Synthesis and electrochemical characterization of electroactive IoNanofluids with high dielectric constants from hydrated ferrous sulphate†
Aswathy
Joseph
 *a,
Marylin Mary
Xavier
b,
Jacek
Fal
c,
Gaweł
Żyła
*a,
Marylin Mary
Xavier
b,
Jacek
Fal
c,
Gaweł
Żyła
 c,
Soorya
Sasi
b,
P.
Radhakrishnan Nair
b,
A. S.
Padmanabhan
a and
Suresh
Mathew
c,
Soorya
Sasi
b,
P.
Radhakrishnan Nair
b,
A. S.
Padmanabhan
a and
Suresh
Mathew
 *ab
*ab
aSchool of Chemical Sciences (SCS), Mahatma Gandhi University, Kottayam, Kerala 686 560, India. E-mail: aswathyj@gmail.com; sureshmathewmgu@gmail.com
bAdvanced Molecular Materials Research Centre (AMMRC), Mahatma Gandhi University, Kottayam 686560, Kerala, India
cDepartment of Physics and Medical Engineering, Rzeszów University of Technology, Rzeszów, Poland
First published on 20th November 2018
Abstract
An iron oxide based-electroactive IoNanofluid with a high dielectric constant, high stability and low viscosity was synthesized from ferrous sulphate heptahydrate via a facile microwave assisted one-step route in 1-butyl-4-methylpyridinium chloride. The IoNanofluid exhibited CE coupled faradaic redox reactions involving reversible chemical reaction and reversible electron transfer steps. A transition from diffusion controlled to surface controlled capacitive processes was observed at varying scan rates. The efficiency of the charge–discharge process was greater than 94% even after 100 cycles.
Ferrous sulphate heptahydrate (FeSO4·7H2O) or melanterite is a major industrial by-product of titanium dioxide and steel surface treatment plants that causes serious environmental issues. Many efforts are being focused on utilizing this excess by-product for purposes like iron recovery, wastewater treatment, production of sodium or potassium ferrates, etc., in an attempt to cover its production volume by these industries.1–3 Recyclable industrial by-products like these often under-utilized in large quantities are an ingenious source of starting materials for nanofluid production. When combined with ionic liquids (ILs), they can offer distinctive and intriguing redox chemistry also. Herein, we investigate the fascinating redox reactions of electroactive IoNanofluids (complex dispersions of nanofluids in ILs) for the first time. The IoNanofluid electrochemistry can be promising if brought to the cognizance of the scientific community. This is mainly due to the wide electrochemical potential window offered by ILs, unlike conventional nanofluid dispersion mediums. In such systems, the IL anion–cation interaction energy, weak inter-ionic forces and nanoparticle–IL surface–interface interactions also have a decisive role in determining the nanofluids’ colloidal stability and electrochemical properties. Here, we present a new aspect of IoNanofluid redox chemistry that involves both coupled reversible chemical reaction (Cr) and reversible electron transfer reaction (Er).
Electroactive nanofluids consist of electrochemically active nanoparticles that can undergo electrochemical redox reactions during the charge–discharge process.4 Iron oxide based-IoNanofluids also exhibit a complicated electroactive nature. The redox processes of iron/iron oxide magnetic nanoparticles (MNPs) and ionic liquids are commonly investigated in many electrochemical systems.5–8 Similarly, nanomaterials of carbonaceous materials, hybrid polyoxometalates, metals, metal alloys, metal oxides, intermetallic or mixed metal oxides, metal phosphates, partially fluorinated metal oxides and phosphates, etc., freely dispersed in electrolytes of Li salts or anchored onto ionic liquid cations like alkyl-ammonium, phosphonium, imidazolium, and pyrrolidinium ions have also been explored as electroactive nanofluids.9–18 The practicality of such systems for implementation in redox flow batteries is also being explored tremendously nowadays.19–23 Characteristics such as very low viscosity, high solid loading, excellent thermal and colloidal stability, superior heat transfer capabilities, maximum discharge capacity, etc., are considered as desirable qualities for an electroactive nanofluid.19,24 Besides, the energy capacity of rechargeable systems working with electroactive nanofluids depends on the mass of electroactive nanomaterials in them.25
In this study, we used FeSO4·7H2O along with 1-butyl-4-methylpyridinium chloride and ammonia solution to prepare the IoNanofluid. For this, about 1 wt% of FeSO4·7H2O was dissolved in 1-butyl-4-methylpyridinium chloride (IL) at 100 °C and added few microlitres of 30 wt% ammonia solution until a pH greater than 10 was achieved. At higher pH, FeSO4·7H2O easily forms solid iron(II) hydroxide colloidal particles. This mixture was then microwave irradiated at 100 °C for 15 minutes in an Anton Paar Monowave 300 microwave reactor (reaction profile shown in Fig. S1 of the ESI†). The resultant IoNanofluid had exceptionally high colloidal stability greater than one year without the use of any stabilizers or surfactants. A compact IL solvation sphere formed around the iron oxide nanoparticles enhanced the dispersion stability as well as the dielectric constant of the IoNanofluid.
