An unprecedented pentanary chalcohalide with Mn atoms in two chemical environments: unique bonding characteristics and magnetic properties†
Yu-Jun
Zheng
a,
Yong-Fang
Shi
a,
Chong-Bin
Tian
a,
Hua
Lin
 *a,
Li-Ming
Wu
*a,
Li-Ming
Wu
 *b,
Xin-Tao
Wu
a and
Qi-Long
Zhu
*b,
Xin-Tao
Wu
a and
Qi-Long
Zhu
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: linhua@fjirsm.ac.cn; qlzhu@fjirsm.ac.cn
bKey Laboratory of Theoretical and Computational Photochemistry, Ministry of Education, College of Chemistry, Beijing Normal University, Beijing 100875, P. R. China. E-mail: liming_wu@fjirsm.ac.cn
First published on 28th November 2018
Abstract
Mixed-anion chalcohalides have attracted significant attention lately, attributable to their unique structure compositions and captivating physicochemical properties. Herein, an unprecedented pentanary chalcohalide, Cs2[Mn2Ga3S7Cl] (1), was discovered by solid-state reaction at 1223 K. It is constructed by alternately stacked layers, each of which is made by a 2D [Ga3S9]9− ribbon embedded with 1D [Mn2S8Cl]13− chains. The coexistence of two Mn-coordinated polyhedra ([MnS6] octahedra and hetero-ligand [MnS3Cl] tetrahedra) in one material is surprisingly observed for the first time in the Mn-containing inorganic chalcogenides or chalcohalides. More interestingly, it exhibits ferrimagnetic (FIM) behaviour, which could be correlated to the magnetic sub-lattice sites with different coordination geometries. This work suggests a new route for designing and searching for unique functional chalcohalides with different chemical environments.
In recent years, low-dimensional chalcogenides with high mobility and rich electronic band structures have received considerable interest because of their fascinating physical properties, especially magnetism and superconductivity.1 In particular, mixed-anion oxychalcogenides or chalcohalides constructed by alternating layers are of great importance. In such layered structures, different electronegative (EN) elements are usually involved where: the more EN elements (e.g., O, F and Cl) prefer to adopt strong ionic-bonds via bonding with highly electropositive (EP) elements (e.g., rare-earth, alkaline-earth and alkali metals), whereas the less EN elements (e.g., S, Se and Te) attempt to form steady covalent-bonds with a less EP element (e.g., p-block elements and transition metals). Such interesting bonding characteristics are important in terms of understanding the formation of compounds and their potential applications.2–5 For instance, iron-lanthanum-oxide-sulfide and -selenide phases La2O3Fe2S2 and La2O3Fe2Se2 feature distinctive 2D layers stacked along the ab-plane in the order Fe2O/Se/La/O/La/Se/Fe2O and show interesting Van-Vleck paramagnetism.2 Oxychalcogenide (Li0.8Fe0.2)OHFeSe contains both conductive layers [FeSe] and spacer layers [(Li0.8Fe0.2)OH], thereby showing superconductivity at 42 K and antiferromagnetic (AFM) ordering at 8.6 K.3 2D [(BaF)2][Fe2−xQ3] (Q = S, Se) is formed by alternating [(BaF)2]2+ and [Fe2−xQ3]2− layers, and this compound exhibits switchable ferromagnetically ordered moments that are dictated mainly by the [Fe2−xQ3]2− layers.4 [Ba4F4][CrIn2S6], which consists of alternate stacking of cationic [Ba2F2]2+ layers and anionic [Cr0.5InS3]2− blocks, exhibits AFM ordering at around 8 K.5 Therefore, the synthesis and discovery of new layered mixed-anion compounds present an enticing prospect for exploring unique physicochemical properties and corresponding applications.
