Transparent supercapacitors of 2 nm ruthenium oxide nanoparticles decorated on a 3D nitrogen-doped graphene aerogel†
Phansiri
Suktha
,
Nutthaphon
Phattharasupakun
and
Montree
Sawangphruk
 *
*
Department of Chemical and Biomolecular Engineering, School of Energy Science and Engineering, Vidyasirimedhi Institute of Science and Technology, Rayong, 21210, Thailand. E-mail: montree.s@vistec.ac.th; Fax: +66-33-01-4445; Tel: +66-33-01-4251
First published on 11th June 2018
Abstract
Although ruthenium oxide nanoparticles (RuO2), graphene, and their composites have been widely used as supercapacitor electrode materials, transparent supercapacitors of these materials have been rarely investigated. In this work, we fabricated high-performance transparent solid-state supercapacitors of 2 nm RuO2 decorated on a 3D nitrogen-doped reduced graphene oxide aerogel (NGA) with 27–54% transparency. The as-fabricated symmetric supercapacitor of RuO2/NGA at a finely tuned mass loading of 16.3 μg cm−2 with a finely tuned transmittance of 34.1% at a wavelength of 550 nm exhibits a maximum areal energy of 0.074 μW h cm−2 and a maximum areal power of 64 μW cm−2. The cycling stability of the device can also be maintained at 100% over 2000 cycles. The high transparent supercapacitor in this work may practically be used in many advanced transparent electrical devices.
1. Introduction
Due to the increasing demand for next-generation energy technologies, transparent and flexible energy storage devices have attracted a lot of interest. They can be used in many advanced applications such as transparent displays, transistors, optical circuits, touch screens, and solar cells.1,2 Recently, supercapacitors providing high power densities (500–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W h kg−1) and high stability (>100
000 W h kg−1) and high stability (>100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) have been developed with the use of transparent substrates.3,4 Although many transparent supercapacitor electrodes have been previously studied, transparent full-cell supercapacitor devices with high charge storage capacity are rather difficult to achieve owing to the poor transparent properties and charge storage capacity of the separators and electrode materials being used in the supercapacitor devices. Generally, conventional supercapacitors can be divided into two types based on their charge storage mechanisms including electrical double layer capacitors (EDLCs) and pseudocapacitors (PCs). EDLCs utilize ion adsorption/desorption at the interface between the electrolyte and the carbon-based electrode such as graphene, activated carbon, and carbon nanotubes.5–7 Meanwhile, PCs store charge via the faradaic redox reaction on the surface of metal oxides or conducting polymers.8
000 cycles) have been developed with the use of transparent substrates.3,4 Although many transparent supercapacitor electrodes have been previously studied, transparent full-cell supercapacitor devices with high charge storage capacity are rather difficult to achieve owing to the poor transparent properties and charge storage capacity of the separators and electrode materials being used in the supercapacitor devices. Generally, conventional supercapacitors can be divided into two types based on their charge storage mechanisms including electrical double layer capacitors (EDLCs) and pseudocapacitors (PCs). EDLCs utilize ion adsorption/desorption at the interface between the electrolyte and the carbon-based electrode such as graphene, activated carbon, and carbon nanotubes.5–7 Meanwhile, PCs store charge via the faradaic redox reaction on the surface of metal oxides or conducting polymers.8
Among carbon-based materials, graphene, a two-dimensional carbon monolayer with sp2 hybridization, has been recently proposed as an effective active material for transparent supercapacitors9–13 due to its good electrical conductivity (2 × 103 S cm−1) and high theoretical surface area (2675 m2 g−1).14,15 However, transparent supercapacitors using graphene sheets still exhibit low charge storage performance (e.g. in-plane CVD graphene (80.7 μF cm−2)14 and laminated ultrathin CVD graphene (4.27 μF cm−2)16) due to the restacking nature of 2D graphene and its low ionic conductivity. Therefore, in this work, a 3D graphene aerogel with nitrogen atom doping (NGA) was used to replace the 2D graphene. The 3D interconnected structure of graphene sheets with their hierarchical porous structure can increase the electron mobility and contact area between the electrode/electrolyte, while nitrogen heteroatoms can generate a pseudocapacitive behaviour and increase the ionic conductivity.17
To further improve the performance of transparent supercapacitors, ruthenium oxide (RuO2) nanoparticles (NPs) were also decorated on the structure of the GA because of their high theoretical capacitance (1450 F g−1 for RuO2 and 1360 F g−1 for RuO2·0.5H2O) due to which it can store charge via the following reaction (1).18
| RuOx(OH)y + δH+ + δe− ↔ RuOx−δ(OH)y+δ | (1) |
Porous carbon/RuO2 nanocomposites have been widely studied as active materials in supercapacitors due to the combination of high pseudocapacitance from RuO2 and electrochemical double layer capacitance from carbon materials.19 For example, F. Z. Amir, et al. reported that the specific capacitance (SC) of RuO2/reduced graphene oxide composites synthesized by an in situ sol–gel deposition method is 500 F g−1 at a current density of 1 A g−1 (ref. 20) and the SC of RuO2/holey reduced graphene oxide (HRGO) is 418 F g−1 at a current density of 1 A g−1.21 R. B. Rakhi and M. L. Lekshmi et al. reported that the SC of RuO2/carbon nanocoil/reduced graphene oxide composites is 725 F g−1 at a scan rate of 20 mV s−1.22 Y. Zhang and S.-J. Park showed that the RuO2/charcoal-derived activated carbon exhibited a SC of 510 F g−1 at a current density of 1 A g−1.23 S. Kong et al. electrodeposited RuO2 on graphene/carbon nanotubes achieving a SC of 480.3 F g−1 at a current density of 0.53 mA cm−2.24 J. Zhang et al. showed that the SC of RuO2 decorated on the C3N4@rGO aerogel is 704.3 F g−1 at a current density of 0.5 A g−1.25 Although there are a few reports on using RuO2 composites as an active material in transparent supercapacitors, the effect of loading contents has not yet been investigated.26–28
In this work, we fabricated a new solid-state supercapacitor of 2 nm ruthenium oxide (RuO2) nanoparticle decorated NGA with 27–54% transparency for which a polymer gel of PVA/H2SO4 was used as the solid-state electrolyte and separator. The as-fabricated symmetric supercapacitor using RuO2/NGA at a mass loading of 16.3 μg cm−2 exhibits the highest electrochemical performance (1.57 mF cm−2 at 0.5 A g−1) with a finely tuned transmittance of 34.1% at a wavelength of 550 nm. The cycling stability of the as-fabricated device can also be maintained at 100% over 2000 cycles.
