Internal standardization as a strategy to overcome non-spectral interferences in the determination of As, Cd and Pb in mineral fertilizers by synchronous vertical dual view (SVDV) ICP OES
Raquel C.
Machado
 *a,
Ana Beatriz S.
Silva
*a,
Ana Beatriz S.
Silva
 ab,
Clarice D. B.
Amaral
ab,
Clarice D. B.
Amaral
 c,
Alex
Virgilio
d and
Ana Rita A.
Nogueira
c,
Alex
Virgilio
d and
Ana Rita A.
Nogueira
 b
b
aGroup for Applied Instrumental Analysis, Department of Chemistry, Federal University of São Carlos, P.O. Box 676, 13565-905, São Carlos, SP, Brazil. E-mail: raquelcm.quim@gmail.com
bEmbrapa Pecuária Sudeste, P.O. Box 339, 13560-970, São Carlos, SP, Brazil
cFederal University of Paraná, Department of Chemistry, 81531-980, Curitiba, PR, Brazil
dSão Paulo State University, UNESP, P.O. Box 355, 14800-060, Araraquara, SP, Brazil
First published on 18th November 2019
Abstract
Elemental determination for fertilizer analysis by optical spectrometric techniques is often prone to non-spectral interferences due to the high content of easily ionizable elements (EIEs). Herein, we verify the use of internal standardization as a strategy for compensating for this matrix effect during As, Cd, and Pb determination in mineral fertilizers by SVDV ICP OES. Bismuth, Ir, Sc, and Y were chosen as internal standards (IS) and their ionic and atomic emission lines were carefully taken into account. The results indicated that the EIEs presence caused signal changes and internal standardization may be a suitable alternative to minimize this interference. By analyzing a certified reference material (CRM NIST 695) the best recoveries (from 94 to 106%) were achieved by applying internal standardization for all analytes. Concerning the elements evaluated as internal standards, Y (360.1 nm) and Sc (424.7 nm) were more suitable for As while Ir (215.8 nm) and Bi (190.2 nm and 195.4 nm) were suitable for Cd. For Pb, Ir (215.8 nm) was the most appropriate internal standard. The limits of detection using ISs were 0.70, 0.03 and 1.00 mg kg−1 for As, Cd and Pb, respectively. The application of internal standardization is an effective strategy to overcome matrix interferences in fertilizer analysis by SVDV ICP OES.
Introduction
Nowadays, nutrient and contaminant determination in fertilizers is extremely relevant because of their growing application in agriculture.1,2 However, the analysis of this type of matrix by analytical spectrometric techniques is not easy, especially those involving plasma sources such as ICP OES, ICP-MS and MIP OES. The main issues are directly correlated to the presence of high amounts of the easily ionizable elements (EIEs) Na, K, Mg and Ba, which can cause non-spectral interferences, such as alteration in transport and aerosol formation and plasma instability, by the modification of their temperature and electron density, which affects the ionization and excitation processes of the analyte. When these effects are neglected and not properly corrected during analysis, a critical bias can occur in the results.3,4O'Hanlon et al. investigated the effect of Na, K, Li and Cs by axially viewed ICP OES. The authors analyzed Mg slurries and solutions in the presence and absence of EIEs and the Mg(II)/Mg(I) ratio was monitored. The results confirmed that the transport efficiency decreased from 10 to 55% when these contaminants are present. Moreover, the presence of potassium in the argon plasma produced alterations in the Boltzmann plot and the relative number density, and as a result, changes in the slopes related to the alteration in excitation temperature were observed.5 Dettman & Olesik also verified that in the presence of Na, Ba, Ca, La and Li, the plasma temperature was reduced as the concentration of these elements was increased.6
Inductively coupled plasma optical emission spectrometry (ICP OES) is one of the most useful analytical techniques employed in the trace analysis of fertilizers due to its main features such as multi-element capability, robustness and good sensitivity. Despite being well established, continuous efforts are being made to develop new instrumental configurations to improve some characteristics. Synchronous Vertical Dual View ICP OES was recently inroduced and unlike conventional dual view ICP OES, this design allows the simultaneous monitoring of radial and axial views due to the use of dichroic spectral combiner (DSC) technology. As a result, higher sample throughputs and, consequently, reduction in argon consumption are the main advantages.7,8
Many strategies such as standard addition or matrix-matching have been reported in the literature to improve accuracy and minimize matrix effects in ICP OES analyses. Furthermore, novel approaches have also been developed such as multi-energy calibration (MEC) and standard dilution analysis (SDA).9–11 In addition to these calibration strategies, the use of internal standardization is well explored in the literature and may also be considered an effective method.12–14 For ICP OES analysis, internal standardization has been employed for several matrices such as food, pharmaceuticals and environmental samples.15–20 Nonetheless, this strategy is still not performed to overcome non-spectral interferences during fertilizer analysis.
