Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly branched poly(N-isopropyl acrylamide) functionalized with an inducer molecule suppresses quorum sensing in Chromobacterium violaceum†
Joanna
Shepherd
 *a,
Thomas
Swift
*a,
Thomas
Swift
 b,
Chien-Yi
Chang
b,
Chien-Yi
Chang
 b,
James R.
Boyne
b,
James R.
Boyne
 c,
Stephen
Rimmer
c,
Stephen
Rimmer
 b and
William H. C.
Martin
b and
William H. C.
Martin
 b
b
aSchool of Clinical Dentistry, University of Sheffield, Sheffield, S10 2TA, UK. E-mail: j.shepherd@sheffield.ac.uk
bSchool of Chemistry and Biosciences, University of Bradford, Bradford, BD7 1DP, UK
cSchool of Applied Sciences, University of Huddersfield, HD1 3DH, UK
First published on 29th July 2019
Abstract
Bacterial quorum sensing has been implicated in a number of pathogenic bacterial processes, such as biofilm formation, making it a crucial target for developing materials with a novel antibiotic mode of action. This paper describes poly(N-isopropyl acrylamide) that has been covalently linked, at multiple chain ends, to homoserine lactone to give a highly branched polymer functionalized with a key messenger molecule implicated in QS. This novel functional material has shown promising anti-QS activity in a Chromobacterium violaceum assay.
Quorum sensing (QS) intercellular communication allows individual bacteria to modify their behaviour collectively in response to the local population density through small molecules termed autoinducers (AI). QS has been implicated in bacterial gene regulatory networks for regulating and coordinating several bacterial behaviours including biofilm formation, virulence expression and antibiotic resistance.1,2 Genes responsible for AI production are also included among the QS-regulated genes leading to an autoinductive signalling feedback loop and the modulation of pathogenic factors.3 A number of different classes of compounds have been identified as acting as inducers in QS.3N-Acyl homoserine lactones (AHL) are major signalling molecules synthesised by Luxl homologs that induces LuxR homologs in Gram-negative bacteria. Examples include as N-butanoyl-homoserine lactone and N-(3-oxododecanoyl)-homoserine lactone produced by pathogenic Pseudomonas aeruginosa.4 Around 10% of downstream genes, including key virulence genes, in the genome of P. aeruginosa are regulated by these two AHL mediated QS systems both directly and indirectly.5,6 In Chromobacterium violaceum the production of violacein, a purple pigment with antibacterial activity, is regulated by the cvil/cviR (luxl/luxR homolog, respectively) QS system through N-hexanoyl-L-homoserine lactone. The C. violaceum CV026 strain, a cviI mutant, only produces violacein in presence of exogenic AHLs.7 This indicative feature makes CV026 a convenient biosensor for detecting exogenic AHL molecules or investigating AHL-based QS inhibitors when supplying exogenic AHLs.8 Due to the regulatory roles of QS in bacterial virulence, the disruption of QS offers a novel way in which to potentially treat bacterial infections. It is particularly attractive since QS is not required for survival of bacteria, so anti-QS therapies are suggested to be unlikely to lead to bacteria developing antimicrobial resistance, although this is hotly contested.1,9 As such, there have been considerable research efforts directed at disrupting the separate stages of the bacterial QS process.10 Approaches include halting the action of the LuxI region responsible for the synthesis of the AHL molecules, sequestering the inducers once they have been released into the intrabacterial matrix, and blocking the LuxR receptor region through the synthesis of small molecule antagonists. Each of these lines of enquiry has showed promising results, but an antimicrobial treatment based on disrupting QS remains elusive.1,10 Functionalized polymers have recently emerged as a potential tool in disrupting QS, with examples reported of the use of linear polymers to sequester AI molecules through covalent and non-covalent interactions. Also, recently Piletska and coworkers have developed molecular imprinted polymers for the passive sequestration of AHL molecules11–13 and others have used polymer films to release AHL based agonists14 or probe furanosyl borate ester (known as autoinducer-2 [AI-2]).15 Other systems known in the literature include studies of polymers functionalized with a diol on the back bone to competitively bind boronic acid for AI-2 production.16 To our knowledge, the direct functionalization of polymers with AIs has not been investigated, but this would seem to offer an alternative mechanism for disrupting QS.
