Amorphous-to-crystalline transformation toward controllable synthesis of fibrous covalent organic frameworks enabling promotion of proton transport†
Weifu
Kong‡
,
Wei
Jia‡
,
Rong
Wang
,
Yifan
Gong
,
Changchun
Wang
 ,
Peiyi
Wu
,
Peiyi
Wu
 and
Jia
Guo
and
Jia
Guo
 *
*
State Key Laboratory of Molecular Engineering of Polymers, Department of Macromolecular Science, Fudan University, Shanghai, 200433, P. R. China. E-mail: guojia@fudan.edu.cn
First published on 26th November 2018
Abstract
Reversible imine exchange is adopted to molecularly re-arrange polyazomethine-based amorphous fibers into covalent organic frameworks (COFs) retaining pristine fibrous characters with periodic and oriented micropore channels. Such fibrous COFs can immobilize the assembled Nafion to form long-range proton conduction pathways, thus largely promoting proton transport.
Covalent organic frameworks (COFs) constitute a fantastic class of crystalline porous polymers that feature periodic pore channels, and designable atomic frameworks arranged in a specific way.1 Two-dimensionally structured COFs have been a fantastic organic platform to integrate and optimize a variety of performances for the energy, environment and biomedical fields.2 With the on-going progress in COF chemistry, the latest attention regarding COFs has been paid to the development of a family of low-dimensional nanomaterials represented by single atomic or few-layered nanosheets.3 In contrast, one-dimensional (1D) COFs have rarely been investigated as the spatially constrained growth along one direction may impair the expansion and alignment of the atomic layers. To our knowledge, only a few publications have studied the synthetic strategy. The template-mediated method relies on inorganic nanofibers such as ZnO and carbon nanotubes to induce the encapsulation of COFs for the formation of 1D hybrids or tubes with elimination of the inner templates.4 A microfluidic technique can produce jelly-like macroscopic fibers by precise control of the reactant fluids under continuous flow conditions.5 Comparably, the template-free strategy is more challenging but promising in that the formed 1D COFs have anisotropic properties that are given by the supramolecular assembly owing to the specific interaction of units such as pyrene6 and triazine.7 During the process, it has often been observed that 1D morphologies are transformed from the primary forms such as spheres and plates.8 Therefore, morphological control has been a tough task because the 1D assembly of COFs from the building blocks consists of an intricate mechanism in a solvothermal system and in turn, is poorly controlled giving rise to a mixture of different shapes. Therefore, the precise manipulation of the 1D morphology of COFs is still to be well explored.
Herein, we apply a strategy of amorphous-to-crystalline transformation by virtue of dynamic imine exchange to prepare fibrous imine-linked COFs. This is a two-step method, involving (1) the reaction-controlled formation of amorphous polyazomethine (PAM) fibers and (2) then solvothermal crystallization of the preformed PAM fibers into imine-linked COFs retaining premier morphology and size. Compared with the previous reports, the amorphous-to-crystalline strategy is template-free while more advantageous in terms of control over the fibrous properties (e.g. length-to-width ratio, surface natures, and functionality). Meanwhile, synthetically versatile PAMs would be a designable platform to constitute a variety of low-dimensional nanomaterials with distinguished functionalities. Taking the structural anisotropy into account, we have engineered the imine-linked COF nanofibers within the proton exchange membrane that was composed of graphene oxide (GO) as the support and Nafion as the proton source. Fibrous COFs are able to provide oriented pore channels as a shortcut for fast proton transport across the immobilized Nafion domains, and also serve as a binder for GO nanosheets giving considerable promotion to the membrane properties in terms of water-proofing behaviour and ductility.
