Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Accelerated recombination of lophyl radicals and control of the surface tension with amphiphilic lophine dimers†
Masaaki
Akamatsu
 *a,
Kazuki
Kobayashi
a,
Kenichi
Sakai
*a,
Kazuki
Kobayashi
a,
Kenichi
Sakai
 ab and
Hideki
Sakai
*ab
ab and
Hideki
Sakai
*ab
aDepartment of Pure and Applied Chemistry, Faculty of Science and Technology, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba, 278-8510, Japan. E-mail: makamatsu@rs.tus.ac.jp; hisakai@rs.noda.tus.ac.jp
bResearch Institute for Science and Technology, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba, 278-8510, Japan
First published on 15th July 2019
Abstract
We report the accelerated photoisomerization of amphiphilic lophine dimers based on the inner environments of molecular assemblies and rapid control of the interfacial properties of aqueous solution with photoirradiation. This novel photoisomerization system enables on-demand controlled release of drugs, perfumes, and other active compounds.
Photochromism, which refers to the reversible isomerization and color variations upon irradiation with light of specific wavelengths, has been utilized for controlling the functions of molecules or assemblies. For example, the photoswitchable morphologies of molecular assemblies facilitate the regulation of solution properties such as surface tension, solubilization capacity, and viscosity.1,2 Photoresponsive molecular assemblies are applied in the controlled release of perfumes and drugs, and regulated heat transfer performance of solutions.3,4 We report the reversible control of solubilization capacities for oily substances, and solution viscosity based on a cationic azobenzene-type surfactant, upon photoirradiation.5–7 Although these properties have been successfully controlled with the photoresponsive compounds, the conventional photoresponsive molecules and molecular assemblies require minutes or hours to induce significant variations in the properties.6,8 In view of the promising applications, the response speed by light irradiation should be fast and should occur at an arbitrary moment. Although kinetics of the photoresponsive surfactant systems have been examined by some research groups,9–11 the acceleration of controlled systems has not yet been investigated.
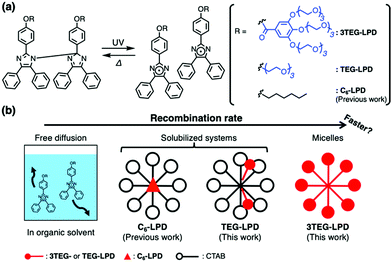
A lophine dimer rapidly dissociates into two lophyl radicals with coloration, upon ultraviolet (UV) irradiation.12 These radical species thermally convert to the initial dimer form through recombination (Fig. 1a). However, this thermal recombination reaction is extremely slow owing to the low collision frequency, because the radicals freely diffuse in the solution. Abe and co-workers accomplished rapid recombination by covalently connecting two lophine moieties through a linker to inhibit the diffusion of the produced radicals.13–15 Strehmel and co-workers reported the accelerated recombination of lophyl radicals in ionic liquids (ILs).16 They assumed that a microscopic domain formed spontaneously by IL molecules serves as a confined space that inhibits the free diffusion of radicals. Furthermore, our group succeeded in accelerating the rate of recombination of lophyl radicals using the inner environment of molecular assemblies as confined nano-space.17 The solubilization of the lipophilic lophine dimer with alkyl chains (C6-LPD), in the micellar solution of cetyltrimethylammonium bromide (CTAB), enhanced the recombination of the lophyl radicals, which is 100-fold higher than that in an organic solvent (Fig. 1a). We demonstrated rapid photoisomerization of the lophine dimer using the spontaneously formed supramolecular space, with a simple lophine dimer analogue. On the basis of this result, we assumed that an increase in the concentration of lophine dimers in micelles further accelerates the recombination. Although, Abe and co-workers reported the formation of vesicles consisting of amphiphilic lophine dimers,18 control of the interfacial properties using photoisomerization of the lophine dimer has not yet been achieved. In this study, we prepared micelles composed of amphiphilic lophine dimers to shorten the diffusion distance of the lophyl radicals and examine the rates of the recombination of lophyl radicals produced by photoirradiation (Fig. 1). Furthermore, rapid control of the interfacial properties of aqueous solution upon photoirradiation was demonstrated. This rapid control is the basis of various applications such as on-demand controlled release of drugs, perfumes, and other active species.
