Integration of a metal–organic framework with zeolite: a highly sustainable composite catalyst for the synthesis of γ-valerolactone and coumarins†
Poonam
Rani
and
Rajendra
Srivastava
 *
*
Department of Chemistry, Indian Institute of Technology Ropar, Rupnagar-140001, India. E-mail: rajendra@iitrpr.ac.in; Fax: +91-1881-223395; Tel: +91-1881-242175
First published on 6th April 2018
Abstract
In this study, two well-known porous materials, zeolite Beta and a metal–organic framework are entangled to form a highly efficient composite catalyst. The composite catalyst contains the characteristic features of both the porous materials and is believed to act as a bridge between these porous materials. Powder X-ray diffraction, nitrogen adsorption, scanning electron microscopy, transmission electron microscopy, thermogravimetric analysis, and Fourier transform infrared spectroscopy techniques confirm the presence of both the frameworks in the composite material. The composite catalyst is employed as a highly efficient and selective catalyst for the synthesis of γ-valerolactone and coumarins. The obtained results reveal that the integration of both the frameworks in the composite catalyst is required to achieve the excellent catalytic activity.
1. Introduction
In recent times, nanocomposite materials have been given more importance due to their multifunctional activity in diverse areas of catalysis ranging from conventional fine chemical synthesis to photocatalysis.1,2 Porous materials play important roles in conventional liquid phase and vapour phase catalysis for the synthesis of fine chemicals due to their selective adsorption and activity.3,4 Inorganic microporous zeolites rule the fine and bulk chemical synthesis market due to their shape selectivity and strong acidity.5 To overcome the diffusion limitation imposed by the microporous zeolites, several synthesis strategies have been developed to prepare mesoporous zeolites.6–9 Specially designed structure directing agents and additives have been used to prepare 2-dimensional (2D) mesoporous zeolites and nanocrystalline zeolites with inter/intra-crystalline mesoporosity to overcome the diffusion limitation.6–9 Mesoporous zeolites provide ample opportunity to functionalize the surface with suitable organic and inorganic functionalities to develop different nanocomposite materials for specific applications.10–12 Since transition metals are difficult to incorporate into the inorganic zeolite framework, attempts have been made to develop a class of porous materials named metal–organic frameworks (MOFs). In recent times, significant progress has been made in developing a wide range of MOFs. MOFs have been prepared using transition metals and non-functional/functional organic linkers.13,14 The uniform microporous structure and high surface area of MOFs are exploited mainly in gas adsorption.15,16 Catalytic applications of MOFs have witnessed only limited success due to their limited activity and structural stability.17,18 In recent times, efforts have been made to prepare functional MOFs via pre- or post-synthesis modification.19–21 In such synthesis strategies, either functional organic ligands have been taken during the synthesis of MOFs or MOF structures have been functionalized via post-synthesis modification using organic groups that can attach to a metal centre via coordinate bonds. Such functionalization has the capability of tuning the pore dimensions, local environment, and functional properties of MOFs.22,23 Although this functionalization could fine-tune the catalytic activity, leaching of functional sites and stability of MOFs are still matters of concern and limit their potential industrial applications.New approaches are being developed to prepare MOF based nanocomposite materials. In such studies, MOF based nanocomposite materials are made with metal nanoparticles, organic polymers, and carbon materials such as graphene and carbon nanotubes.24–27 From what has been discussed above, that robust materials can be prepared using zeolite based inorganic materials, it can be concluded that the development of MOF and zeolite based nanocomposite materials could be of significant importance.28,29 Since zeolite has functional silanol groups, it can be functionalized with a suitable carboxylic acid to grow MOFs on its surface. We anticipate that if a few equivalents of aromatic carboxylic acid grown zeolites are taken along with aromatic di-carboxylic acid compounds, it could produce zeolite–MOF nanocomposite materials with different local environments due to the presence of two different types of –COOH functionalities. In addition to this, it could also provide structural stability due to the incorporation of inorganic zeolites into the nanocomposite material.
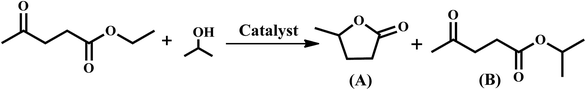
Nanocomposite materials are developed to catalyze those reactions which are of significant socio-economical value. Biomass based products have high socio-economical value. Among the biomass-derived platform chemicals, γ-valerolactone (GVL) is regarded as a versatile building block in a variety of synthetic transformations.30,31 Several catalytic strategies have been reported for the transformation of biomass into γ-valerolactone (GVL), such as reduction of levulinic acid and its ester with molecular H2 or formic acid by using different homogeneous and heterogeneous catalysts.32 Recently, the production of GVL through the Meerwein–Ponndorf–Verley (MPV) reduction using alcohol as a hydrogen donor has received much attention.33 Various metal oxides (ZrO2, Al2O3, MgO, etc.) and metal hydroxides (Cr(OH)3, Zr(OH)4, Al(OH)3, etc.)34 have been used for this catalytic transfer hydrogenation process. Among the crystalline porous materials, different transition metal containing Beta zeolites (Ti, Sn, Zr, and Hf-Beta)35 and MOFs (UiO-66, MOF-808, and MOF-801)36 have been explored for this transformation. The use of such catalysts requires modification (introduction of acidic and basic sites), harsh conditions, or expensive ligands, which limit its industrial scope. Therefore, it is important to develop a synthesis strategy for the preparation of highly sustainable catalysts for the production of GVL. These requirements encouraged us to integrate zeolite and MOF frameworks and develop a composite catalyst for the synthesis of GVL.
We have already reported the catalytic application of Beta zeolite and Zr-BDC-MOF for the formation of different value-added chemicals.37–39 In this study, Beta zeolite was functionalized with surface –COOH groups and this surface functionalized Beta was reacted with Zr-BDC-MOF precursors to prepare a Beta entangled Zr-BDC-MOF based nanocomposite catalyst (hereafter denoted as Beta–Zr-BDC-MOF). To compare the catalytic activity, the parent Beta (without surface functionalization) was also reacted under the same reaction conditions with Zr-BDC-MOF precursors to get a nanocomposite catalyst (hereafter denoted as Beta@Zr-BDC-MOF). To demonstrate the efficiency of the Beta entangled Zr-BDC-MOF structure, physical mixtures of Beta (5 to 20%) and Zr-BDC-MOF were also evaluated in the synthesis of GVL. Furthermore, for a comparative study, Cu-BTC-MOF and Beta entangled Cu-BTC-MOF based nanocomposite catalysts (hereafter denoted as Beta–Cu-BTC-MOF) were also prepared. These catalysts were investigated in the synthesis of GVL by the reaction of ethyl levulinate with a wide range of alcohols. Furthermore, the application of these catalysts was also extended to the synthesis of coumarins by the reaction of hydroxyl benzene and its derivative with ethylacetoacetate.
