Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An eccentric rod-like linear connection of two heterocycles: synthesis of pyridine trans-tetrafluoro-λ6-sulfanyl triazoles†
Prajwalita
Das
a,
Kiyoteru
Niina
a,
Tomoya
Hiromura
a,
Etsuko
Tokunaga
 a,
Norimichi
Saito
b and
Norio
Shibata
a,
Norimichi
Saito
b and
Norio
Shibata
 *ac
*ac
aDepartment of Nanopharmaceutical Sciences and Department of Life Science and Applied Chemistry, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555, Japan. E-mail: nozshiba@nitech.ac.jp
bPharmaceutical Division, Ube Industries, Ltd., Seavans North Bldg, 1-2-1 Shibaura, Minato-ku, Tokyo 105-8449, Japan
cInstitute of Advanced Fluorine-Containing Materials, Zhejiang Normal University, 688 Yingbin Avenue, 321004 Jinhua, China
First published on 14th May 2018
Abstract
The trans-tetrafluoro-λ6-sulfane (SF4) group has been utilized as a unique three-dimensional building block for the linear connection of two independent N-heterocycles, pyridines and triazoles. The linearly connected heterocyclic compounds were synthesized by thermal Huisgen 1,3-dipolar cycloaddition between previously unknown pyridine SF4-alkynes and readily available azides, providing a series of rod-like SF4-connected N-heterocycles in good to excellent yields. X-ray crystallographic analysis of the target products revealed the trans-geometry of the SF4 group, which linearly connects two independent N-heterocycles. This research will open the field of chemistry of SF4-connected heterocyclic compounds.
Introduction
Hypervalent sulfur fluorides belong to an interesting class of compounds, which are popular among both medicinal and material chemists alike.1 The pentafluoro-λ6-sulfane (pentafluorosulfanyl, SF5) group has turned out to be the front-runner of this class of compounds, with chemists realizing the varied potential of this moiety.2 Over the years, a steady increase can be seen in the number of publications related to SF5-containing compounds.2 On the other hand, the tetrafluoro-λ6-sulfane (tetrafluorosulfanyl, SF4) moiety, with equally potent functionality, has not been exploited enough and is highly underdeveloped.3 The SF4 moiety not only has unique physiochemical properties, which make it suitable as a unit of liquid crystals,1,3 but also has interesting geometric features that enable it to connect two independent functional groups via the central hypervalent sulfur atom in either the cis or trans configuration of R-SF4-R′ (Fig. 1a).3,4 Due to the octahedral geometry of the R-SF4-R′ moiety, the trans-SF4 configuration has the ability to function as a building block in the construction of linear structures via its axial bonds, which makes the trans-SF4 configuration highly significant.3,4 The rod-like connection by the trans-SF4 unit potentially suggests new approaches for designing novel pharmaceuticals, while also leading to sought-after fluorine-containing drug candidates. As an extension of our research on fluorine-containing heterocycles,5 we were interested in the development of a novel method to connect two heterocyclic rings via a rod-like linear linker. Linear molecules are currently receiving chemists' attention due to their material and biological applications.6,7 Bicyclo-[1.1.1]pentane (BCP) has gained the most attention in achieving linear connection7 (Fig. 1b). Due to the intrinsically linear framework and lipophilicity of BCP, it has been proposed as a bioisostere of a p-substituted benzene ring7e and as an alkenyl group,7d both of which are found in pharmaceuticals. Our present objective is to propose the trans-SF4 unit as a novel bio-isosteric unit next to BCP and to design rod-like molecules with two independent (hetero)aromatic rings (Fig. 1c).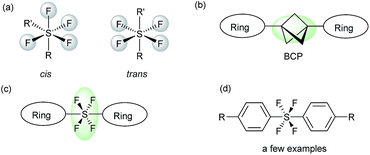 | ||
| Fig. 1 (a) SF4 compounds with cis- and trans-geometry. (b) Rod-like molecules with a BCP moiety. (c) Proposed rod-like molecules with a trans-SF4 moiety. (d) Bisaryltetrafluorosulfuranes. | ||
While the initial idea of SF4-linked diaromatic compounds, Ar-SF4-Ar, appeared in 1973,3a followed by a handful of reports (i.e., 4 papers and 1 patent),3a–e only circumstantial evidence was reported. Kirsch and co-workers in 1999 obviously isolated trans-SF4-linked aromatic building blocks based on direct fluorination of the corresponding bis(aryl)sulfide (Fig. 1d),3d but that method has a serious limitation, i.e., the necessity of substrates substituted by p-nitro groups to deactivate the aromatic moiety, preventing the reaction with fluorine. Therefore, novel methodologies for trans-SF4-linked bis-aromatic compounds are highly desired. Besides, SF4-linked heteroaromatic building blocks have never been reported.
