Organic chemistry students’ interpretations of the surface features of reaction coordinate diagrams
Maia
Popova
 and
Stacey Lowery
Bretz
and
Stacey Lowery
Bretz
 *
*
Miami University, Department of Chemistry & Biochemistry, Oxford, OH, USA. E-mail: bretzsl@miamioh.edu
First published on 9th June 2018
Abstract
Organic chemistry students struggle with understanding the energetics of chemical reactions. Reaction coordinate diagrams are one tool that is widely used in organic chemistry classrooms to assist students with visualizing and explaining the energy changes that take place throughout a reaction. Thirty-six students enrolled in organic chemistry II participated in a qualitative study that used semi-structured interviews to investigate the extent to which students meaningfully extract and integrate information encoded in reaction coordinate diagrams. Results show that students have difficulties explaining the meanings of surface features such as peaks, valleys, peak height, and peak width. Analysis of students’ explanations resulted in four themes that describe students’ challenges with correctly interpreting the features of reaction coordinate diagrams. Students conflated transition states and intermediates, despite being able to recite definitions. Students described the chemical species encoded at points along the x-axis of the reaction coordinate diagrams, while largely ignoring the energies of the species encoded along the y-axis. Implications for teaching organic chemistry are discussed.
Introduction and background
Problem-solving in organic chemistry requires process-oriented thinking, as proposing a reaction mechanism cannot be achieved through memorization (Frey et al., 2017). Multiple studies have investigated students’ approaches to solving mechanistic problems. Students “decorate” reaction equations with arrows, but do not understand the meaning of the arrows (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012). Students also have difficulties grasping the physical processes involved in the transformation of reactants into products, preferring to focus on individual structures rather than the overall mechanism (Bhattacharyya and Bodner, 2005). One implication of this research is that instructors need to shift students’ attention from focusing on specific reaction species to considering the least energetic reaction pathways (Bhattacharyya and Bodner, 2005). Understanding energetics associated with chemical reactions in molecular scale systems has been identified as an anchoring concept for learning organic chemistry (Raker et al., 2013). Reaction coordinate diagrams (RCDs) are widely used as a tool to represent reaction mechanisms, as they have the potential to assist students in their understanding of energetic changes that occur throughout a reaction (Allinger, 1963; Meek et al., 2016). No research, however, has previously investigated students’ understandings of RCDs in organic chemistry. Although some studies regarding students’ misconceptions about reaction mechanisms and kinetics have mentioned a few aspects of RCDs, students’ understandings of RCDs have not been the main focus of this prior research. As part of a study investigating Turkish pre-service teachers’ conceptions about reaction mechanisms, participants were asked to identify where intermediates are encoded on RCDs. While a majority of the pre-service teachers were able to provide a correct definition of an intermediate, they were unable to correctly determine where an intermediate is represented on an RCD. The pre-service teachers also conflated ideas of activated complex and intermediate (Taştan et al., 2010). In a different study, Morrison and colleagues reported that students were able to distinguish between endergonic and exergonic RCDs, but that more research was needed to learn whether students are able to correctly predict relative energies of reaction species for multistep reactions (Morrison et al., 2014). Additional research regarding students’ understandings of kinetics has reported that students hold multiple alternative conceptions regarding the kinetics of reaction mechanisms, including ideas such as “increasing the temperature increases the activation energy,” “no recognition of the slow step as the rate determining step,” and “a catalyst increases activation energy of the reaction” (Çalik et al., 2010; Taştan et al., 2010; Kolomuç and Tekin, 2011; Kaya and Geban, 2012). Recent reviews have called for further research regarding students’ understandings of external representations related to kinetics and reaction mechanisms in order to investigate possible sources of students’ difficulties with respect to the aforementioned concepts (Kirik and Boz, 2012; Bain and Towns, 2016). In this study we investigated students’ thinking regarding the meanings of the features of RCDs.Representational competence
External representations are broadly used in chemistry as they provide simplified depictions of complex, abstract, submicroscopic chemical phenomena (Rouse and Morris, 1986; Davidowitz and Chittleborough, 2009; Prins, 2010). The ability to effectively use external representations to think and communicate about chemical phenomena has been defined as the notion of representational competence (Kozma and Russell, 1997, 2005). Novices with little representational competence are considered to either rely on the surface features of representations in order to solve problems, or to use heuristics that involve the mechanical application of symbolic rules that are grounded in memorization (Chi et al., 1981; Kozma and Russell, 1997, 2005; Weinrich and Talanquer, 2015). Expert chemists with higher levels of representational competence are able to easily translate between multiple modes of representations, conceptually understand the information encoded in different representations, and demonstrate an epistemological understanding of the nature of different representations, including their assumptions and limitations (Kozma and Russell, 2005). In particular, representational competence in chemistry includes the abilities to “use words to identify and analyse features of a particular representation” and “use representations to describe observable chemical phenomena in terms of underling molecular entities and processes” (Kozma and Russell, 2005). Interpreting the features of representations, however, is a cognitively demanding task, as the explicit features often “stand for” chemical species or processes that are implicit and not readily apparent (Elby, 2000; Tufte, 2001). Novices find it challenging to decode otherwise abstract chemical concepts (Seufert and Brunken, 2004). Thus, when developing representational competence, emphasis must be placed on developing discourse and meaning-making between the visible surface features of the representations and the abstract chemical concepts that are encoded in them (Seufert and Brunken, 2004). In order to design instruction to support such meaning making, it is important to understand how students interpret the surface features of representations.Surface features of reaction coordinate diagrams and their underlying meanings
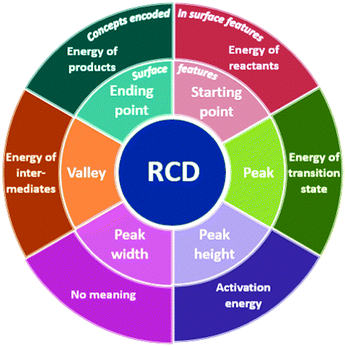
RCDs are an external representation widely used in organic chemistry classrooms to explain the energy changes that occur throughout reaction pathways of different mechanisms (Hulse et al., 1974). These diagrams can be fairly complex in that their surface features represent information about both the kinetic and the thermodynamic considerations of reaction mechanisms. While RCDs may include quantitative information that can be used to calculate ΔH values, in organic chemistry classrooms, RCDs are used primarily for qualitative discussion of reaction mechanisms. Thus, in organic chemistry courses, the axes of an RCD often do not display units and the diagram's features are not necessarily generated to scale.Seufert and Brunken (2004) refer to the explicit features as “the surface features level of representations,” which for an RCD includes the curve itself, the different points along the curve (starting point, peak, valley, ending point), peak height and width, and axes with their corresponding labels. The meanings encoded in each surface feature comprises what Seufert and Brunken (2004) refer to as the deep structure level. Thus, the starting point of an RCD represents the energy of the reactants, a valley represents the energy of an intermediate, and so on (Fig. 1). Conceptual understanding of the information encoded in a representation has been achieved when learners form coherence between the surface feature level and the deep structure level (Seufert and Brunken, 2004). The implicit meanings of the deep structure level for representations are communicated primarily by verbal means.
Therefore, the research question that framed this study was how do students interpret and describe salient features of reaction coordinate diagrams? Specifically, we sought to investigate students’ understandings of the surface features of RCDs, namely, the starting point, peaks, peak height, peak width, valleys, and the ending point.