2. Experimental section
2.1 Synthesis of Beta–Zr-BDC-MOF
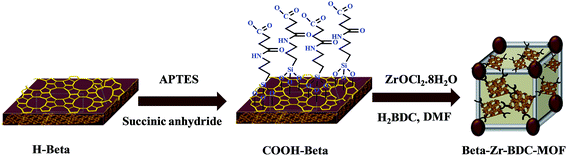
A schematic representation for the synthesis of the Beta entangled MOF catalyst is provided in Scheme 1. For the preparation of Beta–Zr-BDC-MOF, first the external surface of Beta zeolite (Beta was obtained from Sud-Chemie India Pvt Ltd. India) was modified with the carboxylic acid groups. 14 mL of (3-aminopropyl) triethoxysilane (APTES) was added to a solution containing 80 mL of N,N-dimethylformamide (DMF) and 6 g of succinic anhydride and the resultant solution was stirred under ambient conditions for 30 min. Then, 2.0 g of Beta was added to the above reaction mixture and the resultant mixture was stirred under ambient conditions for 24 h under a nitrogen atmosphere. After the reaction, the solid product was filtered and washed with ethanol several times to remove the excess organic compound. The final product was dried in a vacuum oven at 333 K for 12 h to obtain COOH–Beta.In a typical synthesis of Beta–Zr-BDC-MOF, ZrOCl2·8H2O (1.6 g, 5.0 mmol) was dissolved in 25 mL of DMF. Then, 1,4-benzenedicarboxylic acid (H2BDC) (0.49 g, 3.0 mmol) was added to the above reaction mixture and the resultant solution was stirred under ambient conditions for 10 min. Subsequently, 0.27 g of COOH–Beta was added to the resultant solution and the reaction mixture was dispersed for 30 minutes using an ultrasonic bath. In this step, segregation of the COOH–Beta aggregates took place, which provided highly dispersed COOH–Beta particles in the reaction mixture. HCl (0.8 mL, 9.6 mmol) was added drop-wise to the above reaction mixture with vigorous stirring. The resulting mixture was kept in an oil bath at 423 K for 24 h with vigorous stirring. The resultant white product was filtered, washed with DMF and dried in an oven at 373 K for 12 h. To remove the unreacted organic linker, the dried product was taken in 50 mL of DMF and stirred overnight under ambient conditions. To exchange the coordinated solvent in the framework, solvent exchange was carried out thrice with methanol (3 × 10 mL) under ambient conditions for 12 h. The product was filtered and dried in an oven at 373 K for 12 h to obtain Beta–Zr-BDC–MOF. In this study a small amount of Beta is added to form Beta entangled Zr–BDC–MOF. It may be noted that when a large amount of Beta is added, then Beta entangled Zr-BDC-MOF is not formed. This result is consistent with literature reports.29 If the amount of Beta was increased, it hindered the crystal growth of the MOF. Beta–Cu-BTC-MOF was also prepared by using the same strategy using Cu(NO3)2·3H2O as a Cu source.
Beta@Zr-BDC-MOF was prepared by solvothermal synthesis of the MOF in the presence of unmodified Beta. Details of the synthesis of the Beta@Zr-BDC-MOF investigated in this study are provided in the ESI.† For a comparative study, Zr and Cu based MOFs and nanocrystalline zirconosilicate were also prepared by following reported procedures and they are denoted as Zr-BDC-MOF,38 Cu-BTC-MOF40 and Nano-ZrS-1,41 respectively.
The procedure of catalytic reactions and details of the methods and instruments used for the characterization of the different catalysts prepared in this study are provided in the ESI† section.
3. Results and discussion
As indicated in the Introduction section, Lewis–Brönsted acidity and suitable basicity are required for the conversion of ethyl levulinate to γ-valerolactone. Multifunctional catalysts possessing acidic (Lewis and Brönsted acidity) and basic sites are required for the synthesis of γ-valerolactone (GVL) from ethyl levulinate. GVL formation takes place via intermediate 4-hydroxypentanoate. This intermediate can be formed by the catalyst having optimum Lewis acidity and basicity. Such activity is expected to be provided by the Zr-BDC-MOF. Furthermore, cyclization of 4-hydroxypentanoate into GVL is facilitated by the Brönsted acidity, which is expected to be provided by the Beta zeolite. Considering the advantages of both these porous frameworks, the integrated composite structures were designed and prepared. In this study efforts have been made to prepare zeolite Beta entangled Zr-BDC-MOF and Beta supported Zr-BDC-MOF composite catalysts. The concomitant presence of Lewis–Brönsted acidity and basicity in the composite materials is expected to enhance the GVL production.In this study, zeolite–MOF composites were prepared by two strategies. In the first strategy, Beta zeolite was treated with (3-aminopropyl) triethoxysilane (APTES) and succinic anhydride (hereafter represented as COOH–Beta) and then this surface functionalized Beta was reacted with Zr-BDC-MOF precursors to form zeolite entangled Zr-BDC-MOF (Beta–Zr-BDC-MOF). In the second strategy, the parent Beta was treated with the Zr-BDC-MOF precursors directly, without any surface functionalization, and the obtained material is denoted as Beta@Zr-BDC-MOF. COOH–Beta (formed by the functionalization of Beta with APTES and succinic anhydride, Scheme 1) was reacted with the Zr precursor (a solution containing ZrOCl2·8H2O and 1,4-benzenedicarboxylic acid (H2BDC)) to form Beta–Zr-BDC-MOF. Here, the Zr precursor reacted with the carboxylate groups of COOH–Beta and H2BDC and formed zeolite entangled Zr-BDC-MOF in which Beta and the MOF were integrated through the organic linker. The details of catalyst characterization are provided below.