We have recently demonstrated that highly branched (HB) polymer architectures have an advantage over linear polymer systems with regards to biological binding.17 HB-poly(N-isopropylacrylamide) (HB-PNIPAM) polymers can be readily functionalized at the chain end with ligands. In previous work it was shown that placing other biological ligands (vancomycin, polymyxin, GRGDS) at the branched polymer chain ends ensured they were available for binding both above and below the LCST, retaining functionality when the polymer was deosolvated, in contrast to linear analogues where ligands were masked at elevated temperatures.18,19 Thus, the advantage in the use of branched polymers with ligands at the chain ends is that the ligands remain expressed at the outer domains of the polymer coil, at all temperatures and in both bound and non-bound states. Accordingly, desolvation of the polymer from the open chain to the globular form did not prevent HB-PNIPAM with cell-binding end groups binding to interact directly with the bacteria as opposed to merely passively sequestering AI molecules.
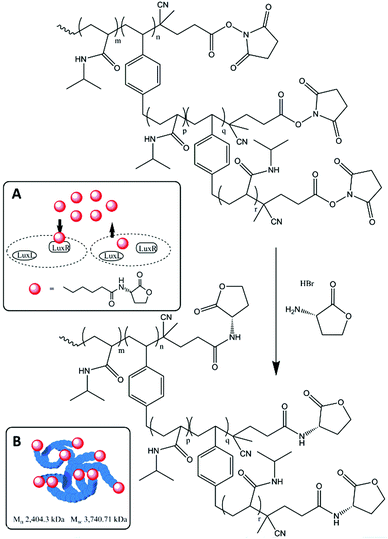
This communication describes the synthesis of a HB-PNIPAM modified with HL on its chain ends and the efficacy of this functionalized material in disruption of QS in C. violaceum. HB-PNIPAM with a ratio of NIPAM to branching units of 25 to 1 was prepared.20 Homo-serine lactone was attached to the chain ends by amidation of the end groups (activated as N-hydroxysuccinimides) as shown in Fig. 1. This produced a material that contained the homoserine lactone ring with a short alkyl linker onto the PNIPAM repeat units.
Full polymer synthesis and characterization is shown in the ESI.† As seen in previous studies of highly branched polymers the self-condensing vinyl polymerization produced a bimodal distribution with high dispersity.20 However, after post functionalization the low molar mass portion is removed (alongside any unreacted residual AHL) via ultra-filtration leaving only the high molar mass fraction of the polymer. Conjugation of chain ends with HL led to a substantial increase in the average molar masses. This was supported by diffusion ordered spectroscopy (DOSY) 1H NMR in chloroform and the hydrodynamic radii of the polymers was shown to have increased by more than 3 nm following functionalization and purification (see ESI† for full details).
Analysis of the HB-PNIPAM, HB-PNIPAM-COOH and HB-PNIPAM-HL polymers, using differential scanning calorimetry, showed that the properties of the LCST had substantially changed due to the modification of the chain end group (see ESI†). This indicated that at temperatures, which span laboratory experimental assays for quorum sensing through to body temperature (around 32 °C for skin temperature), both polymers will be desolvated and exist in a globular state. However, we have recently shown that several different functional end segments of this class of polymer can remain solvated in a core–shell morphology in which core is desovated but the shell is solvated before binding.17,19–21 In this core–shell structure or the fully desolvated state the chain ends of the polymer remain exposed to the solvent, thus ensuring that the HL groups are likely available for binding.
