (CAS number 591-35-5; Merck), potassium dichromate (CAS number 7778-50-9; Sigma-Aldrich), sodium benzoate (CAS number 532-32-1; Sigma-Aldrich), sodium azide (CAS number 26628-22-8; Honeywell Riedel-de Haën®, Seelze, Germany), benzo[a]pyrene (CAS number 50-32-8; ≥96% purity; Sigma-Aldrich) and 4-nitro-o-phenylenediamine (CAS number 99-56-9; 98% purity; Sigma-Aldrich) were used as positive controls in biodegradation, toxicity and mutagenicity testing.Solution preparation
The water/medium solubility of all tested chemicals was screened using the shake-flask method.56 The solutions of chemicals having a water solubility above 100 mg L−1 (all forms of the quinaldine LOHC system as well as all H2-rich forms of the alkylcarbazoles) were prepared by directly weighing and dissolving the compounds in test medium followed by visual inspection of the solution for particles or droplets. The solutions of all remaining chemicals with water solubility lower than 100 mg L−1 (all H2-lean and partially hydrogenated alkylcarbazoles) were prepared using the generator column method.56 Briefly, test substances were deposited on glass beads by vacuum evaporation from the solution in hexane. The beads were placed in the generator column and different test media were circulated through for 24 h in a thermostatic chamber. These solutions (containing the maximum water soluble fraction of the respective LOHC) were then used as stock solutions in the tests as well as for concentration determination. The concentration of the stock solution was determined after liquid–liquid extraction.Analytical methods
The concentration of the quinaldines was measured using GC-FID (HP 6890 series) with splitless injection of 1 μL. The same column type as stated above was used. The GC method parameters were as follows: inlet temperature 250 °C, oven program 40 °C hold for 0.6 min, ramp 20 °C min−1 to 150 °C, ramp 35 °C min−1 to 300 °C hold for 1 min. The detector temperature was set to 320 °C. The concentration of the alkylcarbazoles was measured using the same method used for the qualitative analysis. Limits of detection and quantification (LOD/LOQ) are presented in Table S1 (ESI†). Due to the complex composition and low aqueous solubility of partially hydrogenated samples of alkylcarbazoles, only the most abundant compound in the mixture was used for quantification, namely Car2-12H in Car2-ph samples, Car3-4H in Car3-ph samples and Car4-4H in Car4-ph samples.
Cytotoxicity tests using the IPC-81 cell line
The colorimetric WST-1 assay with the IPC-81 promyelocytic leukemia rat cell line was used to investigate the influence of LOHC chemicals on cell viability. The test is based on the spectrophotometric assessment of the intensity of colour caused by the enzymatic reduction of the WST-1 reagent (sodium 2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium), which is inversely proportional to cytotoxicity. The WST-1 test was carried out in Roswell Park Memorial Institute medium (without NaHCO3, supplemented with 1% penicillin/streptomycin, 1% L-glutamine and, 10% horse serum, pH 7.0) at 37 °C and in an atmosphere containing 5% CO2. The exact procedure is described in detail elsewhere.58 Three independent tests were performed each in three replicates using nine concentrations. Solutions were prepared directly in medium containing 1% (v/v) of DMSO as a co-solvent and diluted in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 series with medium. Testing ranges were 0.5–900 mg L−1 for Quin-2Me, 0.1–300 mg L−1 for Quin-2Me-ph and 0.7–1500 mg L−1 for Quin-2Me-10H. Carbendazim was used as a positive control and tested in regular intervals to ensure the validity of the obtained results.
1 series with medium. Testing ranges were 0.5–900 mg L−1 for Quin-2Me, 0.1–300 mg L−1 for Quin-2Me-ph and 0.7–1500 mg L−1 for Quin-2Me-10H. Carbendazim was used as a positive control and tested in regular intervals to ensure the validity of the obtained results.
Luminescence inhibition test with Vibrio fischeri
This test with the marine luminescent bacterium Vibrio fischeri was performed according to DIN EN ISO 11348-2.65. The freeze-dried bacteria were purchased from Dr Lange (Düsseldorf, Germany). Each substance was tested three times, using independently prepared solutions, with two replicates at each concentration level and accompanied by at least four controls (2% NaCl solution in phosphate buffer). All solutions were prepared directly in phosphate-buffered saline (0.02 M, pH 7.0, including 2% sodium chloride). The tests were performed at 15 °C using thermostats (LUMIStherm; Dr Lange, Düsseldorf, Germany). The freeze-dried bacteria were rehydrated according to the manufacturer's protocol; thereafter, 500 μL aliquots of the bacteria suspension were pre-incubated for 15 min at 15 °C. After measuring the initial luminescence, 500 μL of the samples were added. The bioluminescence was measured again after an incubation time of 30 min using a luminometer (LUMIStox 300, Dr Lange, Düsseldorf, Germany). The toxicity of the samples was expressed as percentage inhibition compared to the controls. Luminescent bacteria assays were conducted with addition of 1% methanol (v/v) as a co-solvent. The range of concentrations tested covered 0.03–240 mg L−1 for Quin-2Me, 0.015–110 mg L−1 for Quin-2Me-ph and 0.035–285 mg L−1 for Quin-2Me-10H. Sodium chloride (7.5 g L−1) was used as a positive control.Growth inhibition test with Raphidocelis subcapitata
The tests were performed following the OECD Test Guideline 20159 under modified light conditions (light–dark cycle of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 h instead of continuous illumination). Cell culture flasks (Nunc EASY flasks, 25 cm2, VWR, Germany) were filled with 20 mL of test solutions or only medium (controls). Algae in the exponential growth phase were exposed to Quin-2Me, Quin-2Me-ph, or Quin-2Me-10H in a range of 0.5–100 mg L−1 prepared in the OECD 201 medium (pH 8.1 ± 0.2). Algae cell counts at the beginning of the test were set to 2.5 × 104 cells mL−1. Samples were placed on orbital shakers at 150 rpm for 72 h in a climate chamber (Thermostatschrank ET 618-4/135, Sanyo) at 22 ± 1 °C. Cell counts after 72 h were recorded using a cell-counting chamber (Neubauer-improved, depth 0.100 mm, surface 0.0025 mm2) and a light microscope (Zeiss, Germany). Eight concentration levels were tested in three replicates with six controls for each test and each test was performed two times. The growth rate of algae at the end of the test was expressed as percentage of cell counts compared to the controls. 3,5-Dichlorophenol was used as a positive control and tested in regular intervals to ensure the validity of the results.
10 h instead of continuous illumination). Cell culture flasks (Nunc EASY flasks, 25 cm2, VWR, Germany) were filled with 20 mL of test solutions or only medium (controls). Algae in the exponential growth phase were exposed to Quin-2Me, Quin-2Me-ph, or Quin-2Me-10H in a range of 0.5–100 mg L−1 prepared in the OECD 201 medium (pH 8.1 ± 0.2). Algae cell counts at the beginning of the test were set to 2.5 × 104 cells mL−1. Samples were placed on orbital shakers at 150 rpm for 72 h in a climate chamber (Thermostatschrank ET 618-4/135, Sanyo) at 22 ± 1 °C. Cell counts after 72 h were recorded using a cell-counting chamber (Neubauer-improved, depth 0.100 mm, surface 0.0025 mm2) and a light microscope (Zeiss, Germany). Eight concentration levels were tested in three replicates with six controls for each test and each test was performed two times. The growth rate of algae at the end of the test was expressed as percentage of cell counts compared to the controls. 3,5-Dichlorophenol was used as a positive control and tested in regular intervals to ensure the validity of the results.