Methods
Sample and setting
The target populations for this study were undergraduate chemistry majors and non-majors enrolled in organic chemistry II at a medium-sized, liberal arts university in the midwestern United States. Prior to beginning the study, an application was submitted to the Institutional Review Board (IRB) to ensure protection of the rights of student participants. Participants were recruited from two second-semester organic chemistry lecture courses, typically taken by the students in the second year of their undergraduate studies. The textbook used for the majors’ course was Organic Chemistry, by Jones and Fleming (2014) and the textbook in the non-majors’ class was Organic Chemistry, by Klein (2012). RCDs were introduced in organic chemistry I in the non-majors’ course in a chapter that reviewed thermodynamics and kinetics and in the majors’ course in a chapter about alkenes and alkynes. RCDs were further used in the context of introducing and explaining substitution, elimination, and addition reactions. Students were asked to analyze relative heights of peaks and determine the rate-determining step both in class and during exams. RCDs were not commonly used when teaching reaction mechanisms in either organic chemistry II course.Students were sent an email that briefly described the study and invited them to participate. The email contained a link to a survey that collected demographic information such as undergraduate year, race, gender, major, and grades earned in previous chemistry courses (first-year university chemistry I & II, as well as organic chemistry I). Thirty-six students were purposefully selected (Patton, 2002; Bretz, 2008) for the study to ensure that the sample included students who had earned a range of grades in organic chemistry I (14 students earned a letter grade of “A”, 14 earned “B”, and 8 earned a “C”). The sample included 15 male and 21 female students, and 6 chemistry majors and 30 non-majors with 8 students enrolled in the major's course and 28 students enrolled in the non-major's course (N.B. Non-major students had the option to enroll in the majors’ course due to schedule conflicts). Pseudonyms were created for all participants in order to protect their identities.
Data collection and analysis
A qualitative semi-structured interview was used to elicit students’ ideas (Drever, 1995). This method allows for follow-up questions to be asked in order to more deeply probe students’ understandings. Interviews took place while students were enrolled in the organic chemistry II lecture courses, starting in the third week of the spring 2016 semester. The interviews were conducted individually and required approximately one hour to complete. Each participant received a $20 gift card as compensation for their time.The interview was both audio- and video-recorded, with concurrent note-taking, using a Livescribe™ Smartpen, audio recorder, and video camera. The Livescribe™ Smartpen was used in order to simultaneously capture everything a student said, wrote, and/or drew on the Livescribe™ dot paper (Linenberger and Bretz, 2012). The audio recorder was used as a backup in case the Livescribe failed during the interview. The video camera was used to record students’ gestures that were subsequently used to annotate the transcripts (e.g., clarification of students’ use of “this” or “that” while pointing to specific features on the RCDs).
Each interview was transcribed verbatim and verified against the video, audio, and Livescribe data. Students’ verbal descriptions, gestures, writings, and drawings were used to augment the transcript. The use of multiple methods of data collection allowed for clarification of the words and phrases that were unclear during the transcription process, thereby ensuring greater fidelity of the final interview transcript.
The transcript data was managed using the NVivo 11 software (Creswell, 2003; Bazeley and Jackson, 2013; QSR International Pty Ltd, 2015). The process of data analysis started with inductive coding by compiling students’ responses about each feature of the RCDs, in order to closely examine participants’ ideas about the meaning of each feature and to look for similarities and differences. The emergent codes from this descriptive qualitative analysis consisted of meaningful words and phrases. Two types of codes were generated: in vivo codes (wording used by the participants themselves in the interview) and constructed codes (codes created by the researcher to summarize a common idea expressed by the study participants) (Bradley et al., 2007). Students described a range of interpretations for each feature, some more accurate than others. Therefore, to analyze the students’ understandings of the meanings encoded in each RCD feature, the data was subsequently deductively coded using the modified concept-evaluation scheme (Abraham et al., 1992) shown in Table 1.
| Level of understanding | Criteria for scoring |
|---|---|
| No understanding | Attempts to answer, but does not know or remember the answer |
| Specific misconception | Responses that include illogical or incorrect information |
| Partial understanding with specific misconception | Responses that show some understanding of the concept, but also make statements which demonstrate a misunderstanding of a concept or a term |
| Partial understanding | Responses that include at least one of the components of the correct response, but not all the components |
| Sound understanding | Responses that include all components of the correct response |
The coding process was also accompanied by writing memos in order to capture the evolution of the researchers’ thoughts about both the raw data and the generated codes, which aided in mapping research activities and communication between the researchers (Birks et al., 2008). The constant comparative method was used to form themes that synthesized the meanings of similar codes (Corbin and Strauss, 1990). To ensure the trustworthiness of the coding process, the authors conducted weekly meetings during which codes were discussed and revised. In addition, the confirmability and credibility of the results were established through periodic external debrief sessions with the other chemistry education researchers at the institution who were uninvolved with the project (Lincoln and Guba, 1985; Creswell, 2003).
Description of interview prompts
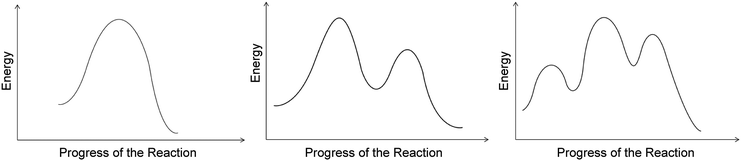
Each interview began with an introduction to the study, a description of the think-aloud protocol, and an explanation of what students would be asked to do during the interview. The students were invited to write and draw on the Livescribe™ dot paper in order to provide more detailed descriptions of their thinking if they wished to do so; students were not required to draw or write during the interview. Students were also given a consent form that described their rights and the treatment of their confidential data. Participants were given an opportunity to read the consent document and ask questions.The interview protocol consisted of four phases. Phase I asked questions to ascertain students’ prior knowledge about bonding, stability, and reactivity of organic structures. Phase II asked students to explain how bonds were formed and broken in one substitution reaction mechanism and one elimination reaction mechanism. Students were asked to comment on the relative stability of chemical species in each step of the reactions. Phase III asked students to explain each feature of three different RCDs that contained one, two, or three transition states (Fig. 2). The RCDs were generated using Adobe® Photoshop® software (Adobe, 1990) and subject to expert validation by three organic chemistry faculty, two of whom were instructors for the courses from which the students were sampled. In Phase IV, students matched reactions from Phase II with RCDs from Phase III in order to elicit the students’ reasoning about the connections between reactions and RCDs. This manuscript presents an analysis of the data from Phase III of the full interview. The full interview protocol and the findings from Phases II and IV of the interview have been reported elsewhere (Popova and Bretz, 2018a, 2018b, 2018c). Colleagues interested in obtaining a copy of the full interview protocol for research purposes should contact the corresponding author.
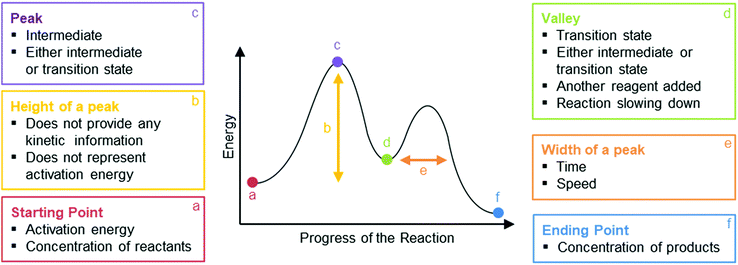
Phase III of the interview began with a general question about what RCDs show, after which students were asked to explain the meaning of each feature of an RCD: the starting point, peak(s), height of peak(s), width of peak(s), valley(s), and the ending point (Fig. 3).
