Open Access Article
Open Access ArticleA high spatiotemporal study of somatic exocytosis with scanning electrochemical microscopy and nanoITIES electrodes†
Theresa M.
Welle
 ,
Kristen
Alanis‡
,
Kristen
Alanis‡
 ,
Michelle L.
Colombo‡
,
Michelle L.
Colombo‡
 ,
Jonathan V.
Sweedler
,
Jonathan V.
Sweedler
 and
Mei
Shen
and
Mei
Shen
 *
*
Department of Chemistry, University of Illinois at Urbana-Champaign, 600 South Matthews Avenue, Urbana, IL 61801, USA. E-mail: mshen233@illinois.edu; Tel: +1-217-265-6290
First published on 15th May 2018
Abstract
Extra-synaptic exocytosis is an essential component of cellular communication. A knowledge gap exists in the exocytosis of the non-redox active transmitter acetylcholine. Using the nano-interface between two immiscible electrolyte solutions and scanning electrochemical microscopy (SECM), a high resolution spatiotemporal study of acetylcholine exocytosis is shown from an individual neuronal soma. The nanoelectrode was positioned ∼140 nm away from the release sites on the soma using an SECM. The quantitative study enables the obtaining of key information related to cellular communication, including extracellular concentration of the neurotransmitter, cellular permeability, Ca2+ dependence on somatic release, vesicle density, number of molecules released and the release dynamics. Measurements were achieved with a high signal to noise ratio of 6–19. The released neurotransmitter with a concentration of 2.7 (±1.0) μM was detected at the nanoelectrodes with radii of 750 nm to 860 nm.
Extra-synaptic release of neurotransmitters, such as somatic exocytosis, is an essential part of neurotransmission and brain communication, playing a critical role in developmental processes in organisms,1 mediation of sensory systems,2 and human health.3,4 Measuring the neurotransmitter release concentration and dynamics simultaneously with high spatiotemporal resolution is important to understand cellular communication. Useful information has been generated via the study of extra-synaptic exocytosis of serotonin, dopamine, noradrenaline, octopamine, and neuropeptides.5–21 However, there is still much to be learned about acetylcholine (ACh) somatic exocytosis. While the possibility of ACh somatic release has been reported,22,23 notably, there has been a lack of knowledge on simultaneous ACh concentration and release dynamics from a living neuronal soma. Besides, the mechanism of neurotransmitter exocytosis from the soma is not fully understood.24 Here, we present an nm spatial and ms temporal resolution study of ACh exocytosis from a living neuronal soma, unveiling its concentration and release dynamics simultaneously, which was achieved using nanometer-resolution scanning electrochemical microscopy (SECM)25 and nanoelectrodes. Other pieces of information, i.e. vesicle density, cellular permeability, and number of transmitter molecules, were generated as well. This fundamental knowledge can offer insights into exocytosis and drug delivery, which may lead to a broader understanding of the chemical aspects of biology.11
The neuronal model in the current study is Aplysia californica, which has been demonstrated as an important model to understand cellular communication and fundamental mechanisms of learning and memory.26–31Aplysia contains a large number of neurons of varying sizes, facilitating its isolation and experimentation at the single cell level.29,31,32 The release of ACh from the living pedal ganglion neurons of this organism in response to chemical stimulation has not been extensively studied; however, the presence of choline acetyltransferase, the enzyme responsible for the synthesis of acetylcholine, has been observed,33,34 indicating cholinergic activity within these neurons.34–36 The study of cholinergic release from Aplysia californica neurons contributes to a greater understanding of the role that acetylcholine plays in cellular communication.
SECM has been useful for biological study under actual physiological conditions, e.g. studying single cells and PC12 neuron cells, enabling biochemical measurements in real-time.37–46 SECM does not require the use of labels/stains and is a non-invasive platform for measuring biochemical release from individual cells, especially while the cell is still alive.47–54 Although SECM has been used in the study of various metabolic and secretory activities, including oxygen consumption or ROS/RNS release,46–48,50,53 our work would be the first demonstration of using SECM in the study of another important signaling molecule, ACh.
