Students’ perceptions of common practices, including some academically dishonest practices, in the undergraduate general chemistry classroom laboratory
K. Christopher
Smith
 *a and
Adrian
Sepulveda
b
*a and
Adrian
Sepulveda
b
aDepartment of Chemistry, University of Texas Rio Grande Valley, 1201 W. University Drive, Edinburg, TX 78539, USA. E-mail: kenneth.smith@utrgv.edu
bSchool of Law, University of Texas at Austin, 110 Inner Campus Drive Austin, TX 78712, USA
First published on 22nd June 2018
Abstract
In this study 635 general chemistry I and general chemistry II students completed a 40-item Likert-scale survey on their opinions of various practices, including some academically dishonest practices, that might occur in the general chemistry laboratory. The practices surveyed were focused on areas including preparation before coming to the laboratory, getting help with the pre-lab assignments, various decisions made by the teaching assistant or laboratory instructor, getting help with the calculations and questions required by the laboratory report, and various methods of obtaining data in the laboratory. An exploratory factor analysis of the results was conducted to identify the underlying factors in the survey, and the scores of the general chemistry I and general chemistry II students along these factors were compared. The findings were generally consistent with results in the literature, but also provided implications for students’ enculturation into chemistry and science as they progressed through their general chemistry coursework.
Introduction
The issue of ethical misconduct in the science laboratory has been up for discussion for centuries, with Charles Babbage defining three types of scientific dishonesty – trimming, cooking, and forging – in 1830 (Babbage, 1830). These aspects of scientific dishonesty were revisited more recently, with Treichel (1999) defining, in more current language, trimming as eliminating particular data from a data set, cooking as manipulating data, and forging as changing data. To investigate students’ perceptions of these forms of scientific dishonesty in the chemistry classroom laboratory, Treichel (1999) administered three short scenarios to undergraduate students, with each scenario focused on one of these forms of scientific dishonesty. In a similar vein, White (2004) administered a modified form of Treichel's scenarios to other undergraduate students. Both Treichel and White found that their students identified forging as the most unacceptable and least defensible form of scientific dishonesty, but were more accepting of trimming and cooking. White also reported that the students made a clear distinction between the research laboratory and the teaching laboratory, and that the students indicated that they would engage in less acceptable practices in the classroom laboratory but not the research laboratory.Del Carlo and Bodner (2004) also examined students’ perceptions of academic dishonesty in the context of the undergraduate chemistry classroom laboratory through a qualitative study. They also found that students viewed the chemistry classroom laboratory as being fundamentally different from an industrial or research laboratory. One main reason reported for this difference was that students viewed dishonesty in industrial or research laboratories as having much greater consequences, such as losing jobs or negatively affecting people or communities, compared to dishonesty in classroom laboratories. In addition, Del Carlo and Bodner found that there were several types of behavior students found as acceptable, including copying prelaboratory assignments, using other students and previous laboratory reports to help complete current laboratory experiments and laboratory reports, and using data from another group of students if their own data were not good. In contrast, the students indicated that copying previous students’ lab reports or other students’ work was not acceptable.
Students’ perceptions of academic dishonesty have also been examined at the secondary level. Rigano and Ritchie (1995) interviewed high school science students and documented several types of academically dishonest behavior reported by the students: making their experimental results fit the expected results, using other class members’ results if their own experimental results were not in agreement with the rest of the class, leaving out experimental results that did not fit the expected results, and making up or acquiring experimental data from other classmates instead of actually carrying out the experiment. Rigano and Ritchie reported that students viewed making up or acquiring experimental data as being not acceptable, whereas the other forms of academic dishonesty were viewed as being acceptable. Similarly, Del Carlo et al. (2006) surveyed high school students on their perceptions of the chemistry laboratory. They found that most of the students surveyed were ambivalent to the idea of another student copying their laboratory work, or found the idea acceptable, with certain justifications.
These studies are among the few studies in the literature examining students’ perceptions of academic dishonesty specifically in the chemistry classroom laboratory. We were also interested in examining students’ perceptions of academic dishonesty in the undergraduate chemistry classroom laboratory, but within the context of several different factors. First, we were interested in examining how students’ perceptions of academic dishonesty, focused on the sourcing, management, and reporting of data, changed as they progressed from their first semester general chemistry lab course to their second semester general chemistry lab course. Would there be any difference in the students’ perceptions? Second, given the current prevalence of online resources, we were interested in investigating how the students’ perceptions were affected by the abundance and availability of online resources. Third, over the years we have had anecdotal reports from students that in some cases their teaching assistants or lab instructors instructed them to run fewer trials than were called for in the experiment, or instructed them to share data from other groups. Therefore, we were also interested in examining how teaching assistants and lab instructors influenced the students’ perceptions. Fourth, we were interested in students’ perceptions of various other practices we had observed in lab or that we had heard reports on, that were led or initiated by students as opposed to teaching assistants or lab instructors. One example of such a practice would be students covertly working in groups of four when at our institution students generally work in pairs to complete the lab experiments, even though such a practice may or may not be viewed as academically dishonest. These last three factors might not necessarily be viewed as academically dishonest, and could be viewed within the context of students’ perceptions of practices involving effort and resources (such as time, supplies, and equipment) associated with the general chemistry classroom laboratory. As such, we were generally interested in examining common chemistry classroom laboratory practices, which included some academically dishonest practices. Our overall research question, incorporating these various contexts, was: what are university general chemistry students’ perceptions of common practices in general chemistry classroom laboratories, including some academically dishonest practices?
