Open Access Article
Open Access ArticleThe challenges of learning and teaching chemical bonding at different school levels using electrostatic interactions instead of the octet rule as a teaching model
Jarkko
Joki
 * and
Maija
Aksela
* and
Maija
Aksela

Department of Chemistry, University of Helsinki, P.O. Box 55, Helsinki, 00014, Finland. E-mail: Jarkko.joki@helsinki.fi
First published on 21st June 2018
Abstract
Teaching chemical bonding using the octet rule as an explanatory principle is problematic in many ways. The aim of this case study is to understand the learning and teaching of chemical bonding using a research-informed teaching model in which chemical bonding is introduced as an electrostatic phenomenon. The study posed two main questions: (i) how does a student's understanding of chemical bonding evolve from lower- to upper-secondary school when an electrostatic model of chemical bonding was used at the lower-secondary level? (ii) How does the teaching of octets/full shells at the upper-secondary level affect students’ understanding? The same students were interviewed after lower-secondary school and again during their first year at upper-secondary school. Their upper-level chemistry teachers were also interviewed. The interview data were analysed using the grounded theory method. The findings showed that the students’ earlier proper understanding of the electrostatic-interactions model at the lower-secondary level did not prevent the later development of less-canonical thinking. Teachers’ pedagogical content knowledge (PCK) of the explanatory principles of chemical bonding and how to use explanations in science education needs to be promoted in both pre-service teacher education and during in-service training.
Introduction
Chemical bonding is a fundamental concept in chemistry education (Levy Nahum et al., 2010). Chemical bonds do not exist ontologically as separate objects, and chemical bonding describes the phenomenon of atoms “sticking together” due to electrostatic interactions and quantum mechanical phenomena (Gillespie, 1997; Gillespie and Robinson, 2007). Due to its abstract and theoretical nature, chemical bonding is a challenging topic in chemistry education (Taber, 2001a; Taber and Coll, 2002; de Jong and Taber, 2014). This challenge needs to be answered by quantum chemistry because bonding cannot be fully understood based only on classical physics and electrostatics (Taber and Coll, 2002; Gonthier et al., 2012). The different historical eras in chemistry as a science and in its teaching have left an imprint on chemistry education practices and teaching models (Croft and de Berg, 2014). The challenges involved in learning about chemical bonding include alternative concepts, misconceptions, and alternative conceptual frameworks (octet framework, Taber, 1998), which have been researched quite comprehensively (for a review, see Özmen, 2004; Unal et al., 2006).The octet framework (Taber, 1998) is an alternative conceptual framework mirrored at least partly in teaching. It is problematic in many widely-discussed ways (de Jong and Taber, 2014). It has been observed that the formation of an octet framework leads to manifold conceptual structures (Taber, 2000b), and that moving away from an inadequate explanatory framework can be a very slow process and perhaps even impossible (Taber, 2003). Taber (2000b) reported that students may use the octet explanatory principle, the minimum-energy explanatory principle and the electrostatic explanatory principle without understanding the connections between them. We examined whether students are resistant to the octet framework when they have been introduced to chemical bonding in a research-informed way. Bergqvist et al. (2016) studied upper-secondary teachers’ knowledge of teaching chemical bonding and found that they were unaware of the difficulties related to teaching and learning chemical bonding, and that their pedagogical content knowledge (PCK) and knowledge of students’ understanding (KSU) should be further developed.
The octet rule is a rule-of-thumb, mnemonic device which can be useful for predicting formulae of simple compounds or ionic charges (mainly second-row elements of the periodic table). The octet rule as a heuristic is still used, especially in organic chemistry, which concentrates on the second-row elements carbon, nitrogen, and oxygen, and in the history of chemistry (Croft and de Berg, 2014). It is nearly impossible for students to avoid encountering the octet rule during their chemistry education, either from a teacher (Bergqvist et al., 2016) or a textbook (Bergqvist et al., 2013). The full-shell explanatory principle uses the octet rule to explain chemical reactions, which occur because atoms “want” to attain a full valence shell. It is therefore important to understand how the octet framework is actually adopted and what factors facilitate its formation. Might the abstract nature of the concepts, the age of the students, or the teachers’ use of metaphors and teaching models be problematic? Why are students unable to connect the minimum-energy principle, electrostatic interactions, and the octet rule (which is based on the idea of quantisation)? To answer these questions, we must divide the principles of minimum energy, octets, and electrostatic interactions into their more detailed components, for example the positive charge of the nucleus, the distance between the outer electrons and the nucleus, and the quantisation of orbitals (see Table 1). There has been no detailed study on how the octet framework is formulated by students on a detailed level and which elements in particular promote it.
| Determination constructs/explanatory schema | Description of schema |
|---|---|
| d1 | Full-outer-shell explanatory principle (Taber, 2002). |
| d2 | Effective attractions of nuclei at the level of the outermost electron shell result from the electron configuration of atoms, which defines how binding electrons are distributed within a bond and the type of the resulting chemical bond. |
| d3 | Bonds are based on coulombic interactions between nuclei and binding electrons. |
| d4 | The outermost electrons’ distance from the nucleus affects their degree of nuclear attraction. |
| d5 | Electronic interactions; positive and negative charges cancel each other out (Boo, 1998). |
| d6 | Nuclear charge affects the attraction experienced by the outermost electrons. |
| d7 | Electrons between the nucleus and the valence electrons shield the latter from nuclear attraction. |
| d8 | Another atom draws electrons to itself. |
| d9 | A structure is unstable if the positive or negative charge is too high. |
| d10 | Electrons repel each other. |
| d11 | Nuclear charge is shared among the residual electrons (Taber, 1998). |
| d12 | Non-charged atoms do not attract each other because there are the same number of protons and electrons and these charges cancel each other out. |
| d13 | Ionic bonds are based on different charges of ions. |
| d14 | There are more charged protons. |
| d15 | Energy levels are quantised. |
Moreover, there has been no detailed research on how teachers’ use of explanations concerning chemical bonding affects students’ understanding, or how the use of the octet rule as an explanatory schema affects students’ understanding.
To prevent alternative conceptual frameworks, different approaches in teaching chemical bonding have been presented (Levy Nahum et al., 2010; Levy Nahum et al., 2013; Dhindsa and Treagust, 2014). In our previous work, we described a teaching model that is already used in lower-secondary schools. The idea of the teaching model is to present electrostatic interactions as a common basis for all kinds of chemical bonding (for more details, see Joki et al., 2015). The same type of bottom-up approach has been used at the upper-secondary level by Kronik et al. (2008). Our primary goal was to introduce electrostatic interactions as a basis for all types of chemical bonding before students adopt an octet framework (Joki et al., 2015). The other purpose was to follow-up on how these students’ conceptual understanding of chemical bonding developed after lower-secondary school.
Research questions
The main research question of the present study is: can a research-informed way of teaching in lower-secondary school prevent students from adopting an octet framework later in upper-secondary school?This main question can be divided into two sub-questions:
1. How does a student's understanding of chemical bonding evolve from lower- to upper-secondary school?
The follow-up interviews revealed that the research-informed teaching model at the lower-secondary level did not prevent students from adopting an octet framework later. The hypothesis was that students would be resistant to the octet framework, but this notion did not conceptualise. Based on this observation, another research question was generated:
2. How does the way a teacher explains octets/full shells affect the student's understanding?
Theoretical background
Research into students’ conceptual understanding, development of conceptual structures and conceptual change has been one of the most important topics in science education research over the past few decades. Research on conceptual change has featured three overlapping phases and involves different approaches to the paradigm. A systemic perspective informed by cognitive science and multiple interactive elements (image schemas, mental models, mathematical representations, etc.), often at different levels of analysis, requires that we distinguish between epistemological and ontological beliefs concerning conceptual knowledge. These different levels of analysis are characteristic of phase three (Amin et al., 2014). Our study is based on a systemic perspective informed by cognitive science (Thagard, 1992; Koponen and Huttunen, 2013; Joki et al., 2015).A model in which the concept structures are broken down into their components and the connections between these pieces, and how different parts activate each other and create a dynamic and connectionist network, may provide fertile ground for understanding conceptual change (diSessa, 1993; diSessa and Sherin, 1998; Thagard, 1992; Brown and Hammer, 2008; Koponen and Huttunen, 2013). In the connectionist model, the concept structures can be seen as dynamic and consisting of different concepts, determining schemas, mnemonics, hypothesis constructions and attributes (Koponen and Huttunen, 2013).
diSessa (1983) created the concept of phenomenological primitives (p-prims), intuitive and mainly subconscious deductions that function as intuitive building blocks for causal schemas and conceptual structures. They can appear as part of naive science or misconceptions, or as part of normative scientific understanding (diSessa, 2014). On the other hand, some p-prims constitute a certain ecological niche as a framework in which certain explanatory schemas can be activated. Activation of causal schemas and different frameworks are also partly dependent on which of the unconscious p-prims will be activated (diSessa, 1993; Perkins and Grotzer, 2005). Modification of conceptual structures is strongly context- and task-dependent (Kokkonen, 2017).
Although research on p-prims has mainly concerned physics education, they have also been mapped out for biology education, and their relationship to the conceptual framework has been observed (Southerland et al., 2001). In chemistry education research, only a few p-prims have so far been mapped out (Taber, 2014), but additional ones may exist; for example, “more is stronger”, “more is bigger” and “natural is healthier” (Toth and Barany, 2016). In our previous research, we suggested that electrostatic interactions could also be related to a possible p-prim, referred to later in this article as “opposites attract”; i.e., opposite charges attract each another, while similar charges repel each other (Joki et al., 2015).
On the other hand, Taber and Garcia-Franco (2010) have questioned whether p-prims will always be an accurate concept for all implicit-knowledge elements in chemistry. Some implicit-knowledge elements are more concerned with the fundamental nature of the material world while others are more like an experiential gestalt of causation (Taber and Garcia-Franco, 2010). Keeping this point in mind, we now propose that diSessa's (1993)p-prim of vacuums impel can also be connected to chemistry education research, particularly to the octet framework (Taber, 1998). This p-prim of vacuums impel helps in understanding why the octet framework is very difficult to root out. Epistemological beliefs and intuitive deductions like p-prims can affect the kind of explanatory schemas a student may connect with particular explanatory principles and frameworks.
Talanquer (2007) and Delgado (2015) addressed the teleological dimensions of models and mnemonics in chemistry teaching and learning. Causal explanations are typical in science; however, students often prefer teleological explanations rather than causal ones (Talanquer, 2013). Taber has criticized the teleological use of the octet rule (2009). When the octet rule is used in a teleological or causal manner, it acquires the status of an explanatory schema (principle). The octet rule can be used as a mnemonic device, or as a heuristic rule for estimating bond order in a limited number of cases, but this technique requires an understanding of the epistemological status of the octet rule. Unfortunately, teachers and textbook-authors are unfamiliar with these pitfalls (Bergqvist et al., 2013, 2016). Bergqvist et al. (2016) proposed that teachers’ PCK (Shulman, 1986) should be developed. We propose that one particular area of PCK that should be developed is teachers’ understanding of instructional explanations and explanatory schemas included in teaching models. This involves the cautious use of teleological explanations and even offers a chance to understand instructional explanations in a teleological way (Watts and Taber, 1996; Talanquer, 2013). Delgado (2015) proposed, for example, that the teleological use of the octet rule or Le Chatelier's principle can be seen as one type of metaconceptual misunderstanding relating to the use of models in explaining certain phenomena. This study focuses only on the explanatory role of the octet rule and the possible causal or teleological status of this explanatory principle.