Growth inhibition test with Lemna minor
This test was conducted according to the OECD Test Guideline 221.60 The plants were grown in Erlenmeyer flasks in sterilised Steinberg medium (pH 5.5 ± 0.2) in a climate chamber at a constant temperature of 24 ± 2 °C and continuously illuminated with a maximum of 125 μE m−2 s−1. The assays were performed in plastic six-well plates incubated under the same conditions for seven days. All substances were tested three times on different days using independently prepared solutions with three replicates at each concentration level and a minimum of six controls (pure Steinberg medium) for each test. The assay was started with one plant having three fronds in each sample. The endpoint was the inhibition of growth rate based on frond area calculated in relation to the controls. The frond area (mm2) was determined using the Scanalyzer software from Lemnatec (Aachen, Germany). The Steinberg medium contained 3.46 mM KNO3, 1.25 mM Ca(NO3)2, 0.66 mM KH2PO4, 0.072 mM K2HPO4, 0.41 mM MgSO4, 1.94 μM H3BO3, 0.63 μM ZnSO4, 0.18 μM Na2MoO4, 0.91 μM MnCl2, 2.81 μM FeCl3, 4.03 μM EDTA and had a pH of 5.5 ± 0.2. The test concentration range was 3.5–2420 mg L−1 for Quin-2Me, 3.4–1290 mg L−1 for Quin-2Me-ph and 1–2000 g L−1 for Quin-2Me-10H. C16-Benzalkonium chloride was used as a positive control and tested in regular intervals to ensure the validity of the results.Immobilisation test with Daphnia magna
The 48 h acute immobilization test with the crustacean Daphnia magna was performed using the commercially available Daphtoxkit F (MicroBioTest Incorporation, Ghent, Belgium) in accordance to ISO standard 6341. The Daphnia neonates were hatched from dormant ephippia at 20 °C under constant illumination. For each replicate, five pre-fed animals, less than 90 h old, were placed in 10 mL of mineral medium (controls) or a solution of test substances in mineral medium. The number of immobilized (dead) organisms was recorded after 24 and 48 h. The toxicity of the test compound was expressed as percentage of not affected organisms compared to the controls. All substances were tested in three independent experiments (five concentrations, five replicates each) in the following test ranges: Quin-2Me 0.3–200 mg L−1, Quin-2Me-ph 0.2–200 mg L−1, Quin-2Me-10H 0.32–500 mg L−1. Potassium dichromate was used as a positive control and tested in regular intervals to ensure the validity of the results.Bacterial reverse mutation assay for mutagenicity (Ames test)
The test solutions were prepared by dissolving the test compounds in DMSO at the highest possible concentration using the shake-flask method (OECD Test Guideline 105)56 and then, when necessary (see below), by further dilution in DMSO in order to obtain working stocks (50 μL per plate). The Ames test was performed as previously described61,62 by using the preincubation method in the presence and absence of a metabolizing system (S9-mix; 10% rat liver homogenate in co-factor buffer) as well as the Salmonella typhimurium strains TA98 and TA100 obtained from Trinova (Gießen, Germany). Sodium azide (TA100 without S9), benzo[a]pyrene (TA98 and TA100 with S9) and 4-nitro-o-phenylenediamine (TA98 without S9) were used as positive controls. Prior to the actual test, the toxicity of the compound dilutions was assessed in strain TA100 by using the change/absence of a bacterial background lawn as well as the reduction of revertants/plate below the level of the spontaneous reversion rate or a complete lack of growth as endpoints.62 In case toxicity was observed, the dilution that produced no or only a slightly toxic effect was used as the highest concentration of the corresponding test compound. LOHCs were tested with both Salmonella strains once in triplicate in three separate batches. Each batch was tested with the corresponding controls. The triplicate determinations (revertants/plate) of the solvent control (7.7% DMSO) were averaged (9 replicates, n = 3) and used to calculate the mutagenic activity (MA), i.e. the number of revertants/plate relative to the solvent control. LOHCs with a revertant/plate count ≥2-fold higher than the corresponding mean solvent control were considered mutagenic.Ultimate biodegradation test
Ultimate biodegradation was measured by the manometric respirometry method according to the OECD Test Guideline 301F using the automated, thermostatically controlled OxiTop® set (WTW, Weilheim, Germany).63 The activated sludge from the municipal wastewater treatment plant in Delmenhorst (Germany) was used as a source of inoculum. The flocs were allowed to settle and the remaining supernatant was aerated for 4–7 days prior to use. The test lasted at least 28 days and was performed in standard OECD medium supplemented with a nitrification inhibitor (allylthiourea). Target compounds were directly weighed and added to the test vessels to yield a biological oxygen demand of 200 mg O2 L−1. Two replicates were run for each compound accompanied by two blanks and two positive controls (sodium benzoate). The test was repeated three times.Data processing and analysis
Dose–response curve parameters and plots were obtained using the drfit package (version 3.1.0) for R (version 3.4; http://www.r-project.org). The LemnaTec software (LemnaLaucher version 6.4.0.19788) was used for plant phenotyping in the Lemna minor test and Marvin (version 6.3.1; ChemAxon http://www.chemaxon.com) was used for drawing, displaying and characterizing chemical structures. Detailed description of data processing is given in the ESI.†Results and discussion
Purity of test compounds
The exact composition of the partially hydrogenated LOHCs was not known. Therefore, the samples were analysed using a GC-MS to confirm their identity and establish their purity (Table 3). All H2-lean and H2-rich samples were single compounds as shown in Table 2. Additionally, the partially hydrogenated sample of the quinaldine-based LOHC system contained only tetrahydroquinaldine (Table 2). On the other hand, the samples of partially hydrogenated alkylcarbazoles were mixtures of several compounds (see Tables 2, 3 and Table S1 for more details, ESI†). For the quantification of the partially hydrogenated samples, the most abundant compound was used, i.e., Car2-12H for Car2-ph, Car3-4H for Car3-ph and Car4-4H for Car4-ph (Tables 4 and 5).| Acronym | Name (abbreviation) | Amounta [%] |
|---|---|---|
| a Calculated on the basis of the GC-MS analysis as percent area under the peak for a given component of the mixture in relation to the summed areas of all components observed. | ||
| Car2-ph | Dodecahydroethylcarbazole (Car2-12H) | 70 |
| Tetrahydroethylcarbazole (Car2-4H) | 13 | |
| Car3-ph | Tetrahydropropylcarbazole (Car3-4H) | 54 |
| Propylcarbazole (Car3) | 11 | |
| Dodecahydropropylcarbazole (Car3-12H) | 7 | |
| Octahydroproplycarbazole (Car3-8H) | 7 | |
| Hexahydropropylcarbazole (Car3-6H) | 5 | |
| Car4-ph | Tetrahydrobutylcarbazole (Car4-4H) | 54 |
| Butylcarbazole (Car4) | 37 | |
| Compound | AChE | IPC-81 | Salmonella thypimurium | |||
|---|---|---|---|---|---|---|
| IC50 and EC50 values in mg L−1 (confidence intervals) | Range of mutagenic activitya | |||||
| TA98 −S9 | TA98 +S9 | TA100 −S9 | TA100 +S9 | |||
| a Mutagenic activity was calculated as the number of revertants/plate relative to the solvent control; the values were obtained at six concentration levels, which are listed in Table S3 (ESI). b The exact EC50 values could not be calculated, since only an approximately 50% effect was achieved at the highest tested concentration; the EC50 value was therefore reported as equal to or higher than that concentration. c No effect was observed and no EC50 values were obtained, the values are the averaged highest test concentrations corresponding to the maximum solubility in the test medium. d Quantified as Car2-12H (Tables 2 and 3). e Quantified as Car3-4H (Tables 2 and 3). f Quantified as Car4-4H (Tables 2 and 3). g Octylmethylimidazolium chloride. h Carbendazim. i 4-Nitro-o-phenylenediamine 5 μg per plate. j Benzo[a]pyrene 5 μg per plate. k Sodium azide 5 μg per plate. l According to ref. 67. m According to ref. 49. n According to ref. 68. o Substance is poorly soluble in medium and was tested at maximum medium solubility. n.a. – data not available. | ||||||
| Quin-2Me | 61 (54–67) | 313 (292–328) | 0.6–0.7 | 0.6–0.8 | 1.1–1.9 | 1.1–1.5 |
| Quin-2Me-ph | ≥147b (n.d.) | ≥480b (n.d.) | 0.6–0.7 | 0.7–0.8 | 1.0–1.7 | 1.0–1.4 |
| Quin-2Me-10H | 90 (80–104) | 842 (734–990) | 0.6–0.8 | 0.7–0.8 | 1.2–1.6 | 0.8–1.4 |
| Car2 | n.d. | >0.92c,o | 0.9–1.0 | 0.9–1.1 | 1.0–1.5 | 1.3–2.4 |
| Car2-ph | n.d. | 9.1d (8.3–10.0) | 0.8–1.1 | 0.9–1.1 | 0.6–1.8 | 1.4–1.5 |
| Car2-12H | n.d. | 60 (45–81) | 0.8–1.0 | 0.9–1.1 | 1.1–1.6 | 1.0–1.8 |
| Car3 | n.d. | >0.24c,o | 0.8–1.2 | 0.7–1.1 | 1.2–1.5 | 1.0–2.1 |
| Car3-ph | n.d. | 0.78e (0.68–0.87) | 0.7–1.0 | 0.9–1.0 | 1.0–1.5 | 1.1–1.4 |
| Car3-12H | n.d. | 72 (62–85) | 0.7 | 0.7–0.9 | 0.9–1.4 | 1.3–1.4 |
| Car4 | n.d. | >0.44c,o | 0.8–1.1 | 0.8 | 1.8–2.3 | 0.9–1.3 |
| Car4-ph | n.d. | 0.85f (0.78–0.94) | 0.6–0.9 | 0.7–0.8 | 1.6–1.9 | 1.0–1.4 |
| Car4-12H | n.d. | 59 (53–68) | 0.7–0.8 | 0.7–0.9 | 1.4–1.9 | 1.3–1.6 |
| Positive control | 9.8 (9.2–10.5)g | 0.33 (0.30–0.36)h | 26.0i | 28.0j | 3.0k | 6.2j |
| Benz | n.a. | n.a. | Not mutagenicl | Not mutagenicl,m | Not mutagenicl | Not mutagenicl,m |
| Chex | n.a. | n.a. | Not mutagenicn | Not mutagenicn | Not mutagenicn | Not mutagenicn |
| Tol | n.a. | n.a. | Not mutagenicl | Not mutagenicl,m | Not mutagenicl | Not mutagenicl,m |
| MChex | n.a. | n.a. | n.a | n.a | n.a | n.a. |
| Compound | Vibrio fischeri | Raphidocelis subcapitata | Lemna minor | Daphnia magna |
|---|---|---|---|---|
| EC50 values in mg L−1 (2.5–97.5% confidence intervals) | ||||
| a Not more than 40% luminescence inhibition was recorded for Quin-2Me-10H at the highest attainable concentration, therefore, the EC50 value was reported to be higher than this concentration. b Averaged maximum solubility in the test medium. c Quantified as Car2-12H (Tables 2 and 3). d Quantified as Car3-4H (Tables 2 and 3). e Quantified as Car4-4H (Tables 2 and 3). f 7.5% (w/w) NaCl (luminescence inhibition between 40 and 60% is expected). g 3,5-Dichlorophenol. h Benzalkonium chloride. i Potassium dichromate. j American Petroleum Institute (API) for automotive diesel oil CAS 68334-30-5.79 k Short-term EC50 for Raphidocelis subcapitata.80 l Short-term EC50 for Daphnia magna or Ceriodaphnia dubia.81,82 m Short-term EC50 for Raphidocelis subcapitata.83 n Short-term EC50 for Daphnia magna.83 o Short-term EC50 for Raphidocelis subcapitata, Chlamydomonas angulosa or Chlorella vulgaris.80,84 p Short-term EC50 for Daphnia magna or Ceriodaphnia dubia.81,82,85,86 q Short-term EC50 for Raphidocelis subcapitata.87 r Short-term EC50 for Daphnia magna.87 s Substance is poorly soluble in medium and was tested at maximum medium solubility. n.d.: value not determined; n.a.: value not available. | ||||
| Quin-2Me | 19 (16–22) | 43 (—) | 42 (36–48) | 56 [ref. 6] (54–59) |
| Quin-2Me-ph | 7.4 (6.2–8.9) | 17 (14–20) | 51 (46–59) | 2.7 [ref. 6] (2.3–3.2) |
| Quin-2Me-10H | >306a | 52 (50–55) | >1000a | 155 (120–191) |
| Car2 | >0.36b,s | n.d. | >0.65b,s | >0.38b,s |
| Car2-ph | 2.5c (2.09–2.82) | n.d. | n.d. | n.d. |
| Car2-12H | >431b,s | n.d. | 258 (254–263) | 60 (50–76) |
| Car3 | >0.06b,s | n.d. | >0.40b,s | >0.36b,s |
| Car3-ph | 4.3d (3.8–4.8) | n.d. | n.d. | n.d. |
| Car3-12H | >440b,s | n.d. | >240 | 10.2 (8.3–12.6) |
| Car4 | >0.03b,s | n.d. | >0.063b,s | >0.16b,s |
| Car4-ph | >2.9d,e,s | n.d. | n.d. | n.d. |
| Car4-12H | >457b,s | n.d. | 112 (n.d.) | 9.6 (7.7–11.8) |
| Reference | See belowf | 1.6g (1.3–1.9) | 6.6h (4.5–10.2) | 1.1i (0.96–1.17) |
| Diesel oil | n.a. | 22–78j | n.a. | 13–210j |
| Benz | n.a. | 29.0k | n.a. | 10.0–17.2l |
| Chex | n.a. | 3.4m | n.a. | 0.9–3.8n |
| Tol | n.a. | 12.5–207o | n.a. | 3.8–14.9p |
| MChex | n.a. | 0.13q | n.a. | 0.33r |
AChE inhibition, cytotoxicity and mutagenicity
The summary of the results of acetylcholinesterase inhibition potential and cytotoxicity expressed as half-maximal effective/inhibitory concentrations (EC50/IC50) and of the mutagenic activity is given in Table 4. The enzyme AChE catalyses the hydrolysis of a neurotransmitter, acetylcholine, in the synaptic clefts of the nervous system. The structure of AChE is highly conserved among species; therefore, AChE inhibition is often used as an endpoint in screening for the neurotoxic potential of chemicals.64 When compared to the positive control 1-octyl-3-methylimidazolium chloride (IC50 = 9.8 mg L−1) or a known inhibitor of AChE the carbamate insecticide aldicarb (IC50 = 0.93 mg L−1),64 the inhibitory potential of the quinaldines was low (IC50 within 61–147 mg L−1), with the sequence of toxicity being Quin-2Me > Quin-2Me-10H > Quin-2Me-ph (Table 4). Interestingly, this order differs from the order of cytotoxic potency (Table 4; see text below).The cytotoxicity tests using cell lines measure the so-called basal cytotoxicity, i.e., the toxicity to common functions and structures of cells. The magnitude of cytotoxic effects is often similar across different cell lines, endpoints and exposure times, sometimes even across species.65 The EC50 values for all tested compounds in this test vary by four orders of magnitude and range from 0.78–842 mg L−1 (Table 4).