Theoretical foundation
The broad foundation used for this research was focused on the burgeoning body of work in the literature on students’ perceptions of their goals and desired outcomes from the chemistry classroom laboratory. Generally, literature reports have focused on students’ desired outcomes that are either somewhat congruent with instructors’ desired outcomes or incongruent with instructors desired outcomes. George-Williams et al. (2018) and Boud et al. (1980) found that students’ desired outcomes for the chemistry classroom laboratory were somewhat in line with instructors’ desired outcomes, and included aspects such as the application of theory and developing practical skills. In contrast, DeKorver and Towns (2015, 2016), and Russell and Weaver (2008) reported that in general, students viewed chemistry classroom laboratories as tasks to be completed as quickly as possible while minimizing frustration, and in such a manner as to obtain a good grade. This second category in which the desired outcomes are incongruent would potentially lead to incidents of academic dishonesty of varying degrees, with one caveat from the literature on academic dishonesty: outright forging and copying without justification are largely unacceptable.The situation is further compounded by the prevalence of traditional verification laboratory exercises, in which students are aware that there is a “right” answer to be reported at the end of the lab. As such, students engaging in traditional laboratory exercises might be motivated to engage in academic dishonesty to get the “right” results (Lawson et al., 1999), which would in turn help them to obtain the desired good grade (Del Carlo et al., 2006).
Methods
Data collection
This research took place in a large public university in the southwest United States, with human subjects research approval from the Institutional Review Board. We developed a survey instrument to examine students’ perceptions of common practices in general chemistry classroom laboratories, including some academically dishonest practices. The survey instrument began with a demographics section including questions on age, gender, student classification, major, minor, and current chemistry laboratory course enrollment. The next section of the survey included forty Likert-scale items with response options of Strongly Disagree, Disagree, Agree, and Strongly Agree. As reported by Nadler et al. (2015), Likert-scale items with 4- to 7-point scales exhibit the strongest validity and reliability, and we chose to use Likert-scale items with a 4-point scale. In addition, we chose not to include a midpoint on the scale so that participants would select a stance as opposed to simply choosing the midpoint (Nadler et al., 2015). Furthermore, we used a 4-point Likert-scale as opposed to a slider scale because on the Likert-scale each response category is labeled, leading to common participant interpretations of the labels, while on a slider scale only the ends of the scales are perhaps labeled, leading to various interpretations of response categories by participants (Weijters et al., 2010). The Likert-scale items were anchored in the results reported by Del Carlo and Bodner (2004), but were expanded to include the contexts described previously, including the sourcing, management, and reporting of data, using online resources, teaching assistant-led decisions, and student-led decisions. Within these contexts, some of the Likert-scale items were about preparation prior to coming to the laboratory, some focused on getting help with the prelaboratory assignment from various sources, some were about doing the lab work, some were focused on the quality of the data collected, and some were about getting help from various sources to write up the laboratory report.After the survey was constructed, as a measure of face validity of the survey, ten current or recent general chemistry students read and examined the survey to provide feedback on wording and their understanding of the survey. The students generally reported that the survey flowed well, that the wording was fine, and that they understood the survey items. The survey was administered to students enrolled in the first semester (General Chemistry Laboratory I) and second semester (General Chemistry Laboratory II) general chemistry laboratory courses viaQualtrics (2017). The general chemistry laboratory experiments at our institution are traditional laboratory exercises, and would generally be described as Level 0: confirmation, according to Buck et al. (2008). The laboratory exercises are scheduled for two hours and forty minutes, and students often require the full time to complete the laboratory exercises. During the laboratory activities students fill in their data in data tables, and the laboratory reports consist of students following prescribed calculations and manipulations of their data. Students generally work in pairs to complete the laboratory exercises, and each laboratory classroom (with a maximum of 22 students) has a teaching assistant or instructor assigned, who facilitates and helps guide students through the laboratory activity. In the semester in which the survey was administered, there were 31 sections of the first semester general chemistry course (with 630 students total) and 26 sections of the second semester general chemistry course (with 452 students total).
Student participation in the survey was voluntary, and students were offered a 5-point bonus credit on their mid-term exam if they completed the survey. The survey was administered just once, shortly before the middle of the semester. In total, 635 of these students completed the survey. The average age of the students was 20.2 years. The distribution of students by gender, course, classification, and major are shown in Table 1; one student did not submit their classification.