3.1. Physico-chemical characterization
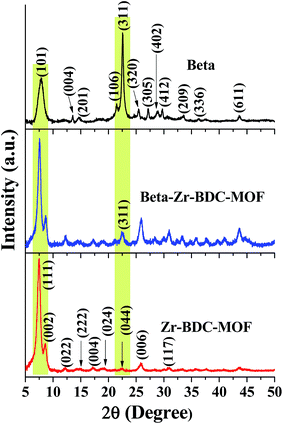
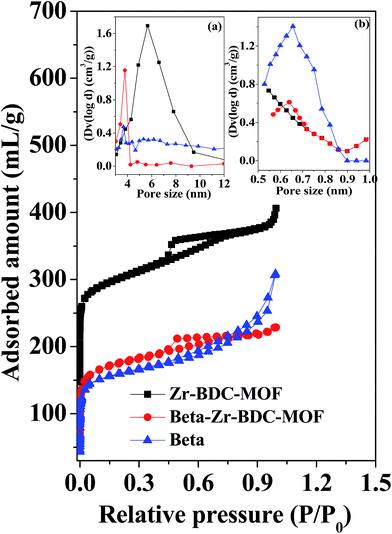
Powder XRD patterns of the various catalysts prepared in this study are shown in Fig. 1 and S1 (ESI†). The XRD patterns of Zr-BDC-MOF and Beta (Fig. 1) are consistent with those reported in the literature for UiO-66 (ref. 37) and Beta,42 respectively. The indexed diffraction peaks in Fig. 1 represent the pure crystalline framework for UiO-66 and Beta reported in the literature. For a comparative study, Cu-BTC-MOF was prepared, and the XRD pattern of Cu-BTC-MOF matches well with the reported XRD pattern of HKUST-1 (Fig. S1b (ESI†)).40 To increase the catalytic efficiency of the porous frameworks, the MOF and zeolite were entangled to form the composite catalyst. The XRD patterns of the Zr based composite catalysts exhibit reflections corresponding to both Zr-BDC-MOF and Beta (Fig. 1 and S1a (ESI†)), which confirms the co-existence of MOF and zeolite Beta structures in the composite catalysts. The characteristic diffraction peaks of Beta zeolite (Fig. 1 and S1a (ESI†)) are merged with Zr-BDC-MOF diffraction peaks in the Zr based composite catalysts. However, one characteristic peak of Beta is clearly visible at 2θ = 22.53° in the Beta–Zr-BDC-MOF and Beta@Zr-BDC-MOF composite catalysts, confirming the incorporation of Beta into the composite catalysts.The textural properties of all the catalysts were evaluated by N2-sorption measurements (Fig. 2 and S2a and b (ESI†)). The N2 adsorption isotherm of the Zr-BDC-MOF is a type IV isotherm with H1 hysteresis. The distinct increase of N2 adsorption in the region of 0.5–0.7 corresponds to the capillary condensation in the intercrystalline mesoporous void space. Beta exhibits a type II isotherm corresponding to the microporous zeolite material. Nonlocal density functional theory (NLDFT) is used to elucidate the micropore size. Zr-BDC-MOF and Beta exhibit micropores with dimensions of 0.55 and 0.65 nm, respectively. The mesopore size distribution is elucidated from the Barret–Joyner–Halenda (BJH) method. Zr-BDC-MOF exhibits a mesopore size distribution in the range of 2–9.5 nm with a peak maximum at 5.6 nm. However, no distinguished mesopore size distribution is observed for Beta. Beta-Zr–BTC–MOF exhibits an isotherm very similar to that of Zr-BDC-MOF but with a low adsorbed volume (Fig. 2 and S2a (ESI†)). The NLDFT micropore size distribution falls in the range of 0.5 to 0.7 nm, confirming the existence of both types of micropores. Beta-Zr-BDC-MOF shows a mesopore size distribution in the range of 2 to 4.0 nm with a peak maximum at 3.5 nm. This clearly shows that during the growth of Zr-BDC-MOF in the presence of COOH–Beta, the generated particles have not created intercrystalline porosity similar to that of Zr-BDC-MOF but have provided a compact porous structure. The adsorption isotherm of Beta@Zr-BDC-MOF is very different from that of Beta–Zr-BDC-MOF. It exhibits an isotherm and hysteresis similar to that of Beta (compare Fig. 2 and S2a (ESI†)). A significant adsorption in the pressure range of 0.8 to 1.0 corresponds to inter-crystalline macropore void space (Fig. S2a (ESI†)). The mesopore size distribution confirms the presence of inter-crystalline porosity. This provides evidence that functionalization of Beta zeolites is responsible for the formation of a compact porous structure. In the absence of functionalization, segregated Beta and Zr-BDC-MOF domains are formed which create different inter-particle porosity. Cu-BTC-MOF exhibits a type I isotherm corresponding to a microporous material (Fig. S2b (ESI†)). The isotherm of Beta–Cu-BTC-MOF is very similar to that of Cu-BTC-MOF but with a low adsorbed volume (Fig. S2b (ESI†)). The textural properties obtained from N2-adsorption–desorption analysis are presented in Table 1. The textural properties of the composite materials are in between those of the zeolite Beta and MOFs, which further reveals the integration of zeolite and MOF structures in the composite materials.
| Catalyst | S BET (m2 g−1) | S EA (m2 g−1) | V total (cm3 g−1) | % composition of Beta in the composite | |
|---|---|---|---|---|---|
| Based on TGA analysis | Based on elemental analysis (Si content) | ||||
| a S BET = total surface area, SEA = external surface area, and Vtotal = total pore volume. b Recycled catalyst obtained after the fifth recycle. | |||||
| Beta | 497 | 144 | 0.47 | — | — |
| Zr-BDC-MOF | 920 | 303 | 0.79 | — | — |
| Beta–Zr-BDC-MOF | 551 | 145 | 0.45 | 5.8 | 6.1 |
| Beta@Zr-BDC-MOF | 512 | 163 | 0.84 | 22.8 | 23.1 |
| Cu-BTC-MOF | 941 | 341 | 0.84 | — | — |
| Beta–Cu-BTC-MOF | 468 | 148 | 0.40 | 16.5 | 16.9 |
| Beta–Zr-BDC-MOFb | 542 | 140 | 0.43 | 5.9 | 6.2 |
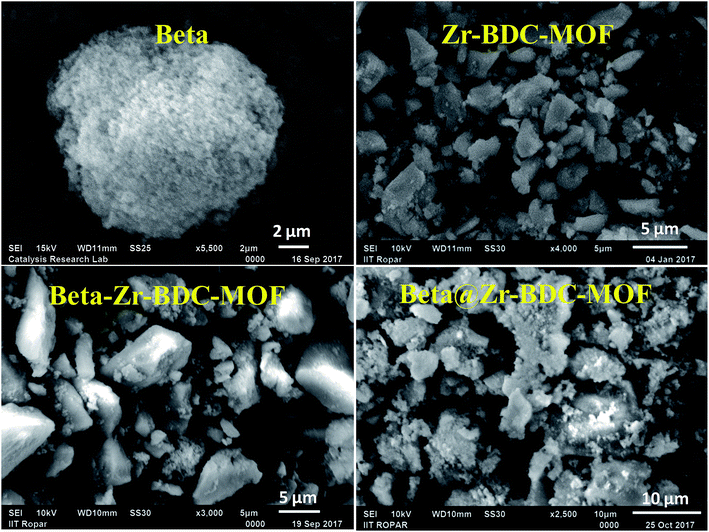
The surface morphology of the bare materials and their composites was analyzed using scanning electron microscopy (SEM) (Fig. 3 and S3 (ESI†)). The SEM image of Beta confirms the aggregated nanometer size zeolite particles. The SEM image of COOH–Beta also exhibits an aggregated morphology similar to Beta but with surface wiggling on the aggregates. Zr-BDC-MOF exhibits an irregular micron size crystal morphology with different dimensions. The SEM image of Beta@Zr-BDC-MOF shows an irregular aggregated (less densely packed and highly densely packed domains) morphology, which is distinct from the Beta–Zr-BDC-MOF morphology (Fig. 3). The morphology of Beta–Zr-BDC-MOF is very similar to the morphology of Zr-BDC-MOF, which confirms that the carboxylate groups of functionalized Beta (COOH–Beta) participate in the crystallization process and produce materials with a similar morphology to that of Zr-BDC-MOF. During the synthesis of Beta–Zr-BDC-MOF, after the addition of COOH–Beta, the solution is ultrasonicated for 30 minutes. In this step, segregation of the COOH–Beta aggregates takes place, providing highly dispersed COOH–Beta particles in the reaction mixture. The carboxylate groups of COOH–Beta interact with Zr metal in a similar way as the carboxylate groups of BDC interact with the Zr metal precursor in the Zr-BDC-MOF framework and induce the growth of Beta entangled Zr-BDC-MOF. However, both the reactions can take place simultaneously with different rates; therefore the random growth of Beta–Zr-BDC-MOF particles takes place, providing Beta–Zr-BDC-MOF with a morphology similar to that of Zr-BDC-MOF, which is clearly visible in the TEM image (Fig. 4). The SEM images of Cu-BTC-MOF and its composite are shown in Fig. S3 (ESI†). Irregular micrometer size (mostly distorted octahedral) Cu-BTC-MOF crystals are observed, whereas Beta–Cu-BTC-MOF exhibits very small size crystals. This further confirms that during synthesis, zeolite particles are segregated and Cu-BTC-MOF crystals have grown around these particles. Furthermore, in this case, BTC molecules (instead of BDC) participate in the synthesis and therefore its morphology is different from the morphology obtained for Beta–Zr-BDC-MOF. The chemical composition of the parent and composite materials was investigated by EDS analysis (Fig. S4 (ESI†)). Zr, O, and C elements are observed in the EDS spectrum of Zr-BDC-MOF whereas two additional peaks corresponding to Si and Al elements are observed in the EDS spectrum of the composite frameworks confirming the incorporation of zeolite Beta into the composite materials.