The principle of the internal standardization strategy considers that interferences affect the analyte and the IS similarly. The element chosen as the IS is added to all samples, blanks and calibration standards, and the data treatment is performed by the ratio of analyte and IS instrumental responses. Furthermore, signal changes and errors are minimized during the analysis. As a general rule, an ideal IS in emission spectroscopy should have an ionization energy, excitation emission line, and type (atomic or ionic) of line similar to those of the analyte.11,21 Nevertheless, finding an element that fulfills all these requirements is not a trivial task. Various studies in the literature report a number of limitations in these requisites, and they consider that the criteria can be influenced by the operational parameters and matrix components.12,22 Besides, there are studies that employ multivariate analysis such as Principal Component Analysis (PCA) for choosing an appropriate IS.23,24 Therefore, considering the complexity of mineral fertilizer matrices, herein we aim to evaluate the use of Bi, Ir, Sc, and Y as ISs for the minimization of non-spectral interferences in the determination of mineral fertilizer contaminants (As, Cd, and Pb) by SVDV ICP OES.
Experimental
Reagents, standard solutions, and samples
All solutions were prepared with ultrapure water (Milli-Q™, Millipore, Bedford, MA, USA). All glassware and polypropylene flasks were decontaminated in 10% (v/v) nitric acid, followed by rinsing with deionized water. Nitric acid (Synth, São Paulo, Brazil) previously purified using a sub-boiling distillation system (Model BSB-939-IR, Distillacid, Berghof, Germany) and hydrogen peroxide (Sigma Aldrich, Germany) were used for acid decomposition. The standard solutions were prepared by appropriate dilution of 1000 mg L−1 As, Cd, and Pb (Qhemis, São Paulo, Brazil) stock solutions. Internal standards (Bi, Ir, Sc and Y) solutions of 2 mg L−1 were added separately to all the working solutions (analytical curves, blanks, and samples).All mineral fertilizer samples were provided by the National Agricultural and Livestock Laboratories (Goiânia, Brazil). The elemental constituents of these samples including EIEs such as Ba, K and Mg were determined by instrumental neutron activation analysis (INAA). They showed a varied composition and the EIE mass fraction ranges were 500–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1 Ba, 5000–14
000 mg kg−1 Ba, 5000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 mg kg−1 K and 750–16
250 mg kg−1 K and 750–16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 mg kg−1 Na. A certified reference material, Trace Elements in Multi-Nutrient Fertilizer, CRM 695 (National Institute of Standards and Technology, Gaithersburg, MD, USA) was analyzed to check the accuracy.
500 mg kg−1 Na. A certified reference material, Trace Elements in Multi-Nutrient Fertilizer, CRM 695 (National Institute of Standards and Technology, Gaithersburg, MD, USA) was analyzed to check the accuracy.