Although the cytotoxicity to IPC-81 cells follows the trend Quin-2Me ≥ Quin-2Me-ph > Quin-2Me-10H in the quinaldine LOHC system, the differences in the EC50 values are rather small (the difference between the EC50 of Quin-2Me and Quin-2Me-H10 is not statistically significant at α = 0.05, please see the ESI† for details regarding data treatment and statistical tests throughout; Table 4). The alkylcarbazoles generally showed a greater range in cytotoxic activity among the three forms tested and were two to three orders of magnitude more toxic than the quinaldines. Among the investigated alkylcarbazoles, the highest EC50 (i.e., the lowest cytotoxicity) was observed for the perhydrogenated forms, with EC50 values within 59–72 mg L−1. All of these values can be considered moderate compared to that of a biocide such as carbendazim (EC50 = 10 mg L−1), which was used as a positive control in the present study, or a common organic solvent such as ethanol (EC50 = 32 g L−1) near the other end of the toxicity spectrum.66 The partially hydrogenated alkylcarbazoles were one to two orders of magnitude more cytotoxic than their perhydrogenated counterparts and, with EC50 values between 0.78–9.1 mg L−1, showed the following trend of toxic potency: Car3-ph ≈ Car4-ph > Car2-ph (only the differences between Car2-ph and Car4-ph is statistically significant at α = 0.05). The partially hydrogenated propyl- and butyl-substituted carbazole species were more toxic than the positive control, which generally indicates high cytotoxicity (difference statistically significant at α = 0.05). The EC50 values for Car2, Car3 and Car4 were not obtained since no effect at the highest tested concentration was observed due to the very low medium solubility of these compounds.
The in vitro bacterial mutagenicity or Ames test is often used as a first step in the mutagenicity screening of chemicals. From a regulatory perspective, a positive result in this test system usually triggers further testing but does not necessarily indicate mutagenicity or carcinogenicity in vivo. Table 4 and Table S3 (ESI†) show the results of the mutagenicity tests using two strains of Salmonella typhimurium (TA98 and TA100) with and without rat liver homogenate (+S9 and −S9) since some chemicals are mutagenic only after metabolic transformation. Compounds producing less than double the number of revertants per plate obtained in the spontaneous revertant controls (SRC see Table S3 and Fig. S1, ESI†), i.e., having mutagenic activity below 2.0, were deemed non-mutagenic.
As shown in Table 4, no mutagenic activity was detected in Salmonella strain TA98 for any of the test compounds. In strain TA100, only treatments with Car2, Car3 and Car4 showed mutagenic activity above two. In particular, in the case of Car2 and Car3, the dose–response relationship was thereby non-monophasic (see Table S3, ESI†), indicating that higher concentrations induce toxic or growth-inhibitory effects that counteract the higher bacterial reversion rate/proliferation induced by their mutagenic activity. Moreover, Car2 and Car3 were only mutagenic in the presence of a rat liver homogenate (Table 4), which suggests that metabolites of these compounds, perhaps hydroxylated derivatives,33,69,70 are the ultimate mutagens, whereas Car4 does not need metabolic activation to be mutagenic. Interestingly, for Car4, the number of revertants/plate decreases in the presence of S9 (Table 4), thereby indicating that N-butylcarbazole is actually detoxified by mammalian enzymes. However, a higher percentage (up to 20–30%) of liver homogenate in the S9-mix than that used in the present study might lead to a considerable increase in the number of revertants, as has been shown, e.g., for quinoline and several of its derivatives.35 The mutagenicity data for N-ethylcarbazole (Car2) presented herein are consistent with previously published work by LaVoie et al. (1982), who showed that Car2 was clearly mutagenic in Salmonella typhimurium strain TA100 only in the presence of S9 and not mutagenic in strain TA98.33 Mutagenicity data regarding N-propylcarbazole (Car3) and N-butylcarbazole (Car4) have, to our knowledge, not been published, although LaVoie et al. tested N-isopropylcarbazole, which was not mutagenic in strain TA100 or TA98 in the presence of a metabolic system33 (possibly due to the branched and rather bulky structure of the isopropyl moiety). Unsubstituted quinoline has been unequivocally shown to exert a mutagenic activity in vitro in conjunction with metabolising enzymes in Salmonella strain TA100.35,36,46,71 However, the picture is not as clear for its 2-methylated counterpart (Quin-2Me). Although its mutagenicity/genotoxicity seems generally lower than that of the parent compound or other methylated quinolines,71,72 some studies have shown that quinaldine has a certain mutagenic activity in TA100 bacteria incubated with S9.46,72 In contrast, other studies report that this molecule is not mutagenic or genotoxic at all.71,73,74 Interestingly, in the present study, quinaldine does not need a metabolic system to induce a sub-threshold (i.e., borderline positive) increase in the number of revertants in strain TA100. A similar finding has been reported by Brinkmann and Eisenträger (2008) using the so-called umu genotoxicity test (performed with Salmonella enterica subsp. enterica), classifying quinaldine as a genotoxic agent not needing metabolic activation.75 In conclusion, for most of the members of the LOHC systems investigated herein, we did not observe mutagenicity in the Ames test using Salmonella typhimurium strains TA100 and TA98 with or without metabolic activation. Even the three mutagenic carbazoles (Car2, Car3 and Car4) have a rather weak potency, especially compared to the well-known mutagens used as positive controls in the present experimental setup. From the mechanistic perspective, the few actually or only weakly mutagenic compounds most likely induce base-pair substitution mutations since the effects were only observed in TA100, a strain known to detect such DNA insults.62,76 Notably, the hydrogenation of the H2-lean LOHCs largely reduces the mutagenic activity.44 Nevertheless, more extensive testing, including other Salmonella strains, higher S9 concentrations, the use of mammalian mutagenicity test systems and an analysis of possibly formed mutagenic metabolites is necessary to further characterise the potential mutagenicity of the presently investigated LOHCs.
Aquatic ecotoxicity
Aquatic organisms at different trophic levels, including luminescent marine bacteria, limnic green algae, aquatic plants and crustaceans, were used to investigate the potential adverse effects of the LOHCs on aquatic organisms (Table 5).The luminescence inhibition assay using Vibrio fischeri, the representative marine organism within our test battery, is a fast, cost-effective and frequently used test with many data available for comparison. The EC50 values for V. fischeri were one to two orders of magnitude lower for Quin-2Me and Quin-2Me-ph compared to the cytotoxicity and AChE inhibition assays (Table 5). Moreover, greater variation was observed between particular members of this LOHC system, with more than one order of magnitude differences in their activity. The potency order of Quin-2Me-ph > Quin-2Me ≫ Quin-2Me-10H follows the order of the substances’ log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values (Table 5). For the alkylcarbazoles, the EC50 values were calculated only for Car2-ph and Car3-ph and were within the same order of magnitude as the EC50 values of Quin-2Me-ph (differences not statistically significant at α = 0.05). Among all other members of the alkylcarbazoles-based LOHC systems, the H2-lean forms had very low solubility in the test medium (below 1 mg L−1), and the H2-rich forms did not exhibit any activity, even at high concentrations (up to 431–457 mg L−1). Therefore, the EC50 values were reported to be higher than the highest concentrations tested.
D values (Table 5). For the alkylcarbazoles, the EC50 values were calculated only for Car2-ph and Car3-ph and were within the same order of magnitude as the EC50 values of Quin-2Me-ph (differences not statistically significant at α = 0.05). Among all other members of the alkylcarbazoles-based LOHC systems, the H2-lean forms had very low solubility in the test medium (below 1 mg L−1), and the H2-rich forms did not exhibit any activity, even at high concentrations (up to 431–457 mg L−1). Therefore, the EC50 values were reported to be higher than the highest concentrations tested.