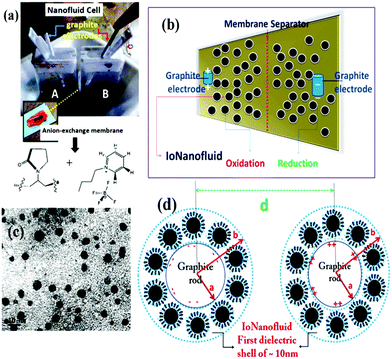
To study the IoNanofluid's electrochemical properties in the static state, we constructed a symmetric IoNanofluid cell, which is demonstrated in Fig. 1a. We used a two-electrode system in which electrochemically cleansed graphite rod electrodes were the positive and negative current collectors. About 1.1 mm2 area of these cylindrical graphite rods were immersed in 4 grams each of the IoNanofluid in the two cell compartments (A & B). To separate these compartments, a conducting anion-exchange thin film separator synthesized from a polymer–IL composite was used. The thin film separator was prepared by the free radical induced thermal polymerization of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of polyvinylpyrrolidone (PVP K-30) and 1-butylpyridinium tetrafluoroborate at 200 °C as previously reported.26 The structure of this IoNanofluid cell is illustrated in Fig. 1b. The nanoparticles formed from 1 wt% FeSO4·7H2O were polydisperse in nature with particle size below 10 nm, which is shown in Fig. 1c. The direct contact of the IL ions with the iron oxide nanoparticles’ surfaces enhanced the efficiency of the redox charge-transfer process. This IoNanofluid had a density of ∼1.025 g mL−1. Increasing the weight percentage of FeSO4·7H2O in the IL medium would increase the iron oxide particle size but decrease the nanofluid stability.27 A maximum of 4 wt% of the precursor could be stably dispersed in 1-butyl-4-methylpyridinium chloride without any surfactants that will give a nanofluid stability of approximately 20 days. However, increasing the amount of precursor dissolved in the IL would give more phase pure γ-Fe2O3 nanoparticles after microwave irradiation. The nanoparticles’ size, morphology and structural features will depend on the weight ratio of FeSO4·7H2O to the IL.
1 mixture of polyvinylpyrrolidone (PVP K-30) and 1-butylpyridinium tetrafluoroborate at 200 °C as previously reported.26 The structure of this IoNanofluid cell is illustrated in Fig. 1b. The nanoparticles formed from 1 wt% FeSO4·7H2O were polydisperse in nature with particle size below 10 nm, which is shown in Fig. 1c. The direct contact of the IL ions with the iron oxide nanoparticles’ surfaces enhanced the efficiency of the redox charge-transfer process. This IoNanofluid had a density of ∼1.025 g mL−1. Increasing the weight percentage of FeSO4·7H2O in the IL medium would increase the iron oxide particle size but decrease the nanofluid stability.27 A maximum of 4 wt% of the precursor could be stably dispersed in 1-butyl-4-methylpyridinium chloride without any surfactants that will give a nanofluid stability of approximately 20 days. However, increasing the amount of precursor dissolved in the IL would give more phase pure γ-Fe2O3 nanoparticles after microwave irradiation. The nanoparticles’ size, morphology and structural features will depend on the weight ratio of FeSO4·7H2O to the IL.