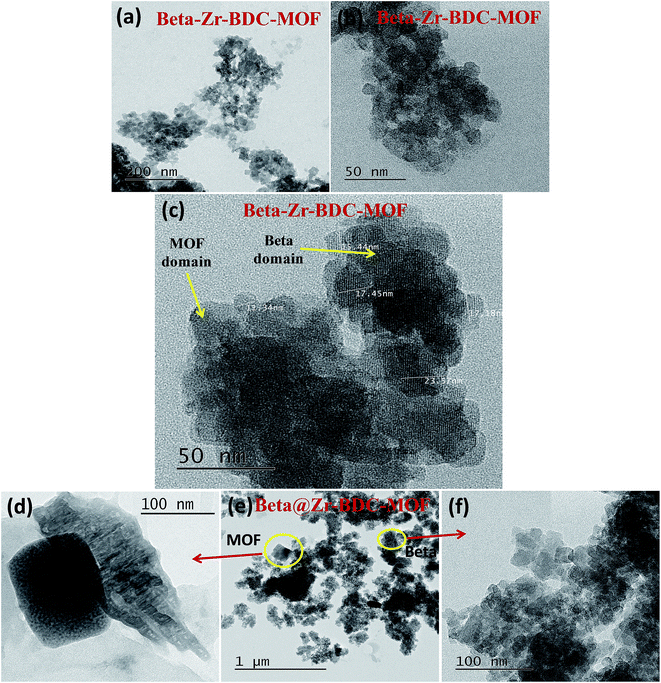
To further analyze the micro-nano architecture of the composite catalysts, transmission electron microscopy (TEM) was used (Fig. 4). The TEM images show that the Beta–Zr-BDC-MOF is composed of very small nanoparticles of 10–20 nm crystals (Fig. 4a and b). Very uniform crystal domains are observed in this sample. The high magnification TEM image clearly shows the highly ordered domains with lattice fringes corresponding to Beta zeolite and less ordered domains (with no lattice fringes) for Zr-BDC-MOF in a very small specimen area of approximately 200 nm length scale for Beta–Zr-BDC-MOF (Fig. 4c). In this case, two microporous structures are linked with each other and therefore no clear domains are visible in the low magnification TEM images. However, the high magnification TEM image of Beta–Zr-BDC-MOF clearly shows different domains of Beta and Zr-BDC-MOF. A clear interface of Zr-BDC-MOF and Beta is shown in the TEM image of Beta@Zr-BDC-MOF (Fig. 4e). Zr-BDC-MOF and Beta zeolite domains are clearly visible and are shown in the representative TEM images (Fig. 4d and f).
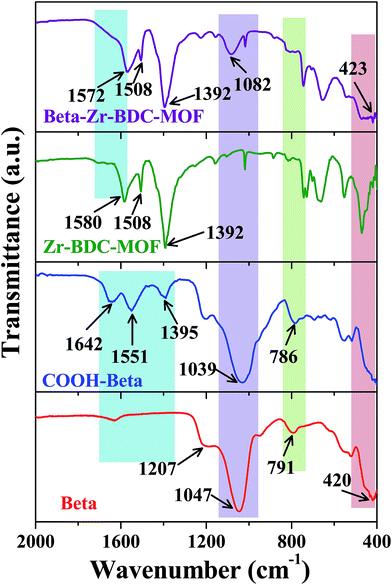
The presence of the MOF and Beta framework in the composite materials was also confirmed by Fourier transform infrared (FTIR) spectroscopy. Fig. 5 and S5a and b (ESI†) show the FT-IR spectra of the parent and the composite materials. The carboxylate linker in the Zr-BDC-MOF produces the asymmetric stretching vibrations at 1508 and 1580 cm−1 and the symmetric stretching vibration at 1392 cm−1. The characteristic peaks of Beta zeolite at 1047 and 1207 cm−1 correspond to the internal and external asymmetric stretching vibrations of O–T–O, respectively (where T = Si or Al). Furthermore, absorption peaks at 420 and 791 cm−1 were also observed, which correspond to the T–O bending mode and O–T–O external symmetric stretching, respectively. The FT-IR spectra of COOH–Beta exhibited new absorption peaks at 1642 cm−1 and 1551 cm−1 that correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band and N–H bending band of the amide linkage, respectively, which confirmed the successful immobilization of –C3H6–NH–CO–C2H4–COOH groups on the surface of Beta. The FT-IR spectra of Beta–Zr-BDC-MOF and Beta@Zr-BDC-MOF exhibit the characteristic peaks of both Zr-BDC-MOF and Beta zeolite, which confirms the presence of both the frameworks in the composite catalyst. Some marginal deviation in the characteristic peaks of Beta zeolite is also observed in the composite frameworks. However, this deviation is higher in Beta–Zr-BDC-MOF (from 1039 cm−1 to 1082 cm−1) than Beta@Zr-BDC-MOF (from 1047 cm−1 to 1064 cm−1), which further confirms the strong interaction of Beta zeolite with the Zr-BDC-MOF framework in the Beta–Zr-BDC-MOF. Similarly, Beta–Cu-BTC-MOF exhibits all the characteristic peaks corresponding to Cu-BTC-MOF and Beta frameworks in the composite catalyst (Fig. S5 (ESI†)).