The green algae are primary producers and are therefore a very important link in the food chains in aquatic environments. Together with crustaceans, they are usually the most sensitive species in the present test battery and in general.77 For that reason, these organisms are often used for regulatory testing purposes. For the quinaldine-based LOHC system, Raphidocelis subcapitata was not more sensitive than the other organisms included in the test battery. The EC50 values obtained in this test render the following order of toxic potency for quinaldines: Quin-2Me-ph > Quin-2Me > Quin-2Me-10H (differences not statistically significant at α = 0.05), which again mirrors their log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values (Table 2). These EC50 values indicate moderate toxicity in comparison to the positive control. Similar EC50 values for the green alga species Desmodesmus subcapitatus were found for structural analogues of Quin-2Me such as quinoline, which lacks a methyl group (EC50 = 60.9 mg L−1), and 6-methylquinoline, which differs in the position of the methyl group (EC50 = 33.2 mg L−1).78
D values (Table 2). These EC50 values indicate moderate toxicity in comparison to the positive control. Similar EC50 values for the green alga species Desmodesmus subcapitatus were found for structural analogues of Quin-2Me such as quinoline, which lacks a methyl group (EC50 = 60.9 mg L−1), and 6-methylquinoline, which differs in the position of the methyl group (EC50 = 33.2 mg L−1).78
Lemna minor (duckweed) is a vascular aquatic plant, and the assay using it was included in the present test battery due to the relatively long assay duration of 7 days, which can be treated as a semi-chronic test. For Quin-2Me and Quin-2Me-ph, EC50 values of 42 mg L−1 and 51 mg L−1, respectively, were observed (differences not statistically significant at α = 0.05). The H2-rich form was considerably less toxic than the other two forms, with a much higher EC50 value of approximately 1000 mg L−1. None of the H2-lean forms of alkylcarbazoles elicited any significant growth inhibition in this test system. However, the test concentrations were notably very low due to the poor water solubility of these compounds. Car2-12H showed a relatively high EC50 value of 258 mg L−1, whereas the exact EC50 value of Car3-12H could not be determined, as this compound did not dissolve well enough to produce a full dose–response curve. Nevertheless, an approximately 50% growth rate inhibition was observed at the highest tested concentration of 240 mg L−1. Although the butyl homologue had a lower medium solubility, the full range of effects was observed, and the EC50 value was determined to be 112 mg L−1. This leads to the following order of toxicity: Car4-12H > Car3-12H > Car2-12H, an order again paralleling the hydrophobicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D, Table 2) of the test compounds.
D, Table 2) of the test compounds.
Clear differences were observed in the toxicity to the water flea (Daphnia magna) for the three quinaldines that were consistent with the order of their log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values. The EC50 value of Quin-2Me was 56 mg L−1, which is within the same order of magnitude as all other organisms of the aquatic test battery (Table 5) and somewhat higher than that measured by Eisentraeger et al. for the structurally similar quinoline and 6-methylquinoline (with EC50 values equal to 14.7 mg L−1 and 8.6 mg L−1, respectively).78 The perhydrogenated form turned out to be an order of magnitude less toxic (EC50 = 155 mg L−1). D. magna seems to be particularly sensitive to the partially hydrogenated quinaldine, as was V. fischeri, with an EC50 value of only 2.7 mg L−1 (statistically significantly different from Quin-2Me and Quin-2Me-H10 at α = 0.05). Perhydro-alkylcarbazoles were moderately to considerably toxic to water fleas with toxicity increasing with the length of the alkyl substituent and EC50 values between 9.6 and 60 mg L−1 (differences statistically significant at α = 0.05), which follows the trend in their log
D values. The EC50 value of Quin-2Me was 56 mg L−1, which is within the same order of magnitude as all other organisms of the aquatic test battery (Table 5) and somewhat higher than that measured by Eisentraeger et al. for the structurally similar quinoline and 6-methylquinoline (with EC50 values equal to 14.7 mg L−1 and 8.6 mg L−1, respectively).78 The perhydrogenated form turned out to be an order of magnitude less toxic (EC50 = 155 mg L−1). D. magna seems to be particularly sensitive to the partially hydrogenated quinaldine, as was V. fischeri, with an EC50 value of only 2.7 mg L−1 (statistically significantly different from Quin-2Me and Quin-2Me-H10 at α = 0.05). Perhydro-alkylcarbazoles were moderately to considerably toxic to water fleas with toxicity increasing with the length of the alkyl substituent and EC50 values between 9.6 and 60 mg L−1 (differences statistically significant at α = 0.05), which follows the trend in their log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values (Table 2). Nevertheless, the EC50 experimentally obtained for Car2-H12 is an order of magnitude higher than the predicted EC50, showing that the models do not perform well for ionisable compounds.6 No effects were observed for H2-lean molecules due to their limited solubility in the test medium.
D values (Table 2). Nevertheless, the EC50 experimentally obtained for Car2-H12 is an order of magnitude higher than the predicted EC50, showing that the models do not perform well for ionisable compounds.6 No effects were observed for H2-lean molecules due to their limited solubility in the test medium.
Range of toxicity and preliminary insight into the mechanism of toxic action
The cytotoxicity of the quinaldines was rather low, with mammalian IPC-81 cells being generally less sensitive than the aquatic organisms. The fully or partially hydrogenated alkylcarbazoles showed an up to three orders of magnitude higher cytotoxicity than any of the quinaldines, and the partially hydrogenated samples were particularly toxic, with EC50 values close to or below 1 mg L−1.The following trend in toxicity was observed in the present test battery: in the aquatic ecotoxicity tests for the quinaldine-based LOHC system, the partially hydrogenated sample was usually the most toxic, with V. fischeri and D. magna being particularly sensitive to this compound (EC50 values of only 4.7 and 2.7 mg L−1, respectively). In the V. fischeri test, the partially hydrogenated samples were also the most toxic forms within the alkylcarbazole-based LOHC systems, which additionally showed the highest toxicity within the entire set of tested chemicals. The H2-rich species were usually the least toxic, and the perhydrogenated alkylcarbazoles were usually more toxic than perhydro-quinaldine (based on the D. magna and L. minor tests at α = 0.05, see ESI† for statistical comparison). The H2-lean species of the alkylcarbazole-based LOHC systems were very poorly soluble in test media and did not elicit any observable effects in any of the tests at maximum medium solubility. The testing of the partially hydrogenated samples shows that the hazard emanating from these compounds might be higher than that expected from the testing of their pure H2-rich and H2-lean homologues. This is especially important for alkylcarbazoles, because partially hydrogenated species will likely be intentionally used in that case. Should this be the case, more detailed tests will probably be needed to determine the toxicity of each particular partially hydrogenated form.
The level of toxicity of the test compounds generally follows the order of hydrophobicity, suggesting a baseline toxicity (i.e., non-specific affinity to and interference with cell membrane integrity and functioning)88 rather than a specific mode of toxic action (i.e., activation of specific cellular pathways). All LOHC chemicals tested here are organic bases. The H2-rich species have predicted dissociation constants (pKb) above pH 10 and are therefore positively charged in all test media (pH from 5.5 to 8.1). As a result of this, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D (which considers ionisation) is significantly lower than the log
D (which considers ionisation) is significantly lower than the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow (which considers the neutral species). Consequently, the H2-rich LOHC compounds are the least hydrophobic and therefore presumably the least toxic. For the quinaldines, the partially hydrogenated species has the highest hydrophobicity, which is also consistent with this compound having the highest toxicity. The situation is more complicated for the partially hydrogenated samples of alkylcarbazoles, which are actually mixtures of several compounds at different levels of hydrogenation and often include H2-rich and H2-lean species. Due to the method of solution preparation used in the present study, each of these forms is theoretically expected to be present at its maximum water solubility at the beginning of the test, in which case the effect of the mixture has to be considered.
Kow (which considers the neutral species). Consequently, the H2-rich LOHC compounds are the least hydrophobic and therefore presumably the least toxic. For the quinaldines, the partially hydrogenated species has the highest hydrophobicity, which is also consistent with this compound having the highest toxicity. The situation is more complicated for the partially hydrogenated samples of alkylcarbazoles, which are actually mixtures of several compounds at different levels of hydrogenation and often include H2-rich and H2-lean species. Due to the method of solution preparation used in the present study, each of these forms is theoretically expected to be present at its maximum water solubility at the beginning of the test, in which case the effect of the mixture has to be considered.
Inhibition of the AChE was the only test system in which the toxic potency of the quinaldines did not reflect the order of hydrophobicity as indicated by log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values (Table 2). Quin-2Me-10H, which was the least toxic compound in most of the aquatic tests, showed higher AChE-inhibiting potential than Quin-2Me-ph, which in turn was usually the most toxic LOHC. Even though Quin-2Me-10H is less hydrophobic, its pKb value is higher than that of the other two forms (Table 2). Under the test pH, Quin-2Me-10H carries a positive charge on the nitrogen atom, whereas the two other forms are uncharged. The quaternary nitrogen in the structure of Quin-2Me-10H might therefore interact with the active site of the AChE, which is supported by the fact that potent AChE inhibitors often possess a positively charged nitrogen in their structure, enabling a better interaction with the active site of the enzyme. This effect was observed for quinolinium cations, which show a much stronger inhibition of AChE than could be explained based solely on their lipophilicity.64
D values (Table 2). Quin-2Me-10H, which was the least toxic compound in most of the aquatic tests, showed higher AChE-inhibiting potential than Quin-2Me-ph, which in turn was usually the most toxic LOHC. Even though Quin-2Me-10H is less hydrophobic, its pKb value is higher than that of the other two forms (Table 2). Under the test pH, Quin-2Me-10H carries a positive charge on the nitrogen atom, whereas the two other forms are uncharged. The quaternary nitrogen in the structure of Quin-2Me-10H might therefore interact with the active site of the AChE, which is supported by the fact that potent AChE inhibitors often possess a positively charged nitrogen in their structure, enabling a better interaction with the active site of the enzyme. This effect was observed for quinolinium cations, which show a much stronger inhibition of AChE than could be explained based solely on their lipophilicity.64
No toxicity up to the solubility limit is reported when there is no observable toxic effect for poorly water-soluble compounds (as in the case of H2-lean alkylcarbazoles) according to the so-called “solubility cut-off”. This concept is based on the belief that highly hydrophobic compounds do not dissolve well enough to cause toxic effects.89 However, even very hydrophobic substances can exert aquatic toxicity, and they definitely can contribute to the overall toxicity when present in mixtures.90 Different issues often cause the “solubility cut-off”, including kinetic aspects (e.g., the short duration of standard aquatic tests), fast excretion, metabolisation of test chemicals or poorly controlled exposure.