Heterocycles, which are common skeletal components of natural products, often exhibit bioactive properties, and are thus extensively used as pharmaceuticals.8 Pyridines and triazoles are two of the most commonly occurring N-heterocycles in medicinal chemistry.9 The use of the trans-SF4 moiety as a rod-like linear linker for pyridine and triazole groups would provide a fascinating novel set of compounds, which should have a very interesting physiochemical profile due to their linear structure6,7 and the fluorinated N-heterocycles.10 Herein, we present the first method for the connection of two independent N-heterocyclic molecules via a rod-like linear trans-SF4 unit, where the axial bonds of the octahedral disubstituted SF4 moiety are responsible for the linear connection (Scheme 1). First, the pyridine tetrafluorosulfanyl chlorides 1 (Py-SF4Cl) were prepared from pyridine disulfides ((Py-S)2) with KF/Cl2 by oxidative chloro-tetrafluorination. It should however be noted that the generation of the tetrafluorosulfanyl chloride group in any organic molecule itself is challenging. The chloro-fluorination needs to be performed under a completely dry and inert atmosphere in FEP bottles, while the isolation of the product requires special equipment.2g,h,j Once the pyridine tetrafluorosulfanyl chlorides 1 were synthesized, based on our previous reports2j,3k their radical addition to alkynes was attempted to furnished pyridine trans-SF4-alkenes 2. Then pyridine trans-SF4-alkenes 2 were converted to previously unknown pyridine SF4-alkynes 3 by treatment with LiOH·H2O. Finally, reaction of the pyridine SF4-alkynes 3 with azides 4 under thermal Huisgen 1,3-dipolar cycloaddition conditions provided the eccentric, three-dimensionally unique trans-SF4 linked pyridine and triazole derivatives 5 in high yields (Scheme 1).11 While a few examples of aryl-SF4-aryl were reported,3 this is the first example for the synthesis of heteroaryl-SF4-heteroaryl systems. An aryl-SF4-heteroaryl system was also accessed by the method. Since both heteroaryl and fluorinated moieties are sought after building blocks for drug candidates, our novel, eccentric heteroaromatic molecules should suggest new fields of drug design.
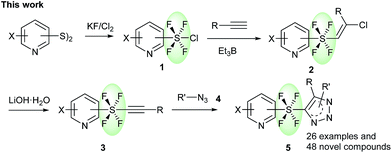 | ||
| Scheme 1 Synthesis of trans-SF4 linked pyridine and triazole compounds via a cycloaddition reaction. | ||
Results and discussion
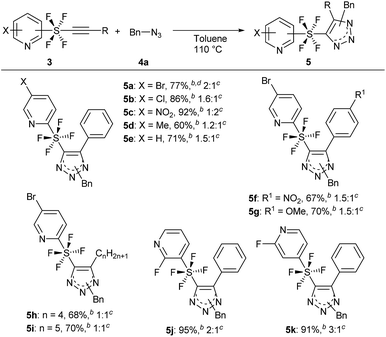
The key precursors, pyridine SF4-alkynes 3, were prepared in good yields from the pyridine SF4-alkenes 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2j,3k by subjecting the latter to dehydrochlorination under basic conditions. Thus, treatment of pyridine SF4 chloroalkenes 2 with an excess amount of LiOH·H2O in DMSO at room temperature furnished the desired pyridine SF4 alkynes 3. A wide variety of functional groups including halogens (Br, Cl, and F), an electron-withdrawing NO2 group, and an electron-donating methyl group in the pyridine ring were well tolerated under these strong basic conditions. All the positions of the SF4 unit on the pyridine ring, namely the ortho-, meta- and para-SF4-pyridines 2, were accepted for this transformation. The desired pyridine SF4 alkynes 3 with an aryl or alkyl group at the terminal position were also obtained in good to excellent yields (Scheme 2).
2j,3k by subjecting the latter to dehydrochlorination under basic conditions. Thus, treatment of pyridine SF4 chloroalkenes 2 with an excess amount of LiOH·H2O in DMSO at room temperature furnished the desired pyridine SF4 alkynes 3. A wide variety of functional groups including halogens (Br, Cl, and F), an electron-withdrawing NO2 group, and an electron-donating methyl group in the pyridine ring were well tolerated under these strong basic conditions. All the positions of the SF4 unit on the pyridine ring, namely the ortho-, meta- and para-SF4-pyridines 2, were accepted for this transformation. The desired pyridine SF4 alkynes 3 with an aryl or alkyl group at the terminal position were also obtained in good to excellent yields (Scheme 2).