The cross-sectional view of graphite electrodes concentrically surrounded by the first dielectric shell constituting iron oxide nanoparticles with an IL double layer is schematically illustrated in Fig. 1d. The value of specific capacitance per unit length of the electrode was estimated to be 0.032 F cm−1 from the equation based on Gauss's law: C = πε0εr[ln(d/a)]−1 for two parallel cylindrical electrodes. ‘a’ is the radius of the electrode placed at a distance ‘d’ as can be seen in Fig. 1d. ε0 and εr are the dielectric constants of the vacuum and IoNanofluid respectively (εr for this calculation was assumed to be 1.5 × 108). Fig. S2a of the ESI† shows the plot of the dielectric constant of the IoNanofluid measured using broadband dielectric spectroscopy (a Concept 80 System with a Quatro Cryosystem) by a parallel plate method. The material could achieve a dielectric constant of the order of 108 under a 0.1 Hz applied field, which is very notable. High dielectric fluid materials can reduce the power loss of energy harvesting systems.28 The capacitance of this IoNanofluid measured from dielectric measurements (shown in Fig. S3 of the ESI†) at 35 °C was about 0.9 F cm−2. Fig. S2b of the ESI† shows the plot of viscosity versus shear rate in the range 0–500 s−1 of the IoNanofluid measured using a HAAKE MARS 2 rheometer with a plate–plate type measuring geometry. The viscosity of the IoNanofluid prepared using 1 wt% FeSO4·7H2O is about 0.15 Pa s at shear rates below 100 s−1. The IoNanofluid showed a Newtonian shear-thinning behaviour until a shear rate of 100 s−1. Besides, unlike other nanofluids, increasing the nanoparticle fraction in the IL by increasing the amount of FeSO4·7H2O up to 15 wt% in the IL would not cause any increase in IoNanofluid viscosity above 0.2 Pa s probably because of the tribological properties of the IL.7 This is a peculiar feature of this IoNanofluid.
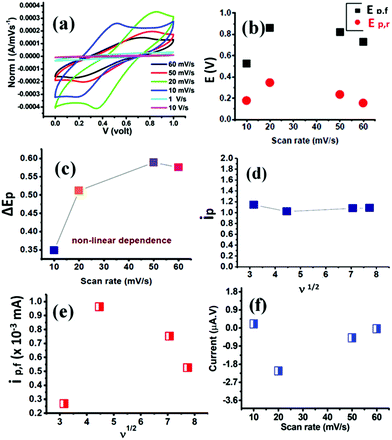
The electrochemical measurements were performed in a Bio-Logic SP-200 workstation with EC Lab software. Cyclic voltammetry (CV) at different scan rates ‘ν’ (10 mV s−1, 20 mV s−1, 50 mV s−1, 60 mV s−1, 1 V s−1 and 10 V s−1) was performed between 0 and 1 V as shown in Fig. 2a. The current measured was normalized with respect to scan rates. The pseudocapacitive nature of the IoNanofluid can be understood from these CV curves. During the forward and reverse scans at 10 and 20 mV s−1 scan rates, redox peaks were observed. These CV curves exhibited faradaic response similar to that of a solution redox couple. The maximum current density was observed at 20 mV s−1 scan rate as can be seen in Fig. 2a. The specific capacitance estimated at 20 mV s−1 scan rate using the equation given in the ESI† was 15 mF g−1 for approximately 0.01 g of γ-Fe2O3 in the IL. The peak oxidation potential (Ep,f) and the peak reduction potential (Ep,r) were detected at 0.84 and 0.35 volts respectively. The E° value calculated from the equation (Ep,f + Ep,r)/2 was 0.59, indicating a single electron involved redox reaction (n = 1). At 50 and 60 mV s−1, the CV curves exhibited a quasi-rectangular shape as the peak current decreased. At very high scan rates of 1 and 10 V s−1, the redox peaks disappeared since the electron transfer step was effectively suppressed by the rapid interconversion of species forming the redox couple. Besides, the current density was observed to be too small during faster scan rates. This confirmed that this high dielectric IoNanofluid generates energy from a faradic redox mechanism unlike in EDLCs.