The detection of ACh is based on the ion transfer across a polarized interface between two immiscible electrolyte solutions (ITIES), where the detection potential follows the Nernst equation (a detailed detection mechanism is included in the Experimental section of the ESI†). ACh detection has been studied using macro- or micro-ITIES pipet electrodes, as well as dual pipet electrodes.55–60 We recently developed nanometer scale ITIES pipet electrodes for the quantitative detection of ACh in vitro,61,62 laying the foundation for studying ACh signaling with nanometer spatial resolution at the single living cell level as presented here. NanoITIES pipet electrodes with radii of 210 nm to 860 nm were used in the current study with the Cyclic Voltammograms (CV) corresponding to ACh detection shown in Fig. 1 (blue dashed curve). The first demonstration of ITIES electrodes in the study of extra-synaptic exocytosis from a living neuronal soma is presented, showing ITIES electrodes as powerful complementary analytical approaches to the conventionally used carbon electrodes in the study of extra-synaptic exocytosis.
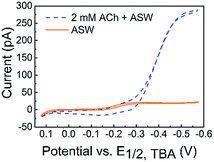 | ||
| Fig. 1 Cyclic voltammograms (CVs) of acetylcholine (ACh) detection in vitro with the nanoITIES pipet electrode in the cellular medium of Aplysia, and artificial seawater (ASW). The electrochemical cell diagram is shown in cell 1. The CV of the ASW background solution is shown as an orange solid line, and the sigmoidal shape of the CV is due to the transfer of ASW ions facilitated by 1 mM DB18C6 inside the pipet (detailed current contributions of ASW ions are included in Fig. S3†). The CV for ACh detection is shown as a sigmoidal blue dashed line. The nanoelectrode radius is ∼370 nm. Cell 1: Pt|1 mM DB18C6 + 5 mM TDDATFAB + 1,2-DCE‖ASW|AgCl|Ag. | ||
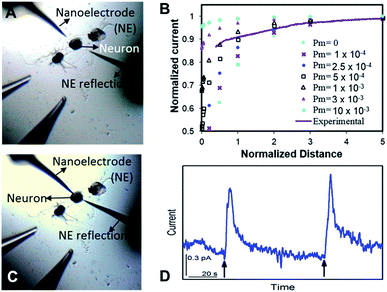
The nm-resolution positioning capabilities of SECM enabled us to place the nanoITIES pipet electrodes at a nanometer distance from the release sites on the soma, where transmitter release was studied before its dilution. SECM also allows non-contact measurements, which prevent artifacts that could interfere with current recordings; this is difficult to achieve by optical microscopy alone. Neurotransmitters are diluted as they diffuse away following their release from the release sites, which poses challenges when studying their release temporal profile. Nanoelectrodes can be positioned at a much closer distance to the neuron surface with an SECM than a macro- or micro-electrode because the feedback distance is proportional to the electrode radius.25,63
Optical images before and after the SECM approach are shown in Fig. 2A and C, respectively. It is challenging to position the nanoelectrode to be at a nanometer distance from the soma surface by visual observation only. Using an SECM, we brought the nanoITIES electrode close (at a nm distance) to the soma surface while simultaneously recording the current measured on the nanoelectrode using a non-invasive electroactive mediator (ASW background ions, solid orange curve in Fig. 1, CVs of ASW and each component that makes up the ASW at nano-ITIES electrodes are shown in Fig. S4†), generating a current–distance curve, i.e. an SECM approach curve (solid purple line, Fig. 2B). The SECM approach curve can be simulated using COMSOL Multiphysics to determine accurately the distance down to the nm scale between the electrode and the cellular surface after the SECM approach; cellular permeability has also been derived from the simulation of the SECM approach curve.50,64–66 Considering that we are approaching a living neuron, it is critical to consider the permeability in the SECM approach curve simulation. The simulated SECM approach curves with different cellular permeabilities to ASW ions are shown in Fig. 2B. As shown in Fig. 2B, without taking into account the cellular permeability (Pm = 0), the simulated approach curve does not fit the experimental approach curve. Based on the fitting between the experimental approach curve and the simulated approach curve, it can be seen that the nanoITIES pipet electrode was positioned ∼140 nm away from the soma surface, and the permeability of the Aplysia californica neuron membrane to ASW ions is between 5 × 10−4 and 1 × 10−3 m s−1.