Instrumental
An inductively coupled plasma optical emission spectrometer operating with Synchronous Vertical Dual View (SVDV) (ICP OES 5100, Agilent Technologies, Mulgrave, Australia) was used in all the experiments. A OneNeb® nebulizer and a cyclonic spray chamber were employed as a sample introduction system. High purity argon (99.999%) (White Martins-Praxair, Sertãozinho, SP, Brazil) was used for plasma generation, nebulization and as the auxiliary gas. The instrument's operating conditions are shown in Tables 1 and 2. An Ethos 1 microwave-assisted digestion system (Milestone, Sorisole, Italy) was used for sample digestion.| Instrumental parameter | Operating condition |
|---|---|
| RF power (kW) | 1.5 |
| Nebulizer gas flow rate (L min−1) | 0.6 |
| Plasma gas flow rate (L min−1) | 12 |
| Auxiliary gas flow rate (L min−1) | 1.0 |
| Integration time (s) | 10 |
| Plasma observation | SVDV |
| Element | Type | Emission line (nm) | Excitation energy (eV) | Ionization energy (eV) | Energy sum (eV) |
|---|---|---|---|---|---|
| a The data were obtained from the NIST database.25 | |||||
| As I | Analyte | 189.042 | 6.56 | 9.78 | 6.56 |
| Cd II | Analyte | 226.502 | 5.47 | 8.99 | 14.46 |
| Pb I | Analyte | 217.000 | 5.71 | 7.42 | 5.71 |
| Bi I | IS | 195.471 | 6.34 | 7.29 | 6.34 |
| Bi I | IS | 306.770 | 4.04 | 7.29 | 4.04 |
| Bi II | IS | 190.241 | 8.63 | 7.29 | 15.92 |
| Ir I | IS | 215.805 | 6.09 | 8.97 | 6.09 |
| Ir II | IS | 215.268 | 6.35 | 8.97 | 15.32 |
| Sc II | IS | 335.372 | 4.01 | 6.56 | 10.57 |
| Sc II | IS | 357.634 | 3.47 | 6.56 | 10.03 |
| Sc II | IS | 424.682 | 3.23 | 6.56 | 9.79 |
| Y II | IS | 324.228 | 4.00 | 6.22 | 10.22 |
| Y II | IS | 360.074 | 3.62 | 6.22 | 9.84 |
| Y II | IS | 371.029 | 3.52 | 6.22 | 9.74 |
Sample preparation
A sample mass of approximately 0.2 g was digested with 6.0 mL of 7.0 mol L−1 HNO3 and 2.0 mL of 30% (m m−1) H2O2. The following microwave heating program was applied to digest the samples: 20 min to reach 200 °C and a 20 min hold at 200 °C (both at a microwave applied a power of 1100 W). After cooling, the solution volumes were made up to 50.0 mL with deionized water. The same procedure was applied for CRM 695.Results and discussion
Effect of EIE concentrations on As, Cd, and Pb signals
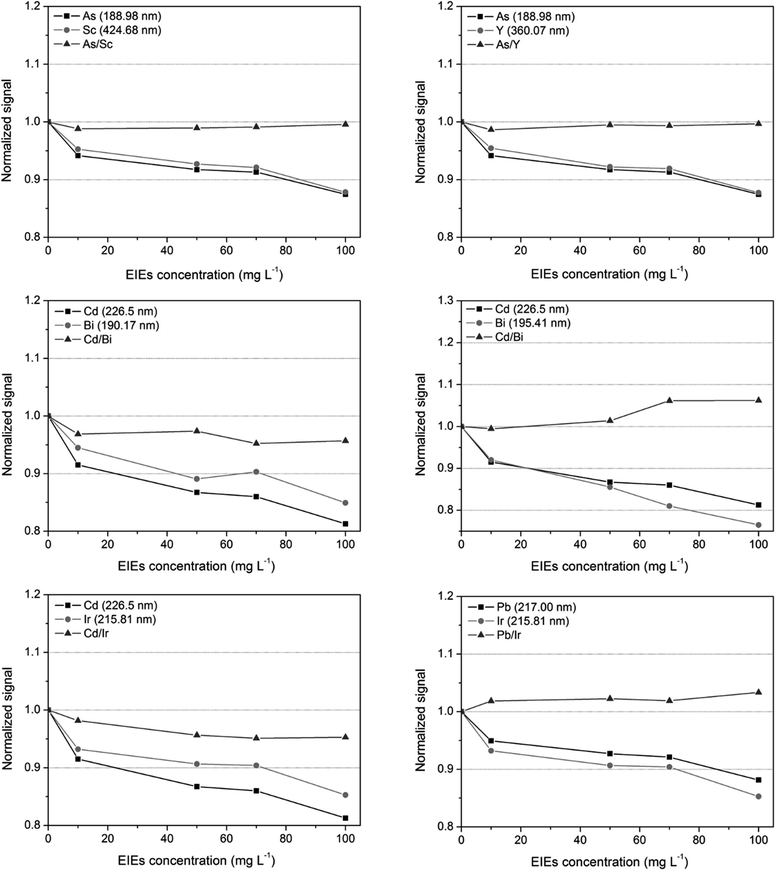
The effect of easily ionizable elements (EIEs) present in the plasma on analyte signals was evaluated with five solutions containing 2 mg L−1 As, Cd, and Pb and crescent concentrations of Ba, K and Na (0, 10, 50, 70 and 100 mg L−1). The EIE concentrations employed in this study were selected based on the values in the real samples (2–44 mg L−1 for Ba, 20–57 mg L−1 for K and 3–66 mg L−1 for Na). The signals were recorded and normalized by the intensities obtained from solutions without EIEs (Fig. 1).According to the results, no considerable effects were observed when the analytes investigated were in the solution containing up to 10 mg L−1 EIEs. On the other hand, when the concentration was increased to 50 mg L−1 or higher, signal suppression was observed for all analytes. This effect was more pronounced for Cd, in which the intensity drifts reached approximately 20%. This signal reduction due to the EIEs present can be attributed to charge transfer reactions between these elements and argon.25,26 As previously mentioned, the introduction of matrices as fertilizers in Ar plasma, which contain high amounts of easily ionizable elements, can directly affect its fundamental parameters such as the temperature and the electron density. Hence, the ionization equilibrium, efficiency or mechanism of analyte excitation are changed.