Due to the high hydrophobicity of H2-lean and partially hydrogenated alkylcarbazoles (see Table 2), we expected that the exposure concentration might decrease, especially in the longer tests such as the WST-1 assay with IPC-81 cells, the immobilisation test with D. magna (both 48 h incubation) and the L. minor growth inhibition test (7 days incubation). In the latter two test systems, the volume of the test solution was sufficiently large for measuring the concentration of the H2-lean compounds at the end of the assay. In all L. minor tests, the concentration at the end of the test fell below the limit of quantification (below 1% of the initial concentration). The losses were lower in the D. magna test, probably as a combined effect of the shorter test duration, lower temperature and lack of illumination, yet still remained above 40%. Unstable exposure, i.e., the test concentration changing by more than 20%, precludes drawing meaningful quantitative conclusions because the effect, or the lack thereof, cannot be clearly assigned to the starting concentration. Nonetheless, the confirmation of the exposure stability is not always performed or is sometimes impossible due to analytic limitations, leading to underestimation of toxicity. In such cases, strategies that limit the fluctuations of exposure (e.g., the use of passive dosing or medium renewal) should be used to confirm the result. Such approaches are, however, time consuming and are not within the scope of our preliminary assessment. As a result, the lack of toxic effects observed for H2-lean forms of alkylcarbazoles cannot be treated as an explicit proof of inherent lack of toxicity. In the assessment for regulatory purposes, however, such results are used due to the lack of data. As an example, Car2 was assigned to the chronic aquatic toxicity category 2 of REACH based on an aquatic exposure fish test conducted in the concentration range of 1–500 mg L−1, even though it is not soluble at such high concentrations.†† Similarly, diesel oil was assessed well above its water solubility as explained below in more detail.
Biodegradability
The biodegradability of all three forms of quinaldine as well as H2-rich and H2-lean alkylcarbazoles was tested according to the manometric respirometry procedure.63 Only Quin-2Me was biodegradable in this test system (Fig. 2). None of the remaining chemicals caused any oxygen consumption in the manometric system, and therefore no degradation was measured (results not shown). In our previous work,6 we used QSAR models to predict the biodegradability of H2-rich/H2-lean forms of LOHC systems based on ethylcarbazole (i.e., Car and Car2-H12), and we have also preliminarily tested the biodegradability of Car2. More comprehensive testing conducted in this work shows that Car2 is not easily biodegradable, contrary to the QSAR prediction, and that Car2-H12 is poorly degradable, which is consistent with QSAR predictions. In four out of six replicates, Quin-2Me actually fulfilled the criterion of ready degradability (at least 60% degradation within a 10 day window).63 In those cases, a lag phase of four to nine days is followed by rapid degradation. One of the replicates did not show any appreciable degradation even though the validity criteria were fulfilled.63 These differences are a natural consequence of variability in the composition of the microbial inoculum. Nevertheless, the extent of degradation was generally high, thereby confirming that Quin-2Me is biodegradable.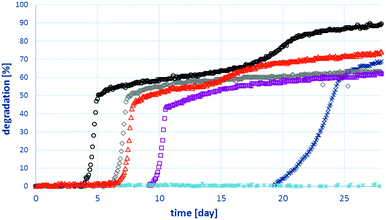 | ||
| Fig. 2 Ultimate biodegradability of Quin-2Me; six replicates tested in three independent tests are shown. | ||
The degradation of methylquinolines under aerobic conditions is much faster than that under denitrifying conditions, which often does not occur or proceeds more slowly and is incomplete.91,92 The degradation pathway of methylquinolines under aerobic conditions often begins with the hydroxylation of the carbon atom adjacent to the nitrogen atom.93 Such a transformation is, however, not possible for 2-methylquinoline (quinaldine) because this position is occupied by the methyl group.91,94 In this case, hydroxylation at position 4 usually occurs, yielding 2-methyl-4-quinolinone.92,95,96 Direct oxidation of the methyl group was also reported by Dembek et al. (1989), leading to the formation of quinaldinic acid and further oxidation.97 After initial hydroxylation, further degradation usually proceeds through the dioxygenolytic cleavage of the benzene ring,98 even though a pathway proceeding through the cleavage of the pyridine ring and the release of a carbon monoxide molecule has been observed.95,96 These results, combined with our findings, suggest that Quin-2Me will be degraded rather quickly and completely. Interestingly, tetrahydro-2-methylquinoline (Quin-2Me-ph), one of the compounds of interest in this study, was detected as one of the main degradation products of 2-methylquinoline (Quin-2Me). Quin-2Me-ph initially accumulated in the degradation batch but was later degraded to some extent, suggesting a higher resistance to degradation but not persistence.92 The conditions used in our work were far less conducive than those in the test performed by Wang et al. (2010; lower biomass, lack of adaptation or active aeration), which allows us to suspect that Quin-2Me-ph is indeed not recalcitrant, even if it did not show any appreciable degradability in our tests. Cyclic alkanes such as Quin-2Me-10H or hydrogenated alkylcarbazoles are usually more resistant to biodegradation than their linear equivalents, but the presence of an alkyl substituent on the cycloalkane ring facilitates biodegradation.99 Cycloalkanes can be co-metabolised, i.e., degraded in the presence of other compounds serving as sources of energy due to the low substrate specificity of the enzymes responsible for degradation.100–102 Therefore, degradability of partially or fully hydrogenated equivalents will be slower, and further research under more favourable conditions would be necessary to exclude the possibility of environmental persistence.
We found no indication of degradation of any alkylcarbazole LOHC chemicals in the standard biodegradation tests performed herein. The structurally simpler homologue carbazole is rather recalcitrant but can be degraded by adapted microbial species/communities.103
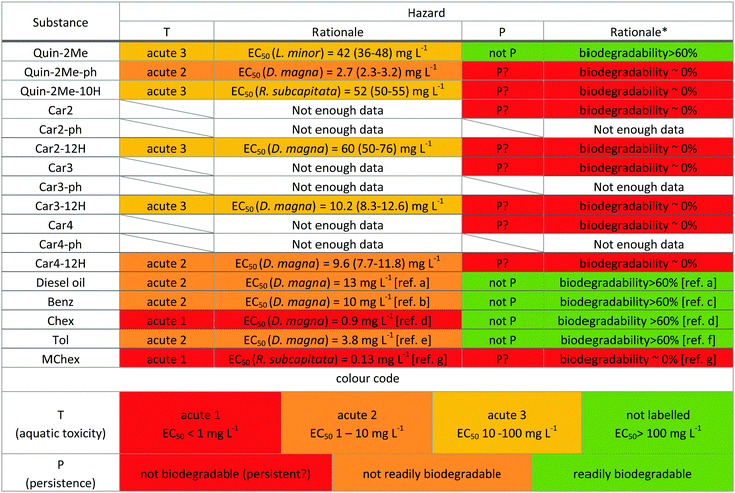
Preliminary hazard assessment
To put the present results in context, we compared the environmental hazard of the six LOHC systems with diesel oil using the thresholds of the globally harmonised system for labelling chemicals (GHS) as a guideline (Table 7). The GHS system is a widely adopted yet voluntary system developed to ensure clear communication of hazards associated with the usage of chemicals. Additionally, many countries have their own regulatory bodies that have the authority to restrict the usage of chemicals if the hazard associated with them is deemed unacceptable. In the European Union, REACH regulation (Regulation [EC] no. 1907/2006) lays out the rules of hazard management.| Compound | Vibrio fischeri | Green algae | Daphnia magna |
|---|---|---|---|
| a Depending on substitution pattern. b Scenedesmus acuminatus. c Desmodesmus supcapitatus. d Scenedesmus pannonicus. | |||
| Carbazole | EC50 > 0.75 mg L−1 [ref. 108] | EC10 > 0.45 mg L−1 [ref. 110]b | EC50 = 3.4 mg L−1 [ref. 78] |
| EC50 > 4.5 mg L−1 [ref. 109] | |||
| Quinoline | EC50 = 6.7 mg L−1 [ref. 110] | EC50 = 123.5 mg L−1 [ref. 110]c | EC50 = 14.7 mg L−1 [ref. 78] |
| EC50 = 100 mg L−1 [ref. 111]c | EC50 = 31.8 mg L−1 [ref. 110] | ||
| Dimethyl-quinoline | EC50 0.36–11 mg L−1 [ref. 112]a | EC50 = 19 mg L−1 [ref. 111]d | EC50 = 38 mg L−1 [ref. 113] |
| EC50 = 31 mg L−1 [ref. 111]d | |||
The GHS entails three categories of acute aquatic toxicity. Substances are assigned to categories based on EC50 values obtained from a test with green algae, and/or aquatic plants (often L. minor) and/or crustaceans and/or fish. The ranges for each category are shown at the bottom of Table 7 with their respective colour code. If data on more than one organism are available, the most sensitive organism is used to assign the compound to its corresponding category. For those cases in which the ecotoxicity was lower than the threshold for the category “acute 3”, we have marked it in green to indicate that labelling is not required. In the absence of chronic toxicity data, the REACH legislation sets a screening threshold on toxicity to aquatic organisms (including algae, crustaceans and fish) at an EC50 value ≤0.1 mg L−1 to identify toxic substances.
For the LOHCs, Daphnia magna is often the most sensitive species (Tables 5 and 6). Using the GHS thresholds, most of the LOHC chemicals tested within this work fall into the “acute 2” or “acute 3” categories. Chex and MChex were previously classified in acute category 1 because the EC50 values for D. magna and R. subcapitata fall below 1 mg L−1 (Table 7). Moreover, EC50 values for Quin-2Me-ph and Tol are near the lower threshold of the GHS highest aquatic toxicity category (“acute 1”). Unlike Tol and alkylcarbazoles, Quin-2Me-ph is not a deliberately introduced component of the LOHC system but could be perceived as an impurity that can be present in a rather low amount as a result of unintentional or incomplete dehydrogenation. Based on the results presented in Table 7, two of the LOHC chemicals, namely Chex and MChex, would need to be labelled as “toxic to aquatic organisms” according to GHS criteria. Additionally, some members, especially the alkylcarbazole LOHC systems, would probably require more testing. For all H2-lean forms of alkylcarbazoles, no EC50 was observed in aquatic test systems; however, due to considerable differences between the nominal and the real exposure concentrations, we decided that the amount of data is not sufficient to assign them to any T category.