This paper provides one possible way to prevent the formation of the octet framework. Rather than avoiding the octet rule altogether, it may be better to increase understanding of the different kinds of explanatory models and to be cautious about explaining phenomena in a teleological way. It is preferable for both students and teachers to recognise when a schema is more like a mnemonic or an explanation. This is necessary since there is still a wide gap between research and practice (Bergqvist et al., 2016) with regard to the teaching of chemical bonding. We must understand the background of the problem relating to the octet framework well enough to solve it.
Previous research has shown how the explanatory principles of the octet rule, minimum energy, and electrostatic interactions can form manifold conceptual structures in which these different models compete with each other (Taber, 2000b, 2001a). Taber (1997) used Mortimer's (1995) model of a conceptual profile without different levels of epistemological sophistication to provide a model for different manifold explanations concerning chemical bonding. In the present research, we investigate how more detailed explanatory schemas are related to each other and to explanatory principles (or profiles, according to Mortimer, 1995), as well as the effects that intuitive and subconscious p-prims have on these explanatory profiles. We analyse how students fluctuate between different principles and different kinds of detailed schemas. In particular, we explore how different p-prims are activated in different situations and how p-prims compete with each other and lead to certain explanatory principles overriding others. We also investigate the schematic structures that teachers relate to these principles and what type of status (explanatory schema or mnemonic device) they apply to these different conceptual constructs.
Settings
This is a qualitative case study that is part of a larger research project developing a teaching model for chemical bonding that aims to prevent students from forming the octet framework. The first part of this research has already been published (Joki et al., 2015), and the research presented here contains data from that study, namely the students’ first interviews during their final year at lower-secondary school (these comprise data set 1). The students had been taught by the first author at this school. The research continues in the present study with follow-up interviews of the students (these comprise data set 2) along with interviews of their teachers at upper-secondary school (these comprise data set 3) (Table 2). The students who participated in the first part of the research (Joki et al., 2015) applied for entry at three different upper-secondary schools after lower-secondary school. Each of these eight students were taught by one of five specific chemistry teachers at the upper-secondary schools. Three of the five teachers agreed to be interviewed (Table 3). The semi-structured telephone interviews with the teachers were recorded and transcribed.| Sample | Description | Participants | Used in answering research questions |
|---|---|---|---|
| Data set 1 | Students’ interviews at the end of basic education | Eight students (analysis of these interviews and research results was reported in Joki et al., 2015) | RQ1 |
| Data set 2 | Students’ follow-up interviews at the upper-secondary level | Eight students | RQ1 and RQ2 |
| See appendix: corpus of follow-up interviews | |||
| Data set 3 | Upper-secondary school teachers’ telephone interviews | Three teachers | RQ1 and RQ2 |
| Student | Upper-secondary school | Teacher |
|---|---|---|
| A1 (participated) | S1 | T1 (participated) |
| A2 (participated) | S2 | T2 (participated) |
| A3 (participated) | S1 | T1 (participated) |
| A4 (participated) | S1 | T3 (did not participate) |
| A5 (participated) | S3 | T4 (participated) |
| A6 (participated) | S3 | T4 (participated) |
| A7 (participated) | S1 | T5 (did not participate) |
| A8 (participated) | S2 | T2 (participated) |
Ethics
This research project received permission from the education office of the City of Espoo, which organises both the lower and upper secondary schools (licence number: 21/2014, 17.03.2014). The parents of the underage students also provided written permission for the interviews. The ethics of the first part of this project were reported in detail by Joki et al. (2015). The students’ interview material was immediately encoded as A1–A8 and that of the teachers was encoded as T1–T5 so that individuals could not be identified.Methods
Rationale behind study design
In the follow-up interviews, if it appeared that a pupil had more strongly adopted the octet framework as a teleological explanation, we attempted to identify the reasons for this. With the help of a continuing professional development (CPD) event, for which we created a preliminary survey to determine how teachers perceive the conceptual structures central to teaching chemical bonding, as well as what significance they give to the octet rule and to electrostatic interactions in this context. During CPD event participants firstly answered to the survey. The material of preliminary survey and recorded discussion during CPD event provided preliminary hints on which to base interviews with these particular pupils' teachers, to determine how they had used the octet rule and electrostatic interactions to explain chemical bonding. The teachers participating in the CPD training had not taught the students involved in the research and were therefore not among the teachers who were interviewed. Data sets 1 and 2 were used to help answer RQ1. Changes in pupils' conceptual understanding of chemical bonding in a less-canonical direction were observed when comparing data sets 1 and 2. Observations on how the teachers’ understanding of the octet rule (data set 3) impacted changes in students’ understanding (data sets 1 and 2) were used to answer RQ2.Sampling
Each interviewed student was a volunteer with a successful background in chemistry. Our intention, especially in the first part of the study, was to test for any weaknesses of a novel teaching model (Joki et al., 2015). To this end, we designed our sampling to provide rich data, and we intentionally chose talented students with good grades in chemistry. Information for data set 1 was collected during May 2014, when the chemistry course had ended at the lower-secondary school. During the 2014–2015 school year, the students were invited back to their former lower-secondary school for follow-up interviews after finishing their first chemistry course at the upper-secondary level. The students were asked to identify their chemistry teachers in the first year of upper-secondary school, and these teachers were invited to be interviewed. Participation was strictly voluntary, and three of the five teachers accepted the invitation.Research approach and design of data collection
The grounded theory approach was used (Glaser and Strauss, 1967; Taber, 2000a). Based on this approach, the research data were collected in stages to support the formation of the grounded theory. Data sets 1 and 2 are based on purposive sampling and data set 3 comes from theoretical sampling (Table 2).The data was collected and recorded using clinical semi-structured interviews of students (Russ et al., 2012), semi-structured telephone interviews of teachers, summaries of the training-event findings, and web-based surveys.
The variety of data and the number of participants are presented in Table 2. Data sets 1, 2 and 3 are connected, making it possible to monitor the development of students’ conceptual structures. In addition, it is possible to estimate how an upper-secondary teacher impacts the students’ conceptual structures. The connections between students’ and teachers’ interview data are presented in Table 3.
Design of instruments in data collection
| Student/question (see interview corpus in Appendix 1) | 20 | 21 | 22 | 23 | 24 | 25 |
|---|---|---|---|---|---|---|
| A5 Lower | a: d6 | b: d6 | Na: d4–d6 | a: d1, d9, d10 | F: d4, d6 | F: d4, d7, d10 |
| A5 Upper | a: d6 | b: d6 | Na: d10 | a: d1, d4, d7, d6, d9 | F: d6, d4 + d7 | F: d4 + d7 |
| A6 Lower | a: d6 | b: d6 | Na: d4 | a: d9, d11 | F: d6 | Br: d6 |
| A6 Upper | a: d4 + d6 | a: d4 + d6 | Na: d4 + d7 + d6 | a: d1, d1–d9, d1 | F: d1 + d6, d12? | F: d4 + d7 |
| A3 Lower | a: d6 | b: d6 | Na: d7, d10 | a: d7, d8, d9 | F: d4, d6 | F: d4, d6 |
| A3 Upper | a: d4 + d6 | b: d4 + d6 | Na: d7 + d4 | a: d9 + d6, d7 + d10, d8 | F: d4 + d6 | F: d4 + d6 + d7 |
In earlier studies, information about the meanings of concepts was obtained by posing questions in which students must adapt certain concepts to certain situations (Taber and Garcia-Franco, 2010). Our interest is in the significance of the octet rule and electrostatic interactions in explaining chemical bonding, so we designed an interview in which pupils were first asked about chemical bonding in general (what does “chemical bonding” mean?) and concepts related to it. They were later asked more specific diagnostic questions requiring the use of concepts to explain particular phenomena in concrete situations (for follow-up interviews, see the Appendix 1).
Design of data collection at the CPD event and teachers’ interviews
We arranged a CPD education event for teachers, whom we surveyed to identify their methods of teaching chemical bonding. First, they completed an online survey in which they mapped out how they currently teach this topic. During the CPD training event, the teachers had small subgroup discussions about central concepts related to the teaching of chemical bonding, and about the significance of these concepts in this context. Next, there was a small recap session, which was recorded. During this session, a teacher from each subgroup briefly explained what the group had discussed, and based on this data, we created an interview for data set 3, in which teachers themselves identified the most significant concepts in the teaching of chemical bonding. The teachers were then asked what significance they gave to these concepts and which type of bonding they connected to them. The interviewers’ strategy was to ask questions in an order that would not direct the teachers’ thoughts in a certain direction. More-general questions were presented first, and depending on the answers, more-specific questions were presented about concepts that the teachers had already introduced (see Appendix 2).The teachers were asked how they used the octet rule to explain chemical bonding and what status they gave it. After the open questions were answered, the teachers were asked to estimate whether the octet rule is used more like a mnemonic device (a heuristic rule) or an explanatory schema. They were also asked to explain the status given to electrostatic interactions when teaching chemical bonding. Data from the CPD event were used to design the teacher interviews, but are not otherwise included in this paper.