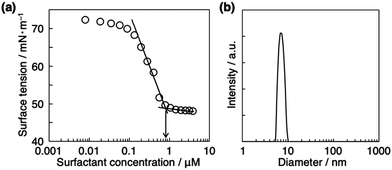
Amphiphilic lophine dimers bearing six or two triethylene glycol (TEG) groups (3TEG-LPD or TEG-LPD) were synthesized as shown in Scheme S1 (ESI†). However, TEG-LPD was insoluble in water. We characterized the interfacial properties of an aqueous solution of water-soluble 3TEG-LPD in the absence of photoirradiation. Fig. 2a shows the static surface tension of aqueous solutions of 3TEG-LPD as a function of various concentrations at 25 °C. The plots indicate a typical surfactant behavior. The critical micellar concentration (cmc) is 0.80 μM, and the surface tension measured at cmc (γcmc) is 46.5 mN m−1. The dynamic light scattering measurement of the aqueous 3TEG-LPD solution at 3.0 mM above the cmc shows that the average particle diameter of the formed micelle is 7.4 nm19 (Fig. 2b), which is consistent with the size of the 3TEG-LPD molecule (ca. 3.1 nm) according to the stabilized structure shown in Fig. S1 (ESI†). These results indicate that the water-soluble lophine dimer derivative exhibits excellent interfacial properties and spontaneously forms the micellar aggregates.
Since water-insoluble TEG-LPD molecules do not form molecular assemblies, TEG-LPD was further solubilized in an aqueous cetyltrimethylammonium bromide (CTAB) solution. The solubilization capability of TEG-LPD in 50 mM aqueous CTAB solution was larger than that of lipophilic C6-LPD. The number of TEG-LPD molecules in the CTAB micelle was estimated to be approximately 2, which is higher than that of lipophilic C6-LPD molecules (less than 1).17 The nuclear Overhauser effect spectroscopy (NOESY) data of the TEG-LPD/CTAB mixture included signals indicative of the protons of TEG units on TEG-LPD that showed correlations with the protons around the hydrophilic group of CTAB (Fig. 3). Furthermore, correlations for the protons of the lophine dimer core and an alkyl chain of CTAB were observed. These results imply that the respective hydrophilic and hydrophobic groups of TEG-LPD or CTAB are closely packed in the micelles. The good affinity of the amphiphilic lophine dimer improved the solubilization capability in CTAB micelles. The introduction of amphiphilicity on the lophine dimer skeleton enabled the accumulation of the lophine dimers in the supramolecular nano-space.
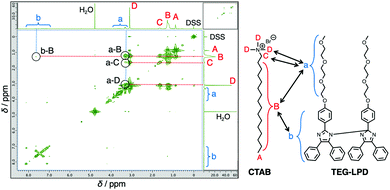 | ||
| Fig. 3 NOESY NMR spectrum of TEG-LPD/CTAB (5 mM/50 mM) in D2O with sodium 3-(trimethylsilyl)-1-propanesulfonate (DSS) as an internal standard. | ||
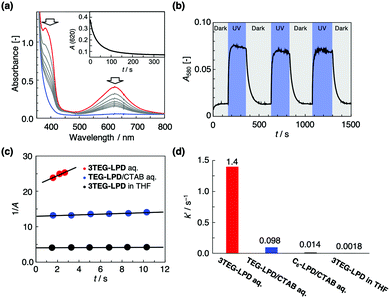
We succeeded in confining and concentrating the amphiphilic lophine dimers by micellization and solubilization for efficient recombination of the lophyl radicals. The photoisomerization of the lophine dimers in the aqueous solutions was evaluated by UV-vis absorption spectroscopy. For the 0.5 mM TEG-LPD/50 mM CTAB mixture in water, the UV irradiation (300–390 nm) resulted in the appearance of new bands at 380 nm and 620 nm, indicative of the lophyl radical (Fig. 4a). When the solution was kept in the dark, the absorption intensity of the band decreased and reached a stationary state in a few minutes, which indicates that the recombination reaction proceeds rapidly (inset of Fig. 4a). The 0.5 mM aqueous solution of 3TEG-LPD, which forms the micelles by itself, also indicated the appearance and disappearance of the band at 580 nm, indicative of the produced lophyl radical due to dimer–monomer photoisomerization (Fig. S2, ESI†). The excellent reversibility of the photoisomerization was confirmed by monitoring the band at 580 nm (Fig. 4b). This result indicates that the decomposition of the lophine dimers and reactions of the reactive lophyl radicals with other reactive molecules, such as water, do not occur during the photoisomerization, which promises low reactivity of the produced radicals with biological molecules when applied in drug delivery systems. In addition, the diameter of the formed micelle remained unchanged after UV light irradiation as shown in Fig. S3 (ESI†) and the formed micelles are stable.