Up to now, group 13 metal chalcohalides containing alkali metals are less extensively investigated; to our knowledge, the existing examples include only 0D-K5(InTe4)(KCl),6 0D-(Cs6Cl)2Cs5[Ga15Ge9Se48],7 2D-(Cs6Cl)6Cs3[Ga53Se96],8 2D-[A3X][Ga3PS8] (A = K, Rb; X = Cl, Br),9 3D-(AX3)[MB12(MS4)3] (A = K, Cs; X = Cl, Br, I; M = Ga, In, Sm, and Gd),10 3D-ABa3Ga5Se10Cl2 (A = K, Rb, and Cs),11 and 3D-Cs2Ba6InxGa10−xSe20Cl4.12 As yet, except for the aforementioned compounds, materials containing magnetic elements are completely unknown in such systems. The aim of this study is to expand the structure and functional diversity of the above-mentioned chalcohalides by introducing magnetic elements. It is proposed that the introduction of magnetic elements is propitious to the formation of low-dimensional magnetic domains in the parent structures and will engender attractive magnetic properties. In this communication, we discover an unprecedented pentanary chalcohalide, Cs2[Mn2Ga3S7Cl] (1),‡ which possesses a unique 2D layered structure and interesting ferrimagnetic (FIM) behaviour.
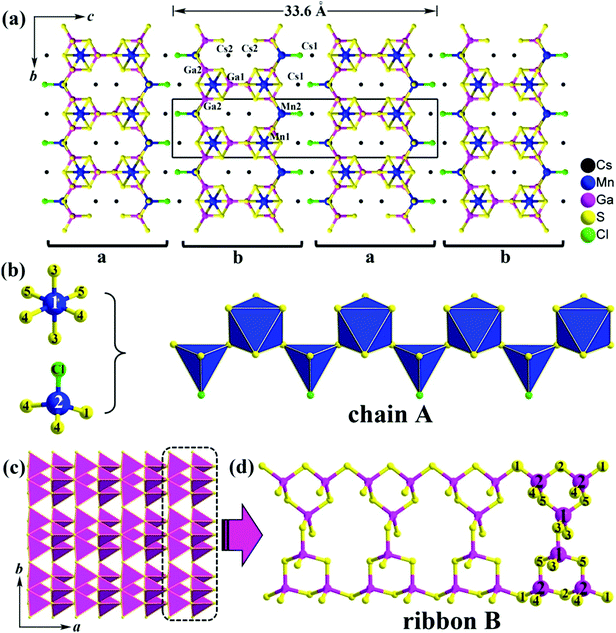
Single-crystal XRD diffraction analysis indicates that 1 crystallizes in the orthorhombic system with a = 6.2467(6) Å, b = 7.3879(8) Å, c = 33.600(3) Å, V = 1550.6(4) Å3 and Z = 4 [space group: Pnma (no. 62); Pearson symbol: oP60; idealized Wyckoff sequence: d3c9]. The asymmetric unit includes 2 crystallographically unique Cs atoms, 2 Mn atoms, 2 Ga atoms, 5 S atoms and 1 Cl atom. Among them, the Ga2, S4 and S5 atoms are at general sites 8d, while other atoms are at the Wyckoff positions 4c with m symmetry (for details see Fig. S1 and Table S1 in ESI†). Because there are no S–S and metal–metal bonds in 1, the formal oxidation states of Cs/Mn/Ga/S/Cl can be assigned as 1+/2+/3+/2−/1−, respectively.