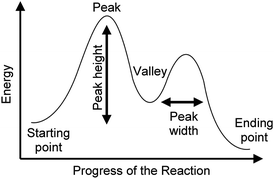 | ||
| Fig. 3 Features of reaction coordinate diagrams that students were asked to describe. The arrows and names of the features were not included on the reaction coordinate diagrams shown to students. | ||
Results and discussion
Students’ descriptions of the RCD features and the meanings encoded in them were analyzed using the coding scheme in Table 1 (Abraham et al., 1992). Exemplars for each level of the coding scheme are provided here.Responses were coded as “no understanding” when students attempted to answer a question but admitted that they did not remember or know the answer. For example, when Alina (second-year kinesiology major) was asked about the meaning of a valley, she responded:
“Yes, they [valleys] do have a meaning… You compare… it's always the bottom of the peak… Okay, there is like, there is k, no, it's not k… I definitely remember learning this. Yes, it has meaning. It does mean something, I just forget what it is.”
Similarly, Aleksei (second-year premedical studies major) also failed to explain the meaning of a valley:
“Um, valleys are um… Um… I am trying to think… I think I know… I know at one point I knew. Um, I was pretty solid on this at one point.”
Responses were coded as “specific misconception” when students’ answers contained only incorrect or illogical information. For instance, multiple students expressed the misconception that peak width represents time:
“It's just like the amount of time that it actually takes to hit that point in a reaction.” Anna (second-year chemical engineering major)
“I guess it [peak width] shows how long it takes for that step to happen.” Elena (second-year microbiology major)
Responses were coded as “partial understanding with specific misconception” when students’ answers were partially correct, but they also included statements that indicated misunderstanding of a concept or a term. Consider this excerpt from Alisa's (second-year chemistry major) interview:
![[I with combining low line]](https://www.rsc.org/images/entities/char_0049_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[v with combining low line]](https://www.rsc.org/images/entities/char_0076_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif) : “What do these peaks represent?”
: “What do these peaks represent?”
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/char_006c_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) : “These are the two intermediates [drew stars above peaks inFig. 4] because reaction goes in two steps.”
: “These are the two intermediates [drew stars above peaks inFig. 4] because reaction goes in two steps.”
![[I with combining low line]](https://www.rsc.org/images/entities/char_0049_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[v with combining low line]](https://www.rsc.org/images/entities/char_0076_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif) : “What is an intermediate?”
: “What is an intermediate?”
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/char_006c_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) : “It's the middle phase when not all of the bonds are completely broken and new ones are formed.”
: “It's the middle phase when not all of the bonds are completely broken and new ones are formed.”
![[I with combining low line]](https://www.rsc.org/images/entities/char_0049_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[v with combining low line]](https://www.rsc.org/images/entities/char_0076_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif) : “And what does the valley represent?”
: “And what does the valley represent?”
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/char_006c_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) : “Um, those are the products of the first step.”
: “Um, those are the products of the first step.”
Alisa knew that the peaks in RCDs represent reaction species in a chemical reaction that persist while bonds are breaking and new bonds are forming, but incorrectly called these species “intermediates”. She also did not remember the term for the species encoded in valley.
Responses were coded as “partial understanding” when students’ answers included at least one component of a complete and correct response, but not all the components. For example, many students described the ending point in an RCD in terms of the products (considering x-axis), but did not comment upon energy (y-axis):
“That [ending point] is when the reaction has reached completion and that is when you have your products.” (Maksim, third-year microbiology major)
Responses were coded as “sound understanding” when students’ answers were both correct and complete. For instance, when asked about the meaning of the starting point, Inga's (second-year biology major) answer integrated both the features of the x-axis and the y-axis:
“It [starting point] shows the original energy of the starting reactants.”
Findings from the deductive coding using the modified concept-evaluation scheme
Students’ descriptions of each feature of RCDs were coded using the modified concept-evaluation scheme. The number of responses in each level were summed and converted into percentages (Fig. 5). Although 36 students were interviewed, only 29 were asked to discuss the feature of peak width as the question about peak width was not originally included in the interview protocol. The eighth research participant Lev (second-year biochemistry major) mentioned during his interview that when analyzing RCDs, chemists pay attention to “the height of the peaks and the width. The width might have, I can be wrong, but width might have something to do with how long it [reaction step] might take.” Subsequent to Lev's interview, the authors chose to ask each student about this feature.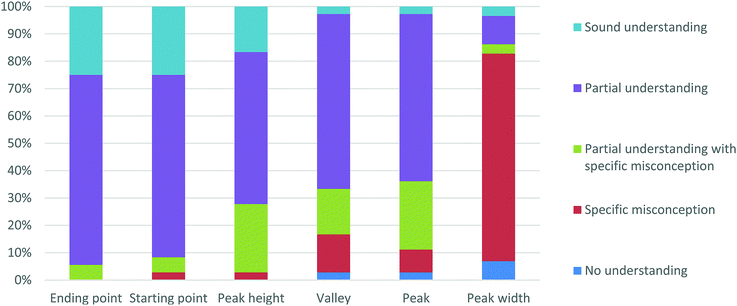 | ||
| Fig. 5 Students’ descriptions regarding the features of reaction coordinate diagrams, coded using Abraham's modified concept-evaluation scheme. | ||
The majority of students’ responses about the meaning of the starting point, ending point, valley, peak, and peak height were coded as “partial understanding.” The majority of responses about the meaning of peak width, however, were coded as “specific misconception.” When examining these responses in light of students’ prior performance in organic chemistry I (i.e., whether the student had earned a grade of ‘A’, ‘B’, or ‘C’), we found no differences. That is to say, ‘A’ students expressed a range of ideas from “no understanding” to “sound understanding” just as the ‘C’ students did. Furthermore, no significant differences were identified when comparing the reasoning of majors to that of non-majors, as both majors and non-majors provided a range of interpretations.
Students had a good grasp of the meanings of the starting and ending points as they provided responses which were primarily coded as “partial understanding.” By contrast, nearly one-third of students had difficulties correctly describing the meanings of peaks, peak heights, and valleys, as indicated by the number of responses coded as “no understanding”, “specific misconception”, or “partial understanding with specific misconception.” Peak width was particularly problematic for students to discuss, as indicated by nearly 85% of students who provided responses coded as either “no understanding”, “specific misconception”, or “partial understanding with specific misconception.”