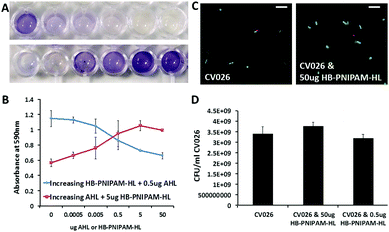
Initial tests using the CV026 biosensor assay indicated HB-PNIPAM-HL was able to block AHL signalling in CV026 bacteria. CV026 was co-incubated with both HB-PNIPAM-HL and AHL. After 48 hours co-incubation, AI signalling was indicated from the increase in intensity at 590 nm (Fig. 2). In the absence of polymer there was an increase in signalling with increasing concentrations of AHL as expected (Fig. 2a). The presence of HB-PNIPAM-HL blocked this signalling in a concentration dependent manner (Fig. 2a and b). It is important to note the lack of signalling observed is not due to any bactericidal effects of the polymer. CV026 incubated with HB-PNIPAM-HL for 48 h remained viable. The live cell counts were comparable to bacteria incubated in LB broth. This was tested via Live/Dead staining, which showed no difference in viable cell numbers between experimental groups (Fig. 2c and d).
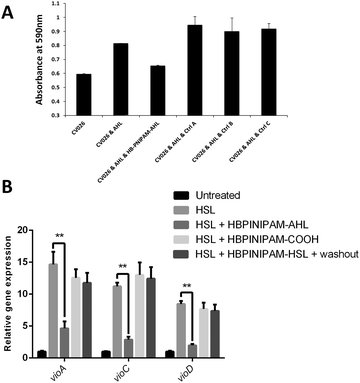
As discussed, previous studies investigating the use of polymeric systems as anti-QS agents have focused on the polymer's ability to sequester AI molecules. Therefore, it was important to examine the capacity of control polymers, not functionalised with AHL, to sequester AHL. As a control experiment HB-PNIPAM, with carboxylic acid end groups was used in place of HB-PNIPAM-HL and this had no effect on violacein production at any concentration (Fig. 3). These data indicated that HB-PNIPAM-HL is not acting as a sequestering agent. Next, we sought to confirm this hypothesis by assessing the impact of HB-PNIPAM-HL on the transcription levels of several CviR-target genes in the presence and absence of HB-PNIPAM-HL. As can be seen in Fig. 3B, co-incubation of CV026 with AHL led to a significant increase in the expression of vioA, vioC and vioD, compared with the untreated control. Strikingly, this increase was dramatically and significantly (p < 0.01) reduced in cells co-treated with AHL and HB-PNIPAM-HL, but not with the control polymer (HB-PNIPAM).
Violacein production in C. violaceum is promoted by a LuxR-type regulator, CviR. After binding to its native ligand, C6-HSL, CviR-AHL complex will be stabilized and form into a dimer to bind to DNA and to interact with RNA polymerase for target gene expression. A study has shown that lengthening of the acyl tail of AHLs enhances the antagonism of C. violaceum quorum sensing.22 Crystal structure data from this work suggests that antagonist binding increases the distance of DNA-binding domains between two subunits of dimeric CviR resulting in unsuccessful DNA binding and RNA polymerase interaction. Also antagonist binding promotes the formation of the closed and inactive conformation of CviR-AHL dimers compared to the predominantly open and competent structure when binding to native ligand.22 The data showed that the highly branched polymer (HB-PNIPAM-HL) inhibited violacein production in C. violaceum and it may also have a strong antagonistic effect on CviR activity.
During auto-induction AHLs cross the cell membrane and bind to LuxR-type proteins in the cytoplasm. The Lux-R-AHL complex then induces transcription of quorum sensing genes. Thus, implicit in the data reported here is the realisation that the HB-PNIPAM-HL is predicted to enter the cytoplasm. The precise mechanism of how this would occur is not available but it is interesting to note that DOSY NMR data of the polymer in water indicates the hydrodynamic radii is ≈17 nm (the size distribution is broad with an interquartile range from 14 to 22 nm – see ESI†) and this seems to be too large for channel mediated transport. More work is required on this aspect but we have previously shown that similar branched amphipathic polymers are also capable of crossing the membranes in eukaryotic cells.23 Also the HB-PNIPAM-HL molecule chain ends are indicated to interact with the LuxR receptors despite the structural difference in the PNIPAM chain compared to the alkyl linker in the small molecule AI.
