Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Boosting axiality in stable high-coordinate Dy(III) single-molecule magnets†
Angelos B.
Canaj
 a,
Mukesh Kumar
Singh
b,
Emma
Regincós Marti
a,
Mukesh Kumar
Singh
b,
Emma
Regincós Marti
 a,
Marko
Damjanović
cd,
Claire
Wilson
a,
Marko
Damjanović
cd,
Claire
Wilson
 a,
Oscar
Céspedes
e,
Wolfgang
Wernsdorfer
a,
Oscar
Céspedes
e,
Wolfgang
Wernsdorfer
 cd,
Gopalan
Rajaraman
cd,
Gopalan
Rajaraman
 *b and
Mark
Murrie
*b and
Mark
Murrie
 *a
*a
aWestCHEM, School of Chemistry, University of Glasgow, University Avenue, Glasgow, G12 8QQ, UK. E-mail: mark.murrie@glasgow.ac.uk
bDepartment of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai, Maharashtra 400076, India. E-mail: rajaraman@chem.iitb.ac.in
cPhysikalisches Institut (PHI), Karlsruhe Institute of Technology, Wolfgang-Gaede-Strasse 1, 76131 Karlsruhe, Germany
dInstitute of Nanotechnology (INT), Karlsruhe Institute of Technology, Hermann-von-Helmholz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
eSchool of Physics and Astronomy, University of Leeds, Leeds LS2 9JT, UK
First published on 29th April 2019
Abstract
A new nine-coordinate, air-stable Dy(III) single-ion magnet has been successfully isolated. Our in silico studies demonstrate that through carefully modulating the ligand electronics, the axiality can be boosted to generate Ucal barriers of over 600 K.
Single-Molecule Magnets (SMMs) are fascinating molecular systems, which display the ability to block magnetisation via an energy barrier, Ueff, resulting in the appearance of magnetic hysteresis of molecular origin.1 Importantly, the design of viable SMMs strongly correlates with the control of the coordination environment at the level of a single metal ion.2 The use of Dy(III) to generate a strong axial magnetic anisotropy,3 a realisation achieved through the vital combination of theory and experiment, has led to a new generation of complexes with impressive energy barriers4 and high blocking temperatures5 with coercivity up to 80 K (i.e. above the boiling point of liquid nitrogen).6 Potential applications will require not only the ability to function at higher temperatures, but also to be chemically stable in air or when exposed to heat.7 However, finding an approach towards coordination environments that promote strong magnetic behaviour in 4f-SMMs and, at the same time, display robust chemical stability under ambient conditions has only recently begun to be addressed. In this regard, we recently reported two pentagonal bipyramidal Dy(III) single-ion magnets (SMMs containing only one metal ion), [Dy(H2O)5(HMPA)2]Cl3·HMPA·H2O and [Dy(H2O)5(HMPA)2]I3·2HMPA (HMPA = hexamethylphosphoramide), with blocking temperatures of ∼10 K, and we emphasized the important role of the second coordination sphere in controlling the magnetisation reversal barrier.8 In fact, complexes with higher coordination numbers (≥9) that also possess high energy barriers are rare and tuning such an environment in a way that promotes high axiality whilst offering good stability to air, heat and moisture is a challenging task. For this reason, we were intrigued by the complexes [LnIIILF](CF3SO3)2·H2O (L = 1,4,7,10-tetrakis(2-pyridylmethyl)-1,4,7,10-tetraaza-cyclododecane) and, in particular, the notable absence of the Dy(III) analogue along with the lack of magnetic data on any of the reported complexes.9 The Dy ion geometry is extremely important in order to obtain higher blocking temperatures and we reasoned that the combination of the pseudo D4d {DyN8} cage with a strong axial ligand should show interesting SMM characteristics.10 Herein, we report the synthesis, magnetic characterisation and ab initio studies of [DyIIILF](CF3SO3)2·H2O (1). Furthermore, we demonstrate an elegant strategy for improving the magnetic behaviour of high-coordinate 4f complexes. By carefully modulating the ligand environment in silico, we show how the calculated energy barrier, Ucal, can be increased, thus making 1 an extremely attractive system to probe the effects of the high-coordinate ligand environment on the dynamics of the magnetisation. Compound 1 (Fig. 1 and Fig. S6, S7, ESI†) was isolated under aerobic conditions from aqueous solution (see ESI†)9 and the stability of 1 to ∼350 °C is verified by TGA analysis (Fig. S5, ESI†).