2. Experimental
2.1 Chemicals and materials
Graphite powder (<20 μm, Aldrich), sodium nitrate (NaNO3, 99% Ajax Finechem), potassium permanganate (KMnO4, 99%, UNIVAR), sulphuric acid (H2SO4, 98%, QRec), nitric acid (HNO3, 65%, QRec), hydrogen peroxide (H2O2, 30%, Chem Supply), hydrochloric acid (HCl, 37%, Qrec), hydrazine (N2H2, 99%, LOBA Chemie), ruthenium(III) chloride trihydrate (RuCl3·3H2O, Aldrich), 1-methyl-2-pyrrolidone (NMP, 99.5%, QRec), and poly(vinyl alcohol) (PVA, Mw = 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–124
000–124![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, 99+% hydrolysed, Aldrich) were of analytical reagent grade and used without further purification. Deionized water from a Millipore system (DI water, 15 MΩ cm) was used in all experiments.
000, 99+% hydrolysed, Aldrich) were of analytical reagent grade and used without further purification. Deionized water from a Millipore system (DI water, 15 MΩ cm) was used in all experiments.
2.2 Synthesis of graphene oxide
Graphene oxide (GO) was prepared using a modified Hummer's method. Briefly, 5.0 g graphite powder and 7.5 g NaNO3 were mixed in 500 ml conc. sulphuric acid in an ice-bath keeping the temperature below 20 °C. 40 g KMnO4 was slowly added into the mixture and stirred for 24 h at ambient temperature. Then, 500 ml DI water and 150 ml H2O2 were subsequently added to the mixture and stirred for 24 h. The mixture was then centrifuged with DI water several times until neutral. Finally, the GO powder was obtained by drying at 60 °C for 72 h.292.3 Synthesis of the nitrogen-doped reduced graphene oxide aerogel (NGA)
The N-rGO aerogel was synthesized by a hydrothermal method using hydrazine hydrate as a reducing agent. Firstly, 300 mg of GO was sonicated in 150 ml of DI water for 2 h. Then, 0.5 M N2H2 was added to the GO solution. The solution was then transferred into a Teflon-lined stainless-steel autoclave and heated at 80 °C for 72 h. The obtained product was then washed with DI water several times and freeze dried for 72 h.302.4 Synthesis of RuO2/NGA nanocomposites
RuO2/NGA nanocomposites were synthesized by chemical oxidation of RuCl3 on the NGA. Firstly, 0.14 g of the N-rGO aerogel powder was mixed with 0.14 g RuCl3·3H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight) in 10 ml of DI water by sonication for 1 h. Then, 73.5 ml of H2O2 solution (13.5 vol%) was slowly added to the mixed solution under magnetic stirring. The final solution was placed in a static water bath at 90 ± 5 °C for 2 h31 and then it was cooled down to room temperature. Finally, the solution was filtered with DI water and dried at 60 °C (Fig. 1).
1 by weight) in 10 ml of DI water by sonication for 1 h. Then, 73.5 ml of H2O2 solution (13.5 vol%) was slowly added to the mixed solution under magnetic stirring. The final solution was placed in a static water bath at 90 ± 5 °C for 2 h31 and then it was cooled down to room temperature. Finally, the solution was filtered with DI water and dried at 60 °C (Fig. 1).