Quin-2Me, Benz,104 Chex83 and Tol105 were shown to be biodegradable when tested according to the OECD 301 “ready biodegradability” test procedure. Other LOHC chemicals did not show appreciable levels of biodegradation when tested according to the OECD301 guideline; therefore, their persistence cannot be excluded. They were marked as potentially persistent and require further testing for a definite classification. Even though MChex was classified as not readily biodegradable there are other non-guideline sources that do report significant levels of biodegradation when adapted or pre-selected inocula are used.83 Among the LOHC chemicals for which we gathered enough data to perform the assessment, Quin-2Me-ph, Car4-H12, Benz and Tol showed a toxicity level comparable to diesel oil, whereas H2-lean and H2-rich quinaldines as well as H2-rich ethyl and butylcarbazoles showed a lower toxicity. On the other hand, Chex and MChex were more toxic to aquatic organisms. In terms of biodegradability, Quin-2Me, Benz, Chex and Tol were readily biodegradable, as is diesel oil. All other LOHC chemicals seem to be less degradable and potentially persistent.
After multiple cycles of hydrogenation–dehydrogenation the LOHCs might undergo degradation. It was shown that the levels of carrier degradation vary depending on the catalyst type/morphology and reaction conditions. The LOHC system based on ethylcarbazole lost less than 2% of the H2 storage capacity during ten cycles of hydrogenation (using Ru on Al2O3 as a catalyst at 180 °C and 80 bar) and dehydrogenation (using Pd on Al2O3 as a catalyst at 178 °C and ambient pressure).106 On the other hand in model systems dealkylation of ethylcarbazole was observed during dehydrogenation using small aggregates of Pt on Al2O3/NiAl support starting at room temperature.107 Using ordered Pt on Al2O3/NiAl support or single crystal Pt as catalysts shifted the onset of dealkylation to 117 °C. During dehydrogenation using Pt on activated carbon as catalyst and temperature of 210 °C quinaldine (Quin-2Me) was shown to undergo dealkylation to quinoline or alkylation to dimethylquinoline.22 To obtain partially/fully hydrogenated forms the carriers examined within this study underwent only one hydrogenation reaction using Ru on Al2O3 at 140 °C and 50 bar H2-pressure. These conditions are milder than those described by Yang et al.106 therefore, we did not expect and did not observe noticeable levels of contamination with degradation products. Nevertheless, because the hydrogenation and dehydrogenation reactions generally lead to formation of decomposition products it is possible that they will be found in the recycled carriers. The effective concentrations for carbazole (main degradation products in Car2 and possibly also Car3 and Car4 LOHC systems), quinoline and dimethylquinoline (main degradation products in Quin-2Me LOHC system) to Vibrio fischeri, different species of green algae and Daphnia magna are shown in Table 6. Based on algae or crustacean toxicity quinoline and dimethylquinoline would need to be classified in acute aquatic toxicity category 3 (as would Quin-2Me and Quin-2Me-H10) and carbazole in category 2 (one category lower than Car2-H12).
Theoretically a catalyst residue might be another source of impurities in H2 carriers but until now nothing is known in this regard. Ru and Pd, the most commonly used catalysts for hydrogenation and dehydrogenation, show no toxic effect at saturation in Daphnia magna acute immobilisation test.114 The spent carriers were not the focus of this work, yet both the stability of carriers during recycling as well as their environmental impacts should be investigated in more detail in the future.
Comparison with fossil fuels
To be considered a greener alterative in terms of environmental hazard, the LOHC systems should be equivalent to or better than the fossil fuels they are meant to replace. Surprisingly, limited scientific data are available regarding the environmental toxicity of fossil fuels, probably due to their complex variable composition and poor water solubility, causing testing to be difficult. The EC50 values (48 h) of automotive diesel oil for D. magna and R. subcapitata were reported as 13–210 mg L−1 and 22–78 mg L−1, respectively, based on the water accommodated fraction (WAF; Table 5).79 The WAF is often used when testing poorly water-soluble compounds, especially mixtures of unknown composition. The WAF is obtained for water or a medium at equilibrium with a certain amount (loading) of the poorly soluble substance and, thus, does not necessarily represent the truly dissolved quantity. When a hydrocarbon mixture, e.g., diesel oil, is added to water in amounts below the solubility limit of the least soluble compound, the aqueous concentration proportionally increases until the saturation concentration of the least soluble component is reached. When more of the substance is added, only the more soluble components continue to dissolve until they reach their own solubility limits. Further addition of the mixture results in an aqueous concentration that is a non-linear function of the amount added. For example, the solubility of diesel oil no. 2 in distilled water, measured as total dissolved hydrocarbons, is 3.2 mg L−1 at 20 °C,115 which is clearly below the EC50 values shown in Table 5. Therefore, the real exposure to diesel oil in these tests was most likely much lower, suggesting also higher toxicity. The toxicity of Benz and the quinaldine LOHC system to green algae and crustaceans is comparable to that of diesel oil, except for the partially hydrogenated form of quinaldine. The EC50 values of Car3-H12, Car4-H12 or Tol but especially Chex and MChex to D. magna are lower than the EC50 values of diesel oil, indicating their higher toxicity.Regarding potential environmental persistence, fossil fuels are generally biodegradable to a high extent116 and are therefore superior to most LOHC chemicals. The rather high biodegradability of diesel oil is due to the fact that it is largely composed of relatively easily biodegradable linear hydrocarbons.117 Nevertheless, after the easily biodegradable fraction is broken down, the less abundant but refractory components, including branched alkanes, terpenoids and larger PAHs, remain and often persist in the environment for a long time.117
Only a few of the LOHC compounds analysed in the present study were (rather weakly) mutagenic, which sets them positively apart from genotoxic/mutagenic (water-soluble) fractions of diesel fuel and diesel engine exhaust particles.118–123 On the other hand, it has been shown that the Benz–Chex and Tol–MChex LOHC systems are either carcinogenic and cause germ cell mutations (Benz) or cause reproductive toxicity (Tol).53,54 Carcinogenicity data are, to the best of our knowledge, available neither for MChex nor for quinaldine or the alkylcarbazoles. Consequently, further studies are needed in order to clarify and compare the carcinogenic potential of various LOHC systems. Nevertheless, it appears that LOHC systems based on Benz and Tol would not be more advantageous over the other LOHC systems presented herein or diesel oil, at least regarding carcinogenicity. In addition to the environmental hazards, physicochemical hazards are also associated with chemicals, e.g., their explosive, oxidising and flammable properties. The LOHC systems based on quinaldine and alkylcarbazoles seem to be less flammable than diesel oil and, therefore, pose a lower risk. On the other hand, LOHC systems based on Benz and Tol are more flammable than diesel (flash points do not exceed 4 °C as compared to a flashpoint of about 50 °C for diesel oil) and therefore pose higher risk.
Because LOHC chemicals have a less complex composition, a much lower batch-to-batch variability in composition is expected than in the case of fossil fuel. This would also mean that the uncertainty of the evaluation is lower. On the other hand, the fact that LOHC chemicals are recycled raises the issue of carrier degradation.
Conclusions
Within the suggested proactive, comparative environmental impact assessment of LOHC systems, an evaluation of six potential LOHC systems was completed. Four systems were tested within this work and for two others literature data were collected. Due to confirmed human carcinogenicity, the use of benzene is restricted under REACH in the EU. Similarly, some uses of toluene are restricted in the EU due to reproductive toxicity. Moreover, high aquatic toxicity of cyclohexane and methylcyclohexane mandates their classification as toxic to aquatic organisms. Lastly, poor biodegradability of methylcyclohexane indicates potential persistence in the environment. Taking all of the above reasons into account, the benzene–cyclohexane and toluene–methylcyclohexane LOHC systems seem to generally present a high hazard to humans as well as the environment and are, in this regard, inferior to other LOHC systems and the currently used energy system based on diesel oil.Low to moderate (eco)toxicity was observed for the LOHC system based on quinaldine. High biodegradability (>60%) was observed for quinaldine (2-methylquinoline, Quin-2Me), whereas no significant degradation occurred for tetrahydroquinaldine (Quin-2Me-ph) and decahydroquinaldine (Quin-2Me-10H). Additionally, low cytotoxicity, moderate AChE inhibition and negligible mutagenic potential were observed for all quinaldines tested. No toxic effect was observed in any of the test systems used in the present study (i.e., IPC-81, Vibrio fischeri, Lemna minor and Daphnia magna) for H2-lean forms of alkylcarbazoles, which does not allow a hazard classification and indicates the need for more advanced testing. Nevertheless, these compounds were the only substances within the entire test set that showed a noticeable mutagenic potential. The H2-rich forms showed a moderate ecotoxicity that was generally higher than that of quinaldines. High cytotoxicity was observed for partially hydrogenated alkylcarbazoles, with the toxic effect increasing with the chain length. The perhydrogenated alkylcarbazoles exerted a moderate cytotoxicity, which was again higher than that observed for quinaldines. None of the tested alkylcarbazoles was biodegradable. The lack of observable biodegradation does not necessarily mean that these LOHC chemicals are persistent, but that additional tests under less stringent conditions than the ready biodegradability test (e.g., with a higher density of (pre-adapted) microbial inoculum or addition of another source of carbon) are needed. Nevertheless, the degradation timeframe will most definitely be longer than that for Quin-2Me or diesel oil.
Based on the results presented above, the quinaldine-based LOHC system can be considered not worse than the currently available energy system based on fossil fuels in terms of (eco)toxicity. Nevertheless, the fact that H2-rich and partially hydrogenated forms did not undergo biodegradation raises concerns of potential persistence, which is a rather significant drawback. The LOHC systems based on alkylcarbazoles are generally more toxic and less biodegradable. Additionally, the considerable hydrophobicity of H2-lean and partially hydrogenated forms of alkylcarbazoles (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D 3.6–4.8) indicates that they might be bioaccumulative.
D 3.6–4.8) indicates that they might be bioaccumulative.
Undeniable benefits come from the fact that LOHC energy systems operate on renewable energy, although they can also be implemented using conventional energy sources. Additionally, certain physicochemical properties of some LOHCs are more favourable in the context of the safety of handling and transportation, e.g., higher boiling points, which means less loss due to evaporation, lower flammability and less inhalatory exposure to potentially toxic vapours. The quinaldine-based LOHC system seems to exhibit a slightly higher environmental hazard than, e.g., automotive diesel oil, based on the potential persistence of its H2-rich and partially hydrogenated forms. In contrast, the alkylcarbazole-based LOHC systems pose a higher hazard than the quinaldine-based LOHC system and diesel oil due to the resistance to biodegradation of all forms and a considerable (eco)toxicity, especially in the case of partially hydrogenated forms. Finally, the benzene and toluene based LOHC systems are more hazardous than diesel oil.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Ms Ulrike Bottin-Weber and Ms Alica Rother for conducting the ecotoxicity tests and GC analysis as well as Ms Nicole Brauer for performing the Ames test. We would also like to acknowledge the financial support of the University of Bremen and the European Union FP7 COFUND within Marie Curie Actions BremenTrac Program (grant agreement no. 600411) and the “M8 Postdoc-Initiatve PLUS”, funded by the German Excellence Initiative.References
- M. S. Dresselhaus and I. L. Thomas, Nature, 2001, 332–337 CrossRef CAS PubMed
.