The remarkably large c-axis, about 5 times larger than the other two axes, adopts a unique stacking of 2D [Mn2Ga3S7Cl]2− anionic layers along the c axis with the counter Cs+ cations locating in between (see Fig. 1a). There are two such layers in the unit cell and these layers are stacked in abab mode along the c-direction (A has a glide to B). Each of the layers consists of Ga–S ribbons (Fig. 1c) embedded with chains A (Fig. 1b) via sharing S1, S2, and S5 atoms. Uniquely, the chain A is built up from the [Mn1S6] octahedra and the hetero-ligand [Mn2S3Cl] tetrahedra. Mn-containing chalcogenides (or chalcohalides) with different coordination environments around Mn have never been reported previously to the best of our knowledge. The shortest distances of Mn1–Mn2, Mn1–Mn1 and Mn2–Mn2 are 4.343 Å, 6.247 Å and 6.247 Å, respectively (Fig. S2, ESI†). Within a ribbon B, the GaS4 tetrahedron serves as the primary building unit, and a Ga3S9 trimer is formed by one Ga1S4 and two Ga2S4 tetrahedra. Each Ga3S9 secondary building unit (SBU) is connected with another 2 neighboring SBUs into a ribbon B (Fig. 1d). Furthermore, such ribbon Bs are interconnected to each other to form a 2D [Ga3S9]9− layer along the a-axis via vertex-sharing (S3 and S4 atoms), as shown in Fig. 1c.
Normally in Mn-containing chalcogenides, the Mn atom is either 4-fold or 6-fold coordinated. Examples are in BaLa2MnS5,13 K10Mn4Sn4S17,14 MnPb4Sb6S14,15 and Ba4F4MnIn2S6.5 In contrast, Mn atoms in oxides usually adopt different coordination numbers (CNs). For instance, the strong antiferromagnetic spin-exchange interactions observed in KMnO4 at the T > 15 K region are attributed to the Mn1 (CN = 6), Mn2 (CN = 6) and Mn3 (CN = 4) sites;16 [NH4]4[Mn9B2(OH)2(HPO4)4(PO4)6] shows canted anti-ferromagnetism below 8.0 K, which originates from the Mn/O layers with Mn atoms of CN = 5, 6.17
Selected bond lengths are provided in Table S3 in the ESI.† Mn1 is in an octahedron sphere with six Mn–S bond lengths between 2.493 and 2.771 Å, which are close to those observed for Mn2+ with a 6-fold coordination, such as 2.464–2.838 Å in Ba4F4In2MnS6,5 and 2.429–2.780 Å in Gd2Mn3Sb4S12.18 Differently, the Mn2 atom is coordinated by 3 S atoms and 1 Cl atom forming a distorted hetero-ligand [MnS3Cl] tetrahedron with bond distances of Mn2–S ranging from 2.369 to 2.458 Å, which are comparable to those of 2.372–2.419 Å in K10Mn4Sn4S17.13 The Mn2–Cl is 2.354 Å, which is similar to those of 2.387–2.962 Å in Mn4(TeO3)(SiO4)Cl2.19 Each Ga atom forms a distorted tetrahedron with four Ga–S lengths varying from 2.271 to 2.297 Å, which are consistent with those in the related chalcohalides, such as (KI3)[SmB12(GaS4)3] (2.242–2.342 Å),10a Ba3GaS4Cl (2.232–2.268 Å),20 and [A3X][Ga3PS8] (2.225–2.357 Å).9
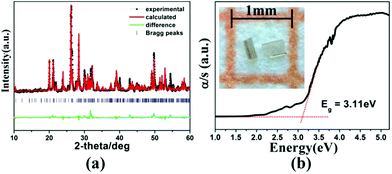
Light-yellow transparent 1 was obtained by mixing the elements Mn, Ga, and S with CsCl flux using solid-state reaction [for details see the ESI†]. Semi-quantitative EDX analyses displayed the presence of Cs, Mn, Ga, S, and Cl, and the average atomic ratio matches well with the single-crystal XRD data (Fig. S3, ESI†). The title crystals were stable in air for several months. The purity of hand-picked crystals was confirmed by powder XRD analyses and impure phases were not discovered within the measurable limits of the instrument (Fig. 2a). Several peaks in the difference curve indicate some deviation of the diffraction intensity, which may be caused by the crystallite orientation preference. Diffuse reflectance spectroscopy showed an optical band-gap of 3.11 eV for 1 (Fig. 2b), which was in agreement with its crystal color and suggests semiconductor behavior. This value is wider than those of Cs2Ba6InxGa10−xSe20Cl4 (2.90–3.01 eV),12 (Cs6Cl)6Cs3[Ga53Se96] (2.74 eV),8 and Ba3GaS4Cl (2.14 eV),20 but smaller than that of [A3X][Ga3PS8] (3.50–3.85 eV).9 Moreover, 1 exhibits high thermal stability up to 950 K (Fig. S4, ESI†).