Once positioned at a nm distance from the soma with the SECM, we employed amperometry to study the release of ACh in response to high concentration K+ stimulation, to learn about its concentration and dynamics (Fig. 2D) with a time resolution on the order of ms. The applied potentials are the diffusion limiting potential for ACh detection, from approximately −0.5 V to −0.6 V vs. E1/2,TBA. At this potential under the condition of cell 1, the nanoITIES pipet electrodes are selective for ACh detection against other substances known to exist or to be released from the animal model in the current study, Aplysia californica, such as GABA, dopamine, glutamate, serotonin, amino acids, and pedal peptide, as well as high concentration K+ in the stimulating solution, and H+ variation accompanying exocytosis, as detailed in our recent study.67 Arrows in Fig. 2D indicate the application of high concentration K+ stimulation. As shown in Fig. 2D, ACh release from the soma was observed immediately in response to high concentration K+ stimulation. Control experiments confirmed that the amperometric peaks in Fig. 2D are due to somatic release of the Aplysia cell, rather than mechanical perturbation of the electrode interface during the stimulation process (Fig. S5†), or from the ions present in the stimulating solution (Fig. S6†). The in situ release result in Fig. 2D was acquired right after the SECM approach with the optical image shown in Fig. 2C; in contrast, there is no significant observation of somatic release (Fig. S7†) before the SECM approach in the optical image shown in Fig. 2A, indicating the low concentration of the transmitter further away from the release sites.
The concentration of ACh released from the single cell measured in situ is 2.7 ± 1.0 μM, calculated from the maximum amperometric peak current (Table 1), using procedures described in the ESI† under the Data acquisition and analysis section. A lower concentration is expected at a location much further away from the cell soma due to dilution that happens when the transmitter diffuses away from the soma after release. The number of moles for each peak event is 80 (±20) × 10−18 mol, corresponding to 4.8 (±1.3) × 107 molecules (Table 1). This amount of transmitter is much higher than the number of ACh molecules estimated by electrophoretic application of ACh in the rat diaphragm,68 iontophoretic application of ACh at the neuromuscular junction,69 and the amount measured with a 15 nm radius ITIES electrode around a single synapse of Aplysia.67 Besides, the observed amperometric half peak width of 9.3 (±3.5) s is significantly higher than that expected for single vesicular events, which is in the order of hundreds of ms.70 The above result suggests the measurements of multiple vesicular contents released from the single neuron soma via nanoITIES electrodes with radii of 750–860 nm during each peak event. Using the content per vesicle as suggested in our recent single synaptic study of acetylcholine release67 from the same animal model, we calculated the releasable vesicle density to be 25 ± 2 vesicles per μm2 (Fig. 3, the calculation procedure is detailed in the ESI†). This number has a similar order of magnitude to the value of 90 vesicles per μm2 obtained in a recent study using 3-D reconstruction of synaptic boutons from serial transmission electron microscopy sections.71
| Total (n = 8) | |
|---|---|
| t 1/2 (s) | 9.3 (±3.5) |
| Concentration (μM) | 2.7 (±1.0) |
| Moles released | 80 (±20) × 10−18 |
| Number of molecules | 4.8 (±1.3) × 107 |
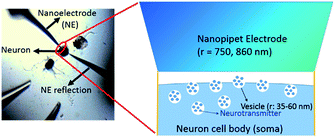 | ||
| Fig. 3 Measurement of vesicle density. The measured vesicle density is ∼25 ± 2 vesicles per μm2. The vesicle radius (r) is reported in ref. 30. | ||
We further examined in situ the role of calcium in the somatic release of acetylcholine (Fig. 4). In these experiments, we replaced the standard ASW cell medium with calcium-free ASW solution, and measured the neurotransmitter release in response to high concentration-K+, Ca2+-free ASW stimulating solution; afterwards, Ca2+ was added back into the cellular medium and stimulating solution, followed by neurotransmitter release measurements. As shown in Fig. 4A, when calcium was not present in the cell medium and stimulating solution, no significant somatic release events were observed. In contrast, very strong release peaks (Fig. 4B) were observed after we added Ca2+ back into the cell medium and stimulating solution. These experiments demonstrated that acetylcholine exocytosis from the soma in Aplysia neurons is calcium-dependent. Our results concur well with the calcium-dependence of somatic release of catecholamines,5–7 and ACh somatic release from other animal models studied with radiochemical assay and excised patch probes22,23 where simultaneous concentration and release dynamics of ACh somatic exocytosis are lacking.