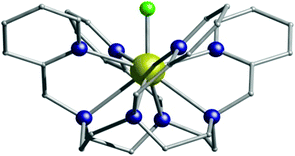 | ||
| Fig. 1 The structure of 1. Dy, gold; N, blue; F, green; C, grey. Hydrogen atoms, counter-ions and the co-crystallised water molecule are omitted for clarity. | ||
We also report the isostructural (but not isomorphous) lanthanum analogue, [LaIIILF](CF3SO3)2·H2O (2) (Table S1 and Fig. S1, S2, S11, ESI†) and the Dy-doped lanthanum analogue (3) (Fig. S4, ESI†). The nine coordinate Dy(III) centre sits on a C2 symmetry axis, coincident with the Dy–F bond. Continuous shape measures analysis,11 which estimates the distortion from the perfect polyhedron, gives a value of 1.429 (where 0 corresponds to the ideal structure) for a muffin geometry (Fig. S8 and Tables S4, S5, ESI†). The ninth coordination site is occupied by a strong electronegative fluoride ion, with a relatively short Dy–F bond length of 2.123(2) Å (Table S3, ESI†). Intermolecular hydrogen bonding between the triflate counter-ions and the water molecule is present (Fig. S9, ESI†). In addition, the C–H⋯F intermolecular interactions between neighbouring molecules of 1 create a 1D columnar structure along the c-axis with the closest Dy⋯Dy distance of 7.757 Å (Fig. S10, ESI†).
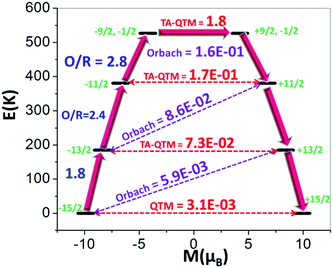
For 1, the eight Kramers Doublets (KDs), corresponding to the 6H15/2 ground state, span an energy range of 808 K (see ESI† for computational details). The transverse components of the ground state (mJ = ±15/2) are found to be negligible (gxx = 0.006, gyy = 0.012, Table S7, ESI†), establishing a strong magnetic anisotropy axis (gzz = 19.837), lying along the Dy–F bond (Fig. S28, ESI†). This can be explained using LoProp12 charges computed using the CASSCF wavefunction. The charge on the axial F atom is found to be nearly three times larger compared to the nitrogen atoms of the cage ligand, (Fig. S28, ESI†) and this dictates the direction of gzz axis. Similarly, the axial nature is also observed for the first and second exited states (mJ = ±13/2, gxx = 0.212, gyy = 0.228, gzz = 16.800 and mJ = ±11/2, gxx = 0.468 gyy = 0.583, gzz = 13.380, respectively, Table S7, ESI†), which are found to lie at 185 K and 381 K, respectively above the ground state. Notably, the larger gxx/gyy values obtained for the third-exited state (mJ = ±9/2; ±1/2, gxx = 5.213 gyy = 5.565, gzz = 8.350) yield a larger magnetic moment matrix element of 1.8 μB (Fig. 2) which is sufficient to promote magnetic relaxation via this state, giving the maximum calculated relaxation barrier (Ucal) of ∼527 K. It is important to note that a small transverse magnetic moment is calculated for the first three KDs (3.1 × 10−3, 7.3 × 10−2 and 1.7 × 10−1μB, respectively), suggesting the presence of weak Quantum Tunnelling of the Magnetisation (QTM) and again, relaxation via the third exited state. The Orbach processes related to the mJ and mJ + 1 excited states of opposite magnetization for the first four KDs are found to be very small (<0.16, Fig. 2). Furthermore, thorough analysis of the g-tensor (Table S7, ESI†) reveals axiality up to the third excited state (KD4).