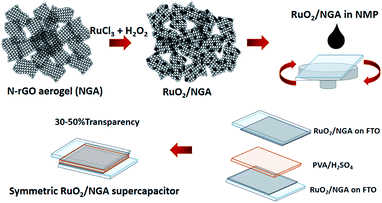 | ||
| Fig. 1 Schematic illustration for the preparation of RuO2/NGA and a transparent solid-state supercapacitor with a PVA/H2SO4 gel electrolyte. | ||
2.5 Morphological and structural characterization
The morphology of the as-prepared materials was characterized by field emission scanning electron microscopy (FE-SEM, JEOL, JSM-7610F) and transmission electron microscopy (TEM, FEI Tecnai G2 20). The crystallographic structure was characterized by X-ray diffraction (XRD, Bruker D8 ADVANCE using Cu-Kα radiation, λ = 1.5418 Å). The specific surface area was measured by nitrogen adsorption/desorption with the BET method (3Flex, Micromeritics). The surface composition was analyzed by X-ray photoelectron spectroscopy (XPS, JEOL, JPS-9010MC). The chemical structure was investigated by Raman spectroscopy (Bruker, an excitation wavelength of 532 nm) and Fourier transform infrared spectroscopy (ATR-FTIR, PerkinElmer). The transmission spectra were recorded by UV-vis spectroscopy (PerkinElmer, Lambda 1050). The thermal gravimetric analysis technique (TGA, STA PT1600, Linseis) was used to measure the amount of RuO2 in the composite. The thickness of the material on FTO was measured with an optical microscope (SteREO Discovery V12, Zeiss).2.6 Fabrication of transparent supercapacitors and electrochemical evaluation
RuO2/NGA nanocomposites were dispersed in NMP at different concentrations by sonication for 1 h. The homogeneous solution was spin-coated on an FTO glass substrate with an active area of 4 cm2 at 1000 rpm for 25 s. Then, the electrode was dried at 60 °C for 24 h. Note that the mass loadings of the RuO2/NGA nanocomposites are 12.5, 16.3, and 22.5 μg cm−2 at the concentrations of 2.5, 5.0, and 10.0 mg ml−1, respectively. Polyvinyl alcohol in sulfuric acid (PVA/H2SO4) was used as the gel electrolyte for which it was prepared by stirring 1 g of PVA in 10 ml of 1 M H2SO4 at 80 °C. The gel electrolyte was spin-coated on the electrode at 2000 rpm for 25 s. Then, the two electrodes were assembled into a symmetric transparent solid-state supercapacitor (see Fig. 1). For electrochemical evaluation, cyclic voltammetry (CV), galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) were performed using a Metrohm AUTOLAB potentiostat (PGSTAT 302N). Note that all calculation details can be found in the ESI.†3. Results and discussion
3.1 Physicochemical characterization
The morphologies of the NGA and RuO2/NGA nanocomposites synthesized from the oxidation of ruthenium chloride (RuCl3) with H2O2 on NGA sheets were investigated by FE-SEM and TEM. Fig. 2a shows the FE-SEM image of the NGA which exhibits a 3D interconnected structure from graphene nanosheets. After combining with RuO2, the small particles of RuO2 were decorated on the graphene layers without destroying the graphene aerogel frameworks (Fig. 2b). The TEM images of the NGA in Fig. 2c and e also show the transparent thin layer of graphene sheets with many wrinkles. Meanwhile for the RuO2/NGA nanocomposites in Fig. 2d and f, it can be clearly observed that tiny RuO2 NPs with a particle size of ca. 2 nm were homogeneously decorated on the surface of the NGA corresponding to the FE-SEM results. The HRTEM image of the RuO2/NGA nanocomposites and their particle size distribution are shown in Fig. S3.† It can be clearly seen that the particle size of RuO2 in the RuO2/NGA nanocomposites is in the range of 1.5–2.0 nm. The STEM image, EDX elemental mapping data, and EDX spectrum of RuO2/NGA are shown in Fig. S4.† The cross-sectional optical micrographs are shown in Fig. S5.† The thicknesses of the NGA and RuO2/NGA at the mass loadings of 12.5, 16.3, and 22.5 μg cm−2 are ca. 10.8, 6.5, 8.7, and 16.8 μm, respectively.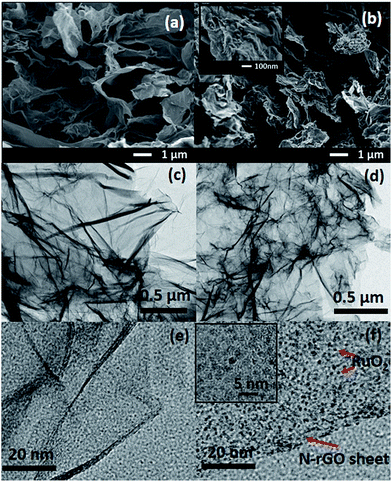 | ||
| Fig. 2 FE-SEM and TEM images of the NGA (a, c, and e) and 2 nm RuO2/NGA nanocomposites (b, d, and f). | ||