Use of internal standardization for overcoming the matrix effect caused by EIEs and performance parameters
Four elements (Bi, Ir, Sc and Y) were investigated as potential ISs. These elements were selected due to their physico-chemical properties being similar to those of the analytes (Table 2). In addition, these internal standards provided a wide range of excitation energies (3.23–8.62 eV) and excitation emission lines (190.2–424.7 nm). Three excitation emission lines were selected considering their sensitivity and the absence of spectral interferences. The accuracy and efficiency of the internal standards were evaluated by the analysis of a certified reference material (CRM NIST 695) and the results are shown in Table 3. Recoveries between 79 and 82% were found when external calibration was employed, indicating poor accuracy due to the occurrence of critical matrix effects.| Internal standard (IS) | As | Cd | Pb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Determined value (mg kg−1) | Rec. (%) | t valueb | Determined value (mg kg−1) | Rec. (%) | t valueb | Determined value (mg kg−1) | Rec. (%) | t valueb | |
| a Certified value (mg kg−1) – As: 200 ± 5; Cd: 16.9 ± 0.2; Pb: 273 ± 17. b t – experimental value. | |||||||||
| Y (324.2 nm) | 196 ± 2 | 98 | 3.5 | 13.4 ± 1.3 | 80 | 3.0 | 178 ± 2 | 65 | 82.3 |
| Y (371.0 nm) | 187 ± 1 | 94 | 11.3 | 13.8 ± 0.2 | 82 | 2.7 | 169 ± 1 | 66 | 90.1 |
| Y (360.1 nm) | 201 ± 1 | 101 | 0.9 | 13.9 ± 0.2 | 80 | 2.6 | 179 ± 1 | 66 | 81.4 |
| Sc (424.7 nm) | 201 ± 2 | 101 | 0.9 | 14.5 ± 0.2 | 86 | 2.1 | 176 ± 2 | 65 | 84.0 |
| Sc (357.6 nm) | 205 ± 1 | 103 | 4.3 | 14.5 ± 0.2 | 86 | 2.1 | 180 ± 1 | 66 | 80.5 |
| Sc (335.7 nm) | 209 ± 2 | 104 | 7.8 | 14.9 ± 0.22 | 82 | 1.7 | 178 ± 2 | 65 | 82.3 |
| Ir (254.4 nm) | 27 ± 0.4 | 14 | 149.8 | — | — | — | — | — | — |
| Ir (215.8 nm) | 239 ± 34 | 120 | 33.8 | 18 ± 3 | 106 | 1.0 | 270 ± 10 | 99 | 2.6 |
| Ir (215.3 nm) | 456 ± 48 | 228 | 221.7 | 29 ± 9 | 169 | 10.5 | 499 ± 71 | 183 | 195.7 |
| Bi (306.8 nm) | 183 ± 12 | 92 | 14.7 | 12 ± 1 | 71 | 4.2 | 134 ± 17 | 49 | 120.4 |
| Bi (195.4 nm) | 212 ± 8 | 106 | 10.4 | 18 ± 1 | 106 | 1.0 | 248 ± 17 | 91 | 21.7 |
| Bi (190.2 nm) | 217 ± 7 | 109 | 14.7 | 16 ± 1 | 94 | 0.8 | 225 ± 9 | 82 | 41.6 |
| Without IS | 163 ± 11 | 82 | 32.0 | 14 ± 1 | 82 | 2.5 | 217 ± 13 | 79 | 48.5 |
Instead, when an IS is used for signal correction, the accuracy for As, Cd and Pb determination was improved. The criteria applied to select the internal standard for each element were based mainly on the recoveries obtained for the CRM NIST 695 with a minor error. For As, the best elements as ISs were Y (360.0 nm) and Sc (424.7 nm), with recoveries of 101% for both ISs. The best ISs for Cd were Bi (190.2 and 195.4 nm) and Ir (215.8 nm), and the recoveries ranged from 94 to 106%. For Pb, the most suitable IS was Ir (215.8 nm), with a recovery of 99%. In all the cases mentioned, according to Student's t-test, the results presented no statistically significant differences at a 95% confidence level (tcrit = 4.30).