| Number of students | % of students | ||
|---|---|---|---|
| Gender | Female | 412 | 64.9 |
| Male | 223 | 35.1 | |
| Course | Gen. Chem. Lab I | 389 | 61.3 |
| Gen. Chem. Lab II | 246 | 38.7 | |
| Classification | Freshman | 158 | 24.9 |
| Sophomore | 266 | 42.0 | |
| Junior | 148 | 23.3 | |
| Senior | 62 | 9.8 | |
| Major | Biology | 286 | 45.0 |
| Nursing | 124 | 19.5 | |
| Chemistry | 49 | 7.7 | |
| Pre-professional | 18 | 2.8 | |
| Psychology | 13 | 2.0 | |
| Other | 145 | 22.8 | |
Data analysis
Once survey data collection was complete, the results were exported into Microsoft Excel (2016). The response option Strongly Disagree was converted to a numerical value of 1, Disagree to 2, Agree to 3, and Strongly Agree to 4. These data were then copied into IBM SPSS Statistics 23 (2015) for further analysis. As one check on the quality of the data, we checked to see how many students had responded in exactly the same way (either Strongly Disagree, Disagree, Agree, or Strongly Agree) to all of the Likert-scale items, possibly indicating that they were not really reading the items and were simply entering responses. There were 11 students who responded Strongly Disagree, 10 students who responded Disagree, and 1 student who responded Agree, respectively, to all of the Likert-scale survey items. While it is possible that students could answer the same way (either Strongly Disagree, Disagree, Agree, or Strongly Agree) to all of the Likert-scale items, we were encouraged that there was just a small proportion of students answering in such a manner. We did not exclude these data from the analysis.We then conducted exploratory factor analysis on the data in IBM SPSS Statistics 23 (2015), followed by reliability analyses of the various factors, and comparisons of means of the various groups on the various factors as well as the factors combined. Exploratory factor analysis is a statistical technique which reduces a large number of variables in a data set into a smaller set of factors, with each of the factors related to a latent construct (Taherdoost et al., 2014), which cannot be directly measured. We chose to apply exploratory factor analysis to our survey data to explore which survey items loaded onto various factors, and to explore the nature of the various factors. In addition, exploratory factor analysis provides evidence of construct validity of self-reporting scales (Taherdoost et al., 2014). As such, exploratory factor analysis would provide evidence of construct validity for our survey, which is a Likert-scale self-reporting survey. Exploratory factor analysis was deemed appropriate as our number of survey participants surpassed the generally recommended number of 300 survey participants for exploratory factor analysis (Taherdoost et al., 2014). Furthermore, our ratio of survey participants to survey items was close to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which surpassed the prevailing minimum recommended ratio of 10
1, which surpassed the prevailing minimum recommended ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Costello and Osborne, 2005). In conducting exploratory factor analysis of the data, we followed the best practices outlined by Costello and Osborne (2005).
1 (Costello and Osborne, 2005). In conducting exploratory factor analysis of the data, we followed the best practices outlined by Costello and Osborne (2005).
To explore the reliabilities of the various factors, internal consistencies were measured by Cronbach's α, with values greater than 0.7 considered acceptable (Nunnally, 1978). To examine difference in survey scores among the different groups (gender, course, classification), analysis of variance (ANOVA) tests were conducted.
Results and discussion
Exploratory factor analysis
Exploratory factor analysis was conducted in IBM SPSS Statistics 23 (2015) using principal axis factoring as the factor extraction method, which allows the data to have a non-normal distribution (Costello and Osborne, 2005). This extraction method was chosen because Shapiro-Wilk tests of the data in IBM SPSS Statistics 23 (2015) indicated that the data were non-normally distributed. The rotation method chosen was an oblique rotation method, promax rotation, with the standard kappa value of 4. This rotation method allows factors to be correlated, which is not uncommon in social sciences research (Costello and Osborne, 2005). After the initial exploratory factor analysis was run, items with loadings of less than 0.32 (low-loading items) on a single factor were dropped, and items with loadings of 0.32 or greater on more than one factor (cross-loading items) were also dropped (Costello and Osborne, 2005). This procedure was repeated until there were no low-loading or cross-loading items remaining, and each factor consisted of three or more items, indicating strong and stable factors (Costello and Osborne, 2005). Additionally, there was one additional item which was dropped due to having a low communality of approximately 0.25 (Costello and Osborne, 2005). Finally, two items were dropped from one of the factors as these two items did not follow a consistent theme as the other items in the factor. In total twenty-two of the original forty survey items were retained, while eighteen of the original survey items were dropped.At this point, the value for the Kaiser–Meyer–Olkin Measure of Sampling Adequacy (KMO) was examined. This measure of sampling adequacy evaluates the degree to which items are correlated; the maximum value of this measure is 1, and values greater than 0.7 are considered adequate, with higher values being preferred (Taherdoost et al., 2014). Our value for the Kaiser–Meyer–Olkin Measure of Sampling Adequacy (KMO) was 0.865. In addition, the p-value for Bartlett's test of sphericity, which tests the hypothesis that the correlation matrix is an identity matrix, was examined. Our results showed p < 0.001, which indicated that the correlation matrix was not an identity matrix and that the items were correlated.
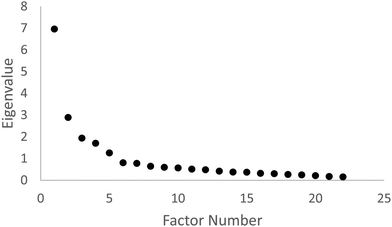
To determine the number of factors to retain from the exploratory factor analysis, the scree test was performed (Costello and Osborne, 2005). The scree test involves inspecting the plot of eigenvalue versus factor number, and examining the plot for a break or bend where the curve flattens out. The number of factors retained would be the number of data points above the break or bend. Fig. 1 shows the scree plot.