Challenges with reading RCDs
The constant comparative analysis of the data corpus resulted in the identification of themes or challenges (Table 2) faced by students when trying to interpret and discuss the salient features of RCDs. Note that the total number of instances in Table 2 is more than the total number of participants in the study because one student could encounter multiple, but different, types of challenges when interpreting features of an RCD. Exemplars of these challenges, and their connections to the analysis regarding the level of understanding (Table 1), are discussed below. Note that Tables 3–6 present the specific codes associated with each challenge. The total number of students in each table is greater than the total number of students who demonstrated a specific challenge reported in Table 2 because in some instances an individual student presented multiple difficulties and therefore was assigned to multiple codes within the same challenge.| Challenge | n |
|---|---|
| (I) Discerning chemistry concepts encoded in RCD feature | 35 |
| (II) Mapping terminology onto RCD feature | 5 |
| (III) Imposing unintended chemistry concepts upon RCD feature | 22 |
| (IV) Differentiating between chemistry concepts | 12 |
| Category | Code | n | |
|---|---|---|---|
| Feature | Meaning/description | ||
| States that a feature does not communicate an intended chemistry concept | Peak height | Does not provide any kinetic information | 3 |
| Does not represent activation energy | 3 | ||
| Describes chemical species associated with a feature without discussing energy | Peak | Reaction step & transition state | 4 |
| Reaction step | 8 | ||
| Transition state | 8 | ||
| Valley | Products of a reaction step | 1 | |
| Intermediates | 21 | ||
| Starting point | Start of a reaction | 1 | |
| Reactants | 21 | ||
| Ending point | End of a reaction | 1 | |
| Products | 21 | ||
| Codes | n | |
|---|---|---|
| Feature | Meaning/description | |
| Peak | Not an actual intermediate | 1 |
| Intermediate step when bonds are not completely broken or formed | 1 | |
| Transition state or intermediate when bonds are breaking or forming | 2 | |
| Valley | Intermediate or transition state when bonds are not breaking or forming | 1 |
| Something like a carbocation, unstable but isolatable species (used term intermediate for peak) | 1 | |
| Products of a step (used term intermediate for peak) | 2 | |
| Codes | n | |
|---|---|---|
| Feature | Meaning/description | |
| Starting point | Activation energy | 1 |
| y-Axis | Concentration of species | 2 |
| Valley | Another reagent added | 1 |
| Reaction slowing down | 2 | |
| Peak width | Speed | 6 |
| Time | 17 | |
| Codes | n |
|---|---|
| Reaction progress vs. reaction time | 5 |
| Transition state vs. intermediate | 10 |
Challenge I: Discerning chemistry concepts encoded in RCD feature (n = 35). Table 3 summarizes the codes that describe students’ thinking regarding this challenge. The codes have been grouped into two categories.
The first category of Challenge I captures instances when students explicitly reported that a specific RCD feature did not communicate an intended chemistry concept. The codes in this category reflect the “partial understanding with specific misconception” level in the modified concept-evaluation scheme (Table 1). For example, when asked about the meaning of the height of the peak, a majority of students (n = 23) responded that this feature showed the amount of activation energy. When discussing the heights of peaks and activation energy, a few students noted the relationship between the height of a peak and the speed of a reaction step. However, three students, when asked whether any information about how fast each reaction step proceeds could be gleaned from an RCD, reported that RCDs do not provide any such information:
“I don’t think that you could tell the speed from it. But you could know, like if these [peaks] were the same scale, you know that this [lower peak] might happen more easily than that [higher peak].” (Vlad, second-year chemical engineering major)
or that only thermodynamic information is encoded in RCDs, not kinetic:
“I don’t think you can get how fast it is. This is more of the thermodynamics I would say.” (Daria, second-year biology major)
Three additional students did not associate activation energy with peak height at all. One student stated that the starting point represents activation energy, one student was indecisive about whether the activation energy was represented by the starting point or by the peak, and a third student reported that the activation energy for the first step was the distance between the valley and the apex of the peak, rather than the distance between the starting point and the apex of the peak.
The second category of Challenge I involves instances when students were able to describe chemical species associated with a specific feature of an RCD, but their description omitted any discussion of energy. The codes in this category reflect “partial understanding” (included at least one component of a correct answer, but not all the components of a complete answer). For instance, when asked about the meaning of a valley, the majority of students (n = 21) reported that this feature represented intermediates, rather than saying that it represented the energy of intermediates:
“This [valley] is the intermediate. It's sort of like a transition state because it also happens throughout the reaction, but it's like more stable, so sometimes you can isolate it.” (Elena, second-year microbiology major)
Such responses demonstrate that students consider the chemical species encoded in the valley feature, but not the energy of these species. That is, students take into consideration the x-axis, but not the y-axis of an RCD. Similarly, eight students reported that peaks represent transition states, another eight students stated that peaks showed reaction steps, and four students discussed both of these concepts in their explanations.
Challenge II: Mapping terminology onto RCD feature (n = 5). Table 4 summarizes the codes that describe students’ thinking regarding this challenge.
Each code in Table 4 was coded as “partial understanding with specific misconception.” This theme depicts instances when students described peaks and valleys in terms of stability of species that were encoded in them, but did not remember what these species were called. Consider the responses of Denis (third-year zoology major):
![[I with combining low line]](https://www.rsc.org/images/entities/char_0049_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[v with combining low line]](https://www.rsc.org/images/entities/char_0076_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif) : “What does this diagram [RCD with two peaks] show?”
: “What does this diagram [RCD with two peaks] show?”
![[D with combining low line]](https://www.rsc.org/images/entities/char_0044_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif) : “It shows the progress of a reaction. So this right here [pointed at the starting point] is the reactants, and this right here [pointed at the peak] will be the intermediate step, and this right here [pointed at ending point] is products. This right here [pointed at valley] will be an actual intermediate product or intermediate stage.”
: “It shows the progress of a reaction. So this right here [pointed at the starting point] is the reactants, and this right here [pointed at the peak] will be the intermediate step, and this right here [pointed at ending point] is products. This right here [pointed at valley] will be an actual intermediate product or intermediate stage.”
![[I with combining low line]](https://www.rsc.org/images/entities/char_0049_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[v with combining low line]](https://www.rsc.org/images/entities/char_0076_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif) : “How is what is in the valley different from what is in the peak?”
: “How is what is in the valley different from what is in the peak?”
![[D with combining low line]](https://www.rsc.org/images/entities/char_0044_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[s with combining low line]](https://www.rsc.org/images/entities/char_0073_0332.gif) : “Well this [valley] is a lot more stable than what is up here [peak]. But this up here is not an actual, it's not anything that formed, it's in the process of breaking one bond and forming another.”
: “Well this [valley] is a lot more stable than what is up here [peak]. But this up here is not an actual, it's not anything that formed, it's in the process of breaking one bond and forming another.”
Denis stated that a peak represents an “intermediate step”, whereas a valley shows “an actual intermediate product or intermediate stage.” An initial reading might suggest that Denis does not understand the chemical concepts that are encoded in a valley and a peak. However, the additional explanation he offers to the interviewer shows that he does indeed understand what these features of RCDs represent, but that he does not remember that one feature is called an intermediate and the other feature is called a transition state.
Likewise, Vlad (second-year chemical engineering major) demonstrated confusion between the terms intermediate and transition state:
![[I with combining low line]](https://www.rsc.org/images/entities/char_0049_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[v with combining low line]](https://www.rsc.org/images/entities/char_0076_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif) : “What does the peak represent?”
: “What does the peak represent?”
![[V with combining low line]](https://www.rsc.org/images/entities/char_0056_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/char_006c_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/char_0064_0332.gif) : “That is when bonds break or something like that happens, and you got atoms with formal charges and stuff like that. They go through this, um, like intermediates or like a transition, where for a while they have more energy as they are forming, or like after they’ve broken a bond. They are trying to form new bonds with something else.”
: “That is when bonds break or something like that happens, and you got atoms with formal charges and stuff like that. They go through this, um, like intermediates or like a transition, where for a while they have more energy as they are forming, or like after they’ve broken a bond. They are trying to form new bonds with something else.”
![[I with combining low line]](https://www.rsc.org/images/entities/char_0049_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[v with combining low line]](https://www.rsc.org/images/entities/char_0076_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif) : “So what is the difference between what is at the peak and at the valley?”
: “So what is the difference between what is at the peak and at the valley?”