Fig. 2b shows the variations of oxidation and reduction peak potentials at different scan rates (10, 20, 50 and 60 mV s−1). The positions of the peak potentials were dependent on scan rates and were not constant. The highest peak potentials were observed at 20 mV s−1 scan rate. The Ep,f at 10 mV s−1 scan rate was lower than the Ep,f at 60 mV s−1 scan rate though the Ep,r values remained nearly the same for both. Fig. 2c shows the plot of ΔEp (i.e., the separation between the two peak potentials) versus the scan rate. Though the ΔEp value increases with an increase in scan rate from 10 to 50 mV s−1, there was no linear correlation between ΔEp and scan rates (ν). This non-linearity indicated the electrochemical reversibility of this pseudocapacitive IoNanofluid. If the electrochemically generated species in the IoNanofluid is stable in the time scale of the scans, the peak current measured for the forward potential scan (ip,f) has to be equal to the peak current measured for the reverse potential scan (ip,r) – i.e., the value of ip = ip,r/ip,f should be equal to 1. However, we measured this value to be 1.14, 1.02, 1.07 and 1.08 at 10, 20, 50 and 60 mV s−1 sweep rates, respectively, as shown in Fig. 2d. Besides, the peak current of the forward scan should linearly increase as a function of the square root of the scan rate (ν1/2) for a reversible electron transfer reaction. However, we observed deviations from this linear relation of ip,f on ν1/2 as shown in Fig. 2e. This indicated some complications in the electron transfer kinetics of the IoNanofluid. Fig. 2f shows that the current response varied directly with sweep rate from 20 mV s−1 and higher. Therefore, the redox reaction could involve a transition from diffusion controlled to surface controlled (capacitive) processes for these scan rates.29 The current function (ip/ν1/2) was also not constant for all these scan rates. The values were calculated to be 0.38, 0.22, 0.15 and 0.14 for scan rates of 10, 20, 50 and 60 mV s−1 respectively.
Complications in the electron transfer kinetics of IoNanofluid arise probably due to chemical changes in the methyl substituted N-butylpyridinium cations rings. The redox chemistry of the IoNanofluid may be influenced by the interaction of the IL with the FeII/FeIII redox couple on the MNPs. The chemical structures of radical species formed from the 1-butyl-4-methylpyridinium cation with inorganic anions by radiation-induced redox reactions have been already reported by Shkrob et al.30 They have also identified the formation of N-butyl radical species for some anions, suggesting a secondary reaction process involving a fragment radical. However, in order to establish the redox mechanism, more detailed studies would be required. Assuming a ‘one electron’ involved redox process, we would like to propose a CrEr reaction mechanism – i.e., a reversible chemical reaction step (Cr: Z ⇌ Ox), followed by a reversible electron transfer (Er: Ox + e− ⇌ Red). In this kind of CE coupled mechanism, the amount of Ox species formed during the Cr process that is available for reduction in the Er process will depend on the equilibrium constant of the Cr process.31 Besides, the reorganization of the IL solvation sphere during electric polarization can also affect the redox chemistry. This is also supported by previous observations that in certain solvent-controlled charge transfer reaction processes the reaction dynamics may be controlled by solvent reorganization.32 The electrical resistance of the IoNanofluid cell was analysed using electrochemical impedance spectroscopy (EIS) in the frequency range from 200 Hz to 100 mHz at a potential amplitude of 1 V. The resulting Nyquist plot of the impedance study is shown in Fig. S4 of the ESI.† The plot did not show any semicircular region indicating charge transfer resistance. Instead, it demonstrated a vertical long tail indicating capacitive behaviour.
The equivalent circuit fitting of this EIS response indicated the double layer capacitance developed at the two electrodes (C1 & C4), the electron transfer resistance arising during charge transfer between the electrode and IoNanofluid (R2 & R4), the solution resistance or electrolyte resistance (R1 & R3) and the Warburg element of slow diffusion or mass transfer of ions (W3) through either the separator membrane or through the medium. Therefore, these observations show the polarization of the bulk EDL region and percolative charge transfer through the IoNanofluid, which enables it to function as a liquid electrolyte.