To better gauge the number of factors to retain from the exploratory factor analysis, the scree test acceleration factors, which emphasize the point on the scree plot at which the slope changes abruptly, were determined (Raîche et al., 2013). The scree test acceleration factor plot graphs the scree test acceleration factor (which is a function of eigenvalue) as a function of factor number from the second to the penultimate eigenvalue. This plot allows for an examination of where the break or bend in the scree plot occurs, enabling a decision on the number of factors retained to be made. Fig. 2 shows the scree test acceleration factor plot.
Based on the scree plot and the scree test acceleration factor plot, five factors were retained. The five factors and corresponding survey items are shown in Table 2.
| Factors and items | Factor loadings | ||||
|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | |
| Factor 1 (F1): Help from Non-Peers | |||||
| It is okay for you to decide to get help with your entire pre-lab assignment from your teaching assistant/lab instructor | 0.87 | 0.04 | −0.12 | 0.00 | −0.04 |
| It is okay for you to decide to get help with your entire pre-lab assignment from a tutor | 0.91 | 0.03 | −0.11 | 0.02 | −0.02 |
| It is okay for you to decide to get help with your entire pre-lab assignment from an online website/source | 0.59 | 0.12 | −0.08 | 0.11 | 0.11 |
| It is okay for you to decide to get help with all the calculations/questions for your lab report from your teaching assistant/lab instructor | 0.68 | −0.10 | 0.22 | −0.11 | 0.04 |
| It is okay for you to decide to get help with all the calculations/questions for your lab report from a tutor | 0.71 | −0.07 | 0.17 | −0.07 | 0.03 |
| Factor 2 (F2): Laboratory Preparation | |||||
| It is okay for you to come to lab without reading the lab experiment | −0.02 | 0.65 | −0.06 | 0.07 | 0.07 |
| It is okay for you to come to lab without feeling completely prepared | 0.02 | 0.77 | 0.08 | −0.11 | −0.01 |
| It is okay for you to come to lab without having an overview of the lab experiment | −0.11 | 0.84 | −0.01 | 0.04 | −0.01 |
| It is okay for you to come to lab without thinking much about what would be done in the lab experiment | 0.03 | 0.84 | 0.04 | −0.04 | −0.04 |
| It is okay for you to come to lab without completing the pre-lab assignment | 0.11 | 0.49 | −0.01 | 0.02 | 0.04 |
| Factor 3 (F3): Teaching Assistant-Led Decisions During Lab | |||||
| It is okay for the teaching assistant/lab instructor to tell you to run one trial of an experiment when the experiment asks you to run two trials | −0.07 | −0.02 | 0.89 | −0.07 | 0.15 |
| It is okay for the teaching assistant/lab instructor to tell you to run two trials of an experiment when the experiment asks you to run three trials | −0.08 | 0.01 | 0.90 | −0.10 | 0.13 |
| It is okay for the teaching assistant/lab instructor to tell you to skip steps in the lab procedure which seem unnecessary | 0.09 | 0.04 | 0.70 | 0.07 | −0.09 |
| It is okay for the teaching assistant/lab instructor to tell you to use data from someone who is not in your lab group to write your lab report if you did not get good data in your lab experiment | 0.08 | 0.06 | 0.52 | 0.17 | −0.06 |
| Factor 4 (F4): Data and the Lab Report | |||||
| It is okay for the teaching assistant/lab instructor to tell you to use data from a student's old lab report from a previous semester to write your lab report if you did not get good data in your lab experiment | 0.06 | −0.02 | 0.25 | 0.64 | −0.09 |
| It is okay for you to decide to change your data so that they fit with the expected results if you did not get good data in your lab experiment | 0.03 | 0.02 | −0.04 | 0.70 | 0.08 |
| It is okay for the teaching assistant/lab instructor to tell you to change your data so that they fit with the expected results if you did not get good data in your lab experiment | 0.02 | −0.02 | 0.29 | 0.73 | −0.23 |
| It is okay for you to decide to turn in a copy of your lab partner's lab report as your own | −0.08 | −0.01 | −0.25 | 0.71 | 0.21 |
| It is okay for the teaching assistant/lab instructor to tell you to turn in a copy of your lab partner's lab report as your own | −0.07 | −0.03 | −0.07 | 0.78 | 0.08 |
| Factor 5 (F5): Student-Led Decisions During Lab | |||||
| It is okay for you to decide to run one trial of an experiment when the experiment asks you to run two trials | 0.01 | 0.00 | 0.06 | 0.08 | 0.72 |
| It is okay for you to decide to run two trials of an experiment when the experiment asks you to run three trials | 0.01 | 0.02 | 0.11 | −0.02 | 0.80 |
| It is okay for lab partners to decide that one lab partner does the lab work and the other lab partner does the questions/calculations for the lab report | 0.10 | 0.03 | 0.05 | 0.22 | 0.36 |
Factor 1 involved students getting help from non-peers, while Factor 2 involved students’ own laboratory preparation, both of which might be viewed within the context of students’ perceptions of effort associated with the general chemistry classroom laboratory. These results indicated that students viewed these aspects as two distinct aspects of the laboratory experience.