![[V with combining low line]](https://www.rsc.org/images/entities/char_0056_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/char_006c_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif)
![[d with combining low line]](https://www.rsc.org/images/entities/char_0064_0332.gif) : “I guess just like, a valley would be, a peak would be um, like in between um… I guess a peak would be when bonds are breaking and reforming. And a valley is when no bonds are changing.”
: “I guess just like, a valley would be, a peak would be um, like in between um… I guess a peak would be when bonds are breaking and reforming. And a valley is when no bonds are changing.”
Vlad contrasted the species that are encoded in the valley against the species that are encoded in the peak that are in the process of breaking and forming bonds. However, he could not tell which species is called an intermediate and which is called a transition state. His confusion with terminology is not surprising as the lexical semantics of both of these terms mean “something in between.”
Challenge III: Imposing unintended chemistry concepts upon RCD feature (n = 22). Most of the codes that describe students’ thinking under Theme III (Table 5) were coded as a “specific misconception,” with a few coded as “partial understanding with specific misconception.”
Theme III captures instances of students providing alternative interpretations by assigning other chemistry concepts to the RCD features. For example, instead of interpreting the starting point as showing the energy of the reactants, Vlad (second-year chemical engineering major) suggested that the starting point represents the activation energy “because [the] reaction, obviously, won’t start without being activated.”
Even though the y-axis in the RCDs shown to students during interviews was labelled “Energy,” two students thought the y-axis depicted concentration. For example, when asked to interpret the meaning of the starting and the ending points, Greta (third-year kinesiology major) indicated that these features showed the concentrations of reactants and products:
“Um, it [starting point] is the concentration of the starting materials… [The ending point is] the concentration of the, like the end product.”
Two different alternative interpretations were provided for the valleys in RCDs. Victor (third-year chemical engineering major) explained that a valley represented the physical addition of a new reagent to a reaction mixture:
“So this valley right here would represent another reagent being added or maybe perhaps a catalyst would have to be added to help continue it all the way through.”
Victor's misconception was connected to his prior knowledge and experiences in the organic chemistry laboratory where he had the physical experience of adding a reagent to a reaction flask that already contained starting material. Two students, including Raisa (third-year biology major) explained that a valley represents a point in time when a reaction slows down:
“In this one [pointing at an RCD with two peaks], it would be, so reaction would have gotten started and then it would have slowed and then something would have activated it again, perhaps one of the products is regenerated, or there was an addition of a catalysts that started it up again.”
Raisa's reasoning is similar to the “ball rolling over a hill” or “roller coaster” analogies that are often used to introduce RCDs in first-year university chemistry textbooks (Brown et al., 2006; Zumdahl and Zumdahl, 2012). In fact, Maksim (third-year microbiology major) specifically mentioned a roller coaster when asked to describe RCDs:
“I think that when you look at a diagram like this, the first activation barrier is what you should look at first… Because that is what you need… Even if it's like a simple step or a two step, that is what you have to initially overcome for your reaction to even start… I think about this as a roller coaster…”
Other students also reported thinking about a ball rolling from a hill: “The, the favorite, the metaphor for describing it [RCD] is like rolling ball from a hill.” (Filipp, second-year biochemistry major)
Raisa, Maksim, and Filipp made connections between the shape of a valley in RCDs and their experiences from everyday life such as riding a roller coaster (Fig. 6) or the changing slopes of the earth at the bottom of a hill. Textbook analogies about roller coasters or balls on hills that are intended to invoke students’ prior knowledge from their everyday lives may in fact interfere with meaningful learning as students learn to decode and discern salient features of RCDs. Rather than inspecting the relative heights of peaks in RCDs to draw conclusions about which step has the largest activation energy and therefore the slower rate, these students chose to focus on the shape of the valley and incorrectly conclude that a valley either indicates another reagent added to the reaction flask or that reaction slowed down.
 | ||
| Fig. 6 Changes in the speed of a roller coaster (Adapted from Clipartix.com, n.d.). | ||
Regarding the width of peaks, students proposed two alternative interpretations. Several students (n = 6) incorrectly interpreted the width of the peak to provide information about the speed of the reaction:
“Um, it tells us the time reaction took to complete. So a narrower peak would indicate that the reaction proceeded at a faster rate, regardless of the amount of energy, because that obviously is determined by the vertical (y-axis). But yeah, width is time.” (Raisa, third-year biology major)
“Um, yeah, I do remember something about, if it's like a really sharp peak, that happens faster than like a peak [that is wide] ( Fig. 7 ). Maybe that means the width is speed. So like this [sharp peak] happens quickly.” Arina (third-year kinesiology major)
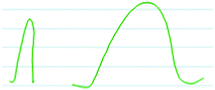 | ||
| Fig. 7 Arina's drawing to explain that more narrow peaks indicate faster reactions (left) while wider peaks indicate slower reactions (right). | ||
Similarly, seventeen students reported that peak width shows time:
“I would think that the width of the peak represents um, the time, um, the time it takes for the reaction to keep going, um, relative to the amount of energy that we are adding.” (Egor, second-year biochemistry major)
“I guess [peak width shows] the duration of time. How fast it reacted. And how long it took to react and change to the next…” (Vera, third-year kinesiology major)
Second-year biology major Ksenia was convinced that the width of the peaks provided numerical values for the length of a reaction. Consider her description of an RCD that was qualitatively similar to that in Fig. 3 (two peaks, with the first peak both taller and wider than the second peak):
“[The width of peaks mean], um, how long it takes from the reactants to intermediate, or from reactants to products. Because if this is a progress of a reaction, then this would have some unit of time. So this [pointing at the middle of the x-axis] is like five minutes, this [pointing at the end of the x-axis] is like 10 minutes or something. So if we look, we can say, we started at time zero, and from here to here [starting point to valley], this is going to be like six minutes. So we know that with this amount of energy it will take six minutes to get from reactants to the intermediate.”
Challenge IV: Differentiating between chemistry concepts (n = 12). Table 6 summarizes the codes that describe students’ thinking regarding this challenge.
Theme IV captures the instances of students who were challenged to differentiate between two chemical concepts that are encoded in or related to RCDs. Five students were unable to differentiate between the label on the x-axis of ‘reaction progress’ and the concept of time:
“That, the x-axis is the progress of a reaction. So that might be time, um, so it would be, basically how long it takes to form that part of the reaction.” (Klava, third-year nutrition major)
“I know that for some reason we don’t use time on x-axis, like when we use progress of the reaction. But like I never understood the difference.” (Elena, second-year microbiology major)
Third-year chemical engineering major Victor drew his own RCD (Fig. 8) and labelled the x-axis as “time frame” because the progress of a reaction and “time frame” meant the same thing to him: “I guess you can read the x-axis more of like the time frame.”
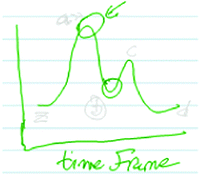 | ||
| Fig. 8 Victor's drawing showing that the x-axis in a reaction coordinate diagram represents time frame. | ||
The progress of the reaction is certainly accompanied by the passage of time; therefore, it is not surprising that students had difficulties distinguishing between these two concepts, just as it was challenging for the seventeen students in Theme III (Table 5) who interpreted peak width as a measure of time.