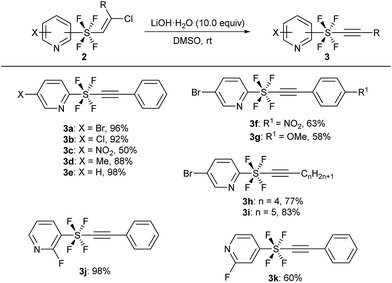 | ||
| Scheme 2 Synthesis of pyridine SF4-alkynes 3 from alkenes 2. aReaction of 2 (1.0 equiv.) was performed in the presence of LiOH·H2O (10.0 equiv.) in DMSO at rt. | ||
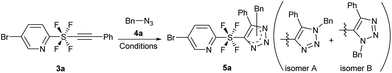
With the precursor pyridine SF4-alkynes 3 in hand, we first examined the reaction conditions that would allow the azide/alkyne cycloaddition to take place (see the ESI for details†). Using alkyne 3a and benzyl azide 4a, initially ruthenium catalyst12 Cp*Ru(PPh3)2Cl2 was used in toluene at 80 °C and 110 °C to give target product 5a in 24% and 32% yield respectively, as a mixture of 1,4- and 1,5-disubstituted isomers (isomer A and isomer B, entries 1 and 2). When the catalyst loading of Cp*Ru(PPh3)2Cl2 was increased, the yield decreased (entry 3) and a subsequent decrease of the catalyst increased the yield to 56% (entry 4). We then realized that our reaction does not require an Ru catalyst, but undergoes a thermal cycloaddition, where the catalyst initially led to the start of material decomposition. Running the reaction in the absence of a catalyst gave the product satisfactorily in 83% yield (entry 5). Being a thermal reaction, the regioisomers A and B were obtained in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.13 The formation of 1,4-disubstituted isomer A was slightly preferred because it avoided the steric repulsions between the bulky SF4 and the benzyl group (Table 1).
1 ratio.13 The formation of 1,4-disubstituted isomer A was slightly preferred because it avoided the steric repulsions between the bulky SF4 and the benzyl group (Table 1).
| Entry | Conditions | 5a |
|---|---|---|
| a Reaction was performed at the 0.1 mmol scale at the given conditions for 24 h. b Total yield of both regioisomers from 19F NMR. c Ratio of two regioisomers A and B. | ||
| 1 | 3a (1.0 equiv.), 4a (3.0 equiv.), Cp*Ru(PPh3)2Cl2 (10 mol%) in toluene at 80 °C | 24%,b 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c |
| 2 | 3a (1.0 equiv.), 4a (3.0 equiv.), Cp*Ru(PPh3)2Cl2 (10 mol%) in toluene at 110 °C | 32%,b 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c |
| 3 | 3a (1.0 equiv.), 4a (3.0 equiv.), Cp*Ru(PPh3)2Cl2 (20 mol%) in toluene at 110 °C | 10%,b 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c |
| 4 | 3a (1.0 equiv.), 4a (3.0 equiv.), Cp*Ru(PPh3)2Cl2 (5 mol%) in toluene at 110 °C | 56%,b 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c |
| 5 | 3a (1.0 equiv.), 4a (3.0 equiv.), in toluene at 110 °C | 83%,b 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c 1c |
With the optimized reaction conditions in hand, we began the substrate screening by modifying the SF4-alkynes 3 (Scheme 3). Changing the halogen on the pyridine ring to Cl gave product 5b in 86% yield with a ratio of 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (isomers A
1 (isomers A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B). Having the electron-withdrawing NO2 group on pyridine gave 5c in an excellent yield of 92%. It is of interest that in this case we observed a reversal in selectivity, with a preference for the 1,5-disubstituted product (isomers A
B). Having the electron-withdrawing NO2 group on pyridine gave 5c in an excellent yield of 92%. It is of interest that in this case we observed a reversal in selectivity, with a preference for the 1,5-disubstituted product (isomers A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B = 1
B = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). 4-Me pyridine SF4-alkyne 3d and unsubstituted pyridine SF4-alkyne 3e both sustained the reaction to give the desired triazole products 5d and 5e in 60% and 71% yield, respectively. Next, we analysed the effect of the electron-withdrawing NO2 and electron-donating OMe on the phenyl ring of the alkyne moiety (3f and 3g). There did not appear to be any drastic effect of the substituents as both alkynes gave products 5f and 5g in 67% and 70% yield, respectively with a 1.5