The XRD patterns of the as-prepared materials are shown in Fig. 3a. The XRD patterns show a broad peak at around 23–25° and a weak peak at around 44.5° corresponding to the (002) and (100) planes of graphene,32 respectively. The (002) plane peak of RuO2/NGA is shifted from 24.3° to 24.6°. The dspacing values of the NGA and RuO2/NGA are 3.66 and 3.62 Å, respectively. These dspacing values are higher than that of graphite (3.35 Å (ref. 33)) indicating the presence of some functional groups on the graphene sheet.34 However, the diffraction peak of RuO2 nanoparticles cannot be seen due to their small particle size and amorphous structure.20,35Fig. 3b shows the nitrogen adsorption–desorption isotherm of both the NGA and RuO2/NGA nanocomposites exhibiting a type-IV isotherm with an H3-hysteresis loop indicating their mesoporous structure.36 The BET specific surface area of the NGA and RuO2/NGA is 133 m2 g−1 and 92 m2 g−1, respectively. Note that the specific surface area of RuO2/NGA is lower than that of the pristine NGA because of the decoration of RuO2 nanoparticles on the NGA surface resulting in the loss of the specific surface area. The FTIR spectra of the NGA and RuO2/NGA are shown in Fig. 3c. The peaks at 2981 and 2885 cm−1 can be attributed to the asymmetric stretching and symmetric vibrations of CH2, respectively,37 whilst the peaks at 1559, 1204, and 1054 cm−1 correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C,38 C–O vibration of C–OH,39 and C–O–C in the epoxy groups,40 respectively. Raman spectroscopy was also carried out to study the defect characteristics of the as-prepared materials, and the results are shown in Fig. 3d. These results show that the major vibration peaks consist of D and G bands. The D band at 1350 cm−1 represents the defects, edges, and disordered carbon while the G band at 1579 cm−1 represents the in-plane vibration of sp2 carbon atoms. The peaks at around 2692 and 2938 cm−1 are the 2D band and the combination mode of G and D bands,41 respectively. The characteristic peak of RuO2 NPs is observed at around 620 cm−1 (ref. 24) (see the inset image of Fig. 3d). In addition, an intensity ratio of D and G bands (ID
C,38 C–O vibration of C–OH,39 and C–O–C in the epoxy groups,40 respectively. Raman spectroscopy was also carried out to study the defect characteristics of the as-prepared materials, and the results are shown in Fig. 3d. These results show that the major vibration peaks consist of D and G bands. The D band at 1350 cm−1 represents the defects, edges, and disordered carbon while the G band at 1579 cm−1 represents the in-plane vibration of sp2 carbon atoms. The peaks at around 2692 and 2938 cm−1 are the 2D band and the combination mode of G and D bands,41 respectively. The characteristic peak of RuO2 NPs is observed at around 620 cm−1 (ref. 24) (see the inset image of Fig. 3d). In addition, an intensity ratio of D and G bands (ID![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IG) of the NGA is 1.12, which is lower than that of the RuO2/NGA (ID/IG = 1.21). This is because the chemical reduction of RuO2 can introduce more lattice defects on the structure of the NGA.42 In addition, the amorphous content can also be calculated by the deconvolution of the amorphous band at around 1520 cm−1 as shown in Fig. S1.†43 The amorphous carbon contents of the NGA and RuO2/NGA are 23 and 21%, respectively. To determine the RuO2 content in the composite, TGA was carried out, and the results are shown in Fig. S6.† The results show a slight weight loss at a temperature lower than 200 °C due to the removal of physically adsorbed water. The weight loss at 200–400 °C is due to the removal of the water molecules of hydrous RuO2, and the large weight loss at >400 °C is due to the removal of the NGA. After 500 °C, the remaining weight remains stable indicating that the RuO2 content in the RuO2/NGA composite is about 20%.
IG) of the NGA is 1.12, which is lower than that of the RuO2/NGA (ID/IG = 1.21). This is because the chemical reduction of RuO2 can introduce more lattice defects on the structure of the NGA.42 In addition, the amorphous content can also be calculated by the deconvolution of the amorphous band at around 1520 cm−1 as shown in Fig. S1.†43 The amorphous carbon contents of the NGA and RuO2/NGA are 23 and 21%, respectively. To determine the RuO2 content in the composite, TGA was carried out, and the results are shown in Fig. S6.† The results show a slight weight loss at a temperature lower than 200 °C due to the removal of physically adsorbed water. The weight loss at 200–400 °C is due to the removal of the water molecules of hydrous RuO2, and the large weight loss at >400 °C is due to the removal of the NGA. After 500 °C, the remaining weight remains stable indicating that the RuO2 content in the RuO2/NGA composite is about 20%.