Two of the other factors, Factors 3 and 5, dealt with decisions during the lab. Factor 3 involved teaching assistant-led decisions, while Factor 5 involved student-led decisions, both of which might be viewed within the context of students’ perceptions of resources (such as time, supplies, and equipment) associated with the general chemistry classroom laboratory. These results indicated that students viewed teaching assistant-led decisions differently than student-led decisions during the lab. These results might indicate that students viewed the teaching assistants and lab instructors as more expert and themselves as more novice, or alternatively, the teaching assistants and lab instructors as having more authority and themselves as having less authority. As such, the teaching assistants’ decisions might carry more weight compared to the students’ decisions.
One interesting item in Factor 3 involved the teaching assistant instructing students to use data from someone not in their lab group if good data were not obtained in the lab experiment. This item loaded onto Factor 3, as opposed to Factor 4, which involved students’ using external lab data, changing data, and turning in copies of their lab partners’ lab reports, which were items most clearly related to academic dishonesty. As Del Carlo and Bodner (2004) noted, sharing data was viewed as being fundamentally different from copying, as sharing data was a means to obtaining good data in the lab. Our results supported the results of Del Carlo and Bodner (2004).
One interesting aspect about Factor 4 was that it was the only factor that involved decisions made by both the students and the teaching assistants. The decisions in Factor 4 focused on sourcing and changing data, and turning in the lab report. These results indicated that students did not view these types of decisions differently depending on whom the decisions came from.
The original forty item survey had four items involving the use of online resources. The final twenty-two item survey had just one item related to the use of online resources. This dearth of use of online resources in the final survey was discussed with an undergraduate student at a recent conference, and the student suggested that it was difficult for students to find their exact laboratory experiment with exactly the same procedures and calculations online. Further, it was suggested that students would be hesitant and unwilling to sift through similar laboratory experiment resources found online to attempt to relate and transfer the resources to the students’ own laboratory experiments. These ideas were discussed with two undergraduate students at our own institution, who both echoed and agreed with the reasoning.
Factor reliabilities
Internal consistencies of the various factors were measured by Cronbach's α. Our goal in measuring Cronbach's α values was to demonstrate the consistency of measurement between the various items within each factor identified by the exploratory factor analysis. This survey falls within the affective domain, and as Taber (2017) argued, “…there is a logic in seeking a high alpha value for scales intended to measure foci that are conceptualised as single constructs, where a range of items are designed to elicit that single construct…” (p. 20). As such, high Cronbach's α values would demonstrate consistency of measurement between items within each factor, with each factor being associated with a particular construct. What constitutes a high value of Cronbach's α? As a very general and often-cited rule of thumb, Cronbach's α values greater than 0.7 are considered acceptable (Nunnally, 1978). The Cronbach's α values of the various factors are shown in Table 3.| Survey items | Cronbach's α |
|---|---|
| Factor 1 (Help from Non-Peers) | 0.87 |
| Factor 2 (Laboratory Preparation) | 0.84 |
| Factor 3 (TA-Led Decisions) | 0.85 |
| Factor 4 (Data and the Lab Report) | 0.83 |
| Factor 5 (Student-Led Decisions) | 0.75 |
These results indicated that the internal consistencies of all the factors surpassed the very general rule of thumb Cronbach's α value of 0.7.
Differences in average survey scores on the different factors
Differences in average survey scores on the different factors were examined using tests of analysis of variance (ANOVA) and a Tukey's HSD post hoc test. The scores were obtained from the response option Strongly Disagree being converted to a numerical value of 1, Disagree to 2, Agree to 3, and Strongly Agree to 4. The minimum average survey score would be 1, while the maximum average survey score would be 4. The results are shown in Table 4.| Survey items | Scores |
|---|---|
| Factor 1 (Help from Non-Peers) | 2.51 |
| Factor 2 (Laboratory Preparation) | 1.64 |
| Factor 3 (TA-Led Decisions) | 2.69 |
| Factor 4 (Data and the Lab Report) | 1.73 |
| Factor 5 (Student-Led Decisions) | 1.95 |
| Entire Survey | 2.09 |
The average survey scores on the different factors were all significantly different at the p = 0.05 level. These results indicated that overall, Factor 3 (TA-Led Decisions) had the highest average score, which indicated that students were most likely to agree to the laboratory practices associated with teaching assistant-led decisions during lab. This finding is noteworthy because the teaching assistants and lab instructors are on the front lines with regards to chemistry laboratory instruction, and students’ enculturation into chemistry and science is impacted by teaching assistants and lab instructors. Factor 1 (Help from Non-Peers) had the next highest average score, while Factor 5 (Student-Led Decisions) and the entire survey had average scores of approximately 2, which corresponded, on average, to a response of “Disagree”.
Factors 2 (Laboratory Preparation) and 4 (Data and the Lab Report) showed the lowest average survey scores overall. These results indicated that students were least likely to agree with the lab practices associated with Factors 2 (Laboratory Preparation) and 4 (Data and the Lab Report), which included students’ own preparation for lab, students’ using external lab data, changing data, and turning in copies of their lab partners’ lab reports.