Transition state and intermediate, however, were the two concepts that were most difficult for students to distinguish between (n = 10). This code differs from the Theme II code of difficulties with remembering the correct names of the species encoded at the peak and the valley of an RCD (Table 4). Here, in Theme IV, students lacked conceptual understanding of the two concepts and often spoke of the two interchangeably:
“The point at which the hill climaxes [is important], it is considered an activation barrier… And then the other important part would be these little dips, because these are transition states in the molecule. So um, this one [RCD with one valley] has like one transition state or intermediate, and this guy [RCD with two valleys] has two… So transition state is just like the point at which during a reaction an intermediate formed.” (Ksenia, second-year biology major)
“So like I am pretty sure that the top, the peak of it is when it reaches its intermediate. Because, um, it's like the intermediate step that is usually the least stable step, so it requires, it's like the most energetically, it's like the most charged, so it has the most energy. So that is why it's an intermediate.” (Alina, second-year kinesiology major)
“…And this [peak] is transition or intermediate state, I don’t remember” (Fig. 9) (Larisa, third-year biology major)
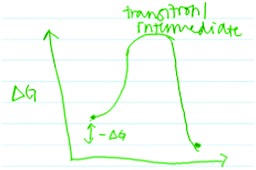 | ||
| Fig. 9 Larisa's drawing showing that the peak represents either a transition state or an intermediate. | ||
Even after being asked to further explain the difference between an intermediate and a transition state, Larisa was unable to do so. Likewise, Arina (third-year kinesiology major) was equally confused:
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) : “I don’t remember which is which. Either these [peaks] are intermediates and that [valley] is a transition state, or those [peaks] are transition states and that [valley] is an intermediate. I think these [peaks] are the intermediates and that [valley] is the transition state. But I’m not hundred percent sure.”
: “I don’t remember which is which. Either these [peaks] are intermediates and that [valley] is a transition state, or those [peaks] are transition states and that [valley] is an intermediate. I think these [peaks] are the intermediates and that [valley] is the transition state. But I’m not hundred percent sure.”
![[I with combining low line]](https://www.rsc.org/images/entities/char_0049_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[v with combining low line]](https://www.rsc.org/images/entities/char_0076_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[w with combining low line]](https://www.rsc.org/images/entities/char_0077_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif) : “Which one, transition state or intermediate, would you think will be higher in energy?”
: “Which one, transition state or intermediate, would you think will be higher in energy?”
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[r with combining low line]](https://www.rsc.org/images/entities/char_0072_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) : “So if I am right and these [peaks] are the intermediates, this [peak] has more energy within it, if that is an intermediate.”
: “So if I am right and these [peaks] are the intermediates, this [peak] has more energy within it, if that is an intermediate.”
Despite being prompted by the interviewer, these students were unable to logically reason through the relative energies of intermediates and transition states when assigning them to valleys and peaks in RCDs. These students were unable to recognize or reason that relatively stable intermediates should be represented by a low energy depression in the curve and that transition states should be depicted by an energy maximum. Similar issues with conflating a transition state and a reaction intermediate with pre-service teachers in general chemistry were previously reported by Taştan and colleagues (Taştan et al., 2010).
Conclusions
Multiple students in these interviews conflated an intermediate with a transition state, even though they could recite definitions for these concepts and even though they knew that intermediates could be isolated in a laboratory setting while transition states cannot. Despite this knowledge, students still had difficulties identifying a transition state and an intermediate on an RCD. Furthermore, students focused almost entirely upon the chemical species and changes to them throughout the reaction along the x-axis, ignoring the changes in energy encoded along the y-axis. This finding contradicts early research regarding chemistry students’ interpretations of graphs (Shah and Carpenter, 1995). Our findings were independent of whether a student was majoring in chemistry or some other science disciplines and independent of whether a students had earned a grade of A, B, or C in the first semester of organic chemistry.The majority of the students’ explanations about the meanings of RCD features were coded as “partial understanding,” “partial understanding with specific misconception,” or “specific misconception,” ranging from 75–90% depending on the feature. Analyses of these responses resulted in the identification of four themes that explain the difficulties that students had with accurately understanding the features of RCDs.
Theme I (n = 35) captures instances where students could not discern the chemistry concepts encoded in RCD's features. Some students were able to accurately describe the chemical species encoded in each feature, but never mentioned the energy associated with these chemical species. This suggests that the students considered the x-axis of the RCD, but not the energy of these species as depicted by the y-axis. Other students expressly indicated that some specific features do not communicate any intended chemistry concept. For example, some students reported that RCDs do not provide any information about how fast a reaction step takes place.
Theme II (n = 5) reflects instances when students were able to describe surface features in terms of the chemical species that are encoded in them, but could not remember what these species are called. The limitations in students’ discourse were manifested either in terms of them using an incorrect term followed by a correct explanation (i.e., saying that a peak represents an intermediate, when bonds are not completely broken/formed) or providing thick explanations to describe the term that they could not recall (i.e., valley is something like a carbocation, stable but isolatable species). Even though these students could not use the correct terminology, they still demonstrated, to some extent, one of the skills identified as the core of representational competence – “ability to use words to identify and analyse features of a particular representation” (Kozma and Russell, 2005).
Theme III (n = 22) encompasses students’ thinking when providing alternative interpretations to the surface features of RCDs. Instances in this theme lacked coherence between the surface feature level and the deep structure level (Seufert and Brunken, 2004), such as students interpreting the width of peaks as representing time or speed. It is particularly concerning that students attribute the meaning of time to the width of the peak immediately after discussing activation energy and the height of the peaks. This suggests that even though students may discuss activation energy when prompted to explain the meaning of peak within an RCD, these students do not have a meaningful mental model of the concept of activation energy and how chemists interpret peak height. Overall, students in this theme failed to demonstrate one of the skills identified as the core of representational competence, namely the “use words to identify and analyse features of a particular representation” (Kozma and Russell, 2005).
Theme IV (n = 12) describes instances where students were unable to differentiate between chemistry concepts that are encoded as different features of RCDs. Students in this theme lacked not only coherence between the surface feature level and deep structure level, but also demonstrated shallow understanding of the chemical concepts that comprise the deep structure level (Seufert and Brunken, 2004). These students were unable to “use words to identify and analyse features of a particular representation,” as well as to “describe observable phenomena in terms of underlying molecular entities and processes” (Kozma and Russell, 2005). For instance, students’ difficulties with correctly explaining the meaning of a peak and a valley in RCDs are primarily attributed to the conflation of the concepts intermediate and transition state themselves, as well as the connotations of stability and energy that accompany them. Several participants demonstrated that they do not realize the difference between an intermediate and a transition state, with some saying that these concepts are different, but unable to reason through which one would be higher in energy.
Several distinct misconceptions were identified in students’ reasonings about the meanings of RCD surface features (statements coded as “partial understanding with specific misconception” and “specific misconception”). These misconceptions are summarized in Fig. 10. These findings align with previous reports that learners make systematic mistakes when interpreting graphs, especially when graphs depict implicit abstract concepts and trends (Leinhardt and Stein, 1990; Guthrie et al., 1993; Shah and Carpenter, 1995; Gattis and Holyoak, 1996; Shah et al., 1999; Novick, 2006; Gültepe, 2016). These difficulties with comprehending graphs are associated with two major factors: the inherent biases of human perception when interpreting the visual characteristics of graphs (e.g., curved line, slope) and poor domain-specific knowledge that is necessary for accurate interpretation of the information that is encoded in the graphs (Cleveland and Mcgill, 1985; Shah and Hoeffner, 2002; Seufert and Brunken, 2004; Novick, 2006). With regard to the first of these two factors, the organic chemistry students in this study incorrectly interpreted multiple visual characteristics of the RCD curve, such as the shape of the valley of an RCD where they attached meanings such as “valley represents addition of another reagent” or “valley represents reaction slowing down.” Students also incorrectly interpreted the width of a peak to represent time, consistent with the idea that novices often impose their expectations when interpreting graphs (Shah and Hoeffner, 2002; Novick, 2006). Because one visual convention of graphs is to depict the independent variable on the x-axis, students in this study might have expected the x-axis of the RCDs to represent the independent variable of time, similar to other graphs they have encountered such as concentration vs. time in a kinetics experiment or a graph of velocity vs. time in a physics class. Our findings differ, however, from those of Shah and Carpenter (1995) who reported that students overly focused on x–y relationships, which lead them to make incorrect interpretations of the data depicted in the graphs. In this study regarding RCDs, while the students often focused on describing species that are encoded along the x-axis, they often ignored discussing the energy that is encoded along the y-axis.