As seen in Fig. 4c, the plots of 1/A vs. t, where A is the absorbance of lophyl radicals at time t, show good linearity, which indicates that the recombination follows a second-order rate reaction. The apparent reaction rates (k′) were calculated from the slope of the 1/A vs. t plots, since the initial concentrations of the lophyl radicals produced could not be determined (Fig. 4d). The recombination rate of 3TEG-LPD in the micelles was 1.4 s−1, which was accelerated by ∼800-fold in comparison to that in tetrahydrofuran (THF), where the micelles are not formed and the produced radicals diffuse freely. The rate is 100-fold higher than that of the reported lipophilic C6-LPD in CTAB micelles.17 The recombination rate of the 0.5 mM TEG-LPD/50 mM CTAB mixture in water is 50-fold higher than that in an organic solvent. The rate enhancement in the 3TEG-LPD micelle was better than that in TEG-LPD, since TEG-LPD was diluted in CTAB micelles, and resulted in free diffusion of lophyl radicals. This observation indicates that the confined and highly concentrated amphiphilic lophine dimer derivatives show rapid recombination of the lophyl radicals produced by UV irradiation.
We examined the rapid control of the static surface tension of the aqueous 3TEG-LPD solution by photoirradiation. The regulation of surface tension with photoirradiation enables the control of micellar formation and dispersion stability of emulsions owing to changes in the adsorption efficiency of the surfactants at interfaces, which provides controlled release of drugs or active compounds. A constant surface tension value of 5.0 mM aqueous 3TEG-LPD solution was observed to be ca. 46.5 mN m−1, in the dark (Fig. 5b). When the solution was irradiated with UV light, the surface tension value decreased within a few seconds. However, after certain duration, the surface tension immediately recovered to the original value in the absence of photoirradiation. These reversible changes were repeatedly observed. The decrease in the surface tension value upon UV irradiation suggested that the produced lophyl radical, which is less bulky than the initial dimer form, efficiently adsorbs at the air/water interface. After the irradiation was halted, the recombination of the radical species readily proceeded in the micelles of the bulk phase or in the Gibbs monolayer formed at the air/water interface. The measurement was carried out above the cmc because the produced lophyl radicals were insoluble in water and the surface tension of the aqueous 3TEG-LPD solution around the cmc was not measured correctly after UV light irradiation. This indicates that the water-insoluble lophyl radicals are located in the micelles and the Gibbs monolayer. As seen in Fig. 5a, in the absence of 3TEG-LPD, there is almost no change in the surface tension value after UV irradiation, however, the value slightly fluctuated within 0.1 mN m−1. Therefore, we achieved reversible and quick control of the surface tension using fast dissociation of the lophine dimer upon photoirradiation and accelerated the recombination of lophyl radicals in the micelles.