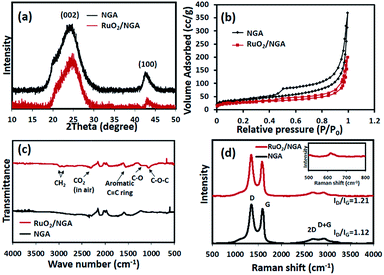 | ||
| Fig. 3 (a) XRD patterns, (b) nitrogen adsorption–desorption isotherms, (c) FTIR spectra, and (d) Raman spectra of the NGA and RuO2/NGA. | ||
To investigate the chemical composition and structure of the NGA and RuO2/NGA, XPS was carried out, and the results are shown in Fig. 4. The wide-scan XPS spectrum of RuO2/NGA in Fig. 4a confirms the existence of RuO2 NPs and nitrogen-containing functional groups of the NGA. In the narrow-scan XPS spectrum of Ru 3p, the RuO2/NGA exhibits the binding energy of Ru 3p3/2 at 463.2 eV and Ru 3p1/2 at 485.5 eV which can be assigned to RuO2.44 The C 1s XPS spectrum of the NGA in Fig. 4c can be deconvoluted into three peaks at 284.7, 285.6, and 286.7 eV which can be attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, and C–O, respectively,45 whilst, the C 1s peak of the RuO2/NGA nanocomposites in Fig. 4d can be deconvoluted into five peaks at 281.8, 284.7, 285.6, 286.7, and 288.5 eV which can be attributed to Ru 3d3/2, C
C, C–N, and C–O, respectively,45 whilst, the C 1s peak of the RuO2/NGA nanocomposites in Fig. 4d can be deconvoluted into five peaks at 281.8, 284.7, 285.6, 286.7, and 288.5 eV which can be attributed to Ru 3d3/2, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N, C–O, and O–C
C, C–N, C–O, and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O,45 respectively. Note that the O–C
O,45 respectively. Note that the O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional group occurs on the surface of RuO2/NGA due to the further oxidation of the NGA in the presence of hydrogen peroxide. The N/C and O/C ratios of the NGA are 0.15 and 0.21 while the N/C, O/C and Ru/C ratios of RuO2/NGA are 0.13, 0.28 and 0.15, respectively.
O functional group occurs on the surface of RuO2/NGA due to the further oxidation of the NGA in the presence of hydrogen peroxide. The N/C and O/C ratios of the NGA are 0.15 and 0.21 while the N/C, O/C and Ru/C ratios of RuO2/NGA are 0.13, 0.28 and 0.15, respectively.
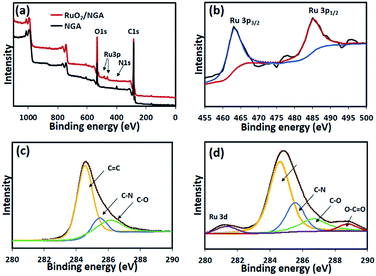 | ||
| Fig. 4 (a) Wide-scan XPS spectra of the NGA and RuO2/NGA, (b) Ru 3p XPS spectra of the NGA and RuO2/NGA, (c) C 1s XPS spectra of the NGA, and (d) C 1s XPS spectra of RuO2/NGA. | ||
3.2 Electrochemical evaluation of transparent solid-state supercapacitors
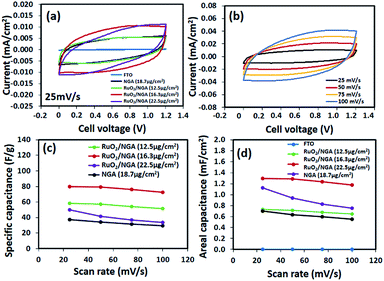
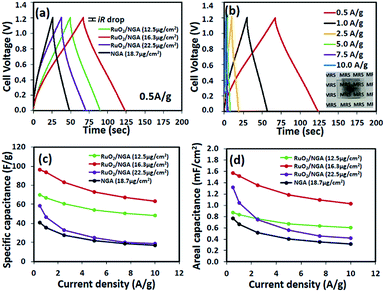
CV was used to measure the electrochemical performance of the as-fabricated supercapacitors. Fig. 5a shows the CVs of the NGA and RuO2/NGA at different mass loadings on FTO glass electrodes and at a scan rate of 25 mV s−1 in the cell voltage range of 0–1.2 V. The CVs of the RuO2/NGA devices show no obvious redox peak of RuO2 due to the fast reversible redox reaction of tiny RuO2 NPs.21 The area under the CV of RuO2/NGA at a mass loading of 16.3 μg cm−2 is larger than those of other samples, indicating its better charge storage performance. The CVs of RuO2/NGA (16.3 μg cm−2) at various scan rates including 25, 50, 75, and 100 mV s−1 are also shown in Fig. 5b. The specific and areal capacitances of RuO2/NGA are higher than those of the pristine NGA due to the combination of pseudocapacitance from RuO2 and electrochemical double layer capacitance from the NGA.35 Among the RuO2/NGA transparent solid-state supercapacitors, the RuO2/NGA at a mass loading of 16.3 μg cm−2 exhibits the highest specific capacitances of 79.6, 79.2, 75.9, and 72.3 F g−1 and areal capacitances of 1.30, 1.29, 1.23, and 1.18 mF cm−2 at a scan rate of 25, 50, 75, and 100 mV s−1, respectively.Fig. 6a shows the galvanostatic charge–discharge (GCD) curves of the NGA and RuO2/NGA supercapacitors at a current density of 0.5 A g−1. These curves show a symmetrical triangular shape with a small iR drop indicating their good charge storage behaviour. The GCD curve of RuO2/NGA at a mass loading of 16.3 μg cm−2 exhibits the highest discharge time, which is in good agreement with the CV results. The GCD curves of RuO2/NGA at a mass loading of 16.3 μg cm−2 at various applied current densities of 0.5–10 A g−1 are shown in Fig. 5b. The RuO2/NGA nanocomposites at a mass loading of 16.3 μg cm−2 exhibit the highest specific (96.4, 93.5, 72.7, 67.2, and 63.4 F g−1) and areal capacitances (1.57, 1.52, 1.35, 1.18, 1.09, and 1.03 mF cm−2) in accordance with the CV results. Note that the areal capacitance of the RuO2/NGA nanocomposites at a mass loading of 22.5 μg cm−2 is higher than that at a mass loading of 12.5 μg cm−2 at low applied current densities. This is because increasing the mass loading (or thickness) of the active material can increase the active surface area of the electrode which facilitates the storage of a large number of ions. However, a higher mass loading will create a longer diffusion pathway for electron transport46,47 reducing the charge storage performance. Therefore, the specific and areal capacitances at a mass loading of 22.5 μg cm−2 are lower than those at a mass loading of 16.3 μg cm−2.