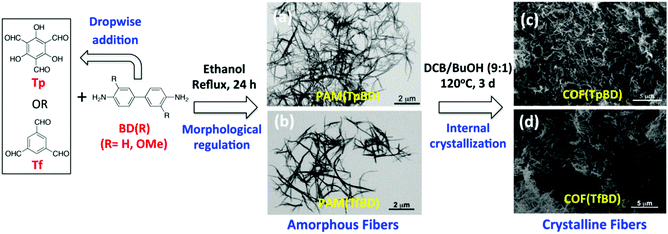
As illustrated in Fig. 1, the first step is the Schiff-base reaction of benzidine (BD) with two aldehyde-containing monomers, i.e. 1,3,5-triformylbenzene (Tf) and 2,4,6-triformylphloroglucinol (Tp), respectively. The control over the reaction process was found to play a crucial role for the oriented growth of PAMs. When the amines were added dropwise into the ethanol solution of aldehydes, the polymerization slowed down and induced the 1D evolution of PAMs. After 24 h ripening, the fibrous product was obtained for both PAM(TpBD) and PAM(TfBD) polymers. Then after the elimination of ethanol, all of the fibrous solids were transferred into a mixture of dichlorobenzene and n-butanol (v/v, 9/1) and a sealed solvothermal reaction was allowed to take place at 120 °C for 3 d, using acetic acid as the catalyst. The evidence of fibrous PAMs and COFs is unequivocally proved by TEM images, as shown in Fig. 1. The preformed PAM(TpBD) appears fibrous with each fibre being several microns in length and ca. 80 nm in width which was confirmed by the magnified image (see Fig. S1 in ESI†). There are nearly no other morphologies appearing in the PAM product. As a control, two monomers were mixed which formed only uniform microspheres without the presence of fibers (see Fig. S2 in ESI†). The results indicate that highly symmetric and rigid structures of building blocks facilitate the shaping of PAMs, while controlled polymerization is responsible for the oriented growth. Successively, the fibrous solids were subjected to high-temperature solvothermal treatment for the transformation of amorphous PAMs into crystalline imine-linked COFs by dynamic imine exchange. As displayed in Fig. 1c and d, the preformed fibers have eventually remained both in size and shape and the crystalline domains could be observed in the HR TEM images of the two cases (see Fig. S3 in ESI†). As a control, irregular solids could only be obtained by the straightforward solvothermal synthesis (see Fig. S4 in ESI†). One can see that aldehyde-containing monomers Tp and Tf have less notable difference in the formation of fibers with BD as well as the corresponding COFs (Fig. 1). Analogously, the fibrous morphology was equally observed when the methoxy-substituted BD derivatives were used as building blocks (see Fig. S5 in ESI†). This provides an opportunity to constitute a variety of functionalized 1D COFs in the future.
The analysis of molecular compositions from the FT IR and solid-state 13C NMR spectra demonstrated that keto-enamine linkages are formed in the fibrous COF(TpBD) (see Fig. S6 and S7 in the ESI†). Thermo-gravimetric analysis exhibited a similar tendency of weight loss for the fibrous COFs and ones synthesized through the direct solvothermal route. Additionally, only ca. 5 wt% loss was found at around 350 °C, which could be ascribed to the decomposition of residual un-reactive groups on the fibrous surfaces (see Fig. S8 in ESI†).
PXRD measurement of the COF(TpBD) reveals remarkable crystallinity irrespective of spherical or fibrous morphologies formed under control. As displayed in Fig. 2, both of them exhibit the same pattern with peaks located at 3.3°, 5.0°, 5.9°, 9.1°, and 26.1°, corresponding to the (100), (110), (200), (220) and (001) facets, respectively, all of which are extremely close to the PXRD pattern of COF(TpBD) directly synthesized by the reported solvothermal route (see Fig. S9 in ESI†).9 The results indicate that the amorphous-to-crystalline transformation is able to implement the atomic re-arrangement followed by the typical hexagonal lattice. However, the crystallinity of fibrous COF(TpBD) is notably attenuated in comparison to the spherical one. In view of the structural inter-conversion occurring within the preformed fibers, it is likely that there is an anisotropic packing of the COF domains due to the 1D spatial confinement. Thus the loss of crystallinity observed may be attributed to the packing orientation, which causes weak diffraction as the alignment of the (100) plane is approximately perpendicular to the optical axis normal vector.10 Additionally, the variable-temperature PXRD was performed at 25 °C and 100 °C, respectively, and almost the same diffraction patterns were obtained for the fibrous COF(TpBD), which proves their high purity without the presence of any unreacted monomers or oligomers (Fig. S10 in ESI†).
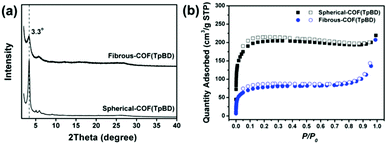 | ||
| Fig. 2 PXRD patterns (a) and N2 sorption isotherms (b) of spherical and fibrous COF(TpBD)s, respectively. | ||
Nitrogen-sorption isotherms of spherical and fibrous COF(TpBD)s both exhibited a rapid uptake at a low pressure of P/P0 < 0.1, followed by a steady plateau and slight increase at P/P0 > 0.8 (Fig. 2c). Those sorption profiles could be described as a type I isotherm, which is characteristic of microporous materials. The BET surface areas and pore volumes were calculated to be 295 m2 g−1 and 0.072 cm3 g−1 for fibrous COFs, and 782 m2 g−1 and 0.248 cm3 g−1 for spherical COFs, respectively (see Table S1 in the ESI†). The moderate BET surface area of the fibrous COFs might be responsible for the eclipsed stacking of few layers in contrast to the spherical counterparts. The pore-size distribution was narrowly populated in the range of 1–3 nm according to the calculation on the NLDFT model (see Fig. S11 in the ESI†).