2). 4-Me pyridine SF4-alkyne 3d and unsubstituted pyridine SF4-alkyne 3e both sustained the reaction to give the desired triazole products 5d and 5e in 60% and 71% yield, respectively. Next, we analysed the effect of the electron-withdrawing NO2 and electron-donating OMe on the phenyl ring of the alkyne moiety (3f and 3g). There did not appear to be any drastic effect of the substituents as both alkynes gave products 5f and 5g in 67% and 70% yield, respectively with a 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (isomers A
1 ratio (isomers A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B). Replacing the aromatic ring by an aliphatic straight chain (n-butyl or n-pentyl) also gave products 5h and 5i in 68% and 70% yield, respectively. We further expanded our reaction by employing m-SF4 pyridine alkyne 3j and p-SF4 pyridine alkyne 3k as substrates. Both alkynes gave products 5j and 5k in excellent yields of 95% and 91%, respectively. The selectivity of the regioisomers increased slightly to 3
B). Replacing the aromatic ring by an aliphatic straight chain (n-butyl or n-pentyl) also gave products 5h and 5i in 68% and 70% yield, respectively. We further expanded our reaction by employing m-SF4 pyridine alkyne 3j and p-SF4 pyridine alkyne 3k as substrates. Both alkynes gave products 5j and 5k in excellent yields of 95% and 91%, respectively. The selectivity of the regioisomers increased slightly to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This reaction could be reproduced at a 1 g scale of alkyne 3a to 5a without any loss of yield.
1. This reaction could be reproduced at a 1 g scale of alkyne 3a to 5a without any loss of yield.
The substrate scope of azides 4 was further investigated for the cycloaddition (Scheme 4). Changing the substituents on the benzene ring of the benzyl azides 4 gave good results. The presence of an electron-withdrawing group was sustained well to give products 5 having NO2 (5l), Br (5m), and F (5n) on the benzene ring in good to excellent yields. Even a CN group was borne to give the triazole product 5o in 75% yield. An electron-donating group also gave products 5p (OMe) and 5q (Me) in 67–68% yields. It is noteworthy that, in the case of 5p, selectivity for the regioisomers increased to 6.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The single crystal X-ray structures of each of the regioisomers of 5m, isomer A and isomer B, clearly revealed the specific regiochemistry of the octahedral sulfur centre, connecting the pyridine and the triazole parts linearly with its axial bonds and four fluorines occupying the equatorial plane. Switching from benzyl to phenyl azide revealed that the 1,4-disubstituted products 5r–t (isomer A) formed exclusively. This was possibly due to the high steric hindrance of the SF4 moiety and phenyl ring in the 1,5-disubstituted product (isomer B). Aliphatic azides 4 having 6 and 8 carbons were used and the respective products 5u and 5v were obtained (67–66% yields) with almost no regioselectivity. Cyclohexyl azide gave product 5w in moderate (52%) yield, while very bulky adamantyl azide gave exclusively the 1,4-disubstituted product 5x (isomer A) regioselectively in 37% yield. The cycloaddition reaction also proceeded well with two azide derivatives, quinine and epiandrosterone, to give products 5y and 5z respectively, having a drug-like structure, in 37% to 67% yields. Isomer A of 5y was in fact positively obtained selectively over isomer B (6.4
1. The single crystal X-ray structures of each of the regioisomers of 5m, isomer A and isomer B, clearly revealed the specific regiochemistry of the octahedral sulfur centre, connecting the pyridine and the triazole parts linearly with its axial bonds and four fluorines occupying the equatorial plane. Switching from benzyl to phenyl azide revealed that the 1,4-disubstituted products 5r–t (isomer A) formed exclusively. This was possibly due to the high steric hindrance of the SF4 moiety and phenyl ring in the 1,5-disubstituted product (isomer B). Aliphatic azides 4 having 6 and 8 carbons were used and the respective products 5u and 5v were obtained (67–66% yields) with almost no regioselectivity. Cyclohexyl azide gave product 5w in moderate (52%) yield, while very bulky adamantyl azide gave exclusively the 1,4-disubstituted product 5x (isomer A) regioselectively in 37% yield. The cycloaddition reaction also proceeded well with two azide derivatives, quinine and epiandrosterone, to give products 5y and 5z respectively, having a drug-like structure, in 37% to 67% yields. Isomer A of 5y was in fact positively obtained selectively over isomer B (6.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