The dc magnetic susceptibility measurements for 1 (Fig. S13, ESI†) show that the experimental χMT value of 14.1 cm3 mol−1 K (at 300 K) is in close agreement with the theoretical value (14.2 cm3 mol−1 K) for a single Dy(III) ion (6H15/2, S = 5/2, L = 5, g = 4/3). Upon cooling, χMT decreases steadily to a value of 11.1 cm3 mol−1 K at 20 K, due to thermal depopulation of the mJ levels, before increasing to 12.0 cm3 mol−1 K at 2.8 K. This low temperature increase is consistent with the presence of weak ferromagnetic intermolecular interactions. Furthermore, the χMT data of the diluted sample 3 instead decreases between 50–2 K, which further supports the hypothesis of weak ferromagnetic intermolecular interactions in 1 (Fig. S14, ESI†). Alternating current (ac) susceptibility measurements between 0.6–800 Hz, under zero external dc field, were performed in order to investigate the dynamics of the magnetisation for 1 and the Dy-doped lanthanum analogue, 3. Under zero external dc field, the out-of-phase, χM′′ magnetic susceptibility data exhibit well-defined maxima as a function of frequency (Fig. 3 and Fig. S17, ESI†) and temperature with χM′′ peaks clearly observable up to 12 K for 1 (Fig. S15, ESI†) and up to 20 K for 3 (Fig. S16, ESI†).
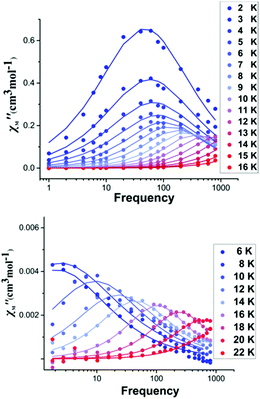 | ||
| Fig. 3 Frequency dependent out-of-phase ac susceptibility signals for complex 1 (upper) and 3 (lower) in zero dc field. The solid lines correspond to the best fit. | ||
For 1, the plots of χM′ and χM′′ vs. temperature show a rapid increase in the low temperature region (Fig. S15, ESI†). Additionally, the low temperature set of peaks in the χM′′(v) curves exhibit little temperature dependence (Fig. 3 upper), which could be attributed to faster relaxation effects (QTM).13 For the diluted sample 3, the χM′′(T) maxima under zero dc field, are shifted to higher temperatures and the signal at lower temperatures is significantly reduced, suggesting a slower relaxation of the magnetisation (Fig. S16, ESI†). The relaxation times, τ, were extracted from the fit of the Argand plots of χM′′ vs. χM′ using the generalized Debye model (Fig. S18, ESI†).14 The α parameters found are relatively large in the range of 0.01–0.29 (2–13 K) for 1, and 0.01–0.41 (6–22 K) for 3, indicating a relatively wide distribution of relaxation times.1 Thus, the Arrhenius plots are fitted considering more than one possible relaxation process yielding an energy barrier of Ueff = ∼110 K for 1 (Fig. S19 and Table S9, ESI†) and Ueff = ∼290 K for the diluted sample 3 (Fig. S20 and Table S9, ESI†). Importantly, it should be noted that these values are among the highest for high-coordinate lanthanide single-ion magnets (see Table S6, ESI†). To explore the role of low-lying vibrational levels that could enhance relaxation and lower the Ucal barrier, we analysed the N–Dy–F bending mode which lies at 115 cm−1 (Fig. S29, ESI†). Calculations performed on selected vibrational modes corresponding to this frequency reveal a significant reduction in the estimated barrier height (see Fig. S29, ESI†). To assess the strength of the intermolecular interactions, further calculations were performed which yielded small dipolar and intermolecular magnetic exchange couplings (0.02 cm−1 and 0.08 cm−1 respectively). These are expected to further enhance relaxation (see Fig. S30, ESI†).15
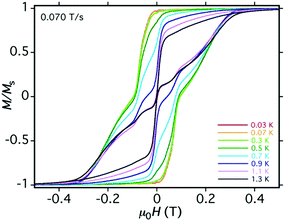
Magnetisation vs. field hysteresis loops were performed on 1 and 3 in light of the large energy barriers observed. Low-temperature hysteresis studies were performed on a single crystal of 1 using an array of micro-SQUIDs.16 The increase in coercivity with decreasing temperature (Fig. 4) and increasing scan rate (Fig. S26, ESI†) confirms 1 to be a SMM.