As demonstrated in this study, the more suitable elements as internal standards did not show similar properties to those of the analytes, such as ionization energy and type of line (Table 2). Therefore, considering that the contaminants can also cause complex and irregular effects on the analyte signal, it is possible to conclude that matrix effects may have several origins. That is, the selection of an IS does not exclusively depend on the analyte physico-chemical properties but matrix composition is also a factor. Similar observations were also found in other studies reported in the literature.13,27
Other experiments were carried out to verify the effect of EIEs on the intensity of analyte signals when they were corrected using the selected internal standards. In this task, five solutions containing 2 mg L−1 analytes (As, Cd, and Pb), 2 mg L−1 internal standards (Bi, Sc, Ir and Y) and increasing concentrations of Ba, K and Na (0, 10, 50, 70 and 100 mg L−1) were analyzed. According to the graphs shown in Fig. 2, the intensities for As normalized using Y and Sc remained constant even when the EIE concentration was 100 mg L−1 and the signal deviation was less than 1%. For Cd, the internal standards (Bi – 190 nm/195 nm and Ir – 215.8 nm) minimized the interferences and the signal decreased by approximately 5%. In addition, Ir (215.8 nm) also shows satisfactory signal correction for Pb and in this case, the variation was less than 3%. It is important to consider that in all the cases, the analyte and internal standard signals presented similar behavior towards the non-spectral interferences, which may explain the good performance of internal standardization.
The limits of detection (LODs) were calculated considering the background equivalent concentration (BEC) and the relative standard deviation (RSD) obtained from ten authentic blanks. The LOD values for As, Cd and Pb were 0.3, 0.04, and 1 mg kg−1 for external calibration and, when internal standardization was used, they were 0.7, 0.03 and 1 mg kg−1, respectively. Besides the aforementioned advantages, for SVDV ICP OES in elemental determination, the LODs obtained using this system (and with the use of the IS) were 7-, 110- and 2-fold lower than the LODs reported by Souza et al.28 for As, Cd, and Pb, respectively during fertilizer analysis by ICP OES. These results indicate that the SVDV mode provides an improvement of sensitivity in comparison with other view modes (axial and radial). This can be related to the equipment configuration, an axial vertical torch position, which contributes to a robust plasma and long term stability. Amaral et al. also reported lower LOD values during rare earth element (REE) determination in fertilizer samples by SVDV ICP OES.28,29
Arsenic, Cd, and Pb in three fertilizer samples were determined by SVDV ICP OES employing the best internal standards for signal correction (Table 4). The mass fractions obtained by external calibration for all fertilizer samples were lower than those determined when the IS was employed, suggesting that the determination of As, Cd and Pb is systematically affected by the fertilizer matrix, which is not properly corrected when external calibration is used. It was observed that the use of Bi (195.4) for Cd quantification in the fertilizer showed a different value when compared to others. In this case, the correction using Bi (195.4) was not effective and this can be associated to contaminants present in this sample that could have affected Bi behavior in this excitation line. Internal standardization is a suitable alternative to overcome non-spectral interferences in fertilizer analysis and the use of SVDV ICP OES provides satisfactory results and adequate sensitivity for contaminant determination in complex samples.