Differences in average survey scores among the different groups
Differences in average survey scores among the different groups (gender, course, classification) were examined using tests of analysis of variance (ANOVA); a Tukey's HSD post hoc test was conducted to examine the differences in survey scores among the various student classifications. The results are shown in Table 5.| Survey items | Scores | |||||||
|---|---|---|---|---|---|---|---|---|
| Gender | Course | Classification | ||||||
| Female | Male | Gen. Chem. Lab I | Gen. Chem. Lab II | Freshman | Sophmore | Junior | Senior | |
| a The average survey score was significantly different within a particular group (gender, course, classification) at the p = 0.05 level. | ||||||||
| Factor 1 (Help from Non-Peers) | 2.48 | 2.57 | 2.49 | 2.54 | 2.49 | 2.55 | 2.45 | 2.54 |
| Factor 2 (Laboratory Preparation) | 1.59a | 1.75a | 1.60a | 1.72a | 1.58 | 1.66 | 1.64 | 1.75 |
| Factor 3 (TA-Led Decisions) | 2.71 | 2.65 | 2.74a | 2.62a | 2.72 | 2.70 | 2.63 | 2.69 |
| Factor 4 (Data and the Lab Report) | 1.68a | 1.84a | 1.75 | 1.71 | 1.71 | 1.76 | 1.72 | 1.73 |
| Factor 5 (Student-Led Decisions) | 1.91a | 2.03a | 2.01a | 1.86a | 1.89 | 1.98 | 1.97 | 1.98 |
| Entire Survey | 2.06a | 2.16a | 2.10 | 2.09 | 2.07 | 2.12 | 2.07 | 2.13 |
The results indicated that on Factors 2 (Laboratory Preparation), 4 (Data and the Lab Report), and 5 (Student-Led Decisions), and on the overall survey, female students scored significantly lower than male students, which indicated that female students were less likely to agree to the various common laboratory practices, compared to male students.
On Factor 2 (Laboratory Preparation), students enrolled in General Chemistry Laboratory I (the first semester general chemistry laboratory course) scored significantly lower than students enrolled in General Chemistry Laboratory II, indicating that students in General Chemistry Laboratory I were less likely to support coming to lab unprepared. This result might be explained by considering that General Chemistry Laboratory I is the first general chemistry laboratory course that students take, so perhaps they were more focused on being prepared for their first college chemistry laboratory experience.
In contrast, students enrolled in General Chemistry Laboratory II scored significantly less than their General Chemistry Laboratory I counterparts on Factors 3 (TA-Led Decisions) and 5 (Student-Led Decisions); these results indicated that General Chemistry Laboratory II students were less likely to support the teaching assistant-led and student-led decisions during lab. These results might be explained by considering that the students in General Chemistry Laboratory II were in their second college general chemistry laboratory course. As such, they would have become more enculturated into the norms of chemistry and science associated with following experimental protocols and procedures, and would thus be more hesitant to engage in the associated teaching assistant-led and student-led decisions during lab. We do acknowledge that context is important, as it is possible that lack of adherence to protocols and procedures might potentially be due to lack of resources, such as time or materials.
One can argue that the last item in Factor 3 (TA-Led Decisions) was more clearly dishonest than the rest of the items in Factor 3 (TA-Led Decisions), so further analysis of this item was warranted. A test of analysis of variance (ANOVA) was conducted and indicated that the average score of the General Chemistry Laboratory I students on this item was 2.60 while the average score of the General Chemistry Laboratory II students on the same item was 2.53; these average scores were not significantly different a the p = 0.05 level. This result is in agreement with the results for Factor 4 (Data and the Lab Report), which included items focused on sourcing and changing data, and which showed no significant differences in the average score among students in General Chemistry Laboratory I and General Chemistry Laboratory II.
Similarly, one can argue that the last item in Factor 5 (Student-Led Decisions) was more clearly dishonest than the rest of the items in Factor 5 (Student-Led Decisions). A test of analysis of variance (ANOVA) was conducted and indicated that the average score of the General Chemistry Laboratory I students on this item was 2.04 while the average score of the General Chemistry Laboratory II students on the same item was 1.87. These average scores were significantly different at the p = 0.05 level, in agreement with the overall average scores on Factor 5 (Student-Led Decisions).
There were no significant differences on any of the Factors nor on the overall survey among students of different classifications.
Implications for teaching
The results of this study carry several implications for instruction in general chemistry laboratories. At our institution, it appears that at this point there is not much need to worry about students sourcing laboratory activity solutions from the internet. In addition, while we do have teaching assistant training focused on content for the general chemistry laboratories, these results imply the need for us to emphasize to teaching assistants the importance of modeling best scientific practices. This survey can also be used by general chemistry laboratory instructors at other institutions to examine students’ perceptions at their own institutions, and to possibly recommend and implement changes in their general chemistry laboratory instruction.One unexpected result was that Factor 2 (Laboratory Preparation) showed the lowest average survey scores overall. Factor 2 (Laboratory Preparation) contained items associated with students’ own preparation for lab and getting help from others’ pre-lab assignments. This result indicated that students were least likely to agree with the practices associated with coming to lab unprepared, or conversely, students felt that it was important to come to lab prepared. In our experience, it has long seemed that students appear quite apathetic about their pre-laboratory preparation, but the results of this study indicate otherwise. In addition, another line of our research is beginning to indicate that students are somewhat dissatisfied with our current pre-laboratory activities, and have several suggestions to make the pre-laboratory activities more engaging and cognitively accessible to them (Tomson and Smith, 2018a, 2018b). As such, the results of this study have shown us that while students might appear apathetic about their pre-laboratory preparation, they do attach importance to it, but the nature of the pre-laboratory activities might contribute to their apparent apathy. This situation is likely common at other institutions, and gives grounds for the examination of pre-laboratory activities currently in use.