The M vs. H loops suggest the presence of QTM and have steps that are somewhat smeared out at low temperatures, possibly due to a distribution of local environments (e.g. disordered solvent molecules, crystals defects) and/or intermolecular interactions. For the diluted powder sample 3 more pronounced butterfly-like loops are observed until 3 K (average sweep rate of ∼10 mT s−1) (Fig. S27, ESI†). The promising magnetic properties and the high stability of 1, combined with the ability to modify either the axial ligand or the polydentate cage ligand, led us to study the effect on the magnetic dynamics of modulating the ligand environment in silico (Fig. 5). To do this, we have created a family of different model systems and used ab initio calculations to show how the ligand electronics can be used to tune and improve the relaxation properties (Fig. S31, S32 and Table S8, ESI†).
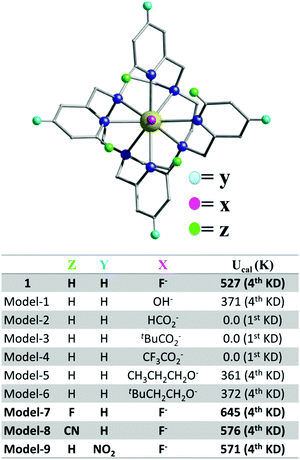 | ||
| Fig. 5 In silico models based on 1 showing how the Ucal barrier can be increased by tuning the ligand electronics. | ||
Substitution of the axial F− ion by monodentate formate or substituted carboxylates (models 2–4 Fig. 5) destroys the Ucal barrier. These weaker axial groups reduce the crystal field splitting significantly, leading to smaller barrier heights (Fig. 5 and Fig. S31, ESI†) and due to smaller charges compared to the nitrogen atoms of the polydentate ligand, the gzz direction changes (Fig. S32, ESI†). Stronger ligands (−OR, models 1, 5 and 6 Fig. 5) maintain the gzz direction, but with smaller Ucal values than 1. This suggests that axial substitution is detrimental in 1 with the F− ion being the most promising, as was recently shown by Norel et al.17 We reasoned that if the coordination by the ligand nitrogen atoms is weakened, this should further enhance the barrier height, by moving towards a pseudo Dy–F environment.18 Hence, we have substituted the -ortho and -para H atoms of the pyridinic ring with strong electron withdrawing groups (models 7–9 Fig. 5). For these models, the computed transverse anisotropy (see Table S8, model-7, model-8 and model-9, ESI†) is found to be significantly reduced, causing an impressive increase to give a Ucal energy barrier of 645 K (for X = F, Z = F, Y = H, see Fig. 5).
Furthermore, the transverse matrix elements for the transition magnetic moments are reduced (see Fig. S31, ESI†) and hence the probability of QTM/TA-QTM is decreased. This suggests a promising route to realise higher barriers for magnetization reversal. Importantly, the models investigated were designed taking into consideration their likely future experimental realisation; i.e. they do not show strong steric hindrance and/or have low coordination numbers. To check further the effect of the transverse field we have rotated the –N4 plane by ±6 degrees in a model complex (Fig. S33, ESI†) and in the presence of the strong axial F ion, changes in the calculated Ucal values are found to be negligible (Fig. S34, ESI†). Efforts are currently underway to synthesise substituted analogues of the polydentate cage ligand in our laboratory and as a proof-of-concept we also report the crystal structure of a new ligand (Fig. S12 and Table S2, ESI†) where the electron withdrawing CN group, has been included in the ortho position of each pyridinic ring (Fig. 5 model-8, Fig. S31, S32 and Table S8, ESI†). Hence, future work will focus on the realisation of the above exciting family of complexes with high energy barriers along with good air and heat stability.
The UK Engineering and Physical Sciences Research Council are thanked for financial support (grant ref. EP/N01331X/1). The data which underpin this work are available at http://dx.doi.org/10.5525/gla.researchdata.789. G. R. thanks the SERB (EMR/2014/00024) for funding. MKS thanks IIT Bombay for a fellowship.