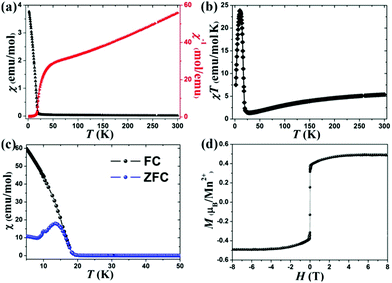
The magnetic susceptibility (χ) of 1 was tested at 1000 Oe in the temperature range from 2 to 300 K. As shown in Fig. 3a, the χ increases with decreasing temperature and a rapid upturn is seen at ∼16 K, indicating the onset of a ferromagnetic (FM) phenomenon. A clear Curie–Weiss behaviour is displayed at the range of 100–300 K, and the calculated Curie constant (C) and Weiss temperature (θ) are 8.6(8) emu mol−1 K−1 and −180.3(4) K, respectively. The negative θ value testifies a dominant AFM interaction among the neighboring Mn2+ ions (intrachain interaction). Moreover, the experimental effective magnetic moment (5.8(9) μB for per Mn2+) is very consistent with the theoretical value (5.916 μB) according to the equation of μeff2 = gS(S + 1). The χT as a function of temperature is shown in Fig. 3b, the value gradually decreases from about 5.38 emu mol−1 K−1 at 300 K to about 1.23 emu mol−1 K−1 at 25 K, and then rapidly increases and reaches the maximum value of 24.15 emu mol−1 K−1 at 16 K, confirming the presence of a ferromagnetic (FM) component, which may lead to the FIM behaviour or spin-canting ferromagnetism in this compound. To further confirm the magnetic behavior of 1, the FC (field-cooling) and ZFC (zero-field-cooling) magnetization data were recorded (Fig. 3c). The disagreement below 16 K suggests the onset of the magnetic phase transition. Furthermore, the strong field-dependent magnetic behaviour can be clearly seen in the FC curves (Fig. S5, ESI†), which testifies to the presence of spontaneous magnetization induced by FIM or spin canting.
The isothermal magnetization was recorded at 2 K (Fig. 3d). The magnetization increases linearly below 1.5 T, and then the magnetization increases slowly and exhibits a plateau above 3 T. The saturation value of 0.49 μB at 8 T, which is far below the saturation value of 5 μB for a high spin Mn2+ ion was obtained. This phenomenon indicates the presence of FIM rather than the spin-canting in 1, as the spin-canting usually results in a linear increase of magnetization with the increase of the magnetic field and the unsaturation magnetization behaviour at higher field. However, the field-induced magnetic phase transition from the FIM state to FM state, as seen in CoV2O621,22 and Co3(BPO4)2(PO4)(OH)3,23 was not observed under our experimental conditions, and a higher field (>8 T) should be required to observe such a magnetic phase transition.
Structurally, two sub-lattice sites are observed in 1, i.e., octahedral site [Mn1S6] and tetrahedral site [Mn2S3Cl]. On the other hand, the crystal field has significant influence on the magnetization – that is, Mn1 and Mn2 atoms may contain un-equivalent magnetization. And thus, the magnetization of Mn1 and Mn2 will not set off against each other when the magnetic interaction between Mn1 and Mn2 is AF type, and as a result the FIM behaviour will occur. In other words, the different magnetic sub-lattice sites with different coordination geometries ([MnS6] octahedron and hetero-ligand [MnS3Cl] tetrahedron) should be responsible for the FIM behaviour.