| Analyte | IS | Fertilizer 1 | Fertilizer 2 | Fertilizer 3 |
|---|---|---|---|---|
| As (mg kg−1) | Y (360.1 nm) | 38.5 ± 1.2 | 34.1 ± 0.5 | 17.9 ± 1.8 |
| Sc (424.7 nm) | 37.7 ± 1.5 | 33.7 ± 0.5 | 17.6 ± 1.8 | |
| External calibration | 31.9 ± 1.2 | 27.9 ± 1.2 | 13.9 ± 1.2 | |
| Cd (mg kg−1) | Ir (215.8 nm) | 40.3 ± 2.4 | 137 ± 2.5 | 4.02 ± 0.45 |
| Bi (195.4 nm) | 30.2 ± 1.8 | 131 ± 0.34 | 5.61 ± 0.34 | |
| Bi (190.2 nm) | 39.3 ± 2.2 | 129 ± 2.4 | 4.86 ± 0.53 | |
| External calibration | 29.4 ± 0.88 | 111 ± 0.96 | 1.29 ± 0.26 | |
| Pb (mg kg−1) | Ir (215.8 nm) | 3823 ± 256 | 5518 ± 77 | 338 ± 26 |
| External calibration | 2712 ± 150 | 4187 ± 51 | 293 ± 20 |
These results were compared with others reported in the literature (Table 5). As observed in the samples employed in this work and in other studies, Pb is present in the greatest amount. In addition, it was verified that the contaminant mass fractions widely varied and are linked to production and fertilizer type.
Conclusion
Due to the EIEs presence, analyte signal suppression was observed during As, Cd, and Pb determination in mineral fertilizers by SVDV ICP OES. The use of internal standardization is considered a strategy to minimize nonspectral interferences. Although the criteria for the use of internal standards involve parameters such as excitation energy, wavelength and line characteristics being similar to those of the analyte, this selection is until a complex task. In addition, this fact is associated with the lack of understanding of the emission signal excitation mechanisms. In this sense, the choice of IS is based more on experimental than on theoretical considerations. Considering the accuracy obtained, the more effective ISs for the correction were Y (360.1 nm) and Sc (424.7 nm) for As, Ir (215.8 nm) and Bi (195.4 nm/195.4 nm) for Cd and Ir (215.8 nm) for Pb.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors kindly acknowledge the Brazilian agencies Fundação de Amparo à Pesquisa do Estado de São Paulo (2015/14488-0) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico for the fellowship provided to A. B. S. S. and the rearch grant (CNPQ, grants 153125/2016-0 and 308178/2018-1). The authors are also thankful to the Agilent Technologies for the technical support and to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Finance Code 001 (CAPES/PNPD – Graduate Program in Chemistry, Federal University of São Carlos).References
- Y. Liu, X. Pan and J. Li, Agron. Sustainable Dev., 2015, 35, 83–93 CrossRef CAS.
- H. Nacke, A. C. Gonçalves, D. Schwantes, I. A. Nava, L. Strey and G. F. Coelho, Arch. Environ. Contam. Toxicol., 2013, 64, 537–544 CrossRef CAS.
- R. C. Machado, A. B. S. Silva, G. L. Donati and A. R. A. Nogueira, J. Anal. At. Spectrom., 2018, 33, 1168–1172 RSC.
- D. C. Gregoire, Spectrochim. Acta, Part B, 1987, 42, 895–907 CrossRef.
- K. O'Hanlon, L. Ebdon and M. Foulkes, J. Anal. At. Spectrom., 1996, 11, 427–436 RSC.
- J. R. Dettman and J. W. Olesik, Spectrochim. Acta, Part B, 2012, 76, 96–108 CrossRef CAS.
- G. L. Donati, R. S. Amais and C. B. Williams, J. Anal. At. Spectrom., 2017, 32, 1283–1296 RSC.
- C. D. B. Amaral, D. Schiavo, J. A. V. A. Barros, R. C. Machado, A. R. A. Nogueira and J. A. Nóbrega, Anal. Lett., 2016, 49, 2092–2098 CrossRef CAS.