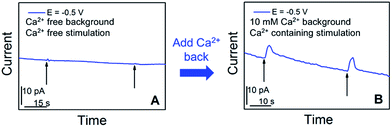 | ||
| Fig. 4 Calcium-dependence of acetylcholine exocytosis from an Aplysia californica neuron in response to high concentration K+ stimulation. (A) Amperometric current response in response to chemical stimulation (Ca2+ free, elevated K+ solution, indicated by black arrows) measured at 50 min after replacing standard ASW with Ca2+-free ASW. (B) Amperometric current response from the cell upon stimulation (Ca2+-containing, elevated K+ solution, indicated by black arrows), 10 min after the addition of Ca2+ solution to a final concentration of 10 mM. The applied potential is the diffusion limited potential for acetylcholine detection shown in Fig. 1. The nanoelectrode radius is 780 nm. | ||
In summary, a high resolution spatiotemporal quantitative study of acetylcholine exocytosis from a single living soma of Aplysia has been demonstrated. To the best of our knowledge, this is the first such report. A nanometer ITIES sensing probe was positioned ∼140 nm vertically from the release sites using scanning electrochemical microcopy; exocytosis events were studied with ms temporal resolution. Due to the nanoscale structure of the electrodes as compared to the approximate 100–150 μm diameter of the Aplysia soma, it is expected that we do not detect all of the transmitter molecules released. However, the scale of the nanoprobes is advantageous because it allows the determination of vesicle density, as well as the nm resolution and non-contact positioning of the sensing probe from the release sites. Key pieces of information related to cellular communication were obtained, including extracellular concentration of acetylcholine during somatic exocytosis, number of molecules released, the dynamics of exocytosis, vesicle density, and Ca2+ dependence on somatic exocytosis. The Ca2+ dependence study contributes to the mechanistic understanding of somatic exocytosis, which is currently not fully understood. When Ca2+ was not present, no exocytosis events were observed in response to high concentration K+ stimulation; in contrast, acetylcholine exocytosis events recovered once Ca2+ had been added back. Ca2+ is known to be involved in the vesicular release of transmitters;11 our findings suggest the vesicular release pathway of acetylcholine somatic exocytosis from Aplysia.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number R21NS085665 (MS) with support from award number P30 DA018310 (JVS), and the National Resource for Aplysia, through award P40 OD010952, to partially defray costs for Aplysia. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Theresa W. is grateful for the support from a Dow Chemical Scholarship for summer research. Michelle L. C. acknowledges the support from the Coleman Fellowship and the NIH Chemical Biology Interface Training Program under training grant number 2T32GM070421-11. We would like to thank Burt Simpson from the Joaquín Rodríguez-López group for the helpful discussion on simulation. The authors thank Xiying Wang for preparing Aplysia cell cultures. We would also like to thank Stanislav Rubakhin for helpful discussion.Notes and references
- S. Jayakumar, S. Richhariya, O. V. Reddy, M. J. Texada and G. Hasan, eLife, 2016, 5, 1 Search PubMed
.