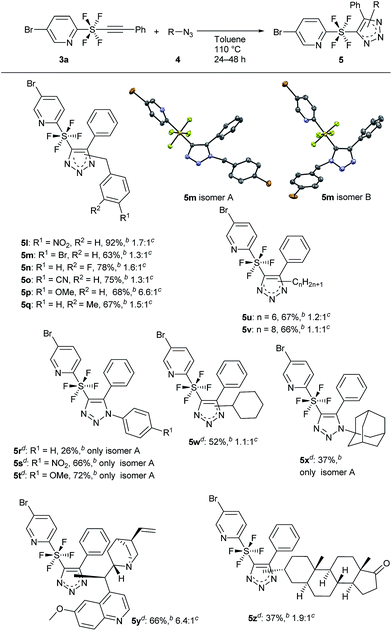 | ||
| Scheme 4 Evaluation of substrate scope by changing azide 3. Molecular structures of 5m with thermal ellipsoids set to 50% probability: 5m isomer A (CCDC 1823578†) and 5m isomer B (CCDC 1823581†). aReaction of 3a (0.5 mmol) was performed with 4 (1.5 mmol) in toluene at 110 °C for 24 h unless otherwise mentioned. bIsolated yield. cRatio of the two regioisomers A and B. dReaction was performed for 48 h. | ||
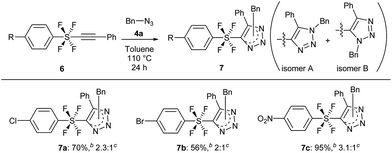
The cycloaddition reaction was also extended to benzene SF4-alkynes 6, which were synthesized according to a reported procedure3i (see the ESI for details†). The reactions of these alkynes 6 were completely feasible with the simple benzyl azide 4a (Scheme 5). While the Br and Cl-benzene SF4-alkyne 6a and 6b gave products 7a and 7b in good yields of 56% and 70% respectively (isomer A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B ratio 2
B ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2.3
1 and 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively), the NO2-benzene SF4-alkyne 6c underwent the reaction to provide product 7c in an excellent yield of 95% with a ratio of 3.1
1 respectively), the NO2-benzene SF4-alkyne 6c underwent the reaction to provide product 7c in an excellent yield of 95% with a ratio of 3.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (isomers A
1 (isomers A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B). Like compounds 5, 7 are also the first examples of aryl-SF4-heteroaryl systems.
B). Like compounds 5, 7 are also the first examples of aryl-SF4-heteroaryl systems.
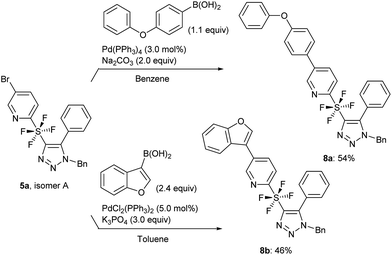
Following the synthesis of the cycloaddition products, we used isomer A of 5a for a further application. Suzuki coupling via the bromo substituent on pyridine was attempted.14 The use of phenoxyphenyl and benzofuran boronic acids, which are aromatic and heteroaromatic substrates, gave the coupled products 8a and 8b in 54% and 46% yield, respectively (Scheme 6).
Conclusion
In conclusion, we designed and synthesized novel tetrafluoro-λ6-sulfanes 5 having a linear connection between pyridine and triazole rings via the trans-tetrafluoro-λ6-sulfane (SF4) moiety. The desired compounds 5 were obtained by thermal Huisgen 1,3-dipolar cycloaddition between pyridine SF4-alkynes 3 and azides 4. Benzene-SF4-triazole product 7 was also synthesized using the same protocol. Further coupling of 5 with boronic acids was also possible. These compounds are eccentric fluorinated heterocycles with potential bioactive and surface properties. Further investigations on the application of 5 are underway.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is partially supported by the Tokyo Chemical Industry Foundation, ACT-C from the JST (JPMJCR12Z7) and the Pesticide Science Society of Japan (ET). We thank Mr Masahiro Takada for his contribution in the early stage of this work.References
-
(a)
D. Lentz and K. Seppelt, in Chemistry of Hypervalent Compounds, ed. K.-Y. Akiba, Wiley-VCH, New York, 1999, p. 295 Search PubMed
; (b) P. Kirsch, M. Bremer, M. Heckmeier and K. Tarumi, Angew. Chem., Int. Ed., 1999, 38, 1989 CrossRef
; (c) P. Kirsch, J. T. Binder, E. Lork and G.-V. Röschenthaler, J. Fluorine Chem., 2006, 127, 610 CrossRef
; (d) P. Kirsch and A. Hahn, Eur. J. Org. Chem., 2005, 2005, 3095 CrossRef
; (e) P. Kirsch and M. Bremer, Chimia, 2014, 68, 363 CrossRef PubMed
; (f) B. A. Lindquist, D. E. Woon and T. H. Dunning Jr, J. Phys. Chem. A, 2014, 118, 1267 CrossRef PubMed
; (g) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315 CrossRef PubMed
; (h) F. Micheli, D. Andreotti, S. Braggio and A. Checchia, Bioorg. Med. Chem. Lett., 2010, 20, 4566 CrossRef PubMed
; (i) J. Welch and D. Lim, Bioorg. Med. Chem., 2007, 15, 6659 CrossRef PubMed
.