Conflicts of interest
There are no conflicts to declare.Notes and references
- A. Dey, P. Kalita and V. Chandrasekhar, ACS Omega, 2018, 3, 9462 CrossRef CAS; A. K. Bar, P. Kalita, M. K. Singh, G. Rajaraman and V. Chandrasekhar, Coord. Chem. Rev., 2018, 367, 163 CrossRef; Z. Zhu, M. Guo, X.-L. Li and J. Tang, Coord. Chem. Rev., 2019, 378, 350 CrossRef; M. Feng and M.-L. Tong, Chem. – Eur. J., 2018, 24, 7574 CrossRef PubMed; S. G. McAdams, A.-M. Ariciu, A. K. Kostopoulos, J. P. S. Walsh and F. Tuna, Coord. Chem. Rev., 2017, 346, 216 CrossRef; B. M. Day, F.-S. Guo and R. A. Layfield, Acc. Chem. Res., 2018, 51, 1880 CrossRef PubMed; S. K. Gupta and R. Murugavel, Chem. Commun., 2018, 54, 3685 RSC.
- L. Sorace, C. Benelli and D. Gatteschi, Chem. Soc. Rev., 2011, 40, 3092 RSC; J. Wu, J. Jung, P. Zhang, H. Zhang, J. Tang and B. Le Guennic, Chem. Sci., 2016, 7, 3632 RSC; K. Katoh, S. Yamashita, N. Yasuda, Y. Kitagawa, B. K. Breedlove, Y. Nakazawa and M. Yamashita, Angew. Chem., Int. Ed., 2018, 57, 9262 CrossRef CAS PubMed; Y.-C. Chen, J.-L. Liu, L. Ungur, J. Liu, Q.-W. Li, L.-F. Wang, Z.-P. Ni, L. F. Chibotaru, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 2829 CrossRef PubMed; J. Liu, Y.-C. Chen, J.-L. Liu, V. Vieru, L. Ungur, J.-H. Jia, L. F. Chibotaru, Y. Lan, W. Wernsdorfer, S. Gao, X.-M. Chen and M.-L. Tong, J. Am. Chem. Soc., 2016, 138, 5441 CrossRef PubMed; S. K. Gupta, T. Rajeshkumar, G. Rajaraman and R. Murugavel, Chem. Sci., 2016, 7, 5181 RSC.
- S. T. Liddle and J. van Slageren, Chem. Soc. Rev., 2015, 44, 6655 RSC; L. Ungur and L. F. Chibotaru, Phys. Chem. Chem. Phys., 2011, 13, 20086 RSC; J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078 RSC.
- S.-D. Jiang, B.-W. Wang, H.-L. Sun, Z.-M. Wang and S. Gao, J. Am. Chem. Soc., 2011, 133, 4730 CrossRef CAS PubMed; Y.-S. Meng, L. Xu, J. Xiong, Q. Yuan, T. Liu, B. W. Wang and S. Gao, Angew. Chem., Int. Ed., 2018, 57, 1 CrossRef; K. L. M. Harriman, J. L. Brosmer, L. Ungur, P. L. Diaconescu and M. Murugesu, J. Am. Chem. Soc., 2017, 139, 1420 CrossRef PubMed; Y.-S. Ding, N. F. Chilton, R. E. P. Winpenny and Y.-Z. Zheng, Angew. Chem., Int. Ed., 2016, 55, 16071 CrossRef PubMed; Y.-S. Ding, K.-X. Yu, D. Reta, F. Ortu, R. E. P. Winpenny, Y.-Z. Zheng and N. F. Chilton, Nat. Commun., 2018, 9, 3134 CrossRef PubMed; S. Demir, M. I. Gonzalez, L. E. Darago, W. J. Evans and J. R. Long, Nat. Commun., 2017, 8, 2144 CrossRef PubMed; M. Gregson, N. F. Chilton, A.-M. Ariciu, F. Tuna, I. F. Crowe, W. Lewis, A. J. Blake, D. Collison, E. J. L. McInnes, R. E. P. Winpenny and S. T. Liddle, Chem. Sci., 2016, 7, 155 RSC; P. E. Kazin, M. A. Zykin, V. V. Utochnikova, O. V. Magdysyuk, A. V. Vasiliev, Y. V. Zubavichus, W. Schnelle, C. Felser and M. Jansen, Angew. Chem., Int. Ed., 2017, 56, 1 CrossRef PubMed; F. Liu, D. S. Krylov, L. Spree, S. M. Avdoshenko, N. A. Samoylova, M. Rosenkranz, A. Kostanyan, T. Greber, A. U. B. Wolter, B. Buchner and A. A. Popov, Nat. Commun., 2017, 8, 16098 CrossRef PubMed.