O stretching band and N–H bending band of the amide linkage, respectively, which confirmed the successful immobilization of –C3H6–NH–CO–C2H4–COOH groups on the surface of Beta. The FT-IR spectra of Beta–Zr-BDC-MOF and Beta@Zr-BDC-MOF exhibit the characteristic peaks of both Zr-BDC-MOF and Beta zeolite, which confirms the presence of both the frameworks in the composite catalyst. Some marginal deviation in the characteristic peaks of Beta zeolite is also observed in the composite frameworks. However, this deviation is higher in Beta–Zr-BDC-MOF (from 1039 cm−1 to 1082 cm−1) than Beta@Zr-BDC-MOF (from 1047 cm−1 to 1064 cm−1), which further confirms the strong interaction of Beta zeolite with the Zr-BDC-MOF framework in the Beta–Zr-BDC-MOF. Similarly, Beta–Cu-BTC-MOF exhibits all the characteristic peaks corresponding to Cu-BTC-MOF and Beta frameworks in the composite catalyst (Fig. S5 (ESI†)).
The amount of individual frameworks in the composite material was calculated by TGA analysis. Fig. 6a and S6 (ESI†) show the thermograms of the parent and composite materials. The difference in the weight loss between Beta and COOH–Beta corresponds to the amount of functionalized linker anchored to the surface of Beta (weight loss for COOH–Beta, 14.4 wt%) (Table 1). In COOH–Beta, the weight losses are due to the removal of physisorbed water, occluded solvent molecules, and most importantly oxobutanoate (CO–C2H4–COOH groups) and propylamine (–C3H6–NH) functionalities. Zr-BDC-MOF exhibits three different weight losses. The first weight loss is related to the removal of physisorbed H2O molecules (between 298 K and 380 K), the second weight loss is attributed to the removal of occluded solvent molecules (between 400 K and 620 K) and the third weight loss is related to the decomposition of the framework (between 620 K and 890 K). Similar to the Zr-BDC-MOF thermogram, the Beta@Zr-BDC-MOF also shows three characteristic weight losses. The difference in the weight loss observed in the case of Zr-BDC-MOF and Beta@Zr-BDC-MOF corresponds to the incorporation of 22.8 wt% Beta into the composite material. The weight loss in the thermogram of Beta–Zr-BDC-MOF corresponds to the decomposition of the functionalized linker on the zeolite as well as the degradation of the MOF framework. The weight loss in the range of 392–653 K involves the removal of the oxobutanoate moiety of COOH–Beta and occluded solvent molecules in the framework. The weight loss in the range of 653–910 K is related to loss of propylamine anchored to the surface of COOH–Beta and the degradation of the Zr-BDC-MOF framework. The difference in the weight loss between Zr-BDC-MOF and Beta–Zr-BDC-MOF corresponds to the incorporation of 5.8 wt% Beta into the composite. The thermogram of Cu-BTC-MOF shows two significant weight losses (Fig. S6 (ESI†)). The first weight loss is related to the removal of physisorbed H2O molecules (between 298 K and 400 K), the second weight loss is attributed to the decomposition of the framework (between 407 and 652 K). The decomposition temperature for Beta–Cu-BTC-MOF is shifted, which confirms the interaction of Beta zeolite with the Cu-BTC-MOF framework. The amount of Beta in Beta–Cu-BTC-MOF is found to be 16.5 wt%.
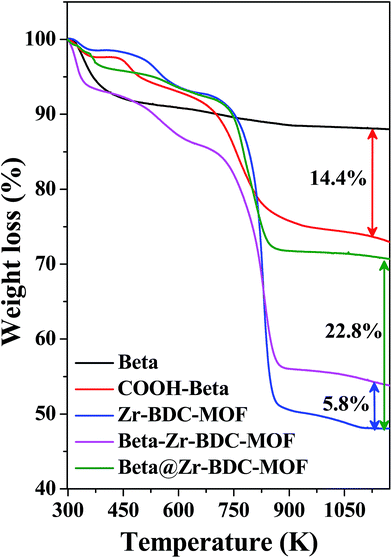 | ||
| Fig. 6 Thermograms of Zr-BDC-MOF, Beta zeolite, COOH–Beta, and their composite catalysts prepared in this study. | ||
3.2. Catalytic activity
When the reaction between ethyl levulinate and isopropanol is investigated using zeolite Beta, the transesterified product is obtained selectively (Table 2, entry 1). However, in the presence of Zr-BDC-MOF, the cyclized GVL product is obtained in higher selectivity (Table 2, entry 2). Under similar reaction conditions, Cu-BTC-MOF and Nano-ZrS-1 are found to be inactive (Table 2, entries 3–4). The composite catalysts are found to be active and produce GVL and transesterified products under the optimum reaction conditions. Among all the catalysts investigated in this study, Beta–Zr-BDC-MOF exhibits the highest activity. The highest selectivity for GVL is obtained using Beta–Zr-BDC-MOF (Table 2, entry 5). These investigations clearly show that Zr metal, MOF framework, and Beta zeolite incorporation all have roles to play in the improvement of GVL yield. The lack of activity of Cu-BTC-MOF shows that Cu does not provide suitable acidity and activity for this reaction. Furthermore, the mere presence of Zr alone is not responsible for this unique activity but the Zr-BDC-MOF framework is required to achieve cyclization to produce GVL. If Zr alone could have caused this reaction then Nano-ZrS-1 should have exhibited some activity towards GVL production. The Beta@Zr-BDC-MOF synthesized in this study is like a physical mixture of Beta and Zr-BDC-MOF. Furthermore, physical mixtures of Beta (5 to 20%) and Zr-BDC-MOF are evaluated in the synthesis of GVL. Physical mixtures (Table 2, entries 7–9) and Beta@Zr-BDC-MOF (Table 2, entry 6) exhibit lower activity when compared to Beta–Zr-BDC-MOF. Based on the comparative catalytic activity of Beta-Zr-BDC-MOF, Beta@Zr-BDC-MOF, and the physical mixtures of Beta and Zr-BDC-MOF, it can be concluded that the entangled catalyst provides the optimum Lewis acidity and basicity required for the formation of intermediate 4-hydroxypentanoate. Furthermore, the optimum Brönsted acidity provided by the entangled Beta is sufficient for the conversion of 4-hydroxypentanoate to GVL.| S. no. | Catalyst | Solvent | Temp. (K) | Time (h) | Conv. (%) | Selectivity (%) | |
|---|---|---|---|---|---|---|---|
| (A) | (B) | ||||||
| a Reaction condition: catalyst (200 mg), ethyl levulinate (1.0 mmol), 2-propanol (6 mL), reaction temperature (413 K), time (6 h). b 2-Propanol (3 mL). c Catalytic data obtained after the fifth recycle. d Remaining is the unidentified product. | |||||||
| 1 | Beta (100 mg) | 2-Propanol | 413 | 6 | 47 | — | 100 |
| 2 | Zr-BDC-MOF | 2-Propanol | 413 | 6 | 96 | 62 | 38 |
| 3 | Cu-BTC-MOF | 2-Propanol | 413 | 6 | Nil | — | — |
| 4 | Nano-ZrS-1 | 2-Propanol | 413 | 6 | Nil | — | — |
| 5 | Beta–Zr-BDC-MOF | 2-Propanol | 413 | 6 | 100 | 75 | 25 |