-
(a)
V. Gouverneur and K. Müller, Fluorine in Pharmaceutical and Medicinal Chemistry: From Biophysical Aspects to Clinical Applications, Imperial College Press, London, 2012 Search PubMed
; (b) P. R. Savoie and J. T. Welch, Chem. Rev., 2015, 115, 1130 CrossRef PubMed
; (c) O. S. Kanishchev and W. R. Dolbier Jr, in Advances in Heterocyclic Chemistry, ed. E. Scriven and C. A. Ramsden, Elsevier, 2016, p. 1 Search PubMed
; (d) P. Das, E. Tokunaga and N. Shibata, Tetrahedron Lett., 2017, 58, 4803 CrossRef
; (e) W. A. Sheppard, J. Am. Chem. Soc., 1962, 84, 3064 CrossRef
; (f) C. Ye, G. L. Gard, R. W. Winter, R. G. Syvret, B. Twamley and J. M. Shreeve, Org. Lett., 2007, 9, 3841 CrossRef PubMed
; (g) T. Umemoto, L. M. Garrick and N. Saito, Beilstein J. Org. Chem., 2012, 8, 461 CrossRef PubMed
; (h) O. S. Kanishchev and W. R. Dolbier Jr, Angew. Chem., Int. Ed., 2015, 54, 280 CrossRef PubMed
; (i) A. Joliton, J.-M. Plancher and E. M. Carreira, Angew. Chem., Int. Ed., 2016, 55, 2113 CrossRef PubMed
; (j) M. Kosobokov, B. Cui, A. Balia, K. Matsuzaki, E. Tokunaga, N. Saito and N. Shibata, Angew. Chem., Int. Ed., 2016, 55, 10781 CrossRef PubMed
; (k) F. W. Friese, A.-L. Dreier, A. V. Matsnev, C. G. Daniliuc, J. S. Thrasher and G. Haufe, Org. Lett., 2016, 18, 1012 CrossRef PubMed
.
-
(a) D. B. Denney, D. Z. Denney and Y. F. Hsu, J. Am. Chem. Soc., 1973, 95, 8191 CrossRef
; (b) I. Ruppert, J. Fluorine Chem., 1979, 14, 81 CrossRef
; (c) K. D. Gupta and J. M. Shreeve, Inorg. Chem., 1985, 24, 1457 CrossRef
; (d) P. Kirsch, M. Bremer, A. Kirsch and J. Osterodt, J. Am. Chem. Soc., 1999, 121, 11277 CrossRef
; (e) X. Ou and A. F. Janzen, J. Fluorine Chem., 2000, 101, 279 CrossRef
; (f) P. Kirsch, J. Krause and K. Tarumi, Ger. Offen., 2000, DE10008505 A1 Search PubMed
; (g) P. Kirsch and A. Hahn, Eur. J. Org. Chem., 2006, 1125 CrossRef
; (h) P. Kirsch and G.-V. Roschenthaler, ACS Symp. Ser., 2007, 949, 221 CrossRef
; (i) L. Zhong, P. R. Savoie, A. S. Filatov and J. T. Welch, Angew. Chem., Int. Ed., 2014, 53, 526 CrossRef PubMed
; (j) L. Zhong, A. S. Filatov and J. T. Welch, J. Fluorine Chem., 2014, 167, 192 CrossRef
; (k) P. Das, M. Takada, E. Tokunaga, N. Saito and N. Shibata, Org. Chem. Front., 2018, 5, 719 RSC
.
-
(a) T. Abe and J. M. Shreeve, Inorg. Nucl. Chem. Lett., 1973, 9, 465 CrossRef
; (b) P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Application, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2013, p. 179 Search PubMed
.