In summary, a novel Mn-based chalcohalide with a distinctive structure type, Cs2[Mn2Ga3S7Cl], is discovered and the single crystal structure and magnetic properties are investigated for the first time. The featured 2D [Mn2Ga3S7Cl]2− anionic layers in this structure are constructed from the [Ga3S9]9− ribbons embedded with infinite chains of [Mn2S8Cl]13−. Specially, the Mn atoms in this compound adopt two types of chemical environments: [MnS6] octahedra and hetero-ligand [MnS3Cl] tetrahedra. This compound exhibits FIM behaviour that is correlated to the two magnetic sub-lattice sites with different coordination geometries. Such unique bonding characteristics and fascinating magnetic properties of Cs2[Mn2Ga3S7Cl] may shed useful light on further exploration. In addition, this work will facilitate the design or prediction of 2D functionalized chalcohalides.
This research was supported by the National Natural Science Foundation of China (21771179, 21571020 and 21301175), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20010200), the support from “Chunmiao Projects” of Haixi Institute of Chinese Academy of Sciences, and the One Thousand Young Talents Program under the Recruitment Program of Global Youth Experts. We thank Prof. Zhang-Zhen He at FJIRSM for helpful discussions.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) E. E. Rodriguez, C. Stock, P. Y. Hsieh, N. P. Butch, J. Paglione and M. A. Green, Chem. Sci., 2011, 2, 1782–1787 RSC; (b) A. K. Ganguli, J. Prakash and G. S. Thakur, Chem. Soc. Rev., 2013, 42, 569–598 RSC; (c) C. D. Malliakas, D. Y. Chung, H. Claus and M. G. Kanatzidis, J. Am. Chem. Soc., 2013, 135, 14540–14543 CrossRef CAS PubMed; (d) J. T. Greenfield, C. Pak, S. Kamali, K. Lee and K. Kovnir, Chem. Commun., 2015, 51, 5355–5358 RSC; (e) M. L. Zhou, W. L. Yin, F. Liang, A. Mar, Z. S. Lin, J. Y. Yao and Y. C. Wu, J. Mater. Chem. C, 2016, 4, 10812–10819 RSC; (f) C. Gong, L. Li, Z. L. Li, H. W. Ji, A. Stern, Y. Xia, T. Cao, W. Bao, C. Z. Wang, Y. Wang, Z. Q. Qiu, R. J. Cava, S. G. Louie, J. Xia and X. Zhang, Nature, 2017, 546, 265–269 CrossRef CAS; (g) X. Zhou, C. Eckberg, B. Wilfong, S. C. Liou, H. K. Vivanco, J. Paglione and E. E. Rodriguez, Chem. Sci., 2017, 8, 3781–3788 RSC.
- J. M. Mayer, L. F. Schneemeyer, T. Siegrist, J. V. Waszczak and R. B. Van Dover, Angew. Chem., Int. Ed. Engl., 1992, 31, 1645–1647 CrossRef.
- X. F. Lu, N. Z. Wang, H. Wu, Y. P. Wu, D. Zhao, X. Z. Zeng, X. G. Luo, T. Wu, W. Bao, G. H. Zhang, F. Q. Huang, Q. Z. Huang and X. H. Chen, Nat. Mater., 2015, 14, 325–329 CrossRef CAS.
- M. Sturaza, J. M. Allred, C. D. Malliakas, D. E. Bugaris, F. Han, D. Y. Chung and M. G. Kanatzidis, Chem. Mater., 2015, 27, 3280–3290 CrossRef.
- Z. Z. Luo, C. S. Lin, W. D. Cheng, Y. B. Li, H. Zhang, W. L. Zhang and Z. Z. He, Dalton Trans., 2013, 42, 9938–9945 RSC.
- S. Sportouch, C. Belin and M. Tillard-Charbonnel, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1994, 50, 1861–1862 CrossRef.
- H. Lin, H. Chen, Z. X. Lin, H. J. Zhao, P. F. Liu, J. S. Yu and L. Chen, Inorg. Chem., 2016, 55, 1014–1016 CrossRef CAS PubMed.