Thanks to the advantages of proton conductivity and mechanical properties, graphene oxide (GO) nanosheets are fabricated on the layered membrane with intercalated polyelectrolytes for use in proton exchange in fuel cells, wherein polyelectrolytes are organized into rod-like aggregates or crosslinking networks for the supply of protons hopping from one site to another.11 However, an aqueous self-assembly of polyelectrolyte is strongly dependent on its concentration and amphiphilic property, which increase the difficulty of acquiring continuous and long-range proton channels. We therefore proposed the utilization of fibrous COFs as a platform to immobilize the sulfonate-terminated Nafion and provide long-range pore channels for proton transport. In contrast to the previous reports that small-molecular proton carriers were impregnated within the COFs,12 macromolecular polyelectrolytes such as Nafion possess a lower risk of leakage and remarkable advantages in design.
In the typical procedure, Nafion was deposited on the fibrous COF(TpBD)s by hydrophobic interaction with the tetrafluoroethylene backbones of Nafion (COF@Nafion), and the mixture of GO and COF@Nafion composites suspended in aqueous solution was subjected to vacuum filtration for preparation of the layered membranes. As shown in Fig. 3a and c, the Nafion/GO membrane without any additives exhibits a flawless top-view surface and a distinct layered cross-sectional structure. With the addition of COF@Nafion, the membrane shows a remarkable difference, wherein a continuous layer consisting of Nafion domains and interpenetrated fibrous COFs is sandwiched between the GO layers (Fig. 3b and d).
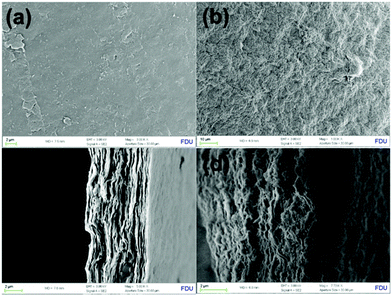 | ||
| Fig. 3 SEM images of the top view and cross-sectional view of the Nafion/GO membrane (a and c), and fibrous-COF@Nafion/GO membrane (b and d), respectively. | ||
The water-proofing property of the composite membrane was examined by immersion in water over 3 days. Due to the amphiphilic properties, the Nafion/GO membrane was re-dispersed in water, thus losing the GO support for proton exchange among Nafion domains (see Fig. S12a in ESI†). Besides, we are aware that the Nafion/GO membrane is too fragile to keep intact while in use. By contrast, incorporation of the sandwiched layer COF(TpBD)@Nafion not only largely improves the flexibility and toughness of the membranes but also ensures the aqueous durability of the membranes against etching of the acidic medium (see Fig. S12b in ESI†).
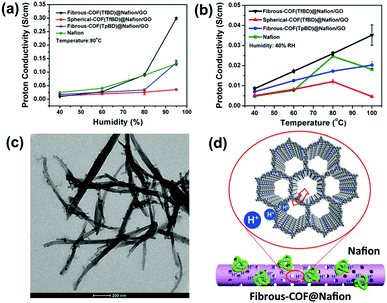
Fig. 4a shows the proton conductivity of the different membranes as a function of humidity at 80 °C. Nafion was used as a control for comparison with the COF-containing Nafion/GO membranes. All of the samples show an increase in humidity-dependent proton conductivity. The maximum value reaches as high as 0.30 S cm−1 at 95% RH (80 °C) for the membranes containing fibrous COF(TfBD), which is over 2-fold that of the commercial Nafion (0.13 S cm−1) as well as fibrous COF(TpBD) (0.13 S cm−1) under the same conditions. Furthermore, it is roughly one order of magnitude higher than that of the spherical COF(TfBD) with the same molecular structure and composition (0.04 S cm−1). When the humidity was kept at 40% RH, the temperature-dependent increase in proton conductivity is depicted in Fig. 4b. The fibrous COF(TfBD) still is the best of the additives for GO/Nafion membranes for proton transport at different temperatures, and its Nyquist plot reflects a typical proton conduction behaviour (see Fig. S13 in ESI†). The activation energy of the fibrous-COF(TfBD)@Nafion/GO was calculated to be as low as 0.27 eV from 40 °C to 80 °C at 40% RH (see Fig. S14 in ESI†). The value is much lower than that of Nafion (0.38 eV), indicating that the COF@Nafion fibers could provide long-range proton conduction channels even at low humidity. The Grotthuss mechanism should be dominant with respect to the vehicle mechanism due to the lower activation energy (<0.4 eV).