-
(a) H. Kawai, Z. Yuan, E. Tokunaga and N. Shibata, Org. Lett., 2012, 14, 5330 CrossRef PubMed
; (b) X.-H. Xu, M. Taniguchi, X. Wang, E. Tokunaga, T. Ozawa, H. Masuda and N. Shibata, Angew. Chem., Int. Ed., 2013, 52, 12628 CrossRef PubMed
; (c) N. Iida, E. Tokunaga, N. Saito and N. Shibata, J. Fluorine Chem., 2014, 168, 93 CrossRef
; (d) B. Cui, M. Kosobokov, K. Matsuzaki, E. Tokunaga and N. Shibata, Chem. Commun., 2017, 53, 5997 RSC
; (e) P. Das, M. Takada, K. Matsuzaki, N. Saito and N. Shibata, Chem. Commun., 2017, 53, 3850 RSC
; (f) B. Cui, S. Jia, E. Tokunaga, N. Saito and N. Shibata, Chem. Commun., 2017, 53, 12738 RSC
.
-
(a)
J. Michl, in Applications of Organometallic Chemistry in the Preparation and Processing of Advanced Materials, ed. J. E. Harrod and R. M. Laine, Kluwer, Dordrecht, The Netherlands, 1995, p. 243 Search PubMed
; (b) A. Matsuyama and T. Kato, Macromolecules, 1995, 28, 131 CrossRef
; (c) J. Hankache and O. S. Wenger, Phys. Chem. Chem. Phys., 2012, 14, 2685 RSC
; (d) M. Messner, S. I. Kozhushkov and A. de Meijere, Eur. J. Org. Chem., 2000, 1137 CrossRef
; (e) R. L. Nos, A. M. Roma, C. J. Garcia-Cervera and H. D. Ceniceros, J. Non-Newtonian Fluid Mech., 2017, 248, 62 CrossRef
; (f) J. Uchida and T. Kato, Liq. Cryst., 2017, 44, 1816 Search PubMed
.
-
(a) J. Kaleta, Z. Janousek, M. Necas and C. MazalI, Organometallics, 2015, 34, 967 CrossRef
; (b) M. Cipolloni, J. Kaleta, M. Masǎt, P. I. Dron, Y. Shen, K. Zhao, C. T. Rogers, R. K. Shoemaker and J. Michl, J. Phys. Chem. C, 2015, 119, 8805 CrossRef PubMed
; (c) J. Kaleta, M. Necas and C. Mazal, Eur. J. Org. Chem., 2012, 4783 CrossRef
; (d) S. Makarov, C. E. Brocklehurst, K. Karaghiosoff, G. Koch and P. Knochel, Angew. Chem., Int. Ed., 2017, 56, 12774 CrossRef PubMed
; (e) Y. P. Auberson, C. Brocklehurst, M. Furegati, T. C. Fessard, G. Koch, A. Decker, L. L. Vecchia and E. Briard, ChemMedChem, 2017, 12, 590 CrossRef PubMed
.
-
(a)
K. C. Majumdar and S. K. Chattopadhyay, Heterocycles in Natural Product Synthesis, Wiley-VCH Verlag GmbH &
Co. KGaA, 2011 Search PubMed
; (b) T. Kosjek and E. Heath, Halogenated Heterocycles as Pharmaceuticals in Halogenated Heterocycles. Topics in Heterocyclic Chemistry ed. J. Iskra, Springer, Berlin, Heidelberg, 2011, Vol 27 Search PubMed
; (c) C. Lamberth and J. Dinges, Bioactive Heterocyclic Compound Classes: Pharmaceuticals, Wiley-VCH Verlag GmbH & Co. KGaA, 2012 Search PubMed
.
- Selected reports for pyridines:
(a)
J. X. Qiao, Heterocyclic Chemistry in Drug Discovery, ed. J. J. Li, Wiley, Hoboken, 2013 Search PubMed
; (b) R. Dua, S. Shrivastava, S. K. Sonwane and S. K. Shrivastava, Adv. Biol. Res., 2011, 5, 120 Search PubMed
; (c) A. A. Altaf, A. Shahzad, Z. Gul, N. Rasool, A. Badshah, B. Lal and E. Khan, Journal of Drug Design and Medicinal Chemistry, 2015, 1, 1 Search PubMed
; (d) A. Chaubey and S. N. Pandeya, Asian J. Pharm. Clin. Res., 2011, 4, 5 Search PubMed
Selected reports for triazoles: (e) E. Bonandi, M. S. Christodoulou, G. Fumagalli, D. Perdicchia, G. Rastelli and D. Passarella, Drug Discovery Today, 2017, 22, 1572 CrossRef PubMed
; (f) D. Dheer, V. Singh and R. Shankar, Bioorg. Chem., 2017, 71, 30 CrossRef PubMed
; (g) P. Nagender, G. M. Reddy, R. N. Kumar, Y. Poornachandra, C. G. Kumar and B. Narsaiah, Bioorg. Med. Chem. Lett., 2014, 24, 2905 CrossRef PubMed
; (h) S. G. Agalave, S. R. Maujan and V. S. Pore, Chem.–Asian J., 2011, 6, 2696 CrossRef PubMed
; (i) J. Hou, X. Liu, J. Shen, G. Zhao and P. G. Wang, Expert Opin. Drug Discovery, 2012, 7, 489 CrossRef PubMed
; (j) R. Kharb, P. C. Sharma and M. S. Yar, J. Enzyme Inhib. Med. Chem., 2011, 26, 1 CrossRef PubMed
.