-
European Comission, A Roadmap for moving to a competitive low carbon economy in 2050, 2011 Search PubMed
.
- S. Satyapal, J. Petrovic, C. Read, G. Thomas and G. Ordaz, Catal. Today, 2007, 120, 246–256 CrossRef CAS
.
- S. Dutta, J. Ind. Eng. Chem., 2014, 20, 1148–1156 CrossRef CAS
.
- A. Züttel, A. Remhof, A. Borgschulte and O. Friedrichs, Philos. Trans. R. Soc., A, 2010, 368, 3329–3342 CrossRef PubMed
.
- M. Markiewicz, Y. Q. Y. Zhang, A. Bösmann, N. Brückner, J. Thöming, P. Wasserscheid and S. Stolte, Energy Environ. Sci., 2015, 8, 1035–1045 RSC
.
- A. A. Ismail and D. W. Bahnemann, Sol. Energy Mater. Sol. Cells, 2014, 128, 85–101 CrossRef CAS
.
- D. J. Durbin and C. Malardier-Jugroot, Int. J. Hydrogen Energy, 2013, 38, 14595–14617 CrossRef CAS
.
- U. Eberle, M. Felderhoff and F. Schüth, Angew. Chem., Int. Ed., 2009, 48, 6608–6630 CrossRef CAS
.
- L. Schlapbach and A. Züttel, Nature, 2001, 414, 353–358 CrossRef CAS
.
- N. Kariya, A. Fukuoka and M. Ichikawa, Appl. Catal., A, 2002, 233, 91–102 CrossRef CAS
.
- P. Preuster, C. Papp and P. Wasserscheid, Acc. Chem. Res., 2017, 50, 74–85 CrossRef CAS PubMed
.
- Y. Okada and M. Yasui, Hyomen Kagaku, 2015, 36, 577–582 CrossRef CAS
.
-
A. C. Cooper, K. M. Campbell and G. P. Pez, 16th World Hydrogen Energy Conference, 2006, vol. 3 Search PubMed
.
-
G. P. Pez, A. R. Scott, A. C. Cooper and H. Cheng, US pat., 7101530, Air Products and Chemicals Inc., 2006 Search PubMed
.
- D. Teichmann, W. Arlt, P. Wasserscheid and R. Freymann, Energy Environ. Sci., 2011, 4, 2767–2773 RSC
.
- K. Stark, P. Keil, S. Schug, K. Müller, P. Wasserscheid and W. Arlt, J. Chem. Eng. Data, 2016, 61, 1441–1448 CrossRef CAS
.
- C. Papp, P. Wasserscheid, J. Libuda and H. P. Steinrück, Chem. Rec., 2014, 14, 879–896 CrossRef CAS
.
- T. He, Q. Pei and P. Chen, J. Energy Chem., 2015, 24, 587–594 CrossRef
.
- S. Mekhilef, R. Saidur and A. Safari, Renewable Sustainable Energy Rev., 2012, 16, 981–989 CrossRef CAS
.
- N. Brückner, K. Obesser, A. Bösmann, D. Teichmann, W. Arlt, J. Dungs and P. Wasserscheid, ChemSusChem, 2013, 7, 229–235 CrossRef PubMed
.
-
N. T. Brückner, PhD thesis, Friedrich-Alexander-University of Erlangen-Nürnberg, 2015, p. 206
.
- D. Teichmann, K. Stark, K. Müller, G. Zöttl, P. Wasserscheid and W. Arlt, Energy Environ. Sci., 2012, 5, 9044–9054 RSC
.
- BP, BP Statistical Review of World Energy About This Review Contents, 2016.
-
P. T. Anastas and J. C. Warner, Green Chemistry, Theory and Practice, Oxford University Press, 2000 Search PubMed
.
- European Chemical Agency, registration dossier (ethylcarbazole), http://echa.europa.eu/.
-
G. Collin and H. Höke, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000 Search PubMed
.
- Globally Harmonized System of Classification and Labelling of Chemicals (GHS): Fourth Revised Edition, 2011.
- B. I. Escher and R. P. Schwarzenbach, Environ. Sci. Technol., 1996, 30, 260–270 CrossRef CAS
.
- M. Kah and C. D. Brown, Chemosphere, 2008, 72, 1401–1408 CrossRef CAS PubMed
.
- E. A. J. Bleeker, S. Wiegman, P. de Voogt, M. Kraak, H. A. Leslie, E. de Haas and W. Admiraal, Rev. Environ. Contam. Toxicol., 2002, 173, 39–83 Search PubMed
.
- R. Benigni, Chem. Rev., 2005, 105, 1767–1800 CrossRef CAS
.
- E. J. LaVoie, G. Briggs, V. Bedenko and D. Hoffmann, Mutat. Res., 1982, 101, 141–150 CAS
.
- M. Nagao, T. Yahagi, Y. Seino, T. Sugimura and N. Ito, Mutat. Res., Fundam. Mol. Mech. Mutagen., 1977, 42, 335–341 CrossRef CAS
.
- M. I. Willems, D. Dubois, D. R. Boyd, R. J. H. Davies, L. Hamilton, J. J. McCullough and P. J. van Bladeren, Mutat. Res., 1992, 278, 227–236 CAS
.
- C. J. Smith, C. Hansch and M. J. Morton, Mutat. Res., Fundam. Mol. Mech. Mutagen., 1997, 379, 167–175 CrossRef CAS
.
- C. Asche and M. Demeunynck, Adv. Anticancer. Agents Med. Chem., 2007, 7, 247–267 CrossRef CAS
.
- T. Helleday, S. Eshtad and S. Nik-Zainal, Nat. Rev. Genet., 2014, 15, 585–598 CrossRef CAS PubMed
.
- M. Hollstein, R. Talcott and E. Wei, J. Natl. Cancer Inst., 1978, 60, 405–410 CrossRef CAS PubMed
.
- W. Yang, T. Jiang, P. J. Davis and D. Acosta, Toxicology, 1991, 68, 217–226 CrossRef CAS
.
- G. Reigh, H. McMahon, M. Ishizaki, T. Ohara, K. Shimane, Y. Esumi, C. Green, C. Tyson and S. Ninomiya, Carcinogenesis, 1996, 17, 1989–1996 CrossRef CAS PubMed
.
- L. L. Furge and F. P. Guengerich, Biochem. Mol. Biol. Educ., 2006, 34, 66–74 CrossRef CAS PubMed
.
- W. Xue and D. Warshawsky, Toxicol. Appl. Pharmacol., 2005, 206, 73–93 CrossRef CAS PubMed
.
- T. Kato, K. Saeki, Y. Kawazoe and A. Hakura, Mutat. Res., Fundam. Mol. Mech. Mutagen., 1999, 439, 149–157 CAS
.
- H. G. Shertzer, M. B. Genter, G. Talaska, C. P. Curran, D. W. Nebert and T. P. Dalton, Carcinogenesis, 2007, 28, 1371–1378 CrossRef CAS PubMed
.
- A. K. Debnath, R. L. Lopez de Compadre and C. Hansch, Mutat. Res., 1992, 280, 55–65 CAS
.
- A. Albert, R. Goldacre and J. Phillips, J. Chem. Soc., 1948, 2240 RSC
.
- J. Mccann, E. Choi, E. Yamasaki and B. N. Ames, Proc. Natl. Acad. Sci. U. S. A., 1975, 72, 5135–5139 CrossRef CAS
.
- E. R. Nestmann, E. G.-H. Lee, T. I. Matula, G. R. Douglas and J. C. Mueller, Mutat. Res., 1980, 79, 203–212 CAS
.
- R. P. Bos, R. M. E. Brouns, R. Van Doorn, J. L. G. Theuws and P. T. Henderson, Mut. Res., 1981, 88, 273–279 CAS
.
- L. E. Kier, D. J. Brusick, A. E. Auletta, E. S. Von Halle, M. M. Brown, V. F. Simmon, V. Dunkel, J. McCann, K. Mortelmans, M. Prival, T. K. Rao and V. Ray, Mutat. Res., Genet. Toxicol., 1986, 168, 69–240 CrossRef CAS
.
- J. Whysner, M. V. Reddy, P. M. Ross, M. Mohan and E. A. Lax, Mutat. Res., Rev. Mutat. Res., 2004, 566, 99–130 CrossRef CAS
.
- Source: European Chemicals Agency, No Title, http://echa.europa.eu/.
- D. Loomis, K. Z. Guyton, Y. Grosse, F. El Ghissassi, V. Bouvard, L. Benbrahim-Tallaa, N. Guha, N. Vilahur, H. Mattock and K. Straif, Lancet Oncol., 2017, 2045, 1–2 Search PubMed
.
- R. Pahlman and O. Pelkonen, Carcinogenesis, 1987, 8, 773–778 CrossRef CAS
.
-
OECD, Guidelines for testing of chemicals 105: water solubility, 1995 Search PubMed
.
- F. Stock, J. Hoffmann, J. Ranke, R. Störmann, B. Ondruschka and B. Jastorff, Green Chem., 2004, 6, 286–290 RSC
.
- J. Ranke, K. Mölter, F. Stock, U. Bottin-Weber, J. Poczobutt, J. Hoffmann, B. Ondruschka, J. Filser and B. Jastorff, Ecotoxicol. Environ. Saf., 2004, 58, 396–404 CrossRef CAS
.
-
OECD, Guidelines for the Testing of Chemicals, 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test, 2011 Search PubMed
.
-
OECD, Guidelines for the Testing of Chemicals 221: Lemna sp. Growth Inhabition Test, 2006 Search PubMed
.
- J. Zhang, M. T. Empl, C. Schwab, M. I. Fekry, C. Engels, M. Schneider, C. Lacroix, P. Steinberg and S. J. Sturla, Toxicol. Sci., 2017, 159, 266–276 CrossRef CAS
.
- K. Mortelmans and E. Zeiger, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2000, 455, 29–60 CrossRef CAS
.