With regard to the second factor of domain-specific knowledge required to accurately interpret graphs, the findings reported herein are consistent with reports in the literature on graph comprehension that learners make systematic mistakes when interpreting graphs because they lack conceptual understanding of the ideas and trends that the graphs are intended to communicate (Shah and Hoeffner, 2002; Novick, 2006; Gültepe, 2016). The students’ lack of understanding regarding the concepts of intermediate and transition state and their accompanying energy transformations negatively affected the students’ abilities to accurately interpret the meanings of peaks and valleys as important features of RCDs.
Limitations and implications
Limitations and implications for research
This research study did not observe the organic chemistry classrooms for every day of the semester to characterize instructional practices with regard to the use of RCDs. The findings reported herein indicate that future research to characterize the instructional practices of faculty regarding RCDs, and then subsequently investigate the impact of instruction upon students’ understanding of the meanings of RCDs’ features, would be warranted.Regarding the students’ thoughts about peak width, this feature in an RCD is not one that chemists typically attend to when creating or interpreting RCDs. Therefore, peak width, is not typically discussed in class nor in textbooks. The number of students (n = 23) who erroneously attached meaning to this feature might be attributed to the fact that the students had not had an opportunity to think about this feature prior to the interview and formed their incorrect responses in situ. However, because the goal of this study was to provide a detailed and rigorous examination of how students interpret the salient features of RCDs, we considered it to be important to add a question about peak width to the interview protocol in response to one of the research participants reporting that the width of the peak depicts how long a reaction step takes.
RCDs are used as a tool to visualize and explain energetic changes that take place throughout a reaction. The task of making meaningful connections between reactions and RCDs merits investigation because, in order to construct knowledge, students need to fluently translate between and integrate information from two modes of representations: symbolic and visual (Kozma and Russell, 2005; Gilbert, 2007). All of the RCDs shown to students in our research study represented exergonic reactions. Additional research should be conducted to identify how students interpret RCDs that depict endergonic reactions and what differences exist, if any, with regard to how students think about the thermodynamic ideas encoded in these two different types of RCDs.
Future research could also target different samples of participants, for example graduate students and teaching assistants, to allow for an exploration of expert-novice differences when interpreting the features of RCDs. Another interesting possibility for a future investigation would be an analysis of how students differentiate the kinetic information encoded in RCDs from the thermodynamic information.
Implications for teaching
The findings reported in this manuscript suggest that students need additional opportunities to decode the surface features of RCDs. In order to engage students in a thorough examination of RCDs and the implicit chemical concepts that are encoded in each of their features, teachers could ask students to choose an appropriate RCD from among several possibilities that would most accurately describe a given reaction, or to generate their own RCD for a given reaction. Both of these activities would benefit from a discussion of the features that the students considered important when selecting/generating an RCD. Students’ attention needs to be directed to the fact that RCDs differ from other Cartesian coordinate graphs, in that the axes in RCDs often do not include units and the diagrams themselves are generally used for qualitative discussion of reaction mechanisms. If teachers emphasized the y-axis and the relative energies of these species, students might not only understand why intermediates can be isolated in the laboratory as opposed to transition states, and they could then correctly determine where these species are encoded on an RCD. In addition to teachers emphasizing the accurate interpretation of features and encoded concepts, teachers should discuss the limitations of what an RCD does not include such as time. Finally, as it has been reported that learners’ ability to map the surface features of a graph with the meaning of those features differs as a function of experience (Shah and Hoeffner, 2002), students could benefit from being taught RCDs in conjunction with mechanisms throughout the entire year of organic chemistry, and not just in the organic chemistry I, as was the case with the students in this study.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Volwiler Family Endowment to the Miami University Department of Chemistry and Biochemistry and by Grant No. 1432466 from the National Science Foundation. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The authors thank the students and instructors of the organic chemistry courses.References
- Abraham M. R., Grzybowski E. B., Renner J. W., and Marek E. A., (1992), Understandings and misunderstandings of eighth graders of five chemistry concepts found in textbooks, J. Res. Sci. Teach., 29(2), 105–120.
- Adapted from Clipartix.com, (n.d.), Roller coaster clipart, accessed June 8, 2018, accessed at: http://clipartix.com/roller-coaster-clipart-image-26090/.
- Adobe, (1990), Adobe Photoshop CC, accessed June 8, 2018, accessed at: https://www.adobe.com/products/photoshop.html?promoid=PC1PQQ5T&mv=other.
- Allinger N. L., (1963), Energy relations in teaching organic chemistry, J. Chem. Educ., 40(4), 201–202.
- Bain K. and Towns M. H., (2016), A review of research on the teaching and learning of chemical kinetics, Chem. Educ. Res. Pract., 17, 246–262.
- Bazeley P. and Jackson K., (2013), in Seaman J. (ed.), Qualitative Data Analysis with NVivo, 2nd edn, Thousand Oaks, CA: SAGE Publications Ltd.
- Bhattacharyya G. and Bodner G., (2005), It gets me to the product: How students propose organic mechanisms, J. Chem. Educ., 82(9), 1402–1407.
- Birks M., Chapman Y., and Francis K., (2008), Memoing in qualitative research: Probing data and processes, J. Res. Nurs., 13(1), 68–75.
- Bradley E. H., Curry L. A., and Devers K. J., (2007), Qualitative data analysis for health services research: Developing taxonomy, themes, and theory, Health Serv. Res., 1758–1772.
- Bretz S. L., (2008), Qualitative research designs in chemistry education research, in Bunce D. M. and Cole R. S. (ed.), Nuts and Bolts of Chemical Education Research, Washington, DC: ACS Symposium Series, pp. 79–99.
- Brown T. E., LeMay E. H., and Bursten B. E., (2006), Chemistry: The Central Science, 10th edn, New York: Pearson.
- Çalik M., Kolomuç A., and Karagölge Z., (2010), The effect of conceptual change pedagogy on students’ conceptions of rate of reaction, J. Sci. Educ. Technol., 19, 422–433.
- Chi M. T. H., Feltovich P. J., and Glaser R., (1981), Categorization and representation of physics problems by experts and novices, Cogn. Sci., 5, 121–152.
- Cleveland W. S. and Mcgill R., (1985), Graphical perception and graphical methods for analyzing scientific data, Science, 229(4716), 828–833.
- Corbin J. and Strauss A., (1990), Grounded Theory Research: Procedures, Canons, and Evaluative Criteria, Qualitative Sociology, San Francisco, CA: Human Sciences Press, Inc.