Analysis
The data were analysed using an inductive content analysis, especially for data sets 1 and 2 (Elo and Kyngäs, 2008). In later phases, the constant comparison method was used according to the grounded theory approach. In the first phase of analysis, we used a classification of conceptual constructs for concepts, mnemonic devices and explanatory schemas (Koponen and Huttunen, 2013; Joki et al., 2015).Analysis of students’ follow-up interviews
For the analysis of the follow-up interviews, we determined whether a student used the octet rule as a teleological explanatory schema and if so, what other explanatory schemas (d1–d15) (Table 1) the student associated with it, especially when asked the reason for choosing a certain schema. We also examined the different schemas (d1–d15) that students used with different explanatory principles (Table 5; for an example of analysis, see Table 6).| Explanatory principle | Description of the principle |
|---|---|
| OP | Octet principle |
| MEP | Minimum-energy principle |
| ESP | Electrostatic principle |
| Excerpt | Coding (for determination constructs, see Table 1) |
|---|---|
| I: Ok, now (in the picture) there are three kinds of sodium: sodium atom, sodium ion Na+ and sodium ion Na7−. What do you think about the stability of these species? How would you arrange them according to stability? | |
| A5: Sodium 7− is the most unstable, if that can be said, and then the sodium atom and then sodium 1+. | |
| I: So, sodium 1+ is the most stable? | |
| A5: Yes, sodium 1+ is the most stable. | |
| I: Could you give a little explanation why? | |
| A5: There is just in this case the well-known octet in the outer shell, and so it is the mostable in terms of energy. | d1 |
| I: So, what do you think about sodium 7−? Are there also eight electrons in the outer shell? | |
| A5: Mm, yes, but maybe now I would say that because in this case there are so many electron shells and electrons compared to the nucleus, the nucleus cannot attract all these electrons in the structure of this species, so electrons will separate from the ion. | d4 + d7 + d6 |
Based on the data analysed according to the grounded theory approach, we developed two classifications: the octet rule can play a role as a mnemonic device or an explanatory schema and can be used as a heuristic rule without causal or teleological meaning, or it can acquire the status of an explanatory schema in which it can hold more or less teleological meaning. We also classified whether or not students gave the octet rule teleological or causal status (see Table 7 for example of analysis). We used Talanquer's (2007) and Wright's (1972) definitions of causal/teleological status. In our examples, teleological status meant that the student thought that the octet is the destination or purpose of the atom, i.e., it is “what atoms want”. If a student could not explain further and thought that the octet rule was the final explanation (i.e., the reason the full octet exists is because the atom “wants” it), the answer was considered teleological (Table 7) (Watts and Taber, 1996). If a student explained that the octet is the most favourable energy state, but could not explain what favourable energy means in this context but used it as a causal explanation for the full octet, this was also considered a teleological explanation. If the student gave an explanation related to electrostatic interactions after a long discussion (see example in Table 8), we classified the status as partially teleological. This means that most of the answers this student gave (see example A5 in Table 8) presented the octet rule as teleological, as a final explanation, but after some consideration the student finally proposed that electrostatic interactions are balanced when there is an octet structure. However, later during the same interview, student A5 again used the octet rule as a teleological explanation and did not connect it to electrostatic interactions. If a student did not use the octet rule at all in their explanation, we classified the status according to the explanatory schema the student gave. The results are presented in Table 9.
| Excerpt | Coding (T means the student gave teleological status to the construct) |
|---|---|
| I: Okay, what is this bond based on, why do sodium and chlorine stick together? | |
| A7: It is based on electric charges. Chlorine has a strong charge and sodium has a weak charge, but they both attract that one electron. This is a bit difficult to explain. | |
| I: Okay, well what about the covalent bond? What is it based on? | |
| A7: Here, both atoms attract the mutual electrons and when they do this, a standoff happens, and they stick together. | |
| I: Why do they attract electrons? | |
| A7: Because electrons are negative and the atoms or the protons of an atom are positive, and there is a charge, which causes the attraction. | ESP |
| I: Why doesn’t sodium attract electrons as strongly? | |
| A7: Well, because sodium has fewer protons than chlorine and therefore it does not attract as strongly. | d6 |
| …. | |
| A7: Mm, but there is still the point that chlorine has one electron missing from a third electron shell, but potassium has a full third electron shell and one electron in the fourth electron shell, which chlorine does not. | |
| I: So, what will the effect be? | |
| A7: Well, then chlorine wants to fill up its third electron shell, but potassium already has a full third electron shell, so potassium wants to donate one electron from the fourth electron shell. | |
| I: Why? | |
| A7: Because they try to get the octet, so that they will have a certain number of electrons. | |
| I: So, why does it try to get the octet? | |
| A7: Mmm, I don’t know. | d1 + T |
| Excerpt | Coding |
|---|---|
| I: So, on what basis is it that some of them receive and some of them donate? | |
| A5: They try to get to a certain energy level and they reach it by donating or receiving. | |
| I: Why do they try to do this? | |
| A5: Well, nature just works like that. They try to reach a certain energy level. | |
| I: I see. What energy level do they try to achieve? | |
| A5: In chemistry it is usually the octet, so that they have eight electrons in the outer shell. It is just a balanced situation which they seem to want to reach. | d1 |
| I: Why do they want to reach it? | OP + MEP |
| A5: Well, I don’t believe that it can be explained further…. it's just so, nature happens to work like that. | T |
| I: So, why do they want to have just eight? | |
| A5: Well, I don’t know, but it happens to be eight, and it then has such a suitable energy level and it just happens to be just eight. There is no more accurately….as I just said…. | MEP, without further explanation → teleological meaning. |
| I: Ok, so can that energy level be something other than just eight? | |
| A5: Well, yes it maybe can be in principle, or can it? | |
| I: So on what basis is it eight? | |
| A5: Why is it just eight? | |
| I: Yes. | |
| A5: Well, maybe it is something…. maybe to the electron shells or to orbitals, orbitals in which there are electrons. | |
| I: In what way is it based on them? | |
| A5: …when they have achieved eight, then they (the orbitals) have a steady energy level. | |
| I: What does a steady energy level mean? | |
| A5: It means that it does not want to receive or donate anymore. | |
| I: The fact that they do not want to receive or donate anymore is based on what? | |
| …….(long silence) | |
| I: So, which force, which force acts on it, so that there is a steady state of energy? Or when does it become unsteady? Which force makes an impact? | (Interviewer gives a hint after long silence, introducing the word “force”) |
| A5: Well, maybe some attractive forces are then in balance. | |
| I: Which forces? | ESP |
| A5: Electromagnetic attractive forces. | (Electrostatic explanatory schema, but appears after long discussion and a hint has been given → partial teleological status) |
| Student | Octet explanatory principle (T = teleological status) | Separation of octet explanatory principle and electrostatic explanatory principle | Student's teacher at upper-secondary school | Teacher's way of using the octet rule | Context in which the teacher uses the electrostatic explanatory principle | Octet explanatory principle (T = teleological status) | Separation of octet explanatory principle and electrostatic explanatory principle |
|---|---|---|---|---|---|---|---|
| Status at lower-secondary school | Status at lower-secondary school | Student's status at upper-secondary school | Student's status at upper-secondary school | ||||
| A1 (participated) | T | Totally | T1 (participated) | Teleological explanatory schema or mnemonic device when the explanatory schema is minimum energy, without interpretation related to electrostatic interactions | Ionic bonding | T | Totally |
| A3 (participated) | — | — | T1 (participated) | — | — | ||
| A2 (participated) | Partially | Partially | T2 (participated) | Teleological explanatory schema | Ionic bonding, atomic radius | T | Totally |
| A8 (participated) | — | — | T2 (participated) | Partially T | — | ||
| A5 (participated) | Partially teleological, two occasions | Partially | T4 (participated) | Teleological explanatory schema | Ionic bonding, intermolecular forces, dipole–dipole-bond | Partially teleological, and more widely used than at lower-secondary school | Partially |
| A6 (participated) | — | — | T4 (participated) | T | Partially | ||
| A7 (participated) | — | — | T5 (did not participate) | T | Totally | ||
| A4 (participated) | T | Totally | T3 (did not participate) | T | Totally | ||
Analysis of teachers’ interviews
Teachers’ interviews were analysed and classified in light of two central questions:1. Does the teacher use the octet rule as a teleological explanatory schema? If not, how does the teacher explain the octet rule to students?
2. Does the teacher use electrostatic interactions as a common explanatory basis for all kinds of chemical bonding? If not, in which cases or bond types would a teacher use electrostatic interactions as an explanatory principle?
The results are presented in Table 9 and excerpts of the teachers’ interviews are presented along with the interpretation of results for trustworthiness of analysis. These questions help to answer our main research question and the second subquestion.
Reliability and validity
Grounded theory-based analysis of data and interpretation was completed iteratively by analysing the different data in order to reach convergence. A sufficient number of excerpts from the transcribed interview data and the analysis are provided as examples so that reliability can be evaluated. The interviews were carried out and transcribed in Finnish, and the excerpts presented here have been translated into English. We selected three excerpts arbitrarily and these excerpts were back-translated into Finnish and compared with the original transcripts by a researcher not involved with this project, for trustworthiness of translation.The validity of the research was increased by careful sampling. We consciously selected motivated and talented students for interviews so that we could test our teaching model and perceive possible incoherence within it. With these students, we obtained very rich material. We carefully planned the interview questions and asked the more-open questions first, followed by more-specific questions. The interviewer (the first author) did not guide the students or teachers toward expected answers. We used telephone interviews for the teachers because of their busy schedules (Stephens, 2007), and so the interviewees could not infer the expected answers from the interviewer's facial expressions. The interviewees could also talk about teaching models vaguely or inaccurately without fear of losing face (Sturges and Hanrahan, 2004; Vogl, 2013). In addition, to confirm that the teachers would understand our questions, we first arranged a CPD training event for other teachers (who would not be interviewed). At this event, we used group discussions to test our preliminary ideas about identifying and classifying concepts based on explanatory status (mnemonic, causal or teleological).
Results
Separation of the octet framework and the electrostatic framework
At the upper-secondary school stage, most of the pupils had adopted an octet framework that was linked to a minimum-energy framework but was not to an electrostatic framework. Only one student (A8) clearly connected an electrostatic framework with an octet framework and a minimum-energy framework through schema d15 (Table 10). Two other pupils implicitly connected an electrostatic framework with an octet and a minimum energy framework (d15) after a long discussion and several more-focused questions.| Excerpt | Coding |
|---|---|
| I: Why has one got higher electronegativity and the other one lower electronegativity? | |
| A8: It depends on the size of the nucleus. If we move to the right on the periodic table, the number of protons will increase by one with every step. Also, there will be an increase in the number of electrons and the size of the atom will increase. However, when we move within the same row, we have the same number of electron shells, but a bigger nucleus. Because the outer electrons are in the same electron shell but there is a bigger nucleus, it attracts more electrons. So that electronegative value is the power; it is how a strong nucleus will attract the outer electrons. | |
| I: Ok, how about the octet? How is it related to this? | |
| A8: Mm…could it be that in some way when an atom has an octet, then the nucleus cannot attract any more electrons. So in a way, the octet is full, then there is no space for more electrons on the shell and also the nucleus cannot attract more electrons. | d1 + d6 + d15? |
| I: Ok. Why not? | |
| A8: Because it (the nucleus) is not big enough. If there were to be still one more proton, it would attract the electron, but then there would also be one more shell and one more electron. | OP + ESP? with d15 |
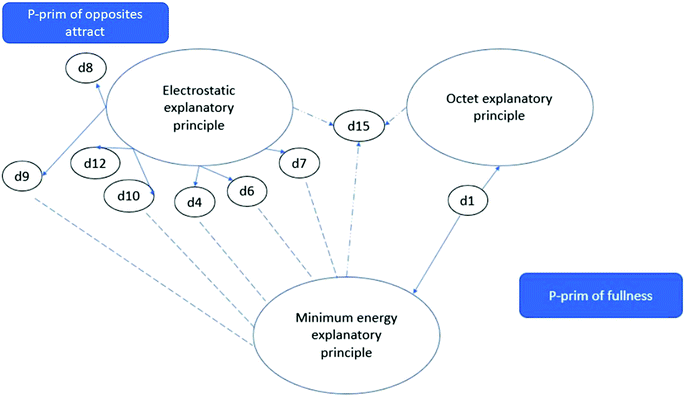
Fig. 1 represents the most common ways detailed explanatory schemas (d) were connected to different frameworks by students. A schema (d15) serving as the link between an octet framework, a minimum-energy framework and an electrostatic framework was expressed explicitly by only one student. Usually, an electrostatic framework and schemas (d8, d9, d12, d10, d4, d6 and d7) were not connected to a minimum-energy explanatory principle nor to an octet explanatory principle (d1) by the students. The minimum-energy principle constitutes a separate schematic framework that does not interact with the electrostatic framework (expressed in Fig. 1 by a dashed line).