The optical transmittance spectra of all transparent solid-state devices are shown in Fig. 7. Note that a symmetric supercapacitor of bare FTO only provides a transmittance of 70% at 550 nm. The RuO2/NGA devices with the mass loadings of 12.5, 16.3, and 22.5 μg cm−2 exhibit the Csp and Cae values of 69.9 F g−1 (0.87 mF cm−2), 96.4 F g−1 (1.57 mF cm−2), and 58.4 F g−1 (1.31 mF cm−2) at a current density of 0.5 A g−1 with transmittances of 48.1%, 34.1%, and 27.0% at 550 nm, respectively. Note that these results are higher than those of the NGA supercapacitor (40.8 F g−1 and 0.77 mF cm−2) which exhibits a transmittance of 53.6% at 550 nm.
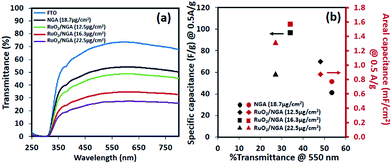 | ||
| Fig. 7 (a) Optical transmittance spectra and (b) specific and areal capacitances as a function of % transmittance@550 nm of the NGA and RuO2/NGA transparent solid-state supercapacitors. | ||
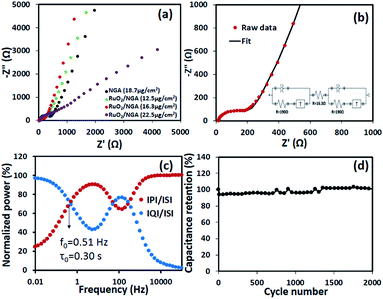
Electrochemical impedance spectroscopy (EIS) of the symmetric supercapacitors using the RuO2/NGA nanocomposites on the FTO glass electrode using PVA–1 M H2SO4 gel electrolyte was further carried out in the frequency range of 10 mHz to 100 kHz at an excitation amplitude of 5 mV, and the results are shown in Fig. 8a. The slope in the low-frequency region approaching the Y-axis indicates the ideal capacitive behaviour and low diffusion resistance of the ions in the electrolyte. The RuO2/NGA at a mass loading of 16.3 μg cm−2 exhibits a nearly vertical line as compared to other devices, whilst the X-intercept of the semicircle in the high-frequency region represents the equivalent series resistance (ESR) related to the resistances of electrode materials, electrolyte, and their contact.48 The ESR values of the NGA and RuO2/NGA at the mass loadings of 12.5, 16.3, and 22.5 μg cm−2 are 23.09, 22.41, 16.22, and 51.22 Ω, respectively. Note that the charge transfer resistance (Rct) or the diameter of the semicircle of all RuO2/NGA devices is smaller than that of the bare NGA indicating that the electrochemical reactions on the interface of RuO2/NGA electrode/electrolyte are more facile.28 In addition, the equivalent circuit model of RuO2/NGA at a mass loading of 16.3 μg cm−2 is also shown in Fig. 8b with an ESR of 16.3 Ω and Rct of 190 Ω. The relaxation time (τ0) which is the minimum time required for the discharge process can also be calculated by using the complex power49 as shown in Fig. 8c. The τ0 of the RuO2/NGA transparent supercapacitor with PVA–1 M H2SO4 gel electrolyte is 0.30 s indicating the high-power capability of the device, whilst the bare NGA transparent supercapacitor exhibits a τ0 of 0.59 s as seen in Fig. S2†. For the cycling stability test, the RuO2/NGA transparent supercapacitor at a mass loading of 16.3 μg cm−2 was tested at an applied current density of 1 A g−1. It exhibits a remarkable capacitance retention of 100% after being tested for 2000 cycles.
To evaluate the performance of the as-fabricated RuO2/NGA transparent solid-state supercapacitor, the Ragone plot was constructed which compares the RuO2/NGA supercapacitor with other transparent/flexible supercapacitors in other previous reports in terms of areal energy and areal power as shown in Fig. 9. The areal energy and areal power of the transparent supercapacitor based on RuO2/NGA at a mass loading of 16.3 μg cm−2 are higher than those of the NGA (Fig. 9). The areal energy of the RuO2/NGA device is 0.074 μW h cm−2 at an areal power of 4.75 μW cm−2 and 0.022 μW h cm−2, and at an areal power of 64 μW cm−2, whilst the areal energy of the GA device is 0.03 μW h cm−2 at an areal power of 5.2 μW cm−2 and 0.002 μW h cm−2, and at an areal power of 28 μW cm−2. These performances are higher than those of other reports based on transparent/flexible devices using PEDOT:PSS/RuO2 (0.018 μW h cm−2 at 1 μW cm−2), reduced multilayer graphene oxide (0.015 μW h cm−2 at 9 μW cm−2), CVD graphene (0.003 μW h cm−2 at 2 μW cm−2), graphene Qdots (0.001 μW h cm−2 at 0.06 μW cm−2), and convex carbon nanocups (0.00005 μW h cm−2 at 0.02 μW cm−2).26 Note that the performance of our transparent supercapacitors in terms of areal capacitance was also compared with those of other previous reports (see the ESI†).