The microstructures of the COF(TfBD)@Nafion composites were examined by TEM measurement. It is observed from Fig. 4c that the spherical nanoparticles formed by the self-assembly of Nafion are discretely attached to the surface of fibrous COF(TfBD) and are retained well. In contrast, the fibrous PAM(TfBD) is amenable to decomposition in the acidic Nafion solution (see Fig. S15 in ESI†), and thus its proton conductivity cannot be estimated.
The possible mechanism of proton transport within the membrane containing fibrous COF(TfBD) is proposed in Fig. 4d. The fibers construct an interpenetrated network between the GO nanosheets, which serves as the matrix to anchor the assemblies of Nafion. The protons of SO3H groups are therefore transported either through the interior openings of the micropore channels or across the external surface of the COF(TfBD) fibers. Within the micropores, water molecules bind with nitrogen of imine linkages by H-bonding interaction for proton hopping. However, β-ketoenamine-linked COF(TpBD) bears the highly stable NH-form salicylideneanilines to give the strong intramolecular H-bonds,13 which may inhibit the activity of proton conduction compared with the imine-linked COF(TfBD). Compared with the previously reported COFs for proton transport, the combination of polyelectrolytes with fibrous COFs is more superior in terms of proton conductivity (Table S2 in ESI†).
In summary, we present an amorphous-to-crystalline strategy for template-free preparation of uniform fibrous COFs by virtue of dynamic imine exchange. The method is advantageous in that (1) Schiff-base polymerization is applicable for flexible manipulation of amorphous PAMs in terms of morphology and size; and (2) solvothermal structural transformation from amorphous PAMs to imine-linked COFs maintains the preformed fibers as well as the combination of porosity and crystallinity. To prove potential in application, Nafion is immobilized on the surface of the fibrous COFs and the composites are knitted as a sandwiched layer within the GO nanosheets for proton exchange. We have corroborated that rapid proton conduction via the COF fibers rather than the spherical form is attributable to the oriented pore channels as well as fibrous morphology, both of which facilitate shuttling of protons between Nafion domains. Also, interpenetration of fibrous COFs is conducive to improving the water-tolerance and ductility of GO-based membranes in use. Our findings develop a reaction-controlled strategy to customize low-dimensional COFs in a template-free fashion and imply necessity of exploiting the desirable assemblies of COFs for optimization of practical performances.
We acknowledge the financial support of the NSFC (21474015 and 51633001), and State Key Project of Research and Development (2016YFC1100300).
Conflicts of interest
There are no conflicts to declare.References
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O’Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 5751 CrossRef CAS; E. Jin, M. Asada, Q. Xu, S. Dalapati, M. A. Addicoat, M. A. Brady, H. Xu, T. Nakamura, T. Heine, Q. Chen and D. Jiang, Science, 2017, 357, 673 CrossRef PubMed.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010 RSC; P. J. Waller, F. Gándara and O. M. Yaghi, Acc. Chem. Res., 2015, 48, 3053 CrossRef CAS PubMed; J. L. Segura, M. J. Mancheno and F. Zamora, Chem. Soc. Rev., 2016, 45, 5635 RSC.