Only one student, A3, did not seem to have an octet framework in mind at all during the follow-up interview. Instead, student A3 proved that he understood the connection between the quantisation of electron configurations (d15) and electrostatic interactions, and he retained a similar explanatory model for chemical bonds based on electrostatic interactions, which he had had at the end of lower-secondary school. At the upper-secondary level, he was able to describe this more closely and broadly. Student A3 was one of the two students with the most adequate and the widest conceptual structure after lower-secondary school. On the other hand, student A7, who had expressed one of the most adequate conceptual structures in the lower-secondary level interviews, had very strongly adopted the octet framework at the upper-secondary level. He used the schema of fullness without connecting it to electrostatic interactions, although he had clearly brought out the meaning of electrostatic interactions at the lower-secondary level (see interview excerpts in Appendix 3) (Table 13).
At the upper-secondary level, most students had moved on to using the octet rule as an explanatory schema. In this research, the octet rule is revealed to be a schema of fullness: atoms aim at acquiring a full outer shell in the easiest way possible; no atom wants to be incomplete. The schema of fullness seems like a type of p-prim, and we propose that it is nearly the same as the p-prim of vacuums impel (diSessa, 1993), such that “emptiness requires filling”. diSessa (1993, page 219) writes: “Consider an extension: How do children explain the fact that sand fills in scooped-out space?” We argue that a schema of fullness relates to the p-prim of vacuums impel because students think that “atoms have an intention to get a full outer shell” by donating, receiving or sharing electrons. Emptiness implies “not full” or “incomplete” in this implementation of the p-prim of vacuums impel. It is notable that students did not connect the octet rule to electrostatic interactions, instead treating it only from the teleological viewpoint of aiming for and reaching a state of fullness. Students used the octet rule and the schema of fullness more widely than did their teachers, with students thinking that in metallic bonds there are also octets because metal atoms have donated outer electrons to the lattice structure (for example, see interview extract 4 [A5]).
We argue that the p-prim of vacuums impel is an intuitive promoter behind the octet explanatory principle and the minimum-energy explanatory principle, causing these to separate from the electrostatic explanatory principle (Table 9; example extract in Table 11).
| Lower-secondary school, Student A2 (first interview) | Upper-secondary school, Student A2 (follow-up interview) |
|---|---|
| I: Okay, yes…If you look at the periodic Table of elements, which has the stronger attraction to electrons, nitrogen or fluorine? | I: Okay, then. You can look at the periodic Table of elements – which has a stronger attraction to electrons, nitrogen or fluorine? |
| A2: Fluorine. | A2: I would say that fluorine has a stronger attraction. |
| I: Yes, why? | I: Why? |
| A2: It has more protons, which attracts electrons towards it. d4 | A2: Because, if I remember correctly, it's so that atoms on the right side of the periodic Table of elements have a stronger attraction so that it would be easier to fill the outer electron shell when it is almost full. d1 |
| I: So, this is the reason? | |
| A2: Yes. | |
| I: Are there any other reasons? | |
| A2: I don’t know how to answer that. |
It was observed that explanatory schema d15, an understanding of quantisation, is a remarkable schema with which a student can understand the connection between the octet rule, the minimum-energy principle and electrostatic interactions. However, we only clearly verified d15 from one student (A3) and got a hint of it from student A8 (see Table 10, counter-example for separation), and from students A5 and A6 only after some time. Student A4 also used the octet explanatory principle (OP) and the minimum energy explanatory principle (MEP) without connecting them to the electrostatic explanatory principle (ESP):
Extract 1
I: Well, okay, what is the most remarkable change in your understanding? What new things have you learnt at upper-secondary school?A4: Concerning bonding?
I: Yes.
A4: I have learnt about the movement of electrons. At lower-secondary school I just knew that there is some kind of bonding, but now I have learnt better to understand why these bonds will be formed.
I: So why are bonds formed?
A4: Well, because atoms want to reach the octet structure in the structure that is most stable.
I: Why the most stable?
A4: …the most stable in respect to energy.
I: What does it mean, the most stable for energy?
A4: It means that it (an atom) does not react willingly with other atoms. It wants to be as it is.
I: But what causes that? Do the atoms understand that now they have an octet?
A4: They don’t understand.
I: So, what is it that caused them to reach the octet structure?
A4: Mmm…I can’t answer that question.
…….
A4: Atoms react in different ways depending on whether they have a lot or a few electrons in the outer shell, because they try to reach the octet in the full outer shell.
I: Why do they try to reach the octet?
A4: Because it has the best state relating to energy.
I: Which things concerning chemical bonding are difficult to understand, in your opinion?
A4: Maybe just that. I know that atoms want to reach the octet, but I don’t know why they try to do this.
As diSessa proposed, p-prims are context-dependent (diSessa, 1993) and the p-prim of vacuums impel (the desire for fullness, the octet) will be activated depending on the context. We observed that the p-prim of vacuums impel is especially related to the conditions under which a student is pondering whether an atom is donating, sharing or receiving electrons, and usually when the focus is only on the so-called “will” of one atom, not the interaction between atoms.
Most students had a proper schema of electrostatic interactions and a proper understanding of electron shells and orbitals connected to energy levels (interview questions 20–25, see corpus of interviews). However, despite having the necessary concepts (Table 4) and required level of understanding, they were not able to explain the basis of the octet rule with the help of electrostatic interactions, quantisation of energy levels and the minimum-energy principle. This observation is analogous with the case reported in the first paper (Joki et al., 2015): in the first interview, student A5 could not explain why different types of chemical bonding exist. However, according to the interview data, student A5 had almost all of the pieces of the puzzle (schemas d4, d6 and d3) related to this concept.
Because the students used electrostatic interactions, the minimum-energy principle and the nucleic charge (effective core charge) adequately in the diagnostic questions (questions 20–25, see interview corpus in Appendix 1), it would seem that the octet rule is an explanatory schema that does not need further explanation or to be connected to other schemas (electrostatic interactions). In teaching this concept, compared to electrostatic interactions, the octet rule has to do with the p-prim of vacuums impel – nothing wants to be incomplete. Perhaps this is one reason teachers do not bind the octet rule to electrostatic interactions, but they intuitively feed the p-prim of vacuums impel.
A few of the students questioned the teaching of the octet rule in chemistry lessons at upper-secondary school. Students also reported that their teacher had not been able to answer questions or claims that disputed the octet rule:
Extract 2 (from the follow-up interview)
I: Have you noticed anything that you had understood incorrectly at lower-secondary school?A2: Not in the first course on chemistry, but when I mentioned a few things about the octet rule that belonged to the second course of chemistry, then…
I: Yes.
A2: There was some disagreement…
I: Well, tell me about it.
A2: Well, our teacher thought that the octet is a really important thing and I remember you saying something different.
I: Yes.
A2: That the octet is so and so, but then we talked about the octet, as it was the common thread.
I: Can you tell me how it is the common thread?
A2: Well, they form bonds in order to reach the octet somehow.
I: Why?
A2: Because their outer electron cloud needs to be full.
I: Why does it need to be full?
A2: I don’t know.
I: Right, well how did you understand the octet before?
A2: I didn’t feel it was important and I changed my opinion constantly on whether the octet was important or not.
The same student had also commented during the first interview:
I: Why do they aim to fill the outer shell?
A2: Well, I’ve been told never to believe in the octet thing, but maybe it has something right. I don’t know.
For student A2, what is inadequate in the octet rule had been unclear since lower-secondary school. For more of this, see Joki et al. (2015). Another student said that having been inspired by the interview during lower-secondary school, his friends had challenged their upper-secondary teacher's views:
Extract 3
I: Well, what things in the theory of chemical bonding are unclear to you or difficult to understand?A5: Well, at the basic level, this is not that difficult, but if we go deeper to explain why this happens, then it is too difficult and actually we presented some questions to our chemistry teacher. John and Simon (the names have been changed) had perhaps written up your questions after the interview at the lower-secondary level and then they asked our upper-secondary school chemistry teacher these questions, who could not really answer them.
I: What questions?
A5: I think that they had recalled the earlier questions from last spring.
I: Okay.
A5: The more difficult questions, which none of us was able to answer.
I: Okay.
A5: Things like why an atom wants to reach the octet.
I: Right.
A5: Then the chemistry teacher was a bit lost and couldn’t explain the question.
I: I see.
A5: If we go deeper into things, it gets really difficult.
I: Ok.
A5: It's not easy to understand.
This comment that “the teacher was a bit lost” was confirmed by that teacher's interview, in which she herself said that she had been challenged by students concerning her use of the octet.
It is noticeable that in the case of this student (A5), the octet rule was not a problem at the time of the lower-secondary school interview, but rather the problem concerned questions about what the different chemical bonds were based on (Joki et al., 2015). In the follow-up interview, he connected the challenges from the previous interview specifically to the octet rule, perhaps after a discussion with friends.
The p-prim of vacuums impel seems to especially hinder the understanding of quantised energy levels. Student A5 became slightly confused when we challenged his answer: “Sodium gives away its electrons to chlorine, because chlorine has a higher charge in its nucleus.” We asked the student why potassium gives up its electrons even though it has an even higher positive charge in its nucleus compared to chlorine. After this, the student moved from the schema of electrostatic interactions back to the p-prim of vacuums impel and retracted his earlier explanation. However, during the diagnostic questions (see questions 20–25 in the interview corpus in Appendix 1), the same student could make a connection between the ease of releasing an electron and the energy level, as well as nucleic charge. Student A5 also connected the octet rule to metallic bonding in the follow-up interview (upper-secondary school):
Extract 4
I: Well, how does the octet relate to this magnesium ribbon?A5: It relates to this in that every magnesium atom…gives up two electrons to be used mutually in the entire lattice structure. The electrons are then delocalised, and they can freely move around in the lattice, and this is how the structure is reached.
I: What keeps the magnesium atoms together?
A5: Well, the electrons there.
I: In what way?
A5: Because they have a negative charge and magnesium has given away two of its electrons and this is why they are positively charged. This electrostatic attraction between them keeps the structure together.
I: How are these now, electrostatic attraction and the octet, related to each other? Are they connected to each other?
A5: They must be connected somehow…
I: In your opinion, how are they connected?
A5: Well … … … … hmm…. I can’t really explain it right, but maybe these electrostatic attractions are in balance in this state, which happens to be the octet. I can’t explain it any clearer.
It is illustrative that this student was unable to explain the connection between the octet rule, electron configuration and electrostatic interactions.