The charge–discharge tests were done using the chronoamperometric method in the E range between −2.5 and 2.5 V and also using the galvanostatic charge–discharge (GCD) method. Fig. S5a of the ESI† shows the plot of charge–discharge capacity versus the number of chronoamperometric cycles. Approximately 0.01 g of iron oxide produced from 1 wt% FeSO4·7H2O dissolved in 1-butyl-4-methylpyridinium chloride could give a discharge capacity greater than 0.01 mA h g−1. The efficiency of the chronoamperometric charge–discharge process was greater than 94% even when reaching 100 cycles as illustrated in Fig. S5b of the ESI.† Fig. S5c of the ESI† shows the galvanostatic charge–discharge curves of the cell at various current densities showing an increase in cell voltage with an increase in current. The chronoamperometric charge–discharge curves measured at constant applied potentials of 0.35 V and 1 V for 10 s shown in Fig. S5d of the ESI† indicate that the electrode capacitance is very small. The first two curves (blue and red) in Fig. S5d of the ESI† represent the current transients during the potential stepping from 0 to 1 and 0 to 0.35 volts. During this stepping, the interfacial charge-transfer reaction occurs very rapidly kinetically so that the equilibrium concentration of the active species during the Cr process is reached very rapidly at the electrode surface. Here, the primary mode of mass transfer at electrodes is non-linear diffusion. The second two curves (blue and red) in Fig. S5d of the ESI† indicate the discharging current during a potential stepping from 1 and 0.35 volts to 0 V. Also, the current density values observed at a constant potential of 1 V was higher than that at 0.35 V. Fig. S5e of the ESI† shows the I–V curve of the IoNanofluid lying in the 4th quadrant, which indicates that this symmetric nanofluid cell produces power from the faradaic pseudocapacitive mechanism and electrical double layer charging. Fig. S5f of the ESI† shows the variation of current and voltage in this cell with respect to time. The current produced by the cell decreases in a sinusoidal manner with the decrease in applied potential.
In conclusion, the present communication has put forward a relatively new concept of using IoNanofluids in electrochemistry. IoNanofluids hold unique electrochemical characteristics brought about by ILs. We have presented here the redox properties of an iron oxide based-IoNanofluid synthesized from FeSO4·7H2O and 1-butyl-4-methylpyridinium chloride. This electroactive IoNanofluid exhibited a remarkably high dielectric constant, apart from moisture stability and low viscosity. The specific capacitance and current density also varied with scan rate. The observed deviations from the linear relation of ip,f to ν1/2 indicated complicated electron transfer kinetics in the IoNanofluid. The material exhibited a complex redox character involving a transition from diffusion controlled to surface controlled (capacitive) processes at different scan rates. The proposed method of IoNanofluid synthesis from hydrated ferrous sulphate offers a sustainable way for nanofluid production. Increasing the amount of nano iron oxide is expected to significantly improve the electrochemical performance of this IoNanofluid. Besides, there also exists a lot of scope for improving its functional properties by further optimizations like nanoparticle surface engineering, use of organic–inorganic hybrid nanoparticles, etc. Furthermore, future research studies aimed at understanding the kinetics of IoNanofluid redox mechanisms involving different IL cations can be helpful to gain more insight into IoNanofluid electrochemistry.
This work was supported by an Indo-Poland Joint research project (2015–2017) – DST/INT/POL/P-06/2014 jointly funded by DST, Govt. of India, and the Polish Ministry of Science and Higher Education. The first author also acknowledges a Jawaharlal Nehru Scholarship for Doctoral Studies (India).
Conflicts of interest
There are no conflicts to declare.References
- D. Filippou and G. Hudon, JOM, 2009, 61, 36–42 CrossRef CAS.
- N. Kanari, O. Evrard, N. Neveux and L. Ninane, JOM, 2001, 53, 32–33 CrossRef CAS.
- N. Kanari, I. Filippova, F. Diot, J. Mochón, I. Ruiz-Bustinza, E. Allain and J. Yvon, Thermochim. Acta, 2014, 575, 219–225 CrossRef CAS.
- E. V. Timofeeva, J. P. Katsoudas, C. U. Segre and D. Singh, Cleantechnol Energy Storage, 2013, 9, 363–366 Search PubMed.
- S. Sen, E. Moazzen, S. Aryal, C. U. Segre and E. V. Timofeeva, J. Nanopart. Res., 2015, 17, 437 CrossRef.
- C. Largeot, C. Portet, J. Chmiola, P. L. Taberna, Y. Gogotsi and P. Simon, J. Am. Chem. Soc., 2008, 130, 2730–2731 CrossRef CAS PubMed.
- R. Chen and R. Hempelmann, Electrochem. Commun., 2016, 70, 56–59 CrossRef CAS.
- S. Zheng, X. Li, B. Yan, Q. Hu, Y. Xu, X. Xiao, H. Xue and H. Pang, Adv. Energy Mater., 2017, 7, 1602733 CrossRef.
- E. V. Timofeeva, J. P. Katsoudas, D. Singh and C. U. Segre, US Pat., 14/889939, 2016 Search PubMed.