-
OECD, Guidelines for testing of chemicals 301: ready Biodegradability, 1992 Search PubMed
.
- J. Arning, S. Stolte, A. Böschen, F. Stock, W.-R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2008, 10, 47 RSC
.
- C. Clemedson and B. Ekwall, Toxicol. In Vitro, 1999, 13, 657–663 CrossRef CAS
.
- S. Stolte, J. Arning, U. Bottin-Weber, A. Müller, W.-R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2007, 9, 760–767 RSC
.
- B. J. Dean, Mutat. Res., Genet. Toxicol., 1985, 154, 153–181 CrossRef CAS
.
- K. Mortelmans, S. Haworth, T. Lawlor, W. Speck, B. Tainer and E. Zeiger, Environ. Mutagen., 1986, 8, 1–52 CrossRef CAS
.
- J. W. Gorrod and D. J. Temple, Xenobiotica, 1976, 6, 265–274 CrossRef CAS
.
- R. Schoeny and D. Warshawsky, Mutat. Res., 1987, 188, 275–286 CAS
.
- E. J. LaVoie, J. Defauw, M. Fealy, B. M. Way and C. A. McQueen, Carcinogenesis, 1991, 12, 217–220 CrossRef CAS PubMed
.
- K. Takahashi, M. Kamiya, Y. Sengoku, K. Kohda and Y. Kawazoe, Chem. Pharm. Bull., 1988, 36, 4630–4633 CrossRef CAS
.
- P. Bowden, K.-T. Chung and A. W. Andrews, J. Natl. Cancer Inst., 1976, 57, 921–924 CrossRef
.
- D. A. Kaden, R. A. Hites and W. G. Thilly, Cancer Res., 1979, 39, 4152–4159 CAS
.
- C. Brinkmann and A. Eisentraeger, Environ. Sci. Pollut. Res. Int., 2008, 15, 211–217 CrossRef CAS PubMed
.
- W. H. Koch, E. N. Henrikson, E. Kupchella and T. A. Cebula, Carcinogenesis, 1994, 15, 79–88 CrossRef CAS PubMed
.
-
ECHA, Guidance on information requirements and chemical safety assessment, European Chemical Agency, 2012 Search PubMed
.
- A. Eisentraeger, C. Brinkmann, H. Hollert, A. Sagner, A. Tiehm and J. Neuwoehner, Environ. Technol. Chem., 2008, 27, 1590–1596 CAS
.
- The American Petroleum Institute Petroleum HPV Testing Group, 2012, 1–152.
-
S. Galassi, M. Mingazzini, L. Vigano, D. Cesareo and M. L. Tosato, Approaches to modeling toxic responses of aquatic organisms to aromatic hydrocarbons, Academic Press, 1988, vol. 16 Search PubMed
.
- B. R. Niederlehner, J. J. Cairns and E. P. Smith, Ecotoxicol. Environ. Saf., 1998, 39, 136–146 CrossRef CAS PubMed
.
- C. R. Janssen and G. Persoone, Environ. Toxicol. Chem., 1993, 12, 711–717 CAS
.
- European Chemicals Bureau, DOI:10.2788/40195.
-
T. C. Hutchinson, J. A. Hellebust, D. Tam, D. Mackay, R. A. Mascarenhas and W. Y. Shiu, Hydrocarbons and Halogenated Hydrocarbons in the Aquatic Environment, Springer US, Boston, MA, 1980, pp. 577–586 Search PubMed
.
- J. Hermens, H. Canton, P. Janssen and R. De Jong, Aquat. Toxicol., 1984, 5, 143–154 CrossRef CAS
.
- A. M. Bobra, W. Y. Shin and D. Mackay, Chemosphere, 1983, 12, 1121–1129 CrossRef CAS
.
-
Finnish Safety and Chemicals Agency, Substance Evaluation Conclusion as required by REACH Article 48 and Evaluation Report for Methylcyclohexane, 2017 Search PubMed
.
- B. I. Escher and J. L. M. Hermens, Environ. Sci. Technol., 2002, 36, 4201–4217 CrossRef CAS
.
- H. Könemann, Toxicology, 1981, 19, 209–221 CrossRef
.
- P. Mayer and F. F. Reichenberg, Environ. Toxicol. Chem., 2006, 25, 2639–2644 CrossRef CAS PubMed
.
- J. Aislabie, A. K. Bej, H. Hurst, S. Rothenburger and R. M. Atlas, Appl. Environ. Microbiol., 1990, 56, 345–351 CAS
.
- L. Wang, Y. Li and D. Yang, Process Biochem., 2010, 45, 919–928 CrossRef CAS
.
- J.-P. Kaiser, Y. Feng and J.-M. Bollag, Microbiol. Rev., 1996, 60, 483–498 CAS
.
- S. S. Johansen, D. Licht, E. Arvin, H. Mosbæk and A. B. Hansen, Appl. Microbiol. Biotechnol., 1997, 47, 292–300 CrossRef CAS
.
- H.-K. Hund, A. de Beyer and F. Lingens, Biol. Chem. Hoppe-Seyler, 1990, 371, 1005–1008 CrossRef CAS
.
- I. Bauer, A. de Beyer, B. Tshisuaka, S. Fetzner and F. Lingens, FEMS Microbiol. Lett., 1994, 117, 299–304 CrossRef CAS
.
- G. Dembek, T. Rommel, F. Lingens and H. Höke, FEBS Lett., 1989, 246, 113–116 CrossRef CAS
.
- S. Fetzner, Appl. Microbiol. Biotechnol., 1998, 49, 237–250 CrossRef CAS
.
-
W. Fritsche and H. Martin, in Biotechnology, Environmnetal processes II, ed. H.-J. Rehm and G. Reed, Wiley-VCH Verlag GmbH, Weinheim, Germany, 2000, vol. 14, pp. 146–164 Search PubMed
.
- S. H. Ko and J.
M. Lebeault, J. Appl. Microbiol., 1999, 87, 72–79 CrossRef CAS PubMed
.
- J. J. Perry, Microbiol. Rev., 1979, 43, 59–72 CAS
.
- H. W. Beam and J. J. Perry, Arch. Mikrobiol., 1973, 91, 87–90 CAS
.
- C. Zhao, Y. Zhang, X. Li, D. Wen and X. Tang, J. Hazard. Mater., 2011, 190, 253–259 CrossRef CAS PubMed
.
-
European Chemicals Bureau, European Union Risk Assessment Report – Benzene, 2008 Search PubMed
.
- K. S. Price, G. T. Waggy and R. A. Conway, J. – Water Pollut. Control Fed., 1974, 46, 63–77 CAS
.
- M. Yang, C. Han, G. Ni, J. Wu and H. Cheng, Int. J. Hydrogen Energy, 2012, 37, 12839–12845 CrossRef CAS
.
- M. Amende, C. Gleichweit, S. Schernich, O. Höfert, M. P. A. Lorenz, W. Zhao, M. Koch, K. Obesser, C. Papp, P. Wasserscheid, H.-P. Steinrück and J. Libuda, J. Phys. Chem. Lett., 2014, 5, 1498–1504 CrossRef CAS PubMed
.
- A. Y. Renouxa, D. Millette, R. D. Tyagi and R. Samson, Water Res., 1999, 33, 2045–2052 CrossRef
.
- R. E. A. Madill, M. T. Orzechowski, G. Chen, B. G. Brownlee and N. J. Bunce, Environ. Toxicol., 2001, 16, 197–208 CrossRef CAS PubMed
.
- J. Neuwoehner, A.-K. Reineke, J. Hollender and A. Eisentraeger, Ecotoxicol. Environ. Saf., 2009, 72, 819–827 CrossRef CAS PubMed
.
- R. Kühn and M. Pattard, Water Res., 1990, 24, 31–38 CrossRef
.
- D. A. Birkholz, R. T. Coutts, S. E. Hrudey, R. W. Danell and W. L. Lockhart, Water Res., 1990, 24, 67–73 CrossRef CAS
.
- J. L. M. Hermens, H. Canton, N. Steyger and R. Wegman, Aquat. Toxicol., 1984, 5, 315–322 CrossRef CAS
.
- A. Okamoto, M. Yamamuro and N. Tatarazako, J. Appl. Toxicol., 2015, 35, 824–830 CrossRef CAS PubMed
.
- W. Y. Shiu, M. Bobra, A. M. Bobra, A. Maijanen, L. Suntio and D. Mackay, Oil Chem. Pollut., 1990, 7, 57–84 CrossRef CAS
.
- R. Marchal, S. Penet, F. Solano-Serena and J. P. Vandecasteele, Oil Gas Sci. Technol., 2003, 58, 441–448 CrossRef CAS
.
- R. M. Atlas, Microbiol. Rev., 1981, 45, 180–209 CAS
.
- D. Schuetzle, F. S.-C. Lee, T. J. Prater and S. B. Tejada, Int. J. Environ. Anal. Chem., 1981, 9, 93–144 CrossRef CAS PubMed
.
- E. T. Wei and H. P. Shu, Am. J. Public Health, 1983, 73, 1085–1088 CrossRef CAS PubMed
.
- M. Sjögren, H. Li, C. Banner, J. Rafter, R. Westerholm and U. Rannug, Chem. Res. Toxicol., 1996, 9, 197–207 Search PubMed
.
- J. Seagrave, J. D. McDonald, A. P. Gigliotti, K. J. Nikula, S. K. Seilkop, M. Gurevich and J. L. Mauderly, Toxicol. Sci, 2002, 70, 212–226 CrossRef CAS PubMed
.
- T. P. Vanzella, C. B. R. Martinez and I. M. S. Cólus, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2007, 631, 36–43 CAS
.
- E. R. Kisin, X. C. Shi, M. J. Keane, A. B. Bugarski and A. A. Shvedova, J. Environ. Eng. Ecol. Sci., 2013, 2 DOI:10.7243/2050-1323-2-3
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ee01696h |
| ‡ Fraunhofer Institute of Industrial Engineering, http://www.iao.fraunhofer.de |
| § Areva GmbH, http://de.areva.com |
| ¶ Advanced Hydrogen Energy Chain Association for Technology Development, http://www.ahead.or.jp |
| || Hynertech, http://www.hynertech.com |
| ** H2-Industries SE, http://www.h2-industries.com |
| †† According to ECHA (details available from http://echa.europe.eu/). |
| This journal is © The Royal Society of Chemistry 2019 |