Student A2 (Table 11) was able to understand that having more electron shells means that more electrons are situated between the nucleus and the outer electrons, which is why these outer electrons are more easily released. This student did not consider the increasing charge of the nucleus (effective core charge) when moving from left to right on the periodic table. However, the same student had done so during lower-secondary school.
In the lower-secondary level interviews, students that used the full-shell principle connected to the principle of electrostatic interactions, and explained the stability of a full shell by stating that a positive nucleus is able to attract a specific number of electrons (Table 12).
| Lower-secondary school, Student A6 (first interview) | Upper-secondary school, Student A6 (follow-up interview) |
|---|---|
| A6: Because non-metals are such that they want to fill the outer electron shells. | I: Alright. Is there now a mutual reason or a factor on which all chemical bonds are based, if we think of all the different bonds, or if we start with the covalent, ionic and metallic bonds? |
| I: Why is that? | A6: All bonds aim to reach the octet. |
| A6: Hmm. Because they are missing only a few electrons from the outer shell and there are many protons in the nucleus that are also able to attract electrons more easily than metals, which have instead only one or a couple of electrons in the outer electron shell. This is why they have a lot of protons pulling the electrons. | I: Okay, and is that the reason that bonds are formed? |
| I: Which have a lot of protons? | A6: Well, yes. |
| A6: Non-metals. | I: Why do they aim to reach the octet? |
| A6: From the viewpoint of energy, it is the most efficient. | |
| I: How is an octet formed in a metallic bond? | |
| A6: They release the electrons into mutual use, away, so that they reach an octet. | |
| I: How does this help metals to stick together? | |
| A6: When they become positively charged, when they give them away and when they go around with a negative charge, they keep the whole system in order. | |
| I: But in that case, the electrons are going around, aren’t they? They are not “away”? | |
| A6: Well, yes. But…how can I say it then? | |
| I: (Laughing) I asked you, (laughing), well, are there any other reasons, besides the octet, why bonds are formed? | |
| A6: The fact that some atoms attract electrons more and in a stronger way than others. | |
| I: What about whether it is connected to this octet thing or whether it is a completely different thing? | |
| A6: It doesn’t specifically have to do with reaching the octet, they just want to get it in a receiving way. |
In addition, at the end of the upper-secondary level interview (Table 12), the student gave a teleological meaning to the achievement of the octet: attraction does not affect whether or not a full octet is achieved, but instead on how it is achieved. The same student pondered the connection between the octet rule and electronegativity in this way:
Extract 5
I: Ok, are these two different things, how to reach an octet and electronegativity? What are they caused by?A6: What was latter question?
I: Sorry, what are they caused by? Are they a separate matter, how to reach an octet and electronegativity? What are they based on?
A6: But the octet does not relate to the electronegativity directly.
I: So where will the octet be connected then?
A6: It would get the most favourable state from the point of view of energy.
I: Ok, is it always this octet that is the most favourable state for all substances?
A6: Yes, I don’t know about those radioactive ones, what adjusts them, but yes otherwise, at least.
I: What things in the theory of chemical bonding are unclear or difficult to understand, in your opinion?
A6: If one reads (chemistry text) books, there are no problems, but some of your questions are such that I will begin to ponder about how these things really are.
I: Which questions?
A6: Well, just these last ones, concerning electronegativity and why just the octet? Why does the atom just want an octet? Why is it just eight and not nine or seven?
I: So, what do you think, what could cause it?
A6: Perhaps some energy thing tries to get a satisfying status.
We also noticed that the explanatory schema of the full-shell principle (d1) was activated when a student wondered about an atom as donating, receiving or sharing electrons. The p-prim of vacuums impel (desire for fullness) also related to a situation in which the student considered a case from the point of view of only one atom and dismissed the fact that there are always at least two atoms involved in any (electrostatic) interaction, when considering bonding. Without another atom, this student imagined the atom to be an intentional being, something endeavouring to achieve fullness.
From lattice structures to molecular ontology
A fascinating hint was acquired from the follow-up interviews that the octet explanatory principle might indeed be linked in the formation of a molecular model for ionic bonding, as proposed by Taber (2001b, 2002). Student A7 represented one of the most adequate conceptual structures in the first interview after lower-secondary school. This student could explain chemical bonds very accurately, based on electrostatic interactions. In his upper-secondary interview, he strongly emphasised the meaning of the octet in the formation of bonds. In the first interview (Fig. 2), he had properly explained and drawn an ionic compound. A significant change can be perceived between the first (Fig. 2) and second interviews (Fig. 3). He had said that ionic compounds also consist of separate molecules; at the follow-up interview, he explained that ionic bonds are stronger bonds and that there are dipole–dipole forces between molecules (Fig. 3).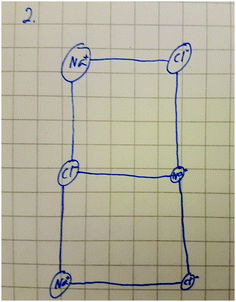 | ||
| Fig. 2 Student A7's understanding of an ionic compound as a lattice structure at lower-secondary school. | ||
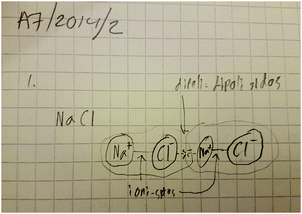 | ||
| Fig. 3 Student A7's understanding of an ionic compound as a molecular structure at upper-secondary school. | ||
Teachers’ understanding of schemas related to chemical bonding
We now turn our attention to the extent to which the p-prim of vacuums impel is behind teachers’ understanding. Almost all the students’ interviews from lower-secondary school emphasised electrostatic interactions, and when students used the full-shell principle, they usually connected it to electrostatic interactions and the structure of electron shells (electron configuration) and within this, to energy levels. Because of this, a strong octet explanatory principle emerged during upper-secondary school, and seemed to have been caused by the teaching at this level. How do teachers affect the formation of students’ notions?Based on interviews with the teachers (n = 3) who had taught the follow-up students (n = 8) at the upper-secondary level, it was observed that teachers’ own understanding about concepts related to chemical bonding and the relationships between them were lacking and bound to particular contexts.
T2 had taught students A2 and A8. She gave a teleological explanatory schema status to the octet rule and did not combine electrostatic interactions with all kinds of chemical bonding:
Extract 6
I: How would you see, for example, the octet rule, the kind of role it plays in explaining chemical bonds?T2: Well, it is quite like a central engine, why atoms start to form bonds with other atoms, this is quite like an explanatory thing.
I: Ok.
T2: There is a specific state that needs to be achieved.
I: What about electrostatic interactions, how would you connect electrostatic attraction and interaction to chemical bonding?
T2: Well, it comes from the interaction of ions of course, and also if we think about the size of atoms and electrons and why the sizes of atoms increase down groups and decrease across periods. Why it is the case that the size of ions of a particular element are in a specific relationship with the size of the element's atom. This is really an explanatory model.
We argue that our observation that for students A2 and A8, a conceptual understanding was developed during upper-secondary school in which the octet principle and the electrostatic explanatory principle became separated. This was a consequence of the upper-secondary level learning and is at least partially due to the teacher, judging from the teacher's own comments about how she used the octet rule and electrostatic interactions to explain chemical bonding. The teacher's comments supported our conclusion.
Teacher T4 taught students A5 and A6, and used the octet rule in her teaching as a teleological explanatory principle. She stated that because she does not explain why the octet is energetically efficient, it is like a mnemonic device, although she uses it more like a teleological explanatory schema in her teaching, based her interview answers:
Extract 7
I: Yes, that's correct. How about the octet rule, what kind of significance does it have?T4: Yes, well, based on the octet rule, we come nearer to whether we have a single, double or triple bond. Here it is possible to start wondering whether students can then draw their own conclusion about which type of bond is formed between atoms.
I: Yes. In this research, I look at these concepts in a way that concepts can be categorised according to whether they are more like mnemonics or explanatory schemas in nature. So, concepts are these kinds of simple mnemonics or explanatory schemas, which are a bit closer to causal causes.
T4: Ok.
I: (Explaining the difference between mnemonics and explanatory schema) Yes, which concepts regarding chemical bonds do you think are more like explanatory conceptual structures?
T4: Well, I think that the octet rule is explanatory, because through it, the type of bond (single, double, triple) is explained and justified, so that we can see what kind of covalent bond is being formed. I always explain this through the octet rule as well as through electronegativity values, so that even though the electronegativity values are not explained, polarity can be explained with it.
I: Yes, what about mnemonics?
T4: Mnemonics…there are not so many in chemistry, but it's not explained why the octet is aimed at and why it's the most energy efficient. Maybe that is the mnemonic – that atoms aim to get eight electrons in their outer shells – and that is more like a mnemonic and not really thoroughly explained.
I: In what way do you bring this out in your teaching? Which category does it belong to, is it a mnemonic or more like an explanatory schema?
T4: I haven’t thought that through, but I would need to. It would be good for the future. I notice that in chemistry there have been a lot of things that I have repeated over and over again, but I don’t classify them.
I: You said earlier that the octet rule is an explanatory schema and now you claim that it might be more like a mnemonic? Could you clarify this?
T4: I meant that atoms try to reach the octet and I think that it is a mnemonic. An octet rule is an explanatory schema for the question of which type of a bond is formed. This is how I often explain that this would be an explanatory rule, whether a single, double or triple bond is formed.
Her students, A5 and A6, also moved toward the teleological use of the octet after lower-secondary school.
This teacher also connected electrostatic interactions only to ionic bonds and to forces between molecules:
Extract 8
I: How do you see electrostatic interactions, what kind of a role do they have in chemical bonds?T4: This is what I teach my students. They (electrostatic interactions) are first revised and then they are brought up again with ionic bonds. Electrostatic interactions also come up when we look at covalent bonds, when we are dealing with polarity. After that we move onto forces between molecules and we compare the difference between partial charges and charges in an ionic bond.
Based on this observation, it is understandable that students A5 and A6 were confused about the role of electrostatic interactions versus the octet rule for explaining chemical bonding. The summary of the findings can be seen in Table 9, in which the results of the analysis of students’ and teachers’ interviews are combined.
Teacher T1 was the most aware of the imperfection of the octet rule, but even he thought that he would not mention the restrictions unless the students questioned the rule or asked “why?” However, teacher T1 also presented a concept concerning electrostatic interactions that confused the students: “Why is an electron pair formed if two similar charges always repel each other?” This was a rhetorical question asked by the teacher and no further explanation was given.
The interviews with teachers confirmed what the students’ comments had revealed (“the teacher didn’t know how to answer”). Teachers do not ponder the schemas of chemical bonds and conceptual structures as an entity, nor on what kind of epistemological status a certain part has. Teachers use schemas and concepts from the viewpoint of a single, particular need, without thinking about it themselves or emphasising to students the meaning of different concepts and schemas concerning the entity: i.e., what is in question here, a memory-helping heuristic rule or an explanatory schema?