- J. W. Colson, A. R. Woll, A. Mukherjee, M. P. Levendorf, E. L. Spitler, V. B. Shields, M. G. Spencer, J. Park and W. R. Dichtel, Science, 2011, 332, 228 CrossRef CAS PubMed; S. Chandra, S. Kandambeth, B. P. Biswal, B. Lukose, S. M. Kunjir, M. Chaudhary, R. Babarao, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 17853 CrossRef PubMed; D. D. Medina, V. Werner, F. Auras, R. Tautz, M. Dogru, J. Schuster, S. Linke, M. Döblinger, J. Feldmann, P. Knochel and T. Bein, ACS Nano, 2014, 8, 4042 CrossRef PubMed; M. A. Khayum, S. Kandambeth, S. Mitra, S. B. Nair, A. Das, S. S. Nagane, R. Mukherjee and R. Banerjee, Angew. Chem., Int. Ed., 2016, 55, 15604 CrossRef PubMed; X. H. Liu, C. Z. Guan, S. Y. Ding, W. Wang, H. J. Yan, D. Wang and L. J. Wan, J. Am. Chem. Soc., 2013, 135, 10470 CrossRef; W. Kong, J. Wan, S. Namuangruk, J. Guo and C. Wang, Sci. Rep., 2018, 8, 5529 CrossRef; S. Haldar, K. Roy, S. Nandi, D. Chakraborty, D. Puthusseri, Y. Gawli, S. Ogale and R. Vaidhyanathan, Adv. Energy Mater., 2018, 8, 1702170 CrossRef; D. Mullangi, S. Shalini, S. Nandi, B. Choksi and R. Vaidhyanathan, J. Mater. Chem. A, 2017, 5, 8376 RSC.
- P. Pachfule, S. Kandmabeth, A. Mallick and R. Banerjee, Chem. Commun., 2015, 51, 11717 RSC; B. Sun, J. Liu, A. Cao, W. Song and D. Wang, Chem. Commun., 2017, 53, 6303 RSC.
- D. Rodriguez-San-Miguel, A. Abrishamkar, J. A. R. Navarro, R. Rodriguez-Trujillo, D. B. Amabilino, R. Mas-Balleste, F. Zamora and J. Puigmarti-Luis, Chem. Commun., 2016, 52, 9212 RSC.
- S. Wang, J. Guo, J. Kim, H. Ihee and D. L. Jiang, Angew. Chem., Int. Ed., 2008, 47, 8826 CrossRef CAS; L. M. Salonen, D. D. Medina, E. Carbo-Argibay, M. G. Goesten, L. Mafra, N. Guldris, J. M. Rotter, D. G. Stroppa and C. Rodriguez-Abreu, Chem. Commun., 2016, 52, 7986 RSC.
- A. Halder, S. Kandambeth, B. P. Biswal, B. Kaur, N. C. Roy, M. Addicoat, J. K. Salunke, S. Banerjee, K. Vanka, T. Heine, S. Verma and R. Banerjee, Angew. Chem., Int. Ed., 2016, 55, 7806 CrossRef CAS.
- B. Gole, V. Stepanenko, S. Rager, M. Grune, D. D. Medina, T. Bein, F. Wurthner and F. Beuerle, Angew. Chem., Int. Ed., 2018, 57, 846 CrossRef CAS; W. Huang, Y. Jiang, X. Li, X. J. Li, J. Y. Wang, Q. Wu and X. K. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 8845 CrossRef; S. Kandambeth, V. Venkatesh, D. B. Shinde, S. Kumari, A. Halder, S. Verma and R. Banerjee, Nat. Commun., 2015, 6, 6786 CrossRef.
- B. P. Biswal, S. Chandra, S. Kandambeth, B. Lukose, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 5328 CrossRef CAS.
- D. A. Vazquez-Molina, G. S. Mohammad-Pour, C. Lee, M. W. Logan, X. Duan, J. K. Harper and F. J. Uribe-Romo, J. Am. Chem. Soc., 2016, 138, 9767 CrossRef CAS.
- G. He, C. Chang, M. Xu, S. Hu, L. Li, J. Zhao, Z. Li, Z. Li, Y. Yin, M. Gang, H. Wu, X. Yang, M. D. Guiver and Z. Jiang, Adv. Funct. Mater., 2015, 25, 7502 CrossRef CAS; W. Jia, B. Tang and P. Wu, ACS Appl. Mater. Interfaces, 2017, 9, 22620 CrossRef.
- S. Chandra, T. Kundu, S. Kandambeth, R. BabaRao, Y. Marathe, S. M. Kunjir and R. Banerjee, J. Am. Chem. Soc., 2014, 136, 6570 CrossRef CAS; H. Ma, B. Liu, B. Li, L. Zhang, Y.-G. Li, H.-Q. Tan, H.-Y. Zang and G. Zhu, J. Am. Chem. Soc., 2016, 138, 5897 CrossRef; X. Hong, S. Tao and D. Jiang, Nat. Mater., 2016, 15, 722 CrossRef.
- J. H. Chong, M. Sauer, B. O. Patrick and M. J. MacLachlan, Org. Lett., 2003, 5, 3823 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods, Fig. S1–S15, and Tables S1 and S2. See DOI: 10.1039/c8cc08590k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2019 |