- S. Sen, E. V. Timofeeva, C. J. Pelliccione, J. P. Katsoudas, D. Singh and C. U. Segre, ECS Meeting Abstracts, 2015, 1, 224 Search PubMed.
- D. P. Dubal, D. Rueda-Garcia, C. Marchante, R. Benages and P. Gomez-Romero, Chem. Rec., 2018, 18, 1–10 CrossRef PubMed.
- J. Lobato, E. Mena and M. Millán, ChemistrySelect, 2017, 2, 8446–8450 CrossRef CAS.
- D. Rueda-Garcia, Z. Cabán-Huertas, S. Sanchez-Ribot, C. Marchante, R. Benages, D. P. Dubal, O. Ayyad and P. Gómez-Romero, Electrochim. Acta, 2018, 281, 594–600 CrossRef CAS.
- J. Gangwar, K. K. Dey, S. K. Tripathi, M. Wan, R. R. Yadav, R. K. Singh and A. K. Srivastava, Nanotechnology, 2013, 24, 415705 CrossRef PubMed.
- P. Leung, X. Li, C. P. de León, L. Berlouis, C. T. John Low and F. C. Walsh, RSC Adv., 2012, 2, 10125–10156 RSC.
- B. Li, P. Gu, Y. Feng, G. Zhang, K. Huang, H. Xue and H. Pang, Adv. Funct. Mater., 2017, 27, 1605784 CrossRef.
- Q. Li, S. Zheng, Y. Xu, H. Xue and H. Pang, Chem. Eng. J., 2018, 333, 505–518 CrossRef CAS.
- X. Li, J. Wei, Q. Li, S. Zheng, Y. Xu, P. Du, C. Chen, J. Zhao, H. Xue, Q. Xu and H. Pang, Adv. Funct. Mater., 2018, 28, 1800886 CrossRef.
- D. P. Dubal and P. Gomez-Romero, 2D Mater., 2016, 3, 031004 CrossRef.
- S. Sen, C. M. Chow, E. Moazzen, C. U. Segre and E. V. Timofeeva, J. Appl. Electrochem., 2017, 47, 593–605 CrossRef CAS.
- B. Kristiawan, A. Wijayanta and W. Juwana, AIP Conf. Proc., 2016, 1717, 030010 CrossRef.
- J. Lobato, E. Mena and M. Millán, ChemistrySelect, 2017, 2, 8446–8450 CrossRef CAS.
- J. Kim and H. Park, Energy, 2018, 160, 192–199 CrossRef CAS.
- Z. Libor, S. A. Wilson and Q. Zhang, J. Mater. Sci., 2011, 46, 5385 CrossRef CAS.
- E. V. Timofeeva, J. P. Katsoudas, C. U. Segre and D. Singh, NSTI-Nanotech, 2013, 2, 679 Search PubMed.
- A. Joseph, M. M. Xavier, G. Żyła, P. R. Nair, A. Padmanabhan and S. Mathew, RSC Adv., 2017, 7, 16623–16636 RSC.
- A. Joseph, J. Fal, G. Żyła and S. Mathew, J. Therm. Anal. Calorim., 2018, 1–8 CAS.
- T. Kim, H. Yong, B. Kim, D. Kim, D. Choi, Y. T. Park and S. Lee, Nat. Commun., 2018, 9, 1437 CrossRef.
- V. Augustyn, P. Simon and B. Dunn, Energy Environ. Sci., 2014, 7, 1597–1614 RSC.
- I. A. Shkrob, T. W. Marin, S. D. Chemerisov, J. L. Hatcher and J. F. Wishart, J. Phys. Chem. B, 2011, 115, 3889–3902 CrossRef CAS PubMed.
- N. Elgrishi, K. J. Rountree, B. D. McCarthy, E. S. Rountree, T. T. Eisenhart and J. L. Dempsey, J. Chem. Educ., 2017, 95, 197–206 CrossRef.
- H. Weingärtner, P. Sasisanker, C. Daguenet, P. J. Dyson, I. Krossing, J. M. Slattery and T. Schubert, J. Phys. Chem. B, 2007, 111, 4775–4780 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Microwave reaction profile of IoNanofluid synthesis. See DOI: 10.1039/c8cc08243j |
| This journal is © The Royal Society of Chemistry 2019 |