| 6 | Beta@Zr-BDC-MOF | 2-Propanol | 413 | 6 | 65 | 48 | 52 |
| 7 | Physical mixture 1 (Beta (5 wt%) + Zr-BDC-MOF (95 wt%)) | 2-Propanol | 413 | 6 | 90 | 58 | 42 |
| 8 | Physical mixture 2 (Beta (10 wt%) + Zr-BDC-MOF (90 wt%)) | 2-Propanol | 413 | 6 | 75 | 53 | 47 |
| 9 | Physical mixture 3 (Beta (20 wt%) + Zr-BDC-MOF (80 wt%)) | 2-Propanol | 413 | 6 | 66 | 45 | 55 |
| 10 | Beta–Cu-BTC-MOF | 2-Propanol | 413 | 6 | 10 | — | 100 |
| 11 | Beta-Zr-BDC-MOFb | 2-Propanol | 413 | 6 | 30 | 41 | 59 |
| 12 | Beta-Zr-BDC-MOFc | 2-Propanol | 413 | 6 | 98 | 73 | 27 |
| 13 | Beta-Zr-BDC-MOF | Methanol | 413 | 6 | 19 | 58 | 42 |
| 14 | Beta-Zr-BDC-MOF | Ethanol | 413 | 6 | 25 | 67d | — |
| 15 | Beta-Zr-BDC-MOF | Cyclohexanol | 413 | 6 | 61 | 100 | — |
| 16 | Beta-Zr-BDC-MOF | t-Butanol | 413 | 6 | 0 | — | — |
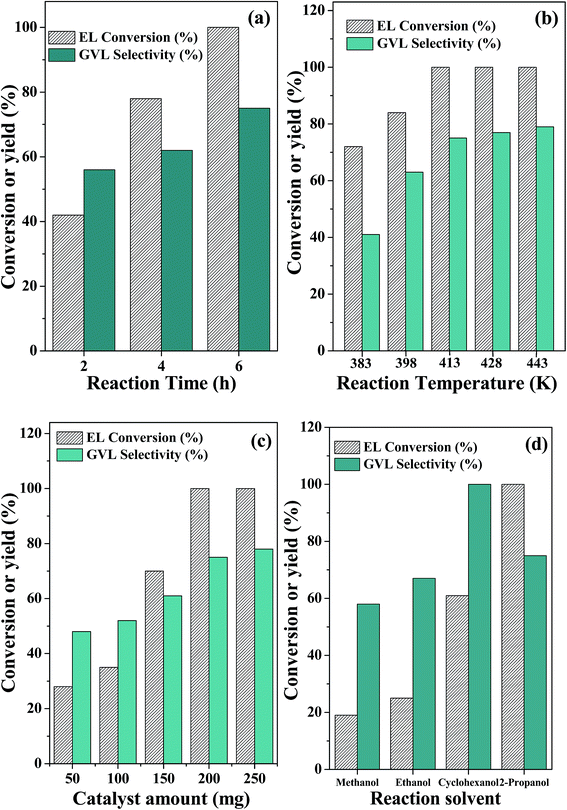
The influence of various reaction parameters is investigated. With an increase in the reaction time, both ethyl levulinate conversion and GVL selectivity are increased (Fig. 7a). With an increase in the reaction temperature from 383 to 413 K, both ethyl levulinate conversion and GVL selectivity are increased (Fig. 7b). With further increase in temperature, only a marginal increase in the GVL selectivity is obtained. Therefore, 413 K was chosen as the optimum temperature for this study. The catalyst amount also plays an important role in achieving higher product yields. With an increase in the catalyst amount from 50 mg to 200 mg, both ethyl levulinate conversion and GVL selectivity are increased (Fig. 7c). With further increase in this amount, no significant improvement is observed. Therefore, a catalyst amount of 200 mg is optimum for this reaction.
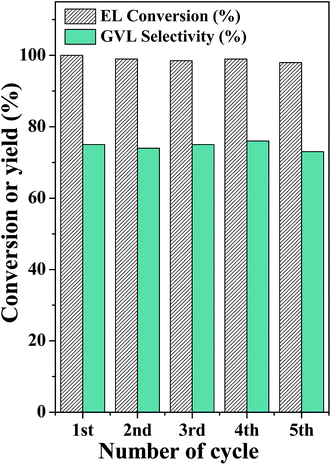
Recycling experiments are performed to confirm the stability of the composite framework. After completion of the reaction, the catalyst is recovered using a centrifuge machine and then washed several times with ethyl acetate and ethanol and dried in an oven at 373 K for 10 h. The catalytic activity of the recycled catalyst is found to be similar to that of the fresh catalyst (Fig. 8). The recycled catalyst exhibits a similar XRD pattern to that of the fresh catalyst (Fig. S7a (ESI†)), which confirms that the framework integrity of Beta–Zr-BDC-MOF is retained. The FT-IR spectrum of the recycled catalyst shows that the characteristic peaks of Beta and Zr-BDC-MOF are retained suggesting the high chemical stability of the catalyst (Fig. S7b (ESI†)). The thermal stability of the recycled catalyst is also investigated by TGA analysis. The thermal stability and % compositions of Beta and Zr-BDC-MOF in the entangled structure of the recycled catalyst are similar to that of the fresh catalyst (Fig. S7c (ESI†)). A leaching experiment is also performed to test the leaching of Zr, Al, and Si species during the reaction. The reaction is performed for 2 h under the optimum reaction conditions. After 2 h, the catalyst is removed from the reaction mixture and progress of the reaction is monitored. No further increase in the reactant conversion occurs after removal of the catalyst (Fig. S8 (ESI†)), demonstrating that no active species was leached during the reaction that could catalyze the reaction in the homogeneous phase. Elemental analysis shows that Zr, Al, and Si species are not detected in the solution after the reaction, which confirms that Zr/Al is not leached into the solution and those Zr/Al sites that are present in the catalyst catalyzed the reaction as a heterogeneous catalyst to produce the desired product. The catalytic activity of Beta–Zr-BDC-MOF is compared with that of other catalysts reported in the literature. The catalyst shows higher or comparable performance with respect to the various reported heterogeneous catalysts (Table S1 (ESI†)).34,36,43–45
Based on the catalytic activity data, the following reaction mechanism is proposed for the formation of GVL (Scheme 2). The bi-functional sites, acidic (Zr4+/Al3+ from zeolite Beta entangled Zr-BDC-MOF) and basic sites (O2− from zeolite Beta entangled Zr-BDC-MOF), present in the catalyst interact with isopropanol and ethyl levulinate to form a six-membered transition state. This six-membered transition state is difficult to form if only acidic sites are present in the catalyst like zeolite Beta. The basicity provided by the O2− from the framework facilitates the formation of 4-hydroxypentanoate via the alkoxide generation from the dissociation of isopropanol and hydride ions attack the carbonyl center of ethyl levulinate. In this process, acetone is generated and it is detected during GC-MS analysis. Furthermore, intramolecular transesterification of 4-hydroxypentanoate into GVL is facilitated in the presence of Beta–Zr-BDC-MOF/Zr-BDC-MOF. In this process, ethanol is obtained as the by-product. GC-MS analysis confirms the formation of acetone, ethanol, and GVL. However, no 4-hydroxypentanoate is observed. This finding suggests that intramolecular cyclization is very facile in the presence of Beta–Zr-BDC-MOF/Zr-BDC-MOF. To gain further mechanistic insight, the influence of H-donor solvents is investigated. Different primary, secondary, and cyclic alcohols are evaluated under the optimum reaction conditions using a highly active catalyst, Beta–Zr-BDC-MOF. Fig. 7d shows that secondary alcohols exhibit higher activity and selectivity for the desired product. Higher selectivity towards GVL is obtained when cyclohexanol is used as the solvent. It may be noted that the reaction is sluggish but the selectivity for the desired GVL product is higher in this case. This confirms that the first step of the reaction is less favourable due to the steric hindrance in which the cyclic transition state is formed. Furthermore, no reaction takes place when t-butanol is chosen as the solvent (Table 2, entry 15). This clearly shows that H-donor sites are required for this reaction to take place. The high activity of Beta–Zr-BDC-MOF when compared to Beta and Zr-BDC-MOF and low acidity of Beta–Zr-BDC-MOF when compared to Beta confirm that Zr incorporation into the entangled porous structure is the most crucial for GVL production.