- L. Rodriguez-Sosa, G. Calderon-Rosete, A. Ortega-Cambranis and F. F. De-Miguel, Comp. Biochem. Physiol., Part A: Mol. Integr. Physiol., 2017, 203, 83 CrossRef PubMed
.
- A. F. Russo, Headache, 2017, 57, 37 CrossRef PubMed
.
- J. Duda, C. Potschke and B. Liss, J. Neurochem., 2016, 139, 156 CrossRef PubMed
.
- K. Suetake, H. Kojima, K. Inanaga and K. Koketsu, Brain Res., 1981, 205, 436 CrossRef PubMed
.
- S. Majdi, E. C. Berglund, J. Dunevall, A. I. Oleinick, C. Amatore, D. E. Krantz and A. G. Ewing, Angew. Chem., Int. Ed., 2015, 54, 13609 CrossRef PubMed
.
- A. Andrews, ACS Chem. Neurosci., 2017, 8, 211 CrossRef PubMed
.
- R. D. Lama, K. Charlson, A. Anantharam and P. Hashemi, Anal. Chem., 2012, 84, 8096 CrossRef PubMed
.
- J. G. Roberts, L. Z. Lugo-Morales, P. L. Loziuk and L. A. Sombers, Methods Mol. Biol., 2013, 964, 275 Search PubMed
.
- E. H. Jaffe, A. Marty, A. Schulte and R. H. Chow, J. Neurosci., 1998, 18, 3548 CrossRef PubMed
.
- C. L. Haynes, L. A. Buhler and R. M. Wightman, Biophys. Chem., 2006, 123, 20 CrossRef PubMed
.
- E. L. Ciolkowski, B. R. Cooper, J. A. Jankowski, J. W. Jorgenson and R. M. Wightman, J. Am. Chem. Soc., 1992, 114, 2815 CrossRef
.
- C. B. Jacobs, T. L. Vickrey and B. J. Venton, Neurotransmission: Measuring Chemical Events, Wiley Encyclopedia of Chemical Biology, Wiley & Sons, Aug 15, 2008, http://onlinelibrary.wiley.com/doi/10.1002/9780470048672.wecb383/pdf, accessed Jul 25, 2016 Search PubMed.
- C. Amatore, S. Arbault, M. Guille and F. Lemaitre, Chem. Rev., 2008, 108, 2585 CrossRef PubMed
.
-
Electrochemical Methods for Neuroscience, ed. A. C. Michael and L. M. Borland, CRC Press/Taylor & Francis, Boca Raton, FL., 2007 Search PubMed
.
- C. Amatore, S. Arbault, I. Bonifas, F. Lemaitre and Y. Verchier, ChemPhysChem, 2007, 8, 578 CrossRef PubMed
.
- M. Perry, Q. Li and R. T. Kennedy, Anal. Chim. Acta, 2009, 653, 1 CrossRef PubMed
.
- K. T. Ngo, E. L. Varner, A. C. Michael and S. G. Weber, ACS Chem. Neurosci., 2017, 8, 329 CrossRef PubMed
.
- S. Ge, S. Koseoglu and C. L. Haynes, Anal. Bioanal. Chem., 2010, 397, 3281 CrossRef PubMed
.
- M. L. Rogers and M. G. Boutelle, Annu. Rev. Anal. Chem., 2013, 6, 427 CrossRef PubMed
.
- P. A. Garris, M. Kilpatrick, M. A. Bunin, D. Michael, Q. D. Walker and R. M. Wightman, Nature, 1999, 398, 67 CrossRef PubMed
.
- D. A. Johnson and G. Pilar, J. Physiol., 1980, 299, 605 CrossRef
.
- Y. A. Sun and M. M. Poo, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 2540 CrossRef
.
- N. T. N. Phan, X. Li and A. G. Ewing, Nat. Rev. Chem., 2017, 1, 0048 CrossRef
.
-
Scanning Electrochemical Microscopy, ed. A. J. Bard and M. V. Mirkin, CRC press/Taylor & Francis Group, Boca Raton, FL., 2nd edn, 2012 Search PubMed
.