Limitations
Our whole study is composed of three case studies related to each other. Because the results are based on particular cases and our sample was very small (only three teachers and eight students), it is clear that much more research is needed to confirm the results and to determine how teachers’ understanding of explanations concerning the basis of chemical bonding has an impact on students’ understanding. We have already collected data from the CPD event (which was used for developing the teachers’ interview corpus) and we have created a preliminary survey for Finnish chemistry teachers, but these results will be presented in a future separate paper.Discussion and implications for education and future research
Unlike earlier research concerning the understanding of chemical bonding, in this research situation, most students first had a research-informed and quite adequate model that emphasised electrostatic interactions as a mutual basis for all types of bonding (Joki et al., 2015). This partially persisted, but at the upper-secondary level, an equally strong, new alternative framework (the octet rule) emerged, which confused some students. The octet framework (Taber, 1998) often led students to a dead end during interviews. In this context, “dead end” means a situation in which a student cannot offer any further clarification because he/she thinks that the octet rule is the final teleological explanation. The same kind of situation is described with the concept of “explanatory gestalt of essence” (Watts and Taber, 1996).Taber reported (2000b, 2001a) that there are three explanatory principles of the framework connected to learning about chemical bonding that students can use in several ways. These principles are:
• the explanatory principle of minimum energy
• the explanatory principle of electrostatic interactions, and
• the explanatory principle of the octet.
We suggest that for students and teachers, the full-shell principle (the wider explanatory principle; Taber, 2001a) is especially connected to the phenomenological primitive (p-prim) of vacuums impel based on diSessa's (1983, 1993) theory. Tentatively, Taber and Garcia-Franco (2010, page 126) considered the role of the full-shell principle in light of diSessa's theory of p-prims:
“As one example, students very commonly make statements that chemical reactions occur so that atoms can obtain full electron shells (Taber, 1998). This is an idea that appears to often derive from a very well-established explicit representation in cognitive structure that may be highly integrated into the students’ conceptual understanding of the subject. This conception might well be so common because its initial development is facilitated by an intuitive knowledge element (about the properties of complete or highly symmetrical configurations), but it becomes represented in cognitive structure in an explicit form.”
Based on evidence from our study, we propose that one reason for the success of the octet framework is the p-prim of vacuums impel (desire for fullness). Support for this argument is that the student does not connect the octet rule or the minimum-energy principle to electrostatic interactions, although he/she can use electrostatic interactions properly in other situations. It seems that the p-prim of vacuums impel and the p-prim of “opposites attract” are exclusive subconscious ideas, and the appearance of one of them will determine which schemas will be activated. Activation of p-prims and schemas is strongly context- and content-dependent (diSessa, 1993). The p-prim of vacuums impel seems like one possible explanation for the fact that once learnt, a student uses the octet rule as an explanatory schema and gladly sticks to it (Taber, 2003). The intuitiveness p-prim of vacuums impel, i.e., aiming to reach fullness (the octet), supports the adoption and permanence of thought. Moving away from this thought has been observed to be slow and perhaps even impossible (Taber, 2003). Based on our research, we claim that there is a more primitive and intuitive idea of fullness (p-prim of vacuums impel) behind the full-shell explanatory principle (Taber, 2000a, 2000b), which competes with the p-prim of “opposites attract”.
Based on our findings, teachers’ awareness and caution about causal or teleological explanations require specific development and attention in teacher training. It is not enough merely to share knowledge as in PCK (e.g. do not use the octet rule); instead, teachers should reach beyond PCK and be aware of the epistemological and historical background of a range of concepts and schemas in their teaching. They should also think about the different explanatory statuses of concepts and schema. Bergqvist et al. (2016) proposed that teachers’ PCK and KSU should be developed accordingly, to teach chemical bonding. Based on our current research, we argue that there is a special need concerning teachers’ skills in understanding the explanatory power of different schemas and epistemological aspects of conceptual constructs regarding chemical bonding. After achieving these skills, teachers can consciously guide students’ understanding about the significance, nature and epistemological status of these concepts and models (Taber, 2010). Epistemological beliefs behind the schemas, like the p-prim of vacuums impel, can restrict particular explanatory schemas to activate, for example, schemas relating to electrostatic interactions (d4, d6, etc.). This assumption is given greater confirmation in this research.
When we compare the change in students’ thinking during their follow-up interviews and in the image of the teachers’ thinking (based on the upper-secondary school teachers’ interviews), we can see indications of the relationship between students’ and teachers’ understanding about explanatory schemas, and teachers’ use of teleological explanations. Two of the upper-secondary teachers (T2, T4) used the octet rule as an explanatory schema and did not connect electrostatic interactions to every bonding type. Teachers connected electrostatic interactions only to ionic and intermolecular bonding. Separation between the electrostatic explanatory principle and the octet explanatory principle (including the minimum-energy explanatory principle) also appeared in the teachers’ interviews. Bergqvist et al. (2016) made similar observations in Sweden, but not with as much detail as in the present study. We argue that teachers should be aware of the pitfalls of unintentionally reinforcing the intuitive and subconscious p-prim of vacuums impel in their teaching.
In the individual interviews, it transpired that the teachers considered the students to be too young and that they should not be told about the epistemological background of concepts too often. We argue that at least older (upper-secondary school) students should be consciously trained to understand the connections between different conceptual structures and schemas and their explanatory power. This might not bear fruit if teachers avoid teaching the octet rule or go around it. Sooner or later, it will come back to the student, either in the teaching material or some other part of the course. Instead, students could be trained to compare different models, even historical ones, and to ponder their area of competence and explanatory power (Croft and de Berg, 2014). However, younger students at lower-secondary schools need to be introduced to chemical bonding with an emphasis on electrostatic interactions as the common basis for bonding; it would be better to avoid emphasising the octet rule at all during lower-secondary school. We would like to provoke a discussion about whether it is necessary that students be able to predict formulae of substances (for example, HF not H2F) at the lower-secondary level. Is this an authentic way that modern scientists do chemistry? We think that molecular modelling and computational chemistry have replaced the necessity for these kinds of simple heuristics. These suggestions unquestionably need more research to identify the best ways to arrange learning pathways with regard to chemical bonding, from lower- to upper-secondary schools and beyond.
Due to the quantisation of orbitals and the stability of full orbitals, the concept of fullness cannot be entirely removed, but it must be made subsidiary or at least parallel to the concept of electrostatic interactions (Taber, 2001b), especially at the lower- and upper-secondary levels, when students have not yet been introduced to the quantum chemical aspects of orbitals. A possible solution is to increase students’ metacognitive awareness of different models and their uses (Delgado, 2015). Electrostatic interactions could be emphasised more than the octet rule in teaching, especially if the teaching features no quantum mechanical framework to explain the quantisation (Gillespie and Robinson, 2007).
Based on this research, an even stronger case could be made that the octet rule is linked to the p-prim of vacuums impel, which does not contain the dimension of electrostatic interactions, both in teachers’ teaching and especially in students’ minds. Because the octet is considered from the viewpoint of fullness and the easiest way to reach it, the minimum-energy principle is not connected to electrostatic interactions but is given characteristics of magical or social energy in a student's mind (Taber, 2001b). Based on our findings, this interpretation by students is not surprising if teachers do not have a clear picture of the overall significance of electrostatic interactions in the background of chemical bonding. Similar results have been reported earlier in Sweden (Bergqvist et al., 2016).
We noted also that the teachers did not want to explain to students, or could not explain, what minimum energy means. If a student asked about the basis of the octet rule, the teachers would answer that it is the most energy-efficient state. This left students to wonder how this energy state is the most efficient. Only electrostatic interactions (an electron's distance from the nucleus, the aim to be as close to the nucleus within the boundaries provided by quantisation) offer a meaningful understanding of the minimum-energy principle. It is essential that students focus on the interactions between atoms and not only on one atom and its behaviour.
Conceptual structures, conceptual constructions and schemas need to be deconstructed and their status (explanatory or mnemonic) in the system needs to be explained. Emphasis on electrostatic interactions is not enough, since quantum mechanical phenomena and the quantisation of energy levels (orbitals) are part of the process of bond formation. It is good to remember that electrostatic interaction by itself creates a possible contradiction in the student's mind when thinking about an electron pair without taking into consideration electron spin, which is connected to quantum mechanics. This is why, in lower- and upper-secondary education, any teaching model for chemical bonds is inevitably a rough compromise, in which reasonable explanatory power is aimed at by a simple conceptual structure (Gillespie and Robinson, 2007; Gonthier et al., 2012). At the same time, such a conceptual structure should be aimed at points where essential explanatory schemas do not need to be entirely forgotten. Teachers’ understanding of different explanations (causal or teleological) and awareness should be promoted, as well as PCK, GPK and KSU (Erman, 2017). Without an understanding of different explanatory schemas, teachers cannot teach a topic as complicated as chemical bonding in an understandable and reasonable way (Oh and Oh, 2011; Delgado, 2015; Papdouris and Constantinou, 2017). Our study presents a critical message: students can regress toward less-canonical thinking if teachers use models and explanations, especially teleological ones, without giving care to an understanding of relationships and difference between explanatory schemas vs. mnemonic devices.
Conclusion
Teaching style has a great impact on students’ conceptual understanding. Unfortunately, it can also lead students toward less-canonical thinking despite previous teaching that introduced them to chemical bonding in a research-informed way. This claim can be justified by the follow-up interview data showing that students adopted an octet-rule explanatory principle after middle school. In addition, interviews revealed that students’ upper-secondary teachers did not have clear insight into the status of the octet rule as a merely mnemonic device, because they use it as a causal or even a teleological explanation.Electrostatic interactions remained an explanatory model in students’ follow-up answers to certain questions. These questions were presented in the first interview and were the type of questions whose content had not received much attention during their upper-secondary level studies (based on comments by the upper-secondary teachers). Instead, broader questions, such as why metals “want” to give up electrons or why electron pairs are formed, led students to use the octet-rule explanatory principle, which led to a dead end.
Based on the follow-up interviews, all students had absorbed most of the building blocks (concepts and schemas, except d15) with which to explain the basis of the octet rule. However, because the octet rule was used vaguely as a teleological cause by their teachers, the students could not use these building blocks to explain the octet rule and were confused by ‘why’ questions, since they thought that the octet rule was the final explanation.
Compared to the previous research, this study included a situation in which students were taught in a research-informed way at the lower-secondary level and their understanding was tracked by interviews. The interview data indicated that most students had conceptual and schematic toolboxes that they used to explain the meaning of the octet rule. Nevertheless, some students gave the octet rule an explanatory teleological meaning, because it was subsequently taught in a certain way at the upper-secondary level. After this, they were unable to understand how the octet rule is connected to the minimum-energy principle or to electrostatic interactions. We also found that teachers appealed to the octet as an energy-minimum, but did not explain the quantisation of electron configuration and electrostatic interactions with the help of the effective core charge. This explains why students did not link minimum energy (linked to the octet) to physical energy, but to social and magical energy.