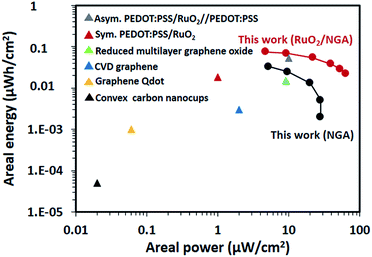 | ||
| Fig. 9 Ragone plot showing the comparison of the NGA and RuO2/NGA devices with other transparent/flexible supercapacitors reported by C. Zhang, et al.26 | ||
4. Conclusions
Tiny 2 nm RuO2 nanoparticles were synthesized by an oxidation reaction of RuCl3 with H2O2 and were decorated on the surface of the NGA. The as-synthesized RuO2/NGA nanocomposites were spin-coated on the FTO substrate and used as the electrode of solid-state transparent supercapacitors. The polymer gel of PVA–H2SO4 was used as the solid-state electrolyte and separator. The as-fabricated symmetric supercapacitor using RuO2/NGA at a finely tuned mass loading of 16.3 μg cm−2 exhibits a maximum areal energy of 0.074 μW h cm−2 and a maximum power density of 64 μW cm−2 with a cycling stability of 100% over 2000 cycles. The supercapacitor with high transparency and charge storage performance in this work may practically be used in many advanced transparent electrical devices.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Vidyasirimedhi Institute of Science and Technology and Thailand Research Fund (RSA6180031). Support from the Frontier Research Centre at VISTEC is also acknowledged.References
- N. Li, X. Huang, H. Zhang, Y. Li and C. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 9763–9771 CrossRef PubMed.
- S. Bellani, L. Najafi, G. Tullii, A. Ansaldo, R. Oropesa-Nuñez, M. Prato, M. Colombo, M. R. Antognazza and F. Bonaccorso, J. Mater. Chem. A, 2017, 5, 25177–25186 RSC.
- A. Yu, I. Roes, A. Davies and Z. Chen, Appl. Phys. Lett., 2010, 96, 253105 CrossRef.
- T. Chen, Y. Xue, A. K. Roy and L. Dai, ACS Nano, 2014, 8, 1039–1046 CrossRef PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef PubMed.
- Z. Li, Z. Li, L. Li, C. Li, W. Zhong and H. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 15557–15565 CrossRef PubMed.
- Z. Li, L. Li, Z. Li, H. Liao and H. Zhang, Electrochim. Acta, 2016, 222, 990–998 CrossRef.
- A. S. Aricò, P. Bruce, B. Scrosati, J.-M. Tarascon and W. van Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef PubMed.
- N. Li, G. Yang, Y. Sun, H. Song, H. Cui, G. Yang and C. Wang, Nano Lett., 2015, 15, 3195–3203 CrossRef PubMed.
- N. Li, X. Huang, H. Zhang, Z. Shi, Y. Li and C. Wang, J. Mater. Chem. A, 2017, 5, 14595–14603 RSC.
- N. Li, Q. Yang, X. Liu, X. Huang, H. Zhang and C. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 42093–42101 CrossRef PubMed.
- N. Li, X. Huang, R. Li, Y. Chen, Y. Li, Z. Shi and H. Zhang, Electrochim. Acta, 2016, 219, 61–69 CrossRef.
- N. Li, C. Zhi and H. Zhang, Electrochim. Acta, 2016, 220, 618–627 CrossRef.
- Z. S. Wu, K. Parvez, X. Feng and K. Müllen, Nat. Commun., 2013, 4 DOI:10.1038/ncomms3487.
- J. J. Yoo, K. Balakrishnan, J. Huang, V. Meunier, B. G. Sumpter, A. Srivastava, M. Conway, A. L. Mohana Reddy, J. Yu and R. Vajtai, Nano Lett., 2011, 11, 1423–1427 CrossRef PubMed.
- P. Xu, J. Kang, J.-B. Choi, J. Suhr, J. Yu, F. Li, J.-H. Byun, B.-S. Kim and T.-W. Chou, ACS Nano, 2014, 8, 9437–9445 CrossRef PubMed.
- N. Li, X. Huang, H. Zhang, Z. Shi and C. Wang, J. Mater. Chem. A, 2017, 5, 16803–16811 RSC.
- V. Augustyn, P. Simon and B. Dunn, Energy Environ. Sci., 2014, 7, 1597–1614 RSC.
- K. S. Yang and B.-H. Kim, Electrochim. Acta, 2015, 186, 337–344 CrossRef.
- F. Z. Amir, V. H. Pham and J. H. Dickerson, RSC Adv., 2015, 5, 67638–67645 RSC.