- D. T. Chiu, S. J. Lillard, R. H. Scheller, R. N. Zare, S. E. Rodriguez-Cruz, E. R. Williams, O. Orwar, M. Sandberg and J. A. Lundqvist, Science, 1998, 279, 1190 CrossRef PubMed
.
-
E. R. Kandel, Behavioral Biology of Aplysia: A Contribution to the Comparative Study of Opisthobranch Molluscs, W. H. Freeman, San Francisco, 1979, vol. xiii Search PubMed
.
- E. R. Kandel, Science, 2001, 294, 1030 CrossRef PubMed
.
- T. J. Comi, T. D. Do, S. S. Rubakhin and J. V. Sweedler, J. Am. Chem. Soc., 2017, 139, 3920 CrossRef PubMed
.
- R. Gillete and B. Pomeranz, J. Neurobiol., 1975, 6, 463 CrossRef PubMed
.
- S. Kang, A. Badea, S. S. Rubakhin, J. V. Sweedler, J. A. Rogers and R. G. Nuzzo, Langmuir, 2017, 33, 8640 CrossRef PubMed
.
- D. B. Sattelle and S. D. Buckingham, Invertebr. Neurosci., 2006, 6, 1 CrossRef PubMed
.
- E. Giller Jr and J. H. Schwartz, J. Neurophysiol., 1971, 34, 93 CrossRef PubMed
.
- R. E. McCaman and S. A. Dewhurst, J. Neurochem., 1970, 17, 1421 CrossRef PubMed
.
- M. Eisenstadt, J. E. Goldman, E. R. Kandel, H. Koike, J. Koester and J. H. Schwartz, Proc. Natl. Acad. Sci. U. S. A., 1973, 70, 3371 CrossRef
.
- J. L. Cohen, K. R. Weiss and I. Kupfermann, J. Neurophysiol., 1978, 41, 157 CrossRef PubMed
.
- J. P. Wilburn, D. Wright and D. E. Cliffel, Analyst, 2006, 131, 311 RSC
.
- M. Ciobanu, D. E. Taylor Jr, J. P. Wilburn and D. E. Cliffel, Anal. Chem., 2008, 80, 2717 CrossRef PubMed
.
- A. J. Bard and R. W. Murray, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11484 CrossRef PubMed
.
- J. Kim, A. Izadyar, N. Nioradze and S. Amemiya, J. Am. Chem. Soc., 2013, 135, 2321 CrossRef PubMed
.
- R. T. Kurulugama, D. O. Wipf, S. A. Takacs, S. Pongmayteegul, P. A. Garris and J. E. Baur, Anal. Chem., 2005, 77, 1111 CrossRef PubMed
.
- J. M. Liebetrau, H. M. Miller, J. E. Baur, S. A. Takacs, V. Anupunpisit, P. A. Garris and D. O. Wipf, Anal. Chem., 2003, 75, 563 CrossRef PubMed
.
- Y. Takahashi, A. I. Shevchuk, P. Novak, B. Babakinejad, J. MacPherson, P. R. Unwin, H. Shiku, J. Gorelik, D. Klenerman, Y. E. Korchev and T. Matsue, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11540 CrossRef PubMed
.
- Y. Takii, K. Takoh, M. Nishizawa and T. Matsue, Electrochim. Acta, 2003, 48, 3381 CrossRef
.
- A. Schulte, M. Nebel and W. Schuhmann, Annu. Rev. Anal. Chem., 2010, 3, 299 CrossRef PubMed
.
-
J. Mauzeroll and S. B. Schougaard, Scanning Electrochemical Microscopy, ed. A. J. Bard and M. V. Mirkin, CRC press/Taylor & Francis Group, Boca Raton, FL., 2nd edn, 2012, p. 12 Search PubMed
.
- P. Sun, F. O. Laforge, T. P. Abeyweera, S. A. Rotenberg, J. Carpino and M. V. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 443 CrossRef PubMed
.