In addition, the teachers did not understand the role of the octet rule as a heuristic mnemonic device in the teaching of chemical bonding. There were also preliminary observations that a teacher's way of using the octet rule as a teleological explanation in relation to other concepts seemed to have quite strong effects on the student's understanding.
These findings partly strengthen Taber's (2000b, 2001a) comprehensive and significant observations, but also provide a more detailed picture of the types of problems and the multidimensionality involved. For this reason, the teaching model for chemical bonding probably cannot be entirely modified to be both intellectually consistent and sufficient for basic education. Instead, increasing teachers’ and students’ instrumental views of how to use explanatory models and understanding the different statuses of conceptual structures (explanatory models versus heuristic mnemonics) helps students avoid attributing teleological meaning to the octet rule (Taber, 2010). Implications from these findings should also be considered in pre-service teacher education.
Conflicts of interest
There are no conflicts of interest to declare.Appendix 1
Follow up interviewBackground information:
the name of the upper secondary school you study at?
the name of the chemistry textbook you used at upper secondary school?
the name of your upper secondary school teacher?
key concepts:
Could you tell me as accurately as possible what the concept of “chemical bonding” means?
What types of chemical bonding are you aware of?
Retrospective part:
How has your understanding of chemical bonding changed during the upper secondary school chemistry course?
Compared to learning at your middle school, what change in your understanding has been most significant in your opinion?
Have you noticed things that you had misunderstood at middle school?
Which things that you learned in middle school led you astray in your understanding of chemical bonding? Are there matters which in the light of your present knowledge seem like misleading simplifications?
What matters in lower secondary school chemistry (connected especially to the chemical bonding and the structure of matter) have supported you in the study of chemistry in the upper secondary school?
In your opinion, what things have provided the most significant basic information in learning about chemical bonding which you learnt in the first chemistry course in the upper secondary school?
Follow up part: (see Joki et al. 2015 )
8. Is there a common reason/factor on which all chemical bonding types are based?
9. Are there other issues that can have an effect on the forming of bonds?
10. Why are there different bonding types? What are they based on?
11. What bonding types are involved in the following materials?
a. Water
b. Diamonds
c. Sodium chloride
d. Magnesium ribbon
12. What factors affect the bonding type that forms in the particular cases above?
a. Why is there a different bonding type in table salt than in water?
13. What matters related to the theory of chemical bonding are still unclear or difficult to understand?
14. Why?
15. Do the theoretical models of chemical bonding help you to understand the properties and structure of the above-mentioned materials?
16. What properties or features do the models not explain? What do the models that you have learnt about failed to explain?
17. What motivates you in your thinking/learning about chemical bonding?
18. Describe some more memorable learning experiences concerning the study of chemical bonding (ionic compounds, covalent bonding, metallic bonding)?
19. What matters reduced your interest in thinking/learning about chemical bonding?
(Questions 20–21; Peterson and Treagust, 1989)
20. Which one of the following best describes the structure of the hydrogen molecule?
(a) H : H (b) H : H
Why?
21. Which one of the following best describes the shared electron pair of hydrogen fluoride?
(a) H : F (b) H : F
Why?
22. Which donates its outermost electron more easily,
(a) lithium or
(b) sodium?
Why?
23. Determine the chemical stability of the following particles:
Na+ ion
Sodium atom
Na7− ion
{These arranged options below are added only for the graphs. Students had to determine the order without having been given any options.
(a) Na+, Na. Na7−
(b) Na, Na+, Na7−
(c) Na+, Na7−, Na
(d) some other order; what kind of order?}
What is the order from the most stable to the least stable structure (Taber, 2000b).
24. Which attracts electrons more strongly, nitrogen or fluorine?
a. Why?
25. Which attracts electrons more strongly, the fluorine atom or the bromine atom?
a. Why?
26. a. HCl is a gas at room temperature. Explain the structure of the molecule. When the gas is introduced into water, the conductance of water will increase. Why? Explain what takes place.
b. When at room temperature, NH3 reacts with HCl so that two gaseous substances produce a solid material. How do you explain this phenomenon?
27. A HArF molecule has been found both experimentally and computationally. How can the molecule be stable?
Appendix 2
Corpus of teachers’ interviews• Explanation of the research project: conceptual structures concerning chemical bonding
• Explanation of anonymity
• Ask permission for tape recording of interview
• Which chemistry text book do you use/have you used in your teaching?
• How do you teach chemical bonding?
• Do you follow the order and method of the text book or do you have your own way of teaching chemical bonding?
• Could you describe briefly how do you process the teaching of chemical bonding?
• What kind of laboratory experiments do you use with students when you are handling the subject area of chemical bonding?
• In which order do you teach the different bonding types?
• What in your opinion are the most central concepts regarding chemical bonding?
….after that in more detail:
○ ionic bonding
○ covalent bonding
○ metallic bonding
○ intermolecular bonding
If the teacher cannot remember the schemas, the interviewer can help: electron pair, shared electrons, octet rule, electrostatic interaction.
• What meaning does the octet rule have in your teaching?
• What meaning does electrostatic interaction have in your teaching?
After that, there is a brief introduction to the idea of different kinds of schema: mnemonic devices and explanatory schemas. For example, an explanatory schema would be conservation of mass when balancing chemical equations and a mnemonic device could be that both sides of an arrow should have the same number of atoms.
• What schemas would the role of an explanatory schema have when you are teaching chemical bonding?
• What schemas would the role of a mnemonic device have when you are teaching chemical bonding?
• How will you explain to students that particular concepts have an explanatory or mnemonic status?
• Have you detected alternative explanations or misconceptions by students? Examples?
Brief summary: what was the intention of the research and how will the data be handled. Ask again for permission to use the data.
Appendix 3
Table 13.| Student A3 | Student A7 | |
|---|---|---|
| Lower secondary school | A3: There is an atom's nucleus and then another atom's nucleus and there are electrons going around. If they come close enough to each other, then they might start attracting the other atom's electrons. | A7: Well, metals naturally want to give up their electrons and then nonmetals want to receive them. |
| T: Okay. Could you write out the charges then? What kinds of charges do these particles have and on what basis do they attract each other? | T: Why is that? | |
| A3: Electric interactions. | A7: This happens when electron shells fill up and nonmetals are then missing only a couple of electrons. This also means that all the electrons are quite close to each other and there are a lot of protons when there is a power which attracts the level of electrons which the atom has. This is quite strong compared to metals, where the opposite happens: there might be a couple of electrons in the outer shell, there are fewer protons and therefore the attraction is not as strong in the level of outer electrons. | |
| T: Okay. Is it so that there are different types of bonds? | ||
| A3: Yes. | ||
| T: Well, what kinds of bonds are there? | ||
| A3: These kinds of covalent bonds, where atoms attract each other's electrons and then there are also ionic bonds. So if an atom loses electrons, then that atom gets an electric charge and it attracts other atoms which have an opposite charge. | ||
| T: Yes, what determines when there is a covalent bond and when there is an ionic bond? What is it based on? | ||
| A3: It is based on the atom's structure how many electrons it has. If we have two atoms, one might receive more electrons and the other might then lose electrons more easily. They can transfer electrons together and that is how they get an electric charge. This is an ionic bond. But if the atoms are equal in a way, they might start to attract the other atom's electrons and therefore create a covalent bond. | ||
| … | ||
| T: Why don’t they give up electrons, why do they form a mutual electron pair? | ||
| A3: Because, in a way, neither of them is able to receive an electron. | ||
| T: Why can’t they receive electrons? | ||
| A3: Or that neither is able to give up an electron. They would have to give up so many electrons that it is easier to attract one another's electrons and not give away one's own electrons. | ||
| T: What affects this? Why can’t they give away electrons? What is it based on that they can’t give up electrons? | ||
| A3: It is based on the fact that an atom or atoms have electron shells, which can hold up a different number of electrons. When the nonmetals’ outer electron shell is almost full, then the electrostatic attraction is much stronger. The electrons are unable to break away or then it would not be energy efficient that the electrons would break away from there. | ||
| Upper secondary school | I: Well, is there a mutual reason or a factor that all chemical bonding is based on? | I: Can you draw a picture of an ionic compound, what kind of a bond is there? |
| A3: Electromagnetism, the number of electrons and interactions between atoms. | A7: Yes …. (drawing) Here, sodium has one valence electron, which is attracted by chlorine. This is why sodium does not attract it as much, it almost becomes the valence electron for chlorine and this is how the bond is formed. | |
| I: Okay, are there any other reasons? | I: Okay, what is this bond based on, why do sodium and chlorine stick together? | |
| A3: Hmm, the mutual interactions between electrons in an atom. | A7: It is based on electric charges. Chlorine has a strong charge and sodium has a weak charge, but they both attract that one electron. This is a bit difficult to explain. | |
| I: Why are there different types of bonds, what is that based on? | I: Okay, well what about the covalent bond? What is it based on? | |
| A3: It is based on the fact that different atoms have a different number of electrons and the other electrons of an atom have a different effect on the valence electrons. | A7: Here, both atoms attract the mutual electrons and when they do this, a standoff happens, and they stick together. | |
| I: What kind of effect does it have? | I: Why do they attract electrons? | |
| A3: For example, in metals, the nucleus does not attract outer electrons as strongly when compared to nonmetal atoms. This is why the metal atoms give off outer electrons more easily. | A7: Because electrons are negative and the atoms or the protons of an atom are positive, there is a charge, which causes the attraction. | |
| I: Why is this the case in metals? | I: Why doesn’t sodium attract electrons as strongly? | |
| A3: Because there are a lot of electrons between the nucleus of an atom and the outer shell of electrons. There are only a couple of electrons and therefore it does not attract them as strongly. | A7: Well, because sodium has fewer protons than chlorine and therefore it does not attract as strongly. | |
| I: Okay, well how do we get the different types of bonds from these, so that the different types of bonds result from this? | I: Well, what about if I show you the periodic Table of elements. Let's see, chlorine is here, and potassium is there. What about potassium, it has more – | |
| A3: Metal atoms give away their outer electrons easily and nonmetals receive them easily. For example, this means that if metal atoms form a bond together, then they usually donate their outer electrons. In the case of a bond between a metal and a nonmetal, the nonmetal atom easily receives the metal atom's outer electrons. | A7: We have to remember that chlorine has a third shell and it is missing one electron and potassium has one electron in the fourth shell, which chlorine does not have. | |
| I: What about if we have two nonmetals? | I: What kind of an effect does this have? | |
| A3: Two nonmetals aim to share the so-called missing electrons so that both atoms would have a full shell. | A7: Chlorine wants to fill up its third shell, but the third shell is full in the case of potassium and therefore the electron wants to get away from the fourth shell. | |
| I: Why do they want a full shell? | I: Why? | |
| A3: Because at this stage, the condition is energy efficient. | A7: Because it aims to reach the octet, so that it would have a specific number of electrons. | |
| I: What is the basis for it being energy efficient? | I: Why does it aim to reach the octet? | |
| A3: It is perhaps the minimum energy that there are electrons on a shell in a suitable proportion and they are all as close to the nucleus as possible. | ||
References
- Amin T. G., Smith C. L. and Wiser M., (2014), Student conceptions and conceptual change, in Lederman N. G. and Abell S. K. (ed.), Handbook of Research on Science Education, New York, NY: Routledge, vol. II.