To our knowledge, this is the first example of an anti-quorum-sensing polymer therapeutic that does not depend on sequestration of small molecules, or blocking metabolic pathways to prevent of AI generation. This is an important observation both in our evolving understanding of the biochemical processes of QS and development of future therapeutics to disperse/prevent biofilm formation.
In conclusion, the first highly branched polymer functionalized with quorum sensing auto inducers has been synthesized. This novel functional material has been shown to act as an anti-quorum sensing compound. Examining the gene expression of bacteria in the presence of polymers showed that the polymer scaffold interrupts the dimerization of LuxR homologs crucial for downstream signalling preventing AHL from inducing QS.
We gratefully acknowledge funding from the MRC/DBT MR/N501888/2. We also thank Dr Jonathan Shaw (The University of Sheffield) for the C. violaceum CV026 bacteria and David Pownall at the University of Bradford for assistance developing polymer synthetic protocols (Wellcome Trust 099800/C/12/Z).
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Whiteley, S. P. Diggle and E. P. Greenberg, Progress in and Promise of Bacterial Quorum Sensing Research, Nature, 2017, 551, 313–320 CrossRef CAS PubMed.
- W.-L. Ng and B. L. Bassler, Bacterial Quorum-Sensing Network Architectures, Annu. Rev. Genet., 2009, 43, 197–222 CrossRef CAS PubMed.
- K. Papenfort and B. L. Bassler, Quorum Sensing Signal-Response Systems in Gram-negative Bacteria, Nat. Rev. Microbiol., 2016, 14, 576–588 CrossRef CAS PubMed.
- C. Fuqua, M. R. Parsek and E. P. Greenberg, Regulation of Gene Expression by Cell-to-Cell Communication: Acyl-Homoserine Lactone Quorum Sensing, Annu. Rev. Genet., 2001, 439–468 CrossRef CAS PubMed.
- P. Williams and M. Cámara, Quorum Sensing and Environmental Adaptation in Pseudomonas Aeruginosa: A Tale of Regulatory Networks and Multifunctional Signal Molecules, Curr. Opin. Microbiol., 2009, 12(2), 182–191 CrossRef CAS PubMed.
- J. Lee and L. Zhang, The Hierarchy Quorum Sensing Network in Pseudomonas Aeruginosa, Protein Cell, 2015, 6(1), 26–41 CrossRef CAS PubMed.
- K. H. Mcclean, M. K. Winson, L. Fish, A. Taylor, S. R. Chhabra, M. Camara, M. Daykin, H. John, S. Swift and B. W. Bycroft, et al., Quorum Sensing and Chromobacterium Violaceum: Exploitation of Violacein Production and Inhibition for the Detection of N-Acyl Homoserine Lactones, Microbiology, 1997, 3703–3711 CrossRef CAS PubMed.
- C. L. Koh, C. K. Sam, W. F. Yin, L. Y. Tan, T. Krishnan, Y. M. Chong and K. G. Chan, Plant-Derived Natural Products as Sources of Anti-Quorum Sensing Compounds, Sensors, 2013, 13(5), 6217–6228 CrossRef CAS PubMed.
- S. P. Diggle, Microbial Communication and Virulence: Lessons from Evolutionary Theory, Microbiology, 2010, 156, 3503–3512 CrossRef CAS PubMed.
- T. Defoirdt, Quorum-Sensing Systems as Targets for Antivirulence Therapy, Trends Microbiol., 2018, 26(4), 313–328 CrossRef CAS PubMed.
- E. Cavaleiro, A. S. Duarte, A. C. Esteves, A. Correia, M. J. Whitcombe, E. V. Piletska, S. A. Piletsky and I. Chianella, Novel Linear Polymers Able to Inhibit Bacterial Quorum Sensing, Macromol. Biosci., 2015, 647–656 CrossRef CAS PubMed.
- E. V. Piletska, G. Stavroulakis, K. Karim, M. J. Whitcombe, I. Chianella, A. Sharma, K. E. Eboigbodin, G. K. Robinson and S. A. Piletsky, Attenuation of Vibrio Fischeri Quorum Sensing Using Rationally Designed Polymers, Biomacromolecules, 2010, 975–980 CrossRef CAS PubMed.