- B. Liu, S. A. Rotenberg and M. V. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 9855 CrossRef
.
- D. Koley and A. J. Bard, Proc. Natl.
Acad. Sci. U. S. A., 2010, 107, 16783 CrossRef PubMed
.
- A. Schulte, M. Nebel and W. Schuhmann, Methods Enzymol., 2012, 504, 237 Search PubMed
.
- Y. Li, K. Hu, Y. Yu, S. A. Rotenberg, C. Amatore and M. V. Mirkin, J. Am. Chem. Soc., 2017, 139, 13055 CrossRef PubMed
.
- D. Polcari, P. Dauphin-Ducharme and J. Mauzeroll, Chem. Rev., 2016, 116, 13234 CrossRef PubMed
.
- S. Bergner, P. Vatsyayan and F. M. Matysik, Anal. Chim. Acta, 2013, 775, 1 CrossRef PubMed
.
- S. Kuss, D. Polcari, M. Geissler, D. Brassard and J. Mauzeroll, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 9249 CrossRef PubMed
.
- P. Vanysek and M. Behrendt, J. Electroanal. Chem., 1981, 130, 287 CrossRef
.
- V. Mareck and Z. Samec, Anal. Lett., 1981, 14, 1241 CrossRef
.
- T. Kakutani, T. Ohkouchi, T. Osakai, T. Kakiuchi and M. Senda, Anal. Sci., 1985, 1, 219 CrossRef
.
- Y. Shao and H. H. Girault, J. Electroanal. Chem., 1991, 282, 59 CrossRef
.
- Y. Shao, J. A. Campbell and H. H. Girault, J. Electroanal. Chem., 1991, 300, 415 CrossRef
.
- Y. Shao, B. Liu and M. V. Mirkin, J. Am. Chem. Soc., 1998, 120, 12700 CrossRef
.
- M. L. Colombo, J. V. Sweedler and M. Shen, Anal. Chem., 2015, 87, 5095 CrossRef PubMed
.
- M. Shen and M. Colombo, Anal. Methods, 2015, 7, 7095 RSC
.
-
A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 2nd edn, 2001 Search PubMed
.
- M. N. Zhang, Z. Ding and Y. T. Long, Analyst, 2015, 140, 6054 RSC
.
- J. Guo and S. Amemiya, Anal. Chem., 2005, 77, 2147 CrossRef PubMed
.
- S. Bergner, J. Wegener and F. M. Matysik, Anal. Methods, 2012, 4, 623 RSC
.
- M. Shen, Z. Qu, J. DesLaurier, T. M. Welle, J. V. Sweedler and R. Chen, Single Synaptic Observation of Cholinergic Neurotransmission on Living Neuron Cells: Concentration and Dynamics, under revision.
- K. Krnjević and R. Miledi, Nature, 1958, 182, 805 CrossRef
.
- S. W. Kuffler and D. Yoshikami, J. Physiol., 1975, 251, 465 CrossRef
.
-
Electrochemical methods for neuroscience, ed. A. C. Michael and L. M. Borland, CRC press/Taylor & Francis Group, Boca Raton, FL., 2007, vol. 14, p. 298 Search PubMed
.
- G. Yang, Y. Gong, K. Gong, W. Jiang, E. Kwon, P. Wang, H. Zheng, X. Zhang, W. Gan and N. Zhao, Neurosci. Lett., 2005, 384, 66 CrossRef PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental description; schematic representation of the experimental setup for neuronal stimulation experiments (and controls); in vitro ACh detection in ASW background solution with the calibration curve; cyclic voltammograms of components of ASW solution; COMSOL simulation of the pipet approach and experimental SECM approach curve to the neuronal soma surface; quantitative and kinetic parameters of release events; control experiments to confirm somatic release instead of physical disturbance that could occur during the stimulation process; results of control experiments demonstrating probe selectivity against stimulating solution; and distance dependence results on acetylcholine somatic release, before and after nano-positioning with an SECM. See DOI: 10.1039/c8sc01131a |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2018 |