- F. Z. Amir, V. Z. Pham, D. W. Mullinax and J. H. Dickerson, Carbon, 2016, 107, 338–343 CrossRef.
- R. B. Rakhi and M. L. Lekshmi, Electrochim. Acta, 2017, 231, 539–548 CrossRef.
- Y. Zhang and S.-J. Park, Carbon, 2017, 122, 287–297 CrossRef.
- S. Kong, K. Cheng, T. Ouyang, Y. Gao, K. Ye, G. Wang and D. Cao, Electrochim. Acta, 2017, 246, 433–442 CrossRef.
- J. Zhang, J. Ding, C. Li, B. Li, D. Li, Z. Liu, Q. Cai, J. Zhang and Y. Liu, ACS Sustainable Chem. Eng., 2017, 5, 4982–4991 CrossRef.
- C. Zhang, T. M. Higgins, S.-H. Park, S. E. O'Brien, D. Long, J. N. Coleman and V. Nicolosi, Nano Energy, 2016, 28, 495–505 CrossRef.
- I. Ryu, M. Yang, H. Kwon, H. K. Park, Y. R. Do, S. B. Lee and S. Yim, Langmuir, 2014, 30, 1704–1709 CrossRef PubMed.
- P. Chen, H. Chen, J. Qiu and C. Zhou, Nano Res., 2010, 3, 594–603 CrossRef.
- M. Sawangphruk, P. Srimuk, P. Chiochan, A. Krittayavathananon, S. Luanwuthi and J. Limtrakul, Carbon, 2013, 60, 109–116 CrossRef.
- T. Pettong, P. Iamprasertkun, A. Krittayavathananon, P. Sukha, P. Sirisinudomkit, A. Seubsai, M. Chareonpanich, P. Kongkachuichay, J. Limtrakul and M. Sawangphruk, ACS Appl. Mater. Interfaces, 2016, 8, 34045–34053 CrossRef PubMed.
- C. Fernández, C. Pezzotta, G. Raj, E. M. Gaigneaux and P. Ruiz, Catal. Today, 2016, 259, 183–191 CrossRef.
- P. Iamprasertkun, A. Krittayavathananon and M. Sawangphruk, Carbon, 2016, 102, 455–461 CrossRef.
- A. H. Palser, Phys. Chem. Chem. Phys., 1999, 1, 4459–4464 RSC.
- S. Yuan, C. Lu, Y. Li and X. Wang, ChemElectroChem, 2017, 4, 2826–2834 CrossRef.
- Y. Yang, Y. Liang, Y. Zhang, Z. Zhang, Z. Li and Z. Hu, New J. Chem., 2015, 39, 4035–4040 RSC.
- Z. Lin, G. H. Waller, Y. Liu, M. Liu and C.-p. Wong, Carbon, 2013, 53, 130–136 CrossRef.
- L. Ma, R. Liu, H. Niu, M. Zhao and Y. Huang, Compos. Sci. Technol., 2016, 137, 87–93 CrossRef.
- B. Gupta, N. Kumar, K. Panda, S. Dash and A. Tyagi, Sci. Rep., 2016, 6, 18372 CrossRef PubMed.
- P. Chettri, V. Vendamani, A. Tripathi, A. P. Pathak and A. Tiwari, Appl. Surf. Sci., 2016, 362, 221–229 CrossRef.
- G. S. Gund, D. P. Dubal, B. H. Patil, S. S. Shinde and C. D. Lokhande, Electrochim. Acta, 2013, 92, 205–215 CrossRef.
- R. Bajpai, S. Roy, J. Rafiee, N. Koratkar and D. Misra, Nanoscale, 2012, 4, 926–930 RSC.
- R. K. Gautam, H. Bhattacharjee, S. V. Mohan and A. Verma, RSC Adv., 2016, 6, 110091–110101 RSC.
- R. Morga, I. Jelonek, K. Kruszewska and W. Szulik, Int. J. Coal Geol., 2015, 144, 130–137 CrossRef.
- G. Yuan, M. Gopiraman, H. J. Cha, H. D. Soo, I.-M. Chung and I. S. Kim, Ind. Eng. Chem. Res., 2017, 46, 279–288 CrossRef.
- Y. Lu, F. Zhang, T. Zhang, K. Leng, L. Zhang, X. Yang, Y. Ma, Y. Huang, M. Zhang and Y. Chen, Carbon, 2013, 63, 508–516 CrossRef.
- P. Kanninen, N. D. Luong, L. H. Sinh, I. V. Anoshkin, A. Tsapenko, J. Seppala, A. G. Nasibulin and T. Kallio, Nanotechnology, 2016, 27, 6 CrossRef PubMed.
- K. Z. Gao, Z. Q. Shao, X. Wu, X. Wang, Y. H. Zhang, W. J. Wang and F. J. Wang, Nanoscale, 2013, 5, 5307–5311 RSC.
- J. H. Kim, S. Lee, J. W. Lee, T. Song and U. Paik, Electrochim. Acta, 2014, 125, 536–542 CrossRef.
- A. Singh and A. Chandra, Sci. Rep., 2015, 5, 15551 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00177d |
| This journal is © The Royal Society of Chemistry 2018 |