- A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Nature, 2017, 548, 439 CrossRef CAS PubMed; A. P. Goodwin, D. Reta, F. Ortu, N. F. Chilton and D. P. Mills, J. Am. Chem. Soc., 2017, 139(51), 18714 CrossRef PubMed; F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamaki and R. A. Layfield, Angew. Chem., Int. Ed., 2017, 56, 11445 CrossRef PubMed; K. R. McClain, C. A. Gould, K. Chakarawet, S. J. Teat, T. J. Groshens, J. R. Long and B. G. Harvey, Chem. Sci., 2018, 9, 8492 RSC.
- F.-S. Guo, B. M. Day, Y.-C. Chen, M.-L. Tong, A. Mansikkamäki and R. A. Layfield, Science, 2018, 362, 1400 CrossRef CAS PubMed.
- R. Vincent, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Nature, 2012, 488, 357 CrossRef CAS PubMed; E. M. Pineda, C. Godfrin, F. Balestro, W. Wernsdorfer and M. Ruben, Chem. Soc. Rev., 2018, 47, 501 RSC; C. Godfrin, A. Ferhat, R. Ballou, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Phys. Rev. Lett., 2017, 119, 187702 CrossRef PubMed.
- A. B. Canaj, M. K. Singh, C. Wilson, G. Rajaraman and M. Murrie, Chem. Commun., 2018, 54, 8273 RSC.
- O. A. Blackburn, A. M. Kenwright, A. R. Jupp, J. M. Goicoechea, P. D. Beer and S. Faulkner, Chem. – Eur. J., 2016, 22, 8929 CrossRef CAS PubMed; L. S. Natrajan, N. M. Khoabane, B. L. Dadds, C. A. Muryn, R. G. Pritchard, S. L. Heath, A. M. Kenwright, I. Kuprov and S. Faulkner, Inorg. Chem., 2010, 49, 7700 CrossRef PubMed; A. Wada, B. Watanabe, Y. Yamanoi and H. Nishihara, Chem. Commun., 2008, 1671 RSC; O. A. Blackburn, N. F. Chilton, K. Keller, C. E. Tait, W. K. Myers, E. J. L. McInnes, A. M. Kenwright, P. D. Beer, C. R. Timmel and S. Faulkner, Angew. Chem., Int. Ed., 2015, 54, 10783 CrossRef PubMed; O. A. Blackburn, A. M. Kenwright, P. D. Beer and S. Faulkner, Dalton Trans., 2015, 44, 19509 RSC.
- T. Gupta and G. Rajaraman, Eur. J. Inorg. Chem., 2018, 3402 CrossRef CAS.
- M. Llunell, D. Casanova, J. Cirera, J. M. Bofill, P. Alemany and S. S. Alvarez, SHAPE (version 2.1), Barcelona, 2013 Search PubMed.
- L. Gagliardi, R. Lindh and G. Karlstrom, J. Chem. Phys., 2004, 121, 4494 CrossRef CAS PubMed.
- J.-L. Liu, Y.-C. Chen and M.-L. Tong, Chem. Soc. Rev., 2018, 47, 2431 RSC.
- D. Gatteschi, R. Sessoli and J. Villain, Molecular Nanomagnets, Oxford Univ. Press, 2006 Search PubMed.
- E. Rousset, M. Piccardo, M.-E. Boulon, R. W. Gable, A. Soncini, L. Sorace and C. Boskovic, Chem. – Eur. J., 2018, 24, 14768 CrossRef CAS PubMed.
- W. Wernsdorfer, Adv. Chem. Phys., 2001, 118, 99 Search PubMed; W. Wernsdorfer, Supercond. Sci. Technol., 2009, 22, 064013 CrossRef CAS; W. Wernsdorfer, N. E. Chakov and G. Christou, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 132413 CrossRef.
- L. Norel, L. E. Darago, B. L. Guennic, K. Chakarawet, M. I. Gonzalez, J. H. Olshansky, S. Rigaut and J. R. Long, Angew. Chem., Int. Ed., 2018, 57, 1 CrossRef PubMed.
- L. Ungur and L. F. Chibotaru, Inorg. Chem., 2016, 55, 10043 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and computational data; CIFs for 1, 4 and 2 CCDC 1889693–1889695. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc00965e |
| This journal is © The Royal Society of Chemistry 2019 |