In addition, Del Carlo and Bodner (2006) argued that traditional “cookbook” laboratory exercises, such as the confirmation laboratories our students engaged in, promote a performance-oriented classroom goal structure. As such, students focus on obtaining a predetermined correct answer or result, and this focus has been cited as a cause for academic dishonesty (Rigano and Ritchie, 1995). Del Carlo and Bodner (2006) suggested inquiry laboratory activities to address this issue; our survey can be used to compare students’ perceptions in traditional laboratory settings versus inquiry laboratory settings.
When considering academic dishonesty in the chemistry laboratory, the existing literature is focused generally on trimming, cooking, and forging. The laboratory experience is a complex one, with many interacting parts. Our results have helped to further delineate the nature of the various interacting parts, and have helped to further shed light on students’ perceptions of the different parts. These results can be used, in general, to consider how students view the different aspects of the laboratory experience, and what can be done to help improve the laboratory experience for students.
Limitations
When constructing surveys, item wording is an important consideration, as it is desirable for the survey respondents to interpret the items as intended by the researchers. The wording of the items in our survey were relevant to the context of the general chemistry laboratories at our institution, and we had general chemistry students read and examine the survey to provide feedback on the wording of the items. Still, it is possible that students may have interpreted some of the items in an unintended fashion. For instance, the items in Factor 3 (TA-Led Decisions) were related to teaching assistant-led decisions during lab, and it is possible that students could have interpreted these decisions from the perspective of the student or the perspective of the teaching assistant. However, the survey instructions directed students to indicate their level of agreement with each statement, so we argue that students were likely responding to the survey items from their perspective.Another limitation related to items in Factor 3 (TA-Led Decisions) is that such teaching-assistant led decisions in some laboratory experiments may conceivably have been related to adjustments made by teaching assistants due to a shortage of materials or time. We argue that even if this scenario were the case, the implicit message being sent to students does not reflect best scientific practices, with the implicit message being that shortcuts are acceptable if justified. It would, however, be interesting for future work to examine the nature of the justifications students consider, other than the justification of not having collected good data during lab, when thinking about the practices discussed in this study. In addition, it would be interesting for future work to include qualitative data collection to corroborate and expand on the practices reported in this manuscript.
Yet another limitation concerns the findings related to students’ enculturation into the norms of chemistry and science associated with following experimental protocols and procedures. As noted previously, both White (2004) and Del Carlo and Bodner (2004) found that students viewed the chemistry classroom laboratory as being fundamentally different from an industrial or research laboratory, and that acts of dishonesty in an industrial or research setting carried more serious weight than in a classroom laboratory setting. These differences were delineated by Martinson et al. (2005) who viewed research misconduct within the context of issues related to practicing, managing, and reporting scientific research, while McCabe and colleagues (McCabe and Treviño, 1993; McCabe and Treviño, 1997; McCabe et al., 2002) focused on academic dishonesty in general as various types of behavior associated with cheating on tests, plagiarism, and collusion, in academic contexts. Furthermore, McCabe et al. (1999) reported that one of the reasons for students’ academic dishonesty was to obtain higher grades. Given that our study occurred within an academic context using traditional laboratory exercises in a performance-oriented classroom goal structure, the findings related to students’ enculturation into the norms of chemistry and science should not be interpreted as enculturation into the norms associated with scientific research. Instead, the interpretation should be limited to students’ enculturation into the norms of chemistry and science associated with following experimental protocols and procedures in an academic setting.
In addition, each institution and its students exists in a unique context. As such, as with any other survey instrument, care must be taken when administering this survey instrument to another student population. Confirmatory factor analysis would be suggested to ensure the consideration of the appropriateness of using this survey with another student population.
Conclusions
The survey we developed focused on general chemistry students’ perceptions of common practices in general chemistry classroom laboratories, including some academically dishonest practices. We were also able to provide evidence and measures for the validity and reliability of the survey. Overall, the results of the survey were generally consistent with results in the published literature. Our survey results also demonstrated the importance of teaching assistants and lab instructors in guiding and enculturating students into chemistry and science. Furthermore, our survey results provided potential evidence for the progress in enculturation as students continued on from their first general chemistry laboratory course onto their second general chemistry laboratory course.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We would like to acknowledge the students for taking part in the study. We would also like to acknowledge Mrs Sylvia Diaz for assisting with the distribution of the surveys, and Drs Ralph Carson and Sophie Wang for useful discussions.References
- Babbage C., (1830), Reflections on the Decline of Science in England, and on Some of Its Causes, Champaign, IL: Project Gutenberg, (republished 2013).
- Boud D. J., Dunn J., Kennedy T. and Thorley R., (1980), The aims of science laboratory courses: a survey of students, graduates and practicing scientists, Eur. J. Sci. Educ., 2(4), 415–428.
- Buck L. B., Bretz S. L. and Towns M. H., (2008), Characterizing the level of inquiry in the undergraduate laboratory, J. Coll. Sci. Teach., 38(1), 52–58.