- Bergqvist A., Drechsler M. and Chang Rundgren S., (2016), Upper secondary teachers' knowledge for teaching chemical bonding models, Int. J. Sci. Educ., 38(2), 298–318, DOI:10.1080/09500693.2015.1125034.
- Bergqvist A., Drechsler M., De Jong O. and Rundgren S. C., (2013), Representations of chemical bonding models in school textbooks – help or hindrance for understanding? Chem. Educ. Res. Pract., 14(4), 589–606, 10.1039/C3RP20159G.
- Boo H. K., (1998), Students' Understandings of Chemical Bonds and the Energetics of Chemical Reactions, J. Res. Sci. Teach., 35(5), 569–581.
- Brown D. E. and Hammer D., (2008), Conceptual change in physics, in Vosniadou S. (ed.), International Handbook of Research on Conceptual Change, New York, NY: Routledge, pp. 127–154.
- Croft M. and de Berg K., (2014), From common sense concepts to scientifically conditioned concepts of chemical bonding: an historical and textbook approach designed to address learning and teaching issues at the secondary school level, Sci. Educ., 23(9), 1733–1761, DOI:10.1007/s11191-014-9683-0.
- De Jong O. and Taber K. S., (2014), Many Faces of High School Chemistry, in Lederman N. G. and Abell S. K (ed.), Handbook of Research on Science Education, New York, NY: Routledge, vol. II.
- Delgado C., (2015), Navigating tensions between conceptual and metaconceptual goals in the use of models, J. Sci. Educ. Technol., 24(2), 132–147, DOI:10.1007/s10956-014-9495-7.
- Dhindsa H. S. and Treagust D. F., (2014), Prospective pedagogy for teaching chemical bonding for smart and sustainable learning, Chem. Educ. Res. Pract., 15(4), 435–446, 10.1039/C4RP00059E.
- diSessa A. A., (1983), Phenomenology and the evolution of intuition, in Gentner D. and Stevens A. (ed.), Mental Models, Hillsdale, NJ: Lawrence Erlbaum, pp. 15–33.
- diSessa A. A., (1993), Toward an epistemology of physics, Cognit. Instr., 10(2), 105–225, retrieved from http://www.jstor.org/stable/3233725.
- diSessa A. A., (2014), The construction of causal schemes: learning mechanisms at the knowledge level, Cognit. Sci., 38(5), 795–850, DOI:10.1111/cogs.12131.
- diSessa A. A. and Sherin B. L., (1998), What changes in conceptual change? Int. J. Sci. Educ., 20(10), 1155–1191, DOI:10.1080/0950069980201002.
- Elo S. and Kyngäs H., (2008), The qualitative content analysis process, J. Adv. Nurs., 62(1), 107–115, DOI:10.1111/j.1365-2648.2007.04569.x.
- Erman E., (2017), Factors contributing to students’ misconceptions in learning covalent bonds, J. Res. Sci. Teach., 54(4), 520–537, DOI:10.1002/tea.21375.
- Gillespie R. J., (1997), The great ideas of chemistry. J. Chem. Educ., 74(7), 862, DOI:10.1021/ed074p862.
- Gillespie R. J. and Robinson E. A., (2007), Gilbert N. Lewis and the chemical bond: the electron pair and the octet rule from 1916 to the present day, J. Comput. Chem., 28(1), 87–97, DOI:10.1002/jcc.20545.
- Glaser B. G. and Strauss A. L., (1967), The Discovery of Grounded Theory: Strategies for Qualitative Research, New York: Aldine de Gruyter.
- Gonthier J. F., Steinmann S. N., Wodrich M. D. and Corminboeuf C., (2012), Quantification of “fuzzy” chemical concepts: a computational perspective, Chem. Soc. Rev., 41(13), 4671–4687, 10.1039/C2CS35037H.
- Joki J., Lavonen J., Juuti K. and Aksela M., (2015), Coulombic interaction in Finnish middle school chemistry: a systemic perspective on students' conceptual structure of chemical bonding, Chem. Educ. Res. Pract., 16(4), 901–917, 10.1039/C5RP00107B.
- Kokkonen T., (2017), Concepts and Concept Learning in Physics: The systemic view, Doctoral dissertation, Report Series in Physics HU-P-D256, Unigrafia, Helsinki.
- Koponen I. T. and Huttunen L., (2013), Concept Development in Learning Physics: The Case of Electric Current and Voltage Revisited, Sci. Educ., 22, 2227–2254.
- Kronik L., Levy Nahum T., Mamlok-Naaman R. and Hofstein A., (2008), A new “bottom-up” framework for teaching chemical bonding, J. Chem. Educ., 85(12), 1680, DOI:10.1021/ed085p1680.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Taber K. S., (2010), Teaching and learning the concept of chemical bonding, Stud. Sci. Educ., 46(2), 179–207, DOI:10.1080/03057267.2010.504548.
- Levy Nahum T., Mamlok-Naaman R. and Hofstein A., (2013), Teaching and learning of the chemical bonding concept: problems and some pedagogical issues and recommendations, in Tsaparlis G. and Sevian H. (ed.), Concepts of matter in science education, Dordrecht: Springer, pp. 373–390.
- Mortimer E. F., (1995), Concpetual change or conceptual profile change? Sci. Educ., 4, 267–285, DOI:10.1007/BF00486624.
- Oh P. S. and Oh S. J., (2011), What teachers of science need to know about models: an overview, Int. J. Sci. Educ., 33(8), 1109–1130, DOI:10.1080/09500693.2010.502191.
- Özmen H., (2004), Some student misconceptions in chemistry: a literature review of chemical bonding, J. Sci. Educ. Technol., 13(2), 147–159, DOI:10.1023/B:JOST.0000031255.92943.6d.
- Papadouris N. and Constantinou C. P., (2017), Integrating the epistemic and ontological aspects of content knowledge in science teaching and learning. Int. J. Sci. Educ., 39, 663–682.
- Perkins D. N. and Grotzer T. A., (2005), Dimensions of causal understanding: the role of complex causal models in students’ understanding of science, Stud. Sci. Educ., 41, 117–166.
- Peterson R. F. and Treagust D. F., (1989), Grade-12 students’ misconceptions of covalent bonding and structure, J. Chem. Educ., 66(6), 459–460.
- Russ R. S., Lee V. R. and Sherin B. L., (2012), Framing in cognitive clinical interviews about intuitive science knowledge: dynamic student understandings of the discourse interaction, Sci. Educ., 96(4), 573–599.
- Shulman L. S., (1986), Those who understand: Knowledge growth in teaching, Educational Researcher, 15, 4–14.
- Southerland S. A., Abrams E., Cummins C. L. and Anzelmo J., (2001), Understanding students' explanations of biological phenomena: conceptual frameworks or p-prims? Sci. Educ., 85(4), 328–348, DOI:10.1002/sce.1013.
- Stephens N., (2007), Collecting data from elites and ultra elites: telephone and face-to-face interviews with macroeconomists, Qual. Res., 7(2),203–216.
- Sturges J. E. and Hanrahan K. J., (2004), Comparing telephone and face-to-face qualitative interviewing: a research note, Qual. Res., 4(1),107–118.
- Taber K. S., (1997), An explanatory model for conceptual development during A-level chemistry, Paper presented at the British Educational Research Association Annual Conference, University of Sussex at Brighton, September 2–5 1999, from: http://www.leeds.ac.uk/educol/documents/00001429.htm.
- Taber K. S., (1998), An alternative conceptual framework from chemistry education, Int. J. Sci. Educ., 20(5), 597–608, DOI:10.1080/0950069980200507.
- Taber K. S., (2000a), Case studies and generalizability: grounded theory and research in science education, Int. J. Sci. Educ., 22(5), 469–487, DOI:10.1080/095006900289732.
- Taber K. S., (2000b), Multiple frameworks? Evidence of manifold conceptions in individual cognitive structure, Int. J. Sci. Educ., 22(4), 399–417, DOI:10.1080/095006900289813.
- Taber K. S., (2001a), Shifting sands: a case study of conceptual development as competition between alternative conceptions, Int. J. Sci. Educ., 23(7), 731–753, DOI:10.1080/09500690010006572.
- Taber K. S., (2001b), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ. Res. Pract., 2, 123–158.
- Taber K. S., (2002), Chemical misconceptions – prevention, diagnosis and cure, Volume I: theoretical background, London: Royal Society of Chemistry.
- Taber K. S., (2003), Lost without trace or not brought to mind? – a case study of remembering and forgetting of college science, Chem. Educ. Res. Pract., 4, 249–277.
- Taber K. S., (2009), College students’ conceptions of chemical stability: the widespread adoption of a heuristic rule out of context and beyond its range of application, Int. J. Sci. Educ., 31,1333–1358.
- Taber K. S., (2010), Straw men and false dichotomies: overcoming philosophical confusion in chemical education, J. Chem. Educ., 87(5),552–558.
- Taber K. S., (2014), The significance of implicit knowledge for learning and teaching chemistry, Chem. Educ. Res. Pract., 15(4), 447–461, 10.1039/C4RP00124A.
- Taber K. S. and Coll R. K., (2002), Bonding, in Gilbert J. K., de Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical Education: Towards Research-based Practice, Kluwer Academic Publishers, pp. 213–234.
- Taber K. S. and Garcia-Franco A., (2010), Learning processes in chemistry: drawing upon cognitive resources to learn about the particulate structure of matter. J. Learn. Sci., 19(1), 99–142.
- Talanquer V., (2007), Explanations and teleology in chemistry education, Int. J. Sci. Educ., 29(7), 853–870, DOI:10.1080/09500690601087632.
- Talanquer V., (2013), When atoms want, J. Chem. Educ., 90, 11, 1419–1424.
- Thagard P., (1992), Conceptual Revolutions, NJ: Princeton University Press.
- Toth Z. and Barany Z. B., (2016), Phenomenological primitives (p-prims) in chemistry, Poster Presentation, ECRICE 2016, Barcelona.
- Ünal S., Çalık M., Ayas A. and Coll R. K., (2006), A review of chemical bonding studies: needs, aims, methods of exploring students’ conceptions, general knowledge claims and students’ alternative conceptions, Res. Sci. Technol. Educ., 24(2), 141–172, DOI:10.1080/02635140600811536.
- Vogl S., (2013), Telephone versus face-to-face interviews: mode effect on semistructured interviews with children, Sociol. Methodol., 43(1),133–177.
- Watts M. and Taber K. S., (1996), An explanatory gestalt of essence: Students’ conceptions of the ‘natura’ in physical phenomena, Int. J. Sci. Educ., 18(8), 939–954, DOI:10.1080/0950069960180806.
- Wright L., (1972), Explanations and teleology, Philos. Sci., 39, 204–218.
| This journal is © The Royal Society of Chemistry 2018 |