- E. V. Piletska, G. Stavroulakis, L. D. Larcombe, M. J. Whitcombe, A. Sharma, S. Primrose, G. K. Robinson and S. A. Piletsky, Passive Control of Quorum Sensing: Prevention of Pseudomonas Aeruginosa Biofilm Formation by Imprinted Polymers, Biomacromolecules, 2011, 1067–1071 CrossRef CAS PubMed.
- A. S. Breitbach, A. H. Broderick, C. M. Jewell, S. Gunasekaran, Q. Lin, D. M. Lynn and H. E. Blackwell, Surface-Mediated Release of a Synthetic Small-Molecule Modulator of Bacterial Quorum Sensing: Gradual Release Enhances Activity, Chem. Commun., 2011, 47, 370–372 RSC.
- A. L. Garner, J. Park, J. S. Zakhari, C. A. Lowery, A. K. Struss, D. Sawada, G. F. Kaufmann and K. D. Janda, A Multivalent Probe for AI-2 Quorum-Sensing Receptors, J. Am. Chem. Soc., 2011, 133, 15934–15937 CrossRef CAS PubMed.
- L. T. Lui, X. Xue, C. Sui, A. Brown, D. I. Pritchard, N. Halliday, K. Winzer, S. M. Howdle, F. Fernandez-Trillo and N. Krasnogor, et al., Bacteria Clustering by Polymers Induces the Expression of Quorum-Sensing-Controlled Phenotypes, Nat. Chem., 2013, 1058–1065 CrossRef CAS PubMed.
- P. Teratanatorn, R. Hoskins, T. Swift, C. W. I. Douglas, J. Shepherd and S. Rimmer, Binding of Bacteria to Poly(N-Isopropylacrylamide) Modified with Vancomycin: Comparison of Behavior of Linear and Highly Branched Polymers, Biomacromolecules, 2017, 18(9), 2887–2899 CrossRef CAS PubMed.
- P. Sarker, K. Swindells, C. W. I. Douglas, S. MacNeil, S. Rimmer and L. Swanson, Förster Resonance Energy Transfer Confirms the Bacterial-Induced Conformational Transition in Highly-Branched Poly(N-Isopropyl Acrylamide) with Vancomycin End Groups on Binding to Staphylococcus Aureus, Soft Matter, 2014, 10(31), 5824 RSC.
- T. Swift, J. Lapworth, K. Swindells, L. Swanson and S. Rimmer, PH Responsive Highly Branched Poly(N-Isopropylacrylamide) with Trihistidine or Acid Chain Ends, RSC Adv., 2016, 6(75), 71345–71350 RSC.
- R. Plenderleith, T. Swift and S. Rimmer, Highly-Branched Poly(N-Isopropyl Acrylamide)s with Core–shell Morphology below the Lower Critical Solution Temperature, RSC Adv., 2014, 4(92), 50932–50937 RSC.
- T. Swift, M. Katsikogianni, R. Hoskins, P. Teratanatorn, I. Douglas, S. MacNeil and S. Rimmer, Highly-Branched Poly(N-Isopropyl Acrylamide) Functionalised with Pendant Nile Red and Chain End Vancomycin for the Detection of Gram-positive Bacteria, Acta Biomater., 2019, 87, 197–206 CrossRef CAS PubMed.
- G. Chen, L. R. Swem, D. L. Swem, D. L. Stauff, C. T. O’Loughlin, P. D. Jeffrey, B. L. Bassler and F. M. Hughson, A Strategy for Antagonizing Quorum Sensing, Mol. Cell, 2011, 199–209 CrossRef CAS PubMed.
- S. Hopkins, S. Carter, L. Swanson, S. MacNeil and S. Rimmer, Temperature-Dependent Phagocytosis of Highly Branched Poly(N-Isopropyl Acrylamide-Co-1,2 Propandiol-3-Methacrylate)s Prepared by RAFT Polymerization, J. Mater. Chem., 2007, 17(38), 4022–4027 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, chemical characterisation and sequestering studies. See DOI: 10.1039/c9cc02524c |
| This journal is © The Royal Society of Chemistry 2019 |