- Creswell J. W., (2003), in Laughton C. D. (ed.), Research Design: Qualitative, Quantitative, and Mixed Methods Approaches, 2nd edn, Thousands Oaks: Sage Publications.
- Davidowitz B. and Chittleborough G., (2009), Linking the macroscopic and sub-microscopic levels: Diagrams, in Gilbert J. K. and Treagust D. F. (ed.), Multiple Representations in Chemical Education, The Netherlands: Springer, pp. 169–191.
- Drever E., (1995), Using semi-structured interviews in small-scale research. A teacher's guide, Edinburgh: The SCRE Centre.
- Elby A., (2000), What students’ learning of representations tells us about constructivism, J. Math. Behav., 19, 481–502.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Frey R. F., Cahill M. J., and Mcdaniel M. A., (2017), Students’ concept-building approaches: A novel predictor of success in chemistry courses, J. Chem. Educ., 94(9), 1185–1194.
- Gattis M. and Holyoak K. J., (1996), Mapping conceptual to spatial relations in visual reasoning, J. Exp. Psychol. Learn. Mem. Cogn., 22(1), 231–239.
- Gilbert J. K., (2007), Visualization: A metacognitive skill in science and science education, in Gilbert J. K. (ed.), Visualization in Science Education, The Netherlands: Springer, pp. 9–27.
- Grove N. P., Cooper M. M., and Rush K. M., (2012), Decorating with arrows: Toward the development of representational competence in organic chemistry, J. Chem. Educ., 89(7), 844–849.
- Gültepe N., (2016), Reflections on high school students’ graphing skills and their conceptual understanding of drawing chemistry graphs, Educ. Sci. Theory Pract., 16(1), 53–81.
- Guthrie J. T., Weber S., and Kimmerly N., (1993), Searching documents: Cognitive processes and deficits in understanding graphs, tables, and illustrations, Contemp. Educ. Psychol., 18, 186–221.
- Hulse J. E., Jackson R. A., and Wright J. S., (1974), Energy surfaces, trajectories, and the reaction coordinate, J. Chem. Educ., 51(2), 78–82.
- Jones M. J. and Fleming S. A., (2014), in Fahlgren, E., (ed.), Organic Chemistry, 5th edn, New York, NY: W. W. Norton & Company.
- Kaya E. and Geban Ö., (2012), Facilitating conceptual change in rate of reaction concepts using conceptual change oriented instruction, Educ. Sci., 37, 216–225.
- Kirik O. T. and Boz Y., (2012), Cooperative learning instruction for conceptual change in the concepts of chemical kinetics, Chem. Educ. Res. Pr., 13, 221–236.
- Klein D., (2012), in Recter P. (ed.), Organic Chemistry as a Second Language, 3rd edn, Hoboken, NJ: John Wiley & Sons, Inc..
- Kolomuç A. and Tekin S., (2011), Chemistry teachers’ misconceptions concerning concept of chemical reaction rate, Eurasian J. Phys. Chem. Educ., 3(2), 84–101.
- Kozma R. and Russell J., (1997), Multimedia and understanding: Expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968.
- Kozma R. and Russell J., (2005), Students becoming chemists: Developing representational competence, in Gilbert J. K. (ed.), Visualization in Science Education, Dordrecht, The Netherlands: Springer, pp. 121–146.
- Leinhardt G. and Stein M. K., (1990), Functions, graphs, and graphing: Tasks, learning, and teaching, Rev. Educ. Res., 60(1), 1–64.
- Lincoln Y. S. and Guba E. G., (1985), Naturalistic Inquiry, Newbury Park, CA: Sage Publications, Inc.
- Linenberger K. J. and Bretz S. L., (2012), A novel technology to investigate students’ understandings of enzyme representations, J. Coll. Sci. Teach., 42(1), 45–49.
- Meek S. J., Pitman C. L., and Miller A. J. M., (2016), Deducing reaction mechanism: A guide for students, researchers, and instructors, J. Chem. Educ., 93, 275–286.
- Morrison R. W., Caughran J. A., and Sauers A. L., (2014), Classroom response systems for implementing interactive inquiry in large organic chemistry classes, J. Chem. Educ., 91, 1838–1844.
- Novick L. R., (2006), The importance of both diagrammatic conventions and domain-specific knowledge for diagram literacy in science: The hierarchy as an illustrative case, in Barker-Plummer D., Cox R. and Swoboda N. (ed.), Diagrams, Stanford, CA: Springer-Verlag, pp. 1–11.
- Patton M. Q., (2002), Qualitative Research & Evaluation Methods, 2nd edn, Thousand Oaks, CA: Sage Publications, Inc.
- Popova M. and Bretz S. L., (2018a), Organic chemistry students’ challenges with coherence formation between reactions and reaction coordinate diagrams, Chem. Educ. Res. Pract., advance article, 10.1039/C8RP00064F.
- Popova M. and Bretz S. L., (2018b), “It's only the major product that we care about in organic chemistry:” An analysis of students’ annotations of reaction coordinate diagrams, J. Chem. Educ., DOI:10.1021/acs.jchemed.8b00153.
- Popova M. and Bretz S.L. (2018c), Organic chemistry students' understandings of what makes a good leaving group, J. Chem. Educ., DOI:10.1021/acs.jchemed.8b00198.
- Prins G. T., (2010), Teaching and Learning of Modelling in Chemistry Education Authentic Practices as Contexts for Learning, The Netherlands: Leersum.
- QSR International Pty Ltd, (2015), NVivo qualitative data analysis Software, Version 11, accessed June 8, 2018, accessed at: http://www.qsrinternational.com/.
- Raker J., Holme T., and Murphy K., (2013), The ACS exams institute undergraduate chemistry anchoring concepts content map II: Organic chemistry, J. Chem. Educ., 90, 1443–1445.
- Rouse W. B. and Morris N. M., (1986), On looking into the black box: Prospects and limits in the search for mental models, Psychol. Bull., 100(3), 349–363.
- Seufert T. and Brunken R., (2004), Supporting coherence formation in multimedia learning, in Gerjets R. J. P., Kirschner P., Elen J. (ed.), Instructional design for effective and enjoyable computer-supported learning: Proceedings of the first joint meeting of the EARLI SIGs Instructional Design and Learning and Instruction with Computers, Tubingen, Germany: Knowledge Media Research Center, pp. 138–147.
- Shah P. and Carpenter P. A., (1995), Conceptual limitations in comprehending line graphs, J. Exp. Psychol. Gen., 124(1), 43–61.
- Shah P. and Hoeffner J., (2002), Review of graph comprehension research: Implications for instruction, Educ. Psychol. Rev., 14(1), 47–69.
- Shah P., Mayer R. E., and Hegarty M., (1999), Graphs as aids to knowledge construction: Signaling techniques for guiding the process of graph comprehension, J. Educ. Psychol., 91(4), 690–702.
- Taştan Ö., Yalçinkaya E., and Boz Y., (2010), Pre-service chemistry teachers’ ideas about reaction mechanism, J. Turkish Sci. Educ., 7(1), 47–60.
- Tufte E. R., (2001), The Visual Display of Quantitative Information, 2nd edn, Cheshire, CT: Graphics Press.
- Weinrich M. L. and Talanquer V., (2015), Mapping students’ conceptual modes when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 16, 561–577.
- Zumdahl S. S. and Zumdahl S. A., (2012), Chemistry: An Atoms First Approach, 1st edn, Belmont: Brooks/Cole, Cengage Learning.
| This journal is © The Royal Society of Chemistry 2018 |