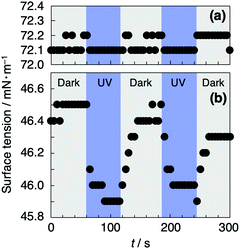 | ||
| Fig. 5 The static surface tension of water or 5.0 mM aqueous 3TEG-LPD solution as a function of time in the absence and presence of UV irradiation (a or b). | ||
In summary, we demonstrated accelerated photoisomerization of amphiphilic lophine dimers using the inside of the micelles and by rapid control of the surface tension. The recombination of the lophyl radicals, which are produced upon UV irradiation, was significantly enhanced in the micelles, formed by the amphiphilic lophine dimer (3TEG-LPD). These results indicate that the confined and concentrated lophine dimers in the micelles exhibit accelerated recombination of the lophyl radicals. The amphiphilic lophine dimer induced quick and reversible variations in the surface tension of the aqueous solution. Currently, we are working on the rapid control of the solubilization capability using the amphiphilic lophine dimer, which leads to on-demand controlled release of drugs, perfumes, and other active compounds.
Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Eastoe and A. Vesperinas, Soft Matter, 2005, 1, 338–347 RSC.
- P. Brown, C. P. Butts and J. Eastoe, Soft Matter, 2013, 9, 2365–2374 RSC.
- F. P. Hubbard, G. Santonicola, E. W. Kaler and N. L. Abbott, Langmuir, 2005, 21, 6131–6136 CrossRef CAS PubMed.
- H. Shi, W. Ge, H. Oh, S. M. Pattison, J. T. Huggins, Y. Talmon, D. J. Hart, S. R. Raghavan and J. L. Zakin, Langmuir, 2013, 29, 102–109 CrossRef CAS PubMed.
- Y. Orihara, A. Matsumura, Y. Saito, N. Ogawa, T. Saji, A. Yamaguchi, H. Sakai and M. Abe, Langmuir, 2001, 17, 6072–6076 CrossRef CAS.
- M. Akamatsu, P. A. Fitzgerald, M. Shiina, T. Misono, K. Tsuchiya, K. Sakai, M. Abe, G. G. Warr and H. Sakai, J. Phys. Chem. B, 2015, 119, 5904–5910 CrossRef CAS PubMed.
- H. Sakai, Y. Orihara, H. Kodashima, A. Matsumura, T. Ohkubo, K. Tsuchiya and M. Abe, J. Am. Chem. Soc., 2005, 127, 13454–13455 CrossRef CAS PubMed.
- H. Oh, A. M. Ketner, R. Heymann, E. Kesselman, D. Danino, D. E. Falvey and S. R. Raghavan, Soft Matter, 2013, 9, 5025–5033 RSC.
- H. Lee, K. K. Diehn, K. Sun, T. Chen and S. R. Raghavan, J. Am. Chem. Soc., 2011, 133, 8461–8463 CrossRef CAS PubMed.
- R. F. Tabor, M. J. Pottage, C. J. Garvey and B. L. Wilkinson, Chem. Commun., 2015, 51, 5509–5512 RSC.
- R. Lund, G. Brun, E. Chevallier, T. Narayanan and C. Tribet, Langmuir, 2016, 32, 2539–2548 CrossRef CAS PubMed.
- T. Hayashi and K. Maeda, Bull. Chem. Soc. Jpn., 1960, 33, 565–566 CrossRef CAS.
- F. Iwahori, S. Hatano and J. Abe, J. Phys. Org. Chem., 2007, 20, 857–863 CrossRef CAS.
- K. Fujita, S. Hatano, D. Kato and J. Abe, Org. Lett., 2008, 10, 3105–3108 CrossRef CAS PubMed.
- H. Yamashita and J. Abe, Chem. Commun., 2014, 50, 8468–8471 RSC.
- S. Berdzinski, J. Horst, P. Straßburg and V. Strehmel, ChemPhysChem, 2013, 14, 1899–1908 CrossRef CAS PubMed.
- M. Akamatsu, T. Suzuki, K. Tsuchiya, H. Masaki, K. Sakai and H. Sakai, Chem. Lett., 2018, 47, 113–115 CrossRef CAS.
- K. Mutoh and J. Abe, Chem. Commun., 2011, 47, 8868–8870 RSC.
- The DLS measurement was performed far above cmc (0. 80 μM) to obtain sufficient scattering intensity. However, we assume that the phase at 3.0 mM is almost the same as that above the cmc because both the solutions showed low viscosity and no birefringence, which suggests that significant growth and association of the micelles do not occur in the concentration region.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9cc04579a |
| This journal is © The Royal Society of Chemistry 2019 |