- S. X. Huangfu, J. N. Shen, H. Lin, L. Chen and L. M. Wu, Chem. – Eur. J., 2015, 21, 9809–9815 CrossRef CAS PubMed.
- B. W. Liu, H. Y. Zeng, X. M. Jiang, G. E. Wang, S. F. Li, L. Xu and G. C. Guo, Chem. Sci., 2016, 7, 6273–6277 RSC.
- (a) S. P. Guo, M. S. Wang, J. P. Zou, H. Y. Zeng, L. Z. Cai, J. S. Huang and G. C. Guo, Chem. Commun., 2009, 4366–4368 RSC; (b) Z. D. Sun, Y. Chi, h. g. Xue and S. P. Guo, Inorg. Chem. Front., 2017, 4, 1841–1847 RSC.
- P. Yu, L. J. Zhou and L. Chen, J. Am. Chem. Soc., 2012, 134, 2227–2235 CrossRef CAS.
- Y. Y. Li, P. F. Liu, H. Lin, M. T. Wang and L. Chen, Inorg. Chem. Front., 2016, 3, 952–958 RSC.
- M. Wakeshima, Y. Hinatsu, K. Oikawa, Y. Shimojob and Y. Morii, J. Mater. Chem., 2000, 10, 2183–2185 RSC.
- O. Palchik, R. G. Iyer, J. H. Liao and M. G. Kanatzidis, Inorg. Chem., 2003, 42, 5052–5054 CrossRef CAS.
- P. Léone, L. Leuch, P. Palvadeau, P. Molinié and Y. Moëlo, Solid State Sci., 2003, 5, 771–776 CrossRef.
- H. B. Yahia, E. Gaudin, C. Lee, M. H. Whangbo and J. Darriet, Chem. Mater., 2007, 19, 5563–5569 CrossRef.
- M. Yang, J. H. Yu, L. Shi, P. Chen, G. H. Li, Y. Chen and R. R. Xu, Chem. Mater., 2006, 18, 476–481 CrossRef CAS.
- H. J. Zhao, L. H. Li, L. M. Wu and L. Chen, Inorg. Chem., 2010, 49, 5811–5817 CrossRef CAS.
- I. Zimmermann, R. K. Kremer and M. Johnsson, J. Solid State Chem., 2014, 218, 6–9 CrossRef CAS.
- K. Feng, W. L. Yin, Z. H. Lin, J. Y. Yao and Y. C. Wu, Inorg. Chem., 2013, 52, 11503–11508 CrossRef CAS.
- Z. Z. He, J. I. Yamaura, Y. Ueda and W. D. Cheng, J. Am. Chem. Soc., 2009, 131, 7554–7555 CrossRef CAS.
- Z. Z. He and W. D. Cheng, J. Magn. Magn. Mater., 2014, 362, 27–30 CrossRef CAS.
- N. N. Wang, Z. Z. He, M. Y. Cui and Y. Y. Tang, Cryst. Growth Des., 2016, 16, 6426–6429 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental section, together with additional figures and tables. CCDC 1848728. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8cc08380k |
‡ Crystal data for Cs2[Mn2Ga3S7Cl] (1) at 293(2) K: orthorhombic; Pnma; Z = 4; a = 6.2467(6) Å, b = 7.3879(8) Å, c = 33.600(3) Å, V = 1550.6(3) Å3. Collection and refinement data: dcalc = 3.62 g cm−3; μ = 12.43 mm−1; 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 245 total reflections; 2117 unique reflections [Fo2 > 2σ(Fo2)]; GOF = 1.092; R1 = 3.81% and wR2 = 9.44% for I > 2σ(I). CCDC: 1848728.† 245 total reflections; 2117 unique reflections [Fo2 > 2σ(Fo2)]; GOF = 1.092; R1 = 3.81% and wR2 = 9.44% for I > 2σ(I). CCDC: 1848728.† |
| This journal is © The Royal Society of Chemistry 2019 |