-
(a)
V. A. Petrov, Fluorinated Heterocyclic Compounds: Synthesis, Chemistry, and Applications, Wiley, Hoboken, 2009 Search PubMed
; (b) A. P. Taylor, R. P. Robinson, Y. M. Fobian, D. C. Blakemore, L. H. Jones and O. Fadeyi, Org. Biomol. Chem., 2016, 14, 6611 RSC
; (c) D. A. DiRocco, K. M. Oberg, D. M. Dalton and T. Rovis, J. Am. Chem. Soc., 2009, 131, 10872 CrossRef PubMed
; (d) W.-B. Yi and C. Cai, Adv. Mater. Res., 2013, 726–731, 165 CrossRef
; (e) R. D. Chambers, Dyes Pigm., 1982, 3, 183 CrossRef
.
-
(a) H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004 CrossRef
; (b) J. E. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249 RSC
; (c) L. Liang and D. Astruc, Coord. Chem. Rev., 2011, 255, 2933 CrossRef
; (d) D. Pasini, Molecules, 2013, 18, 9512 CrossRef PubMed
; (e) W. Tanga and M. L. Becke, Chem. Soc. Rev., 2014, 43, 7013 RSC
; (f) M. S. Singh, S. Chowdhury and S. Koley, Tetrahedron, 2016, 72, 5257 CrossRef
; (g) H. B. Jalani, A. C. Karagoez and S. B. Tsogoeva, Synthesis, 2017, 49, 29 Search PubMed
.
- J. R. Johansson, T. B. Somfai, A. S. Stålsmeden and N. Kann, Chem. Rev., 2016, 116, 14726 CrossRef PubMed
.
- To improve the regioselectivity, we further attempted the reaction under Cu,13a,b Ir13c or Ru13d catalysis, which were reported for regioselective cycloaddition. However,
we didn’t obtain any favorable result and found that the reaction proceeded only via thermal assistance to provide a mixtures of regioisomers. Eventually, the SF4-alkynes are very different from other types of alkynes, presumably due to the combination of the steric bulkiness and electron withdrawing nature of the SF4-unit (see Scheme S1 in the ESI for details†)
(a) F. Himo, T. Lovell, R. Hilgraf, V. V. Rostovtsev, L. Noodleman, K. B. Sharpless and V. V. Fokin, J. Am. Chem. Soc., 2005, 127, 210 CrossRef PubMed
; (b) F.-H. Cui, J. Chen, Z.-Y. Mo, S.-X. Su, Y.-Y. Chen, X.-L. Ma, H.-T. Tang, H.-S. Wang, Y.-M. Pan and Y.-L. Xu, Org. Lett., 2018, 20, 925 CrossRef PubMed
; (c) S. Ding, G. Jia and J. Sun, Angew. Chem., Int. Ed., 2014, 53, 1877 CrossRef PubMed
; (d) P. Destito, J. R. Couceiro, H. Faustino, F. Lopez and J. L. Mascarenas, Angew. Chem., Int. Ed., 2017, 56, 10766 CrossRef PubMed
.
-
(a) S. Reimann, S. Parpart, P. Ehlers, M. Sharif, A. Spannenbergb and P. Langer, Org. Biomol. Chem., 2015, 13, 6832 RSC
; (b) S. He, P. Li, X. Dai, H. Liu, Z. Lai, D. Xiao, C. C. McComas, C. Du, Y. Liu, J. Yin, Q. Dang, N. Zorn, X. Peng, R. P. Nargund and A. Palani, Tetrahedron Lett., 2017, 58, 1373 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1823578 and 1823581. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc01216d |
| This journal is © The Royal Society of Chemistry 2018 |