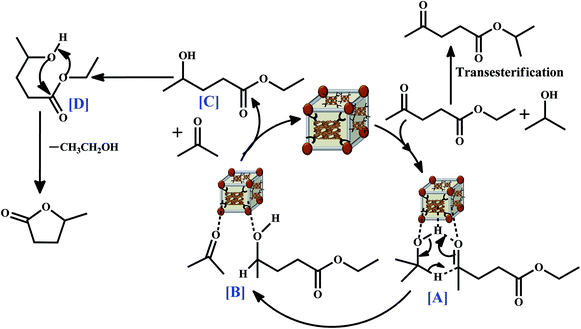 | ||
| Scheme 2 A plausible mechanism for the synthesis of γ-valerolactone by the reaction of ethyl levulinate and alcohols. | ||
To further explore the role of different acid sites in the composite catalyst, a condensation reaction is investigated for the synthesis of coumarin derivatives. Very recently, we have shown the role of the Lewis acid sites of Zr-BDC-MOF in different condensation reactions.37Table 3 shows that Zr-BDC-MOF exhibits better activity when compared to Cu-BTC-MOF in coumarin synthesis. This result is consistent with the results obtained in the Knoevenagel and aldol condensation reactions reported previously by us.37 It is also interesting to note that Nano-ZrS-1 is active for this reaction and produced somewhat better activity when compared to Zr-BDC-MOF. These results clearly suggest that in contrast to GVL synthesis, the Zr present in both nano ZrS-1 and Zr-BDC-MOF provides suitable activity in the condensation reaction for the synthesis of coumarin.
| S. no. | Catalyst | Catalyst amt. (mg) | Reactant | Product yield (%) |
|---|---|---|---|---|
| a Reaction conditions: catalyst (100 mg), phenol (5 mmol), ethylacetoacetate (7.5 mmol), temperature (423 K), and time (4 h). b Phenol conversion. c Catalytic data obtained after the fifth cycle. | ||||
| 1 | Beta | 5.8 | Resorcinol | 25 |
| 2 | Zr-BDC-MOF | 100 | Resorcinol | 19 |
| 3 | Cu-BTC-MOF | 100 | Resorcinol | 2 |
| 4 | Nano-ZrS-1 | 100 | Resorcinol | 23 |
| 5 | Beta–Zr-BDC-MOF | 100 | Resorcinol | 70 |
| 6 | Beta@Zr-BDC-MOF | 100 | Resorcinol | 38 |
| 7 | Beta–Cu-BTC-MOF | 100 | Resorcinol | 23 |
| 8 | Beta–Zr-BDC-MOF | 100 | Catechol | 31 (43)b |
| 9 | Beta–Zr-BDC-MOF | 100 | 1-Naphthol | 17 |
| 10 | Beta–Zr-BDC-MOFc | 100 | Resorcinol | 65 |
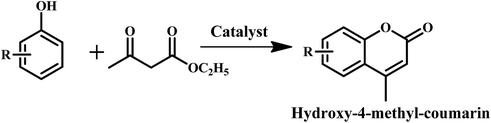
Furthermore, Beta zeolite is found to exhibit good activity in the synthesis of coumarin. Among the two composite materials prepared in this study, Beta–Zr-BDC-MOF exhibits more than three fold higher activity when compared to Zr-BDC-MOF. If we combine the activity of Beta and Zr-BDC-MOF together, even then the activity of Beta–Zr-BDC-MOF is higher than their combined activity. This provides evidence that the Beta and MOF entangled structure is crucial to achieving higher activity. The low activity of Beta@Zr-BDC-MOF also suggests this hypothesis. The applicability of Beta–Zr-BDC-MOF is further investigated with different substrates (Table 3). Coumarin yields are highly dependent on the substrate. Among the different substrates, the composite framework is found to be more active for resorcinol. Scheme S1 (ESI†) presents a plausible mechanism for the preparation of coumarin derivatives. The zeolite framework in the Beta–Zr-BDC-MOF activates the carbonyl group of the Beta–keto ester (intermediate A) and makes it susceptible to nucleophilic attack by resorcinol and produce intermediate B, which further undergoes transesterification, followed by ethanol removal to produce intermediate D. The dehydration of intermediate D produces the desired product and regenerates the acid sites of the catalyst. The catalytic activity of the recycled catalyst is similar to that of the fresh catalyst after five recycles (Table 3 and Fig. S9 (ESI†)). The stability of the recovered catalyst is ascertained using XRD, FT-IR, and TGA analysis (Fig. S10 (ESI†)). The XRD pattern of the recycled catalyst is similar to that of the fresh catalyst, which confirms that no significant crystallographic change is observed after the recycling study (Fig. S10a (ESI†)). The FTIR spectrum and TGA profile recorded for the recovered catalyst further confirm the stability of the material after multiple uses in the Pechmann condensation (Fig. S10b, c (ESI†)).
4. Conclusions
In conclusion, two porous materials, Beta zeolite and Zr-BDC-MOF, were entangled to form a highly efficient and selective Beta–Zr-BDC-MOF composite catalyst. A step-wise synthesis protocol was adopted to prepare the Beta–Zr-BDC-MOF composite. The carboxylate groups in COOH–Beta interacted with the zirconium source and benzene dicarboxylic acid and prompted the growth of an integrated porous network structure of Beta–Zr-BDC-MOF having Zr-BDC-MOF and Beta zeolite frameworks. TEM images confirmed the existence of highly crystalline and less crystalline domains corresponding to Beta and Zr-BDC-MOF frameworks in the Beta–Zr-BDC-MOF nanocomposite. Catalytic investigations confirmed that the Beta–Zr-BDC-MOF composite material exhibited excellent catalytic activity and selectivity for γ-valerolactone and coumarin synthesis. The obtained results clearly demonstrate that the interaction of carboxylate linkers with Zr in the composite material improves the reaction efficiency and selectivity. The optimum bi-functional (acidic and basic sites) activity of both Beta and Zr-BDC-MOF in the nanocomposite is the driving force for the excellent catalytic activity. We believe that this composite framework could open a door to design a wide range of multifunctional catalysts for specific catalytic reactions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful to SERB, Department of Science and Technology, New Delhi (EMR/2016/001408) for financial support. PR acknowledges financial assistance from MHRD, New Delhi.Notes and references
- H. Chang and H. Wu, Energy Environ. Sci., 2013, 6, 3483–3507 CAS.
- R. C. Pawar and C. S. Lee, in Heterogeneous Nanocomposite-Photocatalysis for Water Purification, William Andrew Publishing, Boston, 2015, pp. 1–23 Search PubMed.
- M. E. Davis, Nature, 2002, 417, 813–821 CrossRef CAS PubMed.
- M. H. Sun, S. Z. Huang, L. H. Chen, Y. Li, X. Y. Yang, Z. Y. Yuan and B. L. Su, Chem. Soc. Rev., 2016, 45, 3479–3563 RSC.
- M. H. Rodríguez, J. L. Jorda, F. Rey and A. Corma, J. Am. Chem. Soc., 2012, 134, 13232–13235 CrossRef PubMed.
- R. Srivastava, Catal. Today, 2018, 309, 172–188 CrossRef CAS.
- R. Chal, C. Gerardin, M. Bulut and S. V. Donk, ChemCatChem, 2011, 3, 67–81 CrossRef CAS.
- M. V. Opanasenko, W. J. Roth and J. Cejka, Catal. Sci. Technol., 2016, 6, 2467–2484 CAS.
- W. J. Roth, P. Nachtigall, R. E. Morris and J. Cejka, Chem. Rev., 2014, 114, 4807–4837 CrossRef CAS PubMed.
- B. Kaur and R. Srivastava, New J. Chem., 2015, 39, 6899–6906 RSC.
- O. Vassylyev, J. Chen, G. S. Hall and J. G. Khinast, Microporous Mesoporous Mater., 2006, 92, 101–108 CrossRef CAS.
- B. Sarmah and R. Srivastava, Ind. Eng. Chem. Res., 2017, 56, 8202–8215 CrossRef CAS.
- H. C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- N. Stock and S. Biswas, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- B. Li, H. M. Wen, W. Zhou and B. Chen, J. Phys. Chem. Lett., 2014, 5, 3468–3479 CrossRef CAS PubMed.
- M. Opanasenko, A. Dhakshinamoorthy, J. Cejka and H. Garcia, ChemCatChem, 2013, 5, 1553–1561 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro, P. Concepcion and H. Garcia, Catal. Commun., 2011, 12, 1018–1021 CrossRef CAS.
- Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC.
- C. V. McGuire and R. S. Forgan, Chem. Commun., 2015, 51, 5199–5217 RSC.
- M. Eddaoudi, J. Kim, N. Rosi, D. Vodak, J. Wachter, M. O'Keeffe and O. M. Yaghi, Science, 2002, 295, 469–472 CrossRef CAS PubMed.
- Y. Lin, C. Kong and L. Chen, RSC Adv., 2016, 6, 32598–32614 RSC.
- T. Islamoglu, S. Goswami, Z. Li, A. J. Howarth, O. K. Farha and J. T. Hupp, Acc. Chem. Res., 2017, 50, 805–813 CrossRef CAS PubMed.
- Q. L. Zhu and Q. Xu, Chem. Soc. Rev., 2014, 43, 5468–5512 RSC.
- F. P. Kinik, A. Uzun and S. Keskin, ChemSusChem, 2017, 10, 2842–2863 CrossRef CAS PubMed.
- I. Ahmed and S. H. Jhung, Mater. Today, 2014, 17, 136–146 CrossRef CAS.
- Y. Zhang, X. Feng, S. Yuan, J. Zhou and B. Wang, Inorg. Chem. Front., 2016, 3, 896–909 RSC.
- G. Zhu, R. Graver, L. Emdadi, B. Liu, K. Y. Choi and D. Liu, RSC Adv., 2014, 4, 30673–30676 RSC.
- D. W. Lim, H. Lee, S. Kim, I. H. Cho, M. Yoon and Y. N. Choi, Chem. Commun., 2016, 52, 6773–6776 RSC.
- D. M. Hasko, B. Lengyel, J. M. Tukacs, L. Kollar and L. T. Mika, ChemPlusChem, 2016, 81, 1224–1229 CrossRef.
- Z. Zhang, ChemSusChem, 2016, 9, 156–171 CrossRef CAS PubMed.
- X. Tang, X. Zeng, Z. Li, L. Hu, Y. Sun, S. Liu, T. Lei and L. Lin, Renewable Sustainable Energy Rev., 2014, 40, 608–620 CrossRef CAS.
- M. Chia and J. A. Dumesic, Chem. Commun., 2011, 47, 12233–12235 RSC.
- X. Tang, H. Chen, L. Hu, W. Hao, Y. Sun, X. Zeng, L. Lin and S. Liu, Appl. Catal., B, 2014, 14, 827–834 CrossRef.
- L. Bui, H. Luo, W. R. Gunther and Y. R. Leshkov, Angew. Chem., Int. Ed., 2013, 52, 8022–8025 CrossRef CAS PubMed.
- A. H. Valekar, K. H. Cho, S. K. Chitale, D. Y. Hong, G. Y. Cha, U. H. Lee, D. W. Hwang, C. Serre, J. S. Chang and Y. K. Hwang, Green Chem., 2016, 18, 4542–4552 RSC.
- P. Rani and R. Srivastava, New J. Chem., 2017, 41, 8166–8177 RSC.
- P. Rani and R. Srivastava, RSC Adv., 2015, 5, 28270–28280 RSC.
- B. Sarmah and R. Srivastava, ACS Sustainable Chem. Eng., 2015, 3, 210–215 CrossRef CAS.
- H. Chen, L. Weng, J. Yang and R. T. Yang, J. Phys. Chem. C, 2013, 117, 7565–7567 CAS.
- R. Kore, R. Srivastava and B. Satpati, ACS Catal., 2013, 3, 2891–2904 CrossRef CAS.
- R. Kore, B. Satpati and R. Srivastava, Chem.–Eur. J., 2011, 17, 14360–14365 CrossRef CAS PubMed.
- Y. Kuwahara, H. Kango and H. Yamashita, ACS Sustainable Chem. Eng., 2017, 5, 1141–1152 CrossRef CAS.
- H. Li, Z. Fang and S. Yang, ACS Sustainable Chem. Eng., 2016, 4, 236–246 CrossRef CAS.
- J. Song, B. Zhou, H. Zhou, L. Wu, Q. Meng, Z. Liu and B. Han, Angew. Chem., Int. Ed., 2015, 54, 9399–9403 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8se00098k |
| This journal is © The Royal Society of Chemistry 2018 |