- Costello A. B. and Osborne J. W., (2005), Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis, Pract. Assess., Res. Eval., 10(7), 1–9.
- DeKorver B. K. and Towns M. H., (2015), General chemistry students’ goals for chemistry laboratory coursework, J. Chem. Educ., 92(12), 2031–2037.
- DeKorver B. K. and Towns M. H., (2016), Upper-level undergraduate chemistry students’ goals for their laboratory coursework, J. Res. Sci. Teach., 53(8), 1198–1215.
- Del Carlo D. I. and Bodner G. M., (2004), Students’ perceptions of academic dishonesty in the chemistry classroom laboratory, J. Res. Sci. Teach., 41(1), 47–64.
- Del Carlo D. I. and Bodner G. M., (2006), Dishonesty in the biochemistry classroom laboratory: a synthesis of cause and prevention, Biochem. Mol. Biol. Edu., 34(5), 338–342.
- Del Carlo D. I., Mazzaro D. and Page S., (2006), High school students’ perceptions of their laboratory classroom and the copying of laboratory work, J. Chem. Educ., 83(9), 1362–1367.
- George-Williams S. R., Ziebell A. L., Kitson R. R. A., Coppo P., Thompson C. D. and Overton T., (2018), ‘What do you think the aims of doing a practical chemistry course are?’ A comparison of the views of students and teaching staff across three universities, Chem. Educ. Res. Pract., 19, 463–473.
- IBM SPSS Statistics 23, (2015), Armonk, NY: IBM.
- Lawson A. E., Lewis Jr. C. M. and Birk J. P., (1999), Why do students “cook” data? A case study on the tenacity of misconceptions, J. Coll. Sci. Teach., 29(3), 191–198.
- Martinson B. C., Anderson M. S. and de Vries R., (2005), Scientists behaving badly, Nature, 435(9), 737–738.
- McCabe D. L. and Treviño L. K., (1993), Academic dishonesty: honor codes and other contextual influences, J. High. Educ., 64(5), 522–538.
- McCabe D. L. and Treviño L. K., (1997), Individual and contextual sinfluences on academic dishonesty: a multicampus investigation, Res. High. Educ., 38(3), 379–396.
- McCabe D. L., Treviño L. K. and Butterfield K. D., (1999), Academic integrity in honor code and non-honor code environments: a qualitative investigation, J. High. Educ., 70(2), 211–234.
- McCabe D. L., Treviño L. K. and Butterfield K. D., (2002), Honor codes and other contextual influences on academic integrity: a replication and extension to modified honor code settings, Res. High. Educ., 43(3), 357–378.
- Microsoft Excel 2016, (2016), Redmond, WA: Microsoft.
- Nadler J. T., Weston R. and Voyles E. C., (2015), Stuck in the middle: the use and interpretation of mid-points in items on questionnaires, J. Gen. Psychol., 142(2), 71–89.
- Nunnally J. C., (1978), Psychometric theory, New York, NY: McGraw-Hill, Inc.
- Qualtrics, (2017), Provo, UT: Qualtrics.
- Raîche G., Walls T. A., Magis D., Riopel M. and Blais J.-G., (2013), Non-graphical solutions for Cattell's scree test, Methodology, 9(1), 23–29.
- Rigano D. L. and Ritchie S. M., (1995), Student disclosures of fraudulent practice in school laboratories, Res. Sci. Educ., 25(4), 353–363.
- Russell C. B. and Weaver G., (2008), Student perceptions of the purpose and function of the laboratory in science: a grounded theory study, Int. J. Scholarsh. Teach. Learn., 2(2), 9.
- Taber K. S., (2017), The use of Cronbach's Alpha when developing and reporting research instruments in science education, Res. Sci. Educ., DOI:10.1007/s11165-016-9602-2.
- Taherdoost H., Sahibuddin S. and Jalaliyoon N., (2014), Exploratory factor analysis; concepts and theory, in 2nd International Conference on Mathematical, Computational and Statistical Sciences, Gdansk: WSEAS, pp. 375–382, available at: http://www.wseas.us/e-library/conferences/2014/Gdansk/MATH/MATH-49.pdf, accessed 19 Feb 2018.
- Tomson C. and Smith K. C., (2018a), Student perception of general chemistry pre-laboratory activities, paper presented to the College of Sciences Annual Research Conference at the University of Texas Rio Grande Valley, Edinburg, Texas, 13 April 2018.
- Tomson C. and Smith K. C., (2018b), Student perspectives on general chemistry pre-laboratory activities, paper presented to the 255th American Chemical Society National Meeting & Exposition, New Orleans, Louisana, March 18–22, 2018.
- Treichel P. M., (1999), Ethical conduct in science – the joys of teaching and the joys of learning, J. Chem. Educ., 76(10), 1327–1329.
- Weijters B., Cabooter E. and Schillewaert N., (2010), The effect of rating scale format on response styles: the number of response categories and response category labels, Int. J. Res. Mark., 27, 236–247.
- White H. B., (2004), Ethical conduct in the laboratory: looking the other way, Biochem. Mol. Biol. Educ., 32(5), 348–349.
| This journal is © The Royal Society of Chemistry 2018 |