- D. A. Goncalves, T. McSweeney, M. C. Santos, B. T. Jones and G. L. Donati, Anal. Chim. Acta, 2016, 909, 24–29 CrossRef CAS.
- A. Virgilio, D. A. Gonçalves, T. McSweeney, J. A. Gomes Neto, J. A. Nóbrega and G. L. Donati, Anal. Chim. Acta, 2017, 982, 31–36 CrossRef CAS.
- J. A. Carter, A. I. Barros, J. A. Nóbrega and G. L. Donati, Front. Chem., 2018, 6, 1–25 CrossRef.
- G. A. Zachariadis and C. Vogiatzis, Appl. Spectrosc. Rev., 2010, 45, 220–239 CrossRef CAS.
- W. B. Barnett, V. A. Fassel and R. N. Kniseley, Spectrochim. Acta, Part B, 1970, 25, 139–161 CrossRef CAS.
- A. I. Barros, F. C. Pinheiro and J. A. Nóbrega, Anal. Methods, 2019, 11, 3401–3409 RSC.
- G. L. Scheffler and D. Pozebon, Anal. Methods, 2015, 7, 5180–5185 RSC.
- F. C. Pinheiro, A. I. Barros and J. A. Nóbrega, Microchem. J., 2019, 146, 948–956 CrossRef CAS.
- M. J. Harmse and R. I. McCrindle, J. Anal. At. Spectrom., 2002, 17, 1411–1414 RSC.
- F. A. De Santana, J. T. P. Barbosa, G. D. Matos, M. G. A. Korn and S. L. C. Ferreira, Microchem. J., 2013, 110, 198–201 CrossRef CAS.
- J. Mutic, D. Manojlovic, R. Kovacevic, J. Trifunovic, N. R. Amaizah and L. Ignjatovic, Microchem. J., 2011, 98, 11–14 CrossRef CAS.
- J. C. J. da Silva, S. Cadore, J. A. Nobrega and N. Baccan, Food Addit. Contam., 2007, 24, 130–139 CrossRef CAS PubMed.
- W. B. Barnett, V. A. Fassel and R. N. Kniseley, Spectrochim. Acta, Part B, 1968, 23, 643–664 CrossRef CAS.
- X. Romero, E. Poussel and J. M. Mermet, Spectrochim. Acta, Part B, 1997, 52, 487–493 CrossRef.
- M. Grotti, E. Magi and R. Leardi, J. Anal. At. Spectrom., 2003, 18, 274–281 RSC.
- R. E. S. Froes-Silva, W. B. Neto, C. C. Nascentes and J. B. B. Silva, At. Spectrosc., 2015, 36, 7–14 CAS.
- NIST database, https://physics.nist.gov/PhysRefData/ASD/ionEnergy.html, accessed July 2019 Search PubMed.
- E. Abad-peña, M. E. Villanueva-Tagle and M. Simeón, Spectrosc. Lett., 2018, 51, 1–8 CrossRef.
- G. L. Scheffler and D. Pozebon, Anal. Methods, 2013, 5, 4371–4377 RSC.
- S. O. Souza, S. S. L. Costa, D. M. Santos, J. S. Pinto, C. A. B. Garcia, J. P. H. Alves and R. G. O. Araujo, Spectrochim. Acta, Part B, 2014, 96, 1–7 CrossRef CAS.
- C. D. B. Amaral, R. C. Machado, J. A. V. A. Barros, A. Virgilio, A. R. A. Nogueira and J. A. Nobrega, Spectroscopy, 2017, 32, 32–36 Search PubMed.
- L. S. Teixeira, H. P. Vieira, C. C. Windmöller and C. C. Nascentes, Talanta, 2019, 119, 232–239 CrossRef PubMed.
- L. M. Baika, E. J. Dos Santos, A. B. Herrmann and M. T. Grassi, Anal. Methods, 2016, 8, 1–224 RSC.
- D. F. Andrade and E. R. Pereira-Filho, J. Agric. Food Chem., 2016, 64, 7890–7898 CrossRef CAS PubMed.
- L. C. Nunes, E. R. Pereira-Filho, M. B. B. Guerra and F. J. Krug, Spectrochim. Acta, Part B, 2019, 154, 25–32 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |