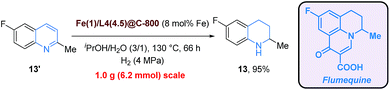Open Access Article
Open Access ArticleA robust iron catalyst for the selective hydrogenation of substituted (iso)quinolones†
Basudev
Sahoo
 a,
Carsten
Kreyenschulte
a,
Carsten
Kreyenschulte
 a,
Giovanni
Agostini
a,
Henrik
Lund
a,
Stephan
Bachmann
b,
Michelangelo
Scalone
b,
Kathrin
Junge
a,
Giovanni
Agostini
a,
Henrik
Lund
a,
Stephan
Bachmann
b,
Michelangelo
Scalone
b,
Kathrin
Junge
 a and
Matthias
Beller
a and
Matthias
Beller
 *a
*a
aLeibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Str. 29a, 18059 Rostock, Germany. E-mail: matthias.beller@catalysis.de
bProcess Chemistry and Catalysis, F. Hoffmann-La Roche Ltd., Grenzacherstrasse 124, 4070 Basel, Switzerland
First published on 6th September 2018
Abstract
By applying N-doped carbon modified iron-based catalysts, the controlled hydrogenation of N-heteroarenes, especially (iso)quinolones, is achieved. Crucial for activity is the catalyst preparation by pyrolysis of a carbon-impregnated composite, obtained from iron(II) acetate and N-aryliminopyridines. As demonstrated by TEM, XRD, XPS and Raman spectroscopy, the synthesized material is composed of Fe(0), Fe3C and FeNx in a N-doped carbon matrix. The decent catalytic activity of this robust and easily recyclable Fe-material allowed for the selective hydrogenation of various (iso)quinoline derivatives, even in the presence of reducible functional groups, such as nitriles, halogens, esters and amides. For a proof-of-concept, this nanostructured catalyst was implemented in the multistep synthesis of natural products and pharmaceutical lead compounds as well as modification of photoluminescent materials. As such this methodology constitutes the first heterogeneous iron-catalyzed hydrogenation of substituted (iso)quinolones with synthetic importance.
Introduction
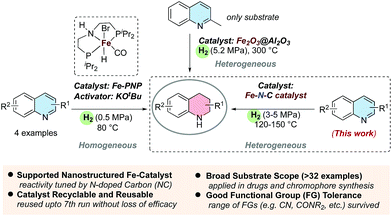
Fundamentally, the controlled reduction of heteroaromatic compounds to their partially or completely saturated congeners holds long persistent interest in streamlining organic synthesis and in producing fine and bulk chemicals.1 As such, molecular hydrogen represents an ideal and clean reducing agent.2 Compared to industrially relevant hydrogenations of benzene and related arenes, the reduction of heteroaromatic compounds, especially N-heteroarenes, continues to be a scientific and technical challenge.3–7 Achieving the desired selectivity in the hydrogenation of (iso)quinolones as well as the tolerance of co-existing reducible functionalities provides additional problems. Notably, the resulting partial hydrogenation product of quinolines (i.e. 1,2,3,4-tetrahydroquinolines) constitutes a ubiquitous motif in numerous bio-active compounds.8 Apart from an array of natural products such as (±)-galipinine,8d,e (±)-cuspareine,8d,eetc., it is found in agrochemicals and synthetic drugs or lead compounds with distinct pharmaceutical activity, e.g. flumequine,8f tubulin polymerization inhibitor,8g histamine-H3-receptor antagonist,8hetc. Furthermore, this scaffold is useful in materials science, e.g. photosensitive9 and even hydrogen storage4a,6c materials.Over the last decade, implementation of low cost, less toxic and earth-abundant metals represents an attractive alternative to the use of scarce and often toxic precious metals in catalytic hydrogenation technologies.10 Despite substantial progress in the hydrogenation of N-heteroarenes by noble metal catalysts (e.g. Pd, Ru, Rh, Ir, Au, etc.),4,5 known homogeneous catalysts sometimes require co-catalysts/additives for substrate or catalyst activation,4 while heterogeneous catalysts, sometimes, lack regioselectivity5h and functional group tolerance.5f Until recently, selective hydrogenations of N-heteroarenes by base metal catalysts (e.g. Co, Ni, etc.) have been explored to a limited extent.6,7 Intriguingly, to chase sustainability and confront environmental concerns, the prime choice of any transition metal in catalysis would be utilizing iron.10g,11a However, it lacks predictability and complete control over its reactivity due to competitive single electron transfer (SET), which requires sophisticated ligand systems in homogeneous catalysis.10,11
In fact, W. D. Jones and co-workers disclosed that a molecularly defined Fe-PNP pincer complex can promote the hydrogenation of N-heteroarenes in the presence of bases (Fig. 1).6c In contrast to these sensitive homogeneous systems, operationally simple and robust heterogeneous iron catalysts are not known, except for one example where Shaw et al. demonstrated the hydrogenation of quinaldine at 300 °C and 5.2 MPa H2 (Fig. 1).12
Recently, substantial efforts have been made to provide advanced materials with tunable structure and properties for catalysis in organic synthesis complementary to molecularly well-defined organometallic catalysts.7a–d,13–15 In this context, we have developed a spectrum of supported nanostructured metal/metal oxide particles modified by a N-doped carbon matrix and applied them in redox reactions, such as the reduction of nitroarenes, nitriles and ketones as well as the oxidation of alcohols.15 The synergistic combination of metal/metal oxide nanoparticles and N-doped carbon, originating from ligands upon pyrolysis, turned out to be crucial for the desired reactivity and excellent selectivity.13c,d,14,15 Since nitrogen doping into the carbon matrix has a substantial influence on the catalytic activity of metal/metal oxide nanoparticles,13d,15d,16 the selection of the N-donor ligand is critical to access high reactivity and it mostly remains limited to 1,10-phenanthroline. Intriguingly, finding alternative ligands to 1,10-phenanthroline would be a significant step forward in the context of the development of catalytically active materials.7b,14b–e,15a–d
Herein, we report a series of novel supported Fe-based materials modified by a N-doped carbon matrix, generated from bidentate N-aryliminopyridine ligands upon pyrolysis. Utilization of the optimal material allowed for the first heterogeneous iron-catalyzed hydrogenation of substituted (iso)quinolones with synthetic value.
Results and discussion
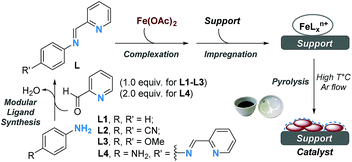
We set out our study with the preparation of a range of Fe-based materials in a sequential process: (a) modular synthesis of N-donor aryliminopyridine ligands via Schiff base condensation; (b) complexation with Fe(OAc)2 (OAc = acetate);17 (c) deposition by wet impregnation of complexes onto suitable supports; and (d) drying and pyrolysis at variable temperatures (500–900 °C) based on thermogravimetric (TG) analysis (Scheme 1 and Fig. S1† respectively).18Having a library of novel Fe-based materials, we began exploring their catalytic activity in the hydrogenation of quinolines. In an initial set of experiments with quinoline (1′) in iPrOH in the presence of H2 (5 MPa) at 140 °C for 28 h, the materials which were prepared from four different ligands (L1–L4) on the carbon support exhibited desired reactivity and the catalyst with L4 remained relatively more efficient (Table 1, entries 1–4). However, altering the support with L4 did not improve the conversion (Table S4,† entries 5–7).18 Additionally, the screening of materials prepared over a wide range of pyrolysis temperatures (500–900 °C) revealed the best efficiency of the catalyst which was prepared at 800 °C (Table S1,† entries 4 and 8–11).18 Testing polar and non-polar solvents alone did not increase product formation (Table S1,† entries 12–14),18 whereas a mixture of iPrOH/H2O (3/1) enhanced the conversion slightly (Table 1, entries 5 and 6). Next, inspired by the metal-to-ligand ratio effect on the catalytic activity in the dehydrogenation of formic acid,19 we synthesized an array of catalysts with various Fe(OAc)2/L4 ratios. To our surprise, the set of four catalysts with different Fe/L4 ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 to 1
1.5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0) exhibited a dramatic enhancement of reaction efficiency (Table 1, entries 6–9). The progress of tetrahydroquinoline (1) formation with time resulted in the following reactivity pattern: Fe(1)/L4(4.5)@C-800 > Fe(1)/L4(6.0)@C-800 > Fe(1)/L4(3.0)@C-800 > Fe(1)/L4(1.5)@C-800 (Fig. 2). Full conversion was achieved at 150 °C and 5 MPa hydrogen pressure after 30 h (Table 1, entries 10). Nevertheless, hydrogenation to 1 (87% GC yield) is also possible at lower temperature and pressure for a prolonged reaction time (Fe(1)/L4(4.5)@C-800 (12 mol% Fe) in iPrOH/H2O (3/1), H2 (4 MPa), 130 °C, 56 h) (Table 1, entry 13). For the success of this hydrogenation reaction, the presence of the ligand and metal as well as the pyrolysis process remains crucial. Consequently, no reactivity was observed in the presence of Fe@C-800, L4@C-800 and unpyrolyzed Fe(1)/L4(4.5)/C composites (Table 1, entries 14–16). No conversion is observed carrying out the reaction in the presence of nitrogen (4 MPa) used instead of hydrogen, which excludes transfer hydrogenation with isopropanol (Table 1, entry 17).
6.0) exhibited a dramatic enhancement of reaction efficiency (Table 1, entries 6–9). The progress of tetrahydroquinoline (1) formation with time resulted in the following reactivity pattern: Fe(1)/L4(4.5)@C-800 > Fe(1)/L4(6.0)@C-800 > Fe(1)/L4(3.0)@C-800 > Fe(1)/L4(1.5)@C-800 (Fig. 2). Full conversion was achieved at 150 °C and 5 MPa hydrogen pressure after 30 h (Table 1, entries 10). Nevertheless, hydrogenation to 1 (87% GC yield) is also possible at lower temperature and pressure for a prolonged reaction time (Fe(1)/L4(4.5)@C-800 (12 mol% Fe) in iPrOH/H2O (3/1), H2 (4 MPa), 130 °C, 56 h) (Table 1, entry 13). For the success of this hydrogenation reaction, the presence of the ligand and metal as well as the pyrolysis process remains crucial. Consequently, no reactivity was observed in the presence of Fe@C-800, L4@C-800 and unpyrolyzed Fe(1)/L4(4.5)/C composites (Table 1, entries 14–16). No conversion is observed carrying out the reaction in the presence of nitrogen (4 MPa) used instead of hydrogen, which excludes transfer hydrogenation with isopropanol (Table 1, entry 17).
| Entry | Catalyst (mol%) | Solvent | H2 (MPa) | T (°C) | t (h) | Conv.(yield)b,c (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol) and Fe-catalyst (12 mol% Fe) in solvent (1 mL) heated in the presence of H2. b Determined by GC analysis using hexadecane as the internal standard. c GC yield reported in parentheses. d Reaction was performed in the presence of nitrogen (4 MPa). | ||||||
| 1 | Fe(1)/L1(3.0)@C-800 (12) | iPrOH | 5 | 140 | 28 | 16(16) |
| 2 | Fe(1)/L2(3.0)@C-800 (12) | iPrOH | 5 | 140 | 28 | 12(12) |
| 3 | Fe(1)/L3(3.0)@C-800 (12) | iPrOH | 5 | 140 | 28 | 15(15) |
| 4 | Fe(1)/L4(1.5)@C-800 (12) | iPrOH | 5 | 140 | 28 | 55(50) |
| 5 | Fe(1)/L4(1.5)@C-800 (12) | iPrOH | 5 | 140 | 24 | 45(45) |
| 6 | Fe(1)/L4(1.5)@C-800 (12) | iPrOH/H2O(3/1) | 5 | 140 | 24 | 52(52) |
| 7 | Fe(1)/L4(3.0)@C-800 (12) | iPrOH/H2O(3/1) | 5 | 140 | 24 | 77(70) |
| 8 | Fe(1)/L4(4.5)@C-800 (12) | iPrOH/H2O(3/1) | 5 | 140 | 24 | 96(84) |
| 9 | Fe(1)/L4(6.0)@C-800 (12) | iPrOH/H2O(3/1) | 5 | 140 | 24 | 91(82) |
| 10 | Fe(1)/L4(4.5)@C-800 (12) | iPrOH/H2O(3/1) | 5 | 140 | 30 | >99(85) |
| 11 | Fe(1)/L4(4.5)@C-800 (12) | iPrOH/H2O(3/1) | 5 | 130 | 30 | 80(73) |
| 12 | Fe(1)/L4(4.5)@C-800 (12) | iPrOH/H2O(3/1) | 5 | 120 | 30 | 47(45) |
| 13 | Fe(1)/ L4 (4.5)@C-800 (12) | i PrOH/H 2 O(3/1) | 4 | 130 | 56 | >99(87) |
| 14 | Fe(1)/L4/C (∼12) | iPrOH/H2O(3/1) | 4 | 130 | 56 | — |
| 15 | Fe@C-800 (12) | iPrOH/H2O(3/1) | 4 | 130 | 56 | — |
| 16 | L4@C-800 | iPrOH/H2O(3/1) | 4 | 130 | 56 | — |
| 17d | Fe(1)/L4(4.5)@C-800 (12) | iPrOH/H2O(3/1) | — | 130 | 56 | — |
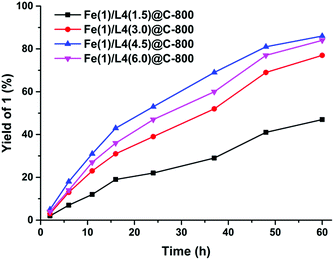 | ||
| Fig. 2 Product yield/time profile for the comparison between Fe-catalysts with different metal-to-ligand ratios under standard conditions. | ||
The composition of the catalysts was determined by elemental analysis. Interestingly, an ascending order of nitrogen amount and a descending order of iron content were noticed when the metal-to-ligand ratio was changed in the preparation process from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 to 1
1.5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
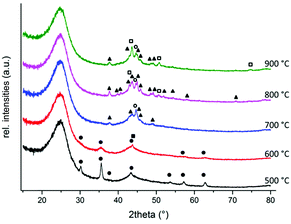
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.0 (Table S2†).18 The structural characteristics of selected materials were investigated by means of various techniques, such as powder XRD, STEM, XPS and Raman spectroscopy. Investigating the bulk phase of the materials, the powder X-ray diffraction measurements demonstrated the presence of iron carbide (Fe3C) as a major iron constituent along with additional metallic Fe(0) and FeNx in both the catalysts: Fe(1)/L4(4.5)@C-800 and Fe(1)/L4(1.5)@C-800, while the main components in the almost inactive Fe(1)/L4(1.5)@C-500 are iron oxides (e.g. Fe3O4) along with the obvious reflections for carbon in all these materials (Fig. 3 and S2†).18 Interestingly, the iron oxide (Fe3O4) phase was formed at lower pyrolysis temperatures (500–600 °C), while the formation of metallic Fe(0) and iron carbide (Fe3C) was observed at higher pyrolysis temperatures (700–900 °C), likely stemming from the iron oxides (Fig. 3).20
6.0 (Table S2†).18 The structural characteristics of selected materials were investigated by means of various techniques, such as powder XRD, STEM, XPS and Raman spectroscopy. Investigating the bulk phase of the materials, the powder X-ray diffraction measurements demonstrated the presence of iron carbide (Fe3C) as a major iron constituent along with additional metallic Fe(0) and FeNx in both the catalysts: Fe(1)/L4(4.5)@C-800 and Fe(1)/L4(1.5)@C-800, while the main components in the almost inactive Fe(1)/L4(1.5)@C-500 are iron oxides (e.g. Fe3O4) along with the obvious reflections for carbon in all these materials (Fig. 3 and S2†).18 Interestingly, the iron oxide (Fe3O4) phase was formed at lower pyrolysis temperatures (500–600 °C), while the formation of metallic Fe(0) and iron carbide (Fe3C) was observed at higher pyrolysis temperatures (700–900 °C), likely stemming from the iron oxides (Fig. 3).20
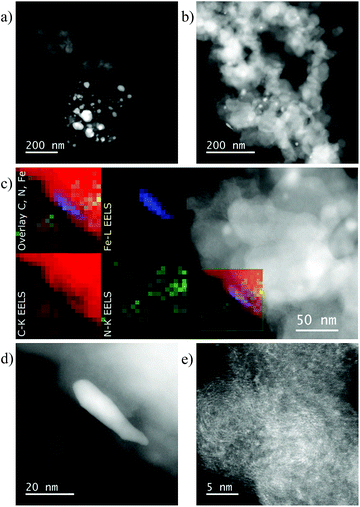
The electronic structure and surface elemental composition were determined by X-ray photoelectron spectroscopy (XPS) (Table S3†).18 The N1s spectra for the above-mentioned materials displayed two different peaks (Fig. S7a†). Due to similar electron binding energy (B.E.) a univocal assignment is not possible. The lower peak can be ascribed to the pyridinic nitrogen and/or nitrogen bound to the metal ion (Fe–Nx), while the higher one resembles graphitic and/or pyrrolic nitrogen.21,22a Additionally, Fe2p spectra of all samples exhibited two wide peaks centered around 711.5 and 725 eV due to 2p3/2 and 2p1/2 contributions, respectively (Fig. 4). Broadening of the peak is a hint of heterogeneity of the Fe phase present on the surface of the sample. According to TEM and literature data, this contribution is ascribed to the Fe–N bond but other Fe species in high oxidation states cannot be excluded.20,22 In addition, Fe(1)/L4(4.5)@C-800 and Fe(1)/L4(1.5)@C-800 samples clearly exhibit a contribution at a lower binding energy around 709 eV attributed to iron carbide (Fe3C).20 The aberration corrected scanning transmission electron microscopy (STEM) high angle annular dark field (HAADF) overview images of less active Fe(1)/L4(1.5)@C-800 (Fig. 5a) and most active Fe(1)/L4(4.5)@C-800 (Fig. 5b) catalysts were acquired together with electron energy loss spectroscopy (EELS) and energy dispersive X-ray spectroscopy (EDXS). In both catalysts, the iron containing particles, probably iron carbide according to XRD, are generally surrounded by a few to many graphene layers forming a seemingly tight enclosure. Additionally, there is a carbon phase probably stemming from the ligand, as it also contains small amounts of nitrogen (Fig. 5c and d, and S10–12†).18 In the Fe(1)/L4(1.5)@C-800 catalyst, the additional nitrogen containing carbon structure seems to contain larger clusters of iron atoms (Fig. S12†) than in the Fe(1)/L4(4.5)@C-800 catalyst, where aberration corrected high resolution STEM-HAADF images suggest the presence of finely distributed atoms and very small clusters of a heavier kind than carbon (Fig. 5e, S10 and S11†),18 attributed to Fe. Moreover, carbon nanotubes could be observed in the Fe(1)/L4(4.5)@C-800 catalyst, while they were rarely noticed in the Fe(1)/L4(1.5)@C-800 catalyst (Fig. S11 and S12†).18 The STEM data of almost inactive Fe(1)/L4(1.5)@C-500, prepared by pyrolysis at 500 °C, showed the parallel occurrence of nitrogen and iron in fine distribution in some regions. Also, iron oxide particles and a few iron/iron oxide core shell type particles were present (Fig. S13†).18 In further analysis by Raman spectroscopy, two peaks were observed at ∼1590 cm−1 (G band) and ∼1343 cm−1 (D band) in each material (Fig. S15 and Table S4†).18,23 Notably, the occurrence of the D band signified the presence of defects in the graphitic carbon matrix.
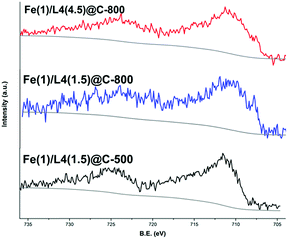 | ||
| Fig. 4 Fe2p XPS spectra of Fe-based materials: Fe(1)/L4(4.5)@C-800 (red), Fe(1)/L4(1.5)@C-800 (blue) and Fe(1)/L4(1.5)@C-500 (black). | ||
With the optimal reaction conditions in hand, we sought to explore the synthetic scope and limitations of this transformation, which are summarized in Scheme 2. A variety of quinolines with diverse substitution patterns and functionalities were employed under the developed protocol for the selective hydrogenation. At first, 2-substituted quinolines, for example, quinaldine and 2-phenylquinoline were selectively hydrogenated to corresponding 1,2,3,4-tetrahydroquinaldine (2), an intermediate for the synthesis of antitrypanosomal lead compounds,24 and 2-phenyl-1,2,3,4-tetrahydroquinoline (3), respectively, in excellent yields (both 98%). Furthermore, 3-methyl-1,2,3,4-tetrahydroquinoline (4, 90%) and 3-(4-methoxyphenyl)-1,2,3,4-tetrahydroquinoline (5, 91%) were successfully prepared from the corresponding quinoline substrates. More interestingly, a 2,3-disubstituted quinoline was hydrogenated to afford 1,2,3,4-tetrahydroquinoline derivative 6 in good yield (77%; d.r. = 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), whereas 2,4-dimethylquinoline was converted to 2,6-dimethyl-1,2,3,4-tetrahydroquinoline (7) in good yield (77%) and moderate diastereoselectivity (d.r. = 4.5
1), whereas 2,4-dimethylquinoline was converted to 2,6-dimethyl-1,2,3,4-tetrahydroquinoline (7) in good yield (77%) and moderate diastereoselectivity (d.r. = 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
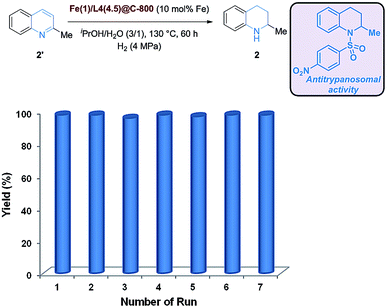
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) without arene ring hydrogenation. Aside from the pyridine ring, different substituents at the 6-position of quinoline survived under the employed hydrogenation conditions. Here, 6-isopropylquinoline was converted to 8 in moderate yield (67%). Furthermore, quinoline derivatives featuring 4-fluorophenyl and 4-trifluoromethoxyphenyl and a pyrazole moiety were successfully hydrogenated to the corresponding 1,2,3,4-tetrahydroquinoline congeners 9–11 in good to excellent yields. Gratifyingly, substituted quinaldine substrates have been proven to be suitable candidates for this selective hydrogenation reaction, too. Electron-rich 6-methoxyquinaldine and electron-deficient 6- and 7-fluoroquinaldine were selectively converted to pyridine core hydrogenation products 12 (95%), 13 (94%) and 14 (88%), respectively, with almost equal efficiency. Furthermore, sterically hindered 8-methyl-1,2,3,4-tetrahydroquinaldine (15) was also furnished in very good yield (88%). In addition to the ethyl protected 8-quinolinol, which was easily converted to the reduced product 16 (92%) in excellent yield, a more challenging substrate 8-quinolinol with a free hydroxyl group was also transformed into the corresponding 1,2,3,4-quinolin-8-ol (17, 64%), which constitutes a substructure of nicainoprol.25 Notably, the more challenging lepidine substrate was also hydrogenated to the corresponding 1,2,3,4-tetrahydrolepidine (18, 65%), which serves as a precursor for the synthesis of a central nervous system (CNS) depressant agent.26 Furthermore, 2-(4-(tert-butyl)phenyl)-6-isopropyl-1,2,3,4-tetrahydroquinoline (19), a key intermediate for the synthesis of a neuronal Na+-channel antagonist,27 was also afforded in high yield (93%). Interestingly, a quinoline substrate bioconjugated with (+)-α-tocopherol (vitamin E) was also successfully employed to furnish the selective hydrogenation product 20 in good yield (76%). Besides quinolines, this novel hydrogenation method could also be extended to benzannulated quinolines, such as 3-methylbenzo[f]quinoline and acridine, and even (iso)quinolone to deliver the corresponding hydrogenated products 1, 21–23 in moderate to excellent yields.
1) without arene ring hydrogenation. Aside from the pyridine ring, different substituents at the 6-position of quinoline survived under the employed hydrogenation conditions. Here, 6-isopropylquinoline was converted to 8 in moderate yield (67%). Furthermore, quinoline derivatives featuring 4-fluorophenyl and 4-trifluoromethoxyphenyl and a pyrazole moiety were successfully hydrogenated to the corresponding 1,2,3,4-tetrahydroquinoline congeners 9–11 in good to excellent yields. Gratifyingly, substituted quinaldine substrates have been proven to be suitable candidates for this selective hydrogenation reaction, too. Electron-rich 6-methoxyquinaldine and electron-deficient 6- and 7-fluoroquinaldine were selectively converted to pyridine core hydrogenation products 12 (95%), 13 (94%) and 14 (88%), respectively, with almost equal efficiency. Furthermore, sterically hindered 8-methyl-1,2,3,4-tetrahydroquinaldine (15) was also furnished in very good yield (88%). In addition to the ethyl protected 8-quinolinol, which was easily converted to the reduced product 16 (92%) in excellent yield, a more challenging substrate 8-quinolinol with a free hydroxyl group was also transformed into the corresponding 1,2,3,4-quinolin-8-ol (17, 64%), which constitutes a substructure of nicainoprol.25 Notably, the more challenging lepidine substrate was also hydrogenated to the corresponding 1,2,3,4-tetrahydrolepidine (18, 65%), which serves as a precursor for the synthesis of a central nervous system (CNS) depressant agent.26 Furthermore, 2-(4-(tert-butyl)phenyl)-6-isopropyl-1,2,3,4-tetrahydroquinoline (19), a key intermediate for the synthesis of a neuronal Na+-channel antagonist,27 was also afforded in high yield (93%). Interestingly, a quinoline substrate bioconjugated with (+)-α-tocopherol (vitamin E) was also successfully employed to furnish the selective hydrogenation product 20 in good yield (76%). Besides quinolines, this novel hydrogenation method could also be extended to benzannulated quinolines, such as 3-methylbenzo[f]quinoline and acridine, and even (iso)quinolone to deliver the corresponding hydrogenated products 1, 21–23 in moderate to excellent yields.
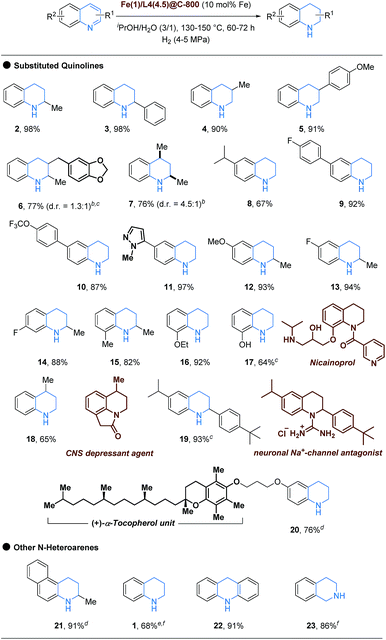 | ||
| Scheme 2 Selective hydrogenation of N-heteroarenesa. aReaction conditions: substrate (0.32–0.50 mmol) and Fe(1)/L4(4.5)@C-800 (10 mol% Fe) in iPrOH/H2O (3/1) (2–2.5 mL), heated at 130–150 °C in the presence of H2 (4–5 MPa), all yields are isolated unless otherwise mentioned (see ESI† for details); bd.r. was determined by GC analysis; cFe(1)/L4(4.5)@C-800 (12 mol% Fe) was used; dFe(1)/L4(4.5)@C-800 (15 mol% Fe) was used; equinoline-N-oxide was used as the substrate; fGC yield was determined by GC analysis using hexadecane as the internal standard. | ||
To address the often asked question of scalability, a gram scale synthesis of 6-fluoro-1,2,3,4-tetrahydroquinaldine (13), which is a key intermediate for the synthesis of flumequine (antibiotic),8f starting from 6-fluoroquinaldine (13′) was executed without hampering efficiency under standard conditions with an even reduced catalyst amount (8 mol% Fe loading) (Scheme 3).
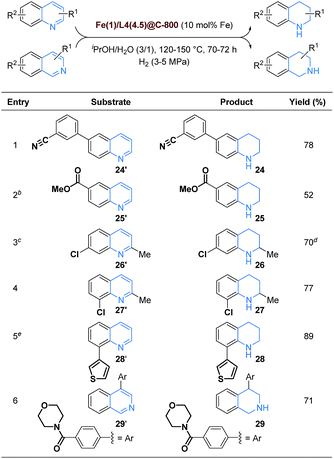
Obviously, chemoselective reduction of (iso)quinolones in the presence of other reducible functional groups constitutes a major challenge in catalytic hydrogenations, especially for life science molecules.5 Intrigued by this task, we tested several substrates bearing redox-sensitive nitriles, esters, amides, halogens, and heteroarenes (Table 2).
| a Reaction conditions: substrate (0.25–0.40 mmol) and Fe(1)/L4(4.5)@C-800 (10 mol% Fe) in iPrOH/H2O (3/1) (1.2–2.0 mL), heated at 120–150 °C for 70–72 h in the presence of H2 (3–5 MPa), all yields are isolated unless otherwise mentioned (see ESI for details). b Reaction was performed in MeOH/H2O (3/1). c Reaction was carried out at 120 °C and H2 (3 MPa). d Hydrodechlorination product 2 could be detected in 7% yield. e Fe(1)/L4(4.5)@C-800 (15 mol% Fe) was used. |
|---|
|
Delightfully, a 6-arylquinoline substrate featuring a nitrile group was successfully converted to the corresponding 1,2,3,4-tetrahydroquinoline congener 24 in good yield (78%) keeping nitrile intact. Methyl 1,2,3,4-tetrahydroquinoline-6-carboxylate (25) could also be obtained without reduction of the ester group, albeit in moderate yield, while an (iso)quinolone derivative featuring a tertiary amide moiety was successfully hydrogenated to 1,2,3,4-tetrahydroisoquinoline 29 in 71% yield without amide hydrogenation. Interestingly, quinaldines bearing chlorine substituents at 6- and 7-positions furnished 1,2,3,4-tetrahydroquinaldines 26 and 27 in 70 and 77% yield, respectively. However, small amounts of the hydrodechlorination product 2 (7%) were detected in the case of 26, too. Notably, a quinoline substrate featuring a thiophene unit at the 8-position was also hydrogenated to 8-(thiophen-3-yl)-1,2,3,4-tetrahydroquinoline (28, 89%) in very good yield.
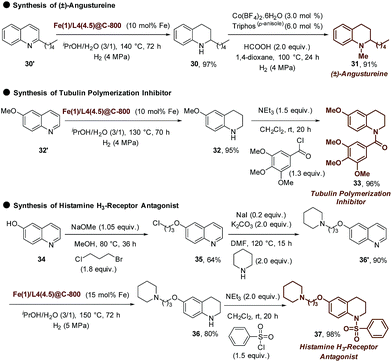
Aside from exploring the general scope, the multistep synthesis of natural products as well as medicinally important lead compounds was performed as a proof-of-applicability. Hence, the Fe-catalyzed hydrogenation was utilized as a key step in converting 2-n-pentylquinoline (30′) to (±)-angustureine (31)8e in two steps (Scheme 4). Furthermore, commercially available 6-methoxyquinoline (32′) was transformed to 6-methoxy-1,2,3,4-tetrahydroquinoline (32, 95%), which was further converted to a tubulin polymerization inhibitor (33)8g (Scheme 4). Finally, the pharmaceutically important lead compound 37 of histamine H3-receptor antagonist was synthesized in four steps starting from readily available 6-quinolinol, which represents an attractive alternative to the noble metal catalyzed process (Scheme 4).8h
Additionally, the developed technology proved to be a useful tool to convert 6-pyrenequinoline-based photoluminescent materials (38′ and 39′)28 to analogous congeners (38 and 39, respectively) (Fig. 6). As noticed in the UV-Vis absorption as well as emission spectra, a clear bathochromic-shift of absorption and emission wavelengths altering photophysical properties was observed due to selective hydrogenation (Fig. 6).
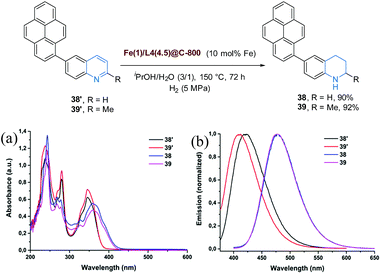 | ||
| Fig. 6 Applications in the conversion of photoactive materials (a) UV-Vis spectra and (b) Emission spectra of compounds 38′ (20 μM), 39′ (20 μM), 38 (20 μM) and 39 (20 μM) in dichloromethane. | ||
Since the recycling and reusability of the catalyst constitute a crucial advantage in heterogeneous catalysis, the optimal Fe-catalyst, Fe(1)/L4(4.5)@C-800, was employed seven times for the hydrogenation of quinaldine (4.0 mmol scale). To our delight, the catalyst could be recycled and reused without a significant drop of reactivity to obtain the desired 1,2,3,4-tetrahydroquinaldine (2) in almost quantitative yield (Fig. 7 and Table S5†). Notably, some Fe3O4 particles appeared in the reused catalyst compared to the fresh catalyst (Fig. S3†).18
Conclusions
We have developed the first heterogeneous Fe-catalyst for the selective hydrogenation of quinolines and (iso)quinolones using molecular hydrogen as a clean reducing agent. The optimal catalyst is easily prepared from commercially available iron acetate and N-aryliminopyridines. This robust material can be conveniently handled and is stable towards air and moisture, which provides the basis for simple catalyst recycling. A variety of quinolines with diverse substitution patterns and functionalities were successfully converted to 1,2,3,4-tetrahydroquinolines in moderate to excellent yields with high selectivity, even in the presence of other reducible functional groups, such as nitriles, halogens, esters, amides and heteroarenes. The value of this methodology is showcased by the synthesis of bioactive molecules as well as the modification of photoactive materials. It is worth mentioning that the presented Schiff base condensation of 2-pyridinecarboxaldehyde with commercially available anilines allows for the design of an infinite number of new potential catalysts for various other applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the state of Mecklenburg Vorpommern and F. Hoffmann-La Roche AG. We thank Dr Wolfgang Baumann, Dr Dario Formenti, Dr Wu Li, Dr Annette-Enrica Surkus, Kathiravan Murugesan and Thirusangumurugan Senthamarai (all LIKAT Rostock) for experimental assistance and helpful discussions. We also thank Dr Nils Rockstroh, Dr C. Fischer, S. Buchholz, S. Schareina, Mrs Anja Simmula, Mrs Astrid Lehmann and Mr Alexander Wolzka (all LIKAT Rostock) for experimental and analytical assistance.References
- Selected reviews and books: (a) A. Gualandi and D. Savoia, RSC Adv., 2016, 6, 18419 RSC; (b) T. J. Donohoe, R. Garg and C. A. Stevenson, Tetrahedron: Asymmetry, 1996, 7, 317 CrossRef; (c) T. J. Donohoe, C. R. Jones, and C. Winter, in Comprehensive Organic Synthesis II, Elsevier, Amsterdam, 2nd edn, 2014 Search PubMed.
- (a) P. G. Andersson, and I. J. Munslo, Modern Reduction Methods, Wiley, New York, 2008 CrossRef; (b) J. G. De Vries and C. J. Elsevier, Handbook of Homogeneous Hydrogenation, Wiley-VCH, Weinheim, Germany, 2007 Search PubMed; (c) P. N. Rylander, Catalytic Hydrogenation in Organic Synthesis, Acadamic Press, New York, 1979 Search PubMed.
- Selected reviews: (a) Z.-P. Chen and Y.-G. Zhou, Synthesis, 2016, 48, 1769 CrossRef; (b) B. Balakrishna, J. L. Nùñez-Rico and A. Vidal-Ferran, Eur. J. Org. Chem., 2015, 5293 CrossRef; (c) D.-S. Wang, Q.-A. Chen, S.-M. Lu and Y.-G. Zhou, Chem. Rev., 2012, 112, 2557 CrossRef PubMed; (d) Z. Yu, W. Jin and Q. Jiang, Angew. Chem., Int. Ed., 2012, 51, 6060 CrossRef PubMed; (e) F. Glorius, Org. Biomol. Chem., 2005, 3, 4171 RSCAccount: (f) Y.-E. Luo, Y.-M. He and Q.-H. Fan, Chem. Rec., 2016, 16, 2697 CrossRef PubMed; (g) Y.-G. Zhou, Acc. Chem. Res., 2007, 40, 1357 CrossRef PubMed.
- Selected reports on noble metal homogeneous catalysts for N-heteroarene hydrogenation: (a) Á. Vivancos, M. Beller and M. Albrecht, ACS Catal., 2018, 8, 17 CrossRef; (b) C. Schlepphorst, M. Wiesenfeldt and F. Glorius, Chem.–Eur. J., 2018, 24, 354 CrossRef PubMed; (c) Z. Yang, F. Chen, S. Zhang, Y. He, N. Yang and Q.-H. Fan, Org. Lett., 2017, 19, 1458 CrossRef PubMed; (d) R. Kuwano, Y. Hashiguchi, R. Ikeda and K. Ishizuka, Angew. Chem., Int. Ed., 2015, 54, 2393 CrossRef PubMed; (e) T. Wang, L.-G. Zhuo, Z. Li, F. Chen, Z. Ding, Y. He, Q.-H. Fan, J. Xiang, Z.-X. Yu and A. S. C. Chan, J. Am. Chem. Soc., 2011, 133, 9878 CrossRef PubMed; (f) G. E. Dobereiner, A. Nova, N. D. Schley, N. Hazari, S. J. Miller, O. Eisenstein and R. H. Crabtree, J. Am. Chem. Soc., 2011, 133, 7547 CrossRef PubMed; (g) S. Urban, N. Ortega and F. Glorius, Angew. Chem., Int. Ed., 2011, 50, 3803 CrossRef PubMed.
- Selected reports on noble metal heterogeneous catalysts for N-heteroarene hydrogenation: (a) S. Zhang, Z. Xia, T. Ni, Z. Zhang, Y. Ma and Y. Qu, J. Catal., 2018, 359, 101 CrossRef; (b) X. Wang, W. Chen, L. Zhang, T. Yao, W. Liu, Y. Lin, H. Ju, J. Dong, L. Zheng, W. Yan, X. Zheng, Z. Li, X. Wang, J. Yang, D. He, Y. Wang, Z. Deng, Y. Wu and Y. Li, J. Am. Chem. Soc., 2017, 139, 9419 CrossRef PubMed; (c) T.-N. Ye, J. Li, M. Kitano and H. Hosono, Green Chem., 2017, 19, 749 RSC; (d) Y.-G. Ji, K. Wei, T. Liu, L. Wu and W.-H. Zhang, Adv. Synth. Catal., 2017, 359, 933 CrossRef; (e) A. Karakulina, A. Gopakumar, İ. Akçok, B. L. Roulier, T. LaGrange, S. A. Katsyuba, S. Das and P. J. Dyson, Angew. Chem., Int. Ed., 2016, 55, 292 CrossRef PubMed; (f) Y. Zhang, J. Zhu, Y.-T. Xia, X.-T. Sun and L. Wu, Adv. Synth. Catal., 2016, 358, 3039 CrossRef; (g) M. Niu, Y. Wang, P. Chen, D. Du, J. Jiang and Z. Jin, Catal. Sci. Technol., 2015, 5, 4746 RSC; (h) M. Fang and R. A. Sánchez-Delgado, J. Catal., 2014, 311, 357 CrossRef; (i) D. Ren, L. He, L. Yu, R.-S. Ding, Y.-M. Liu, Y. Cao, H.-Y. He and K.-N. Fan, J. Am. Chem. Soc., 2012, 134, 17592 CrossRef PubMed; (j) N. A. Beckers, S. Huynh, X. Zhang, E. J. Luber and J. M. Buriak, ACS Catal., 2012, 2, 1524 CrossRef.
- Reports on non-noble metal homogeneous catalysts for N-heteroarene hydrogenation: (a) R. Adam, J. R. Cabrero-Antonino, A. Spannenberg, K. Junge, R. Jackstell and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 3216 CrossRef PubMed; (b) R. Xu, S. Chakraborty, H. Yuan and W. D. Jones, ACS Catal., 2015, 5, 6350 CrossRef; (c) S. Chakraborty, W. W. Brennessel and W. D. Jones, J. Am. Chem. Soc., 2014, 136, 8564 CrossRef PubMed.
- Reports on non-noble metal heterogeneous catalysts for N-heteroarene hydrogenation: (a) I. Sorribes, L. Liu, A. Doménech-Carbó and A. Corma, ACS Catal., 2018, 8, 4545 CrossRef; (b) J. Li, G. Liu, X. Long, G. Gao, J. Wu and F. Li, J. Catal., 2017, 355, 53 CrossRef; (c) Z. Wei, Y. Chen, J. Wang, D. Su, M. Tang, S. Mao and Y. Wang, ACS Catal., 2016, 6, 5816 CrossRef; (d) F. Chen, A.-E. Surkus, L. He, M.-M. Pohl, J. Radnik, C. Topf, K. Junge and M. Beller, J. Am. Chem. Soc., 2015, 137, 11718 CrossRef PubMed; (e) C. Liu, Z. Rong, Z. Sun, Y. Wang, W. Du, Y. Wang and L. Lu, RSC Adv., 2013, 3, 23984 RSC.
- (a) V. Sridharan, P. A. Suryavanshi and J. C. Menéndez, Chem. Rev., 2011, 111, 7157 CrossRef PubMed; (b) K. W. Bentley, Nat. Prod. Rep., 2006, 23, 444 RSC; (c) A. R. Katritzky, S. Rachwal and B. Rachwal, Tetrahedron, 1996, 52, 15031 CrossRefselected examples: (d) P. J. Houghton, T. Z. Woldemariam, Y. Watanabe and M. Yates, Planta Med., 1999, 65, 250 CrossRef PubMed; (e) A. O'Byrne and P. Evans, Tetrahedron, 2008, 64, 8067 CrossRef; (f) J. Bálint, G. Egri, E. Fogassy, Z. Böcskei, K. Simon, A. Gajáry and A. Friesz, Tetrahedron: Asymmetry, 1999, 10, 1079 CrossRef; (g) J.-P. Liou, Z.-Y. Wu, C.-C. Kuo, C.-Y. Chang, P.-Y. Lu, C.-M. Chen, H.-P. Hsieh and J.-Y. Chang, J. Med. Chem., 2008, 51, 4351 CrossRef PubMed; (h) C. D. Jesudason, L. S. Beavers, J. Cramer, J. Dill, D. R. Finley, C. W. Lindsley, F. C. Stevens, R. A. Gadski, S. W. Oldham, R. T. Pickard, C. S. Siedem, D. K. Sindelar, A. Singh, B. M. Watson and P. A. Hipskind, Bioorg. Med. Chem. Lett., 2006, 16, 3415 CrossRef PubMed.
- R. Chen, X. Yang, H. Tian, X. Wang, A. Hagfeldt and L. Sun, Chem. Mater., 2007, 19, 4007 CrossRef.
- Selected reviews: (a) G. A. Filonenko, R. van Putten, E. J. M. Hensen and E. A. Pidko, Chem. Soc. Rev., 2018, 47, 1459 RSC; (b) F. Kallmeier and R. Kempe, Angew. Chem., Int. Ed., 2018, 57, 46 CrossRef PubMedselected accounts: (c) P. J. Chirik, Acc. Chem. Res., 2015, 48, 1687 CrossRef PubMed; (d) R. H. Morris, Acc. Chem. Res., 2015, 48, 1494 CrossRef PubMed; (e) T. Zell and D. Milstein, Acc. Chem. Res., 2015, 48, 1979 CrossRef PubMed; (f) S. Chakraborty, P. Bhattacharya, H. Dai and H. Guan, Acc. Chem. Res., 2015, 48, 1995 CrossRef PubMedperspective: (g) R. M. Bullock, Science, 2013, 342, 1054 CrossRef PubMed.
- (a) A. Fürstner, ACS Cent. Sci., 2016, 2, 778 CrossRef PubMed; (b) O. R. Luca and R. H. Crabtree, Chem. Soc. Rev., 2013, 42, 1440 RSC; (c) P. J. Chirik and K. Wieghardt, Science, 2010, 327, 794 CrossRef PubMed.
- J. E. Shaw and P. R. Stapp, J. Heterocycl. Chem., 1987, 24, 1477 CrossRef.
- Selected reviews: (a) L. Liu and A. Corma, Chem. Rev., 2018, 118, 4981 CrossRef PubMed; (b) Z. Ye, P. Zhang, X. Lei, X. Wang, N. Zhao and H. Yang, Chem.–Eur. J., 2018, 24, 8922 CrossRef PubMed; (c) D. Wang and D. Astruc, Chem. Soc. Rev., 2017, 46, 816 RSC; (d) Y. Cao, S. Mao, M. Li, Y. Chen and Y. Wang, ACS Catal., 2017, 7, 8090 CrossRef; (e) L. He, F. Weniger, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2016, 55, 12582 CrossRef PubMed; (f) M. Li, F. Xu, H. Li and Y. Wang, Catal. Sci. Technol., 2016, 6, 3670 RSC; (g) E. Pérez-Mayoral, V. Calvino-Casilda and E. Soriano, Catal. Sci. Technol., 2016, 6, 1265 RSC.
- Selected reports: (a) W. Liu, L. Zhang, X. Liu, X. Liu, X. Yang, S. Miao, W. Wang, A. Wang and T. Zhang, J. Am. Chem. Soc., 2017, 139, 10790 CrossRef PubMed; (b) P. Zhou, L. Jiang, F. Wang, K. Deng, K. Lv and Z. Zhang, Sci. Adv., 2017, 3, e1601945 CrossRef PubMed; (c) J. Xie, K. Yin, A. Serov, K. Artyushkova, H. N. Pham, X. Sang, R. R. Unocic, P. Atanassov, A. K. Datye and R. J. Davis, ChemSusChem, 2017, 10, 359 CrossRef PubMed; (d) F. Chen, B. Sahoo, C. Kreyenschulte, H. Lund, M. Zeng, L. He, K. Junge and M. Beller, Chem. Sci., 2017, 8, 6239 RSC; (e) J. Shi, Y. Wang, W. Du and Z. Hou, Carbon, 2016, 99, 330 CrossRef; (f) W. Zhong, H. Liu, C. Bai, S. Liao and Y. Li, ACS Catal., 2015, 5, 1850 CrossRef; (g) L. Zhang, A. Wang, W. Wang, Y. Huang, X. Liu, S. Miao, J. Liu and T. Zhang, ACS Catal., 2015, 5, 6563 CrossRef.
- (a) R. Ferraccioli, D. Borovika, A.-E. Surkus, C. Kreyenschulte, C. Topf and M. Beller, Catal. Sci. Technol., 2018, 8, 499 RSC; (b) R. V. Jagadeesh, K. Murugesan, A. S. Alshammari, H. Neumann, M.-M. Pohl, J. Radnik and M. Beller, Science, 2017, 358, 326 CrossRef PubMed; (c) B. Sahoo, A.-E. Surkus, M.-M. Pohl, J. Radnik, M. Schneider, S. Bachmann, M. Scalone, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 11242 CrossRef PubMed; (d) D. Formenti, F. Ferretti, C. Topf, A.-E. Surkus, M.-M. Pohl, J. Radnik, M. Schneider, K. Junge, M. Beller and F. Ragaini, J. Catal., 2017, 351, 79 CrossRef; (e) F. Chen, C. Kreyenschulte, J. Radnik, H. Lund, A.-E. Surkus, K. Junge and M. Beller, ACS Catal., 2017, 7, 1526 CrossRef; (f) F. Chen, C. Topf, J. Radnik, C. Kreyenschulte, H. Lund, M. Schneider, A.-E. Surkus, L. He, K. Junge and M. Beller, J. Am. Chem. Soc., 2016, 138, 8781 CrossRef PubMed; (g) R. V. Jagadeesh, H. Junge, M.-M. Pohl, J. Radnik, A. Brückner and M. Beller, J. Am. Chem. Soc., 2013, 135, 10776 CrossRef PubMed; (h) R. V. Jagadeesh, A.-E. Surkus, H. Junge, M.-M. Pohl, J. Radnik, J. Rabeah, H. M. Huan, V. Schunemann, A. Brückner and M. Beller, Science, 2013, 342, 1073 CrossRef PubMed; (i) F. A. Westerhaus, R. V. Jagadeesh, G. Wienhöfer, M.-M. Pohl, J. Radnik, A.-E. Surkus, J. Rabeah, K. Junge, H. Junge, M. Nielsen, A. Brückner and M. Beller, Nat. Chem., 2013, 5, 537 CrossRef PubMed.
- (a) Y. Sun, L. Chen, Y. Bao, G. Wang, Y. Zhang, M. Fu, J. Wu and D. Ye, Catal. Today, 2018, 307, 212 CrossRef; (b) R. Shi, J. Zhao, S. Liu, W. Sun, H. Li, P. Hao, Z. Li and J. Ren, Carbon, 2018, 130, 185 CrossRef; (c) C. E. Chan-Thaw, A. Villa, G. M. Veith and L. Prati, ChemCatChem, 2015, 7, 1338 CrossRef.
- (a) W. Meng, B. Breiner, K. Rissanen, J. D. Thoburn, J. K. Clegg and J. R. Nitschke, Angew. Chem., Int. Ed., 2011, 50, 3479 CrossRef PubMed; (b) D. Wilson, B. Djukic and M. T. Lemaire, Transition Met. Chem., 2014, 39, 17 CrossRef.
- See ESI.†.
- C. Tang, A.-E. Surkus, F. Chen, M.-M. Pohl, G. Agostini, M. Schneider, H. Junge and M. Beller, Angew. Chem., Int. Ed., 2017, 56, 16616 CrossRef PubMed.
- F. Bonnet, F. Ropital, P. Lecour, D. Espinat, Y. Huiban, L. Gengembre, Y. Berthier and P. Marcus, Surf. Interface Anal., 2002, 34, 418 CrossRef.
- F. Jaouen, J. Herranz, M. Lefèvre, J.-P. Dodelet, U. I. Kramm, I. Herrmann, P. Bogdanoff, J. Maruyama, T. Nagaoka, A. Garsuch, J. R. Dahn, T. Olson, S. Pylypenko, P. Atanassov and E. A. Ustinov, ACS Appl. Mater. Interfaces, 2009, 1, 1623 CrossRef PubMed.
- (a) X. Xin, H. Qin, H.-P. Cong and S.-H. Yu, Langmuir, 2018, 34, 4952 CrossRef PubMed; (b) R. Cao, R. Thapa, H. Kim, X. Xu, M. G. Kim, Q. Li, N. Park, M. Liu and J. Cho, Nat. Commun., 2013, 4, 2076 CrossRef PubMed; (c) Y. Zhao, K. Watanabe and K. Hashimoto, J. Am. Chem. Soc., 2012, 134, 19528 CrossRef PubMed.
- Y. S. Ponosov, M. A. Uimin, A. E. Ermakov, N. N. Shchegoleva and A. A. Mysik, Phys. Solid State, 2013, 55, 1528 CrossRef.
- R. J. Pagliero, S. Lusvarghi, A. B. Pierini, R. Brun and M. R. Mazzieri, Bioorg. Med. Chem., 2010, 18, 142 CrossRef PubMed.
- L. R. Bush, Cardiovasc. Drug Rev., 1991, 9, 247 CrossRef.
- G. E. Hardtmann, US Pat., 4015005, 1977.
- M. C. Maillard, M. E. Perlman, O. Amitay, D. Baxter, D. Berlove, S. Connaughton, J. B. Fischer, J. Q. Guo, L.-Y. Hu, R. N. McBurney, P. I. Nagy, K. Subbarao, E. A. Yost, L. Zhang and G. Durant, J. Med. Chem., 1998, 41, 3048 CrossRef PubMed.
- P. Kathirgamanathan and S. Surendrakumar, US Pat., 112854, 2009.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8sc02744g |
| This journal is © The Royal Society of Chemistry 2018 |