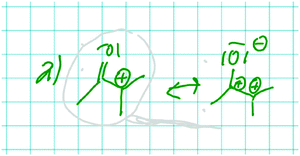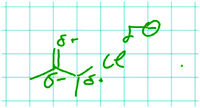Resolving the complexity of organic chemistry students' reasoning through the lens of a mechanistic framework
I.
Caspari
 ,
D.
Kranz
,
D.
Kranz
 and
N.
Graulich
and
N.
Graulich
 *
*
Justus-Liebig-University Giessen, Institute of Chemistry Education, Heinrich-Buff-Ring 17, 35392 Giessen, Germany. E-mail: Nicole.Graulich@didaktik.chemie.uni-giessen.de
First published on 20th June 2018
Abstract
Research in organic chemistry education has revealed that students often rely on rote memorization when learning mechanisms. Not much is known about student productive resources for causal reasoning. To investigate incipient stages of student causal reasoning about single mechanistic steps of organic reactions, we developed a theoretical framework for this type of mechanistic reasoning. Inspired by mechanistic approaches from philosophy of science, primarily philosophy of organic chemistry, the framework divides reasoning about mechanisms into structural and energetic accounts as well as static and dynamic approaches to change. In qualitative interviews, undergraduate organic chemistry students were asked to think aloud about the relative activation energies of contrasting cases, i.e. two different reactants undergoing a leaving group departure step. The analysis of students’ reasoning demonstrated the applicability of the framework and expanded the framework by different levels of complexity of relations that students constructed between differences of the molecules and changes that occur in a leaving group departure. We further analyzed how students’ certainty about the relevance of their reasoning for a claim about activation energy corresponded to their static and dynamic approaches to change and how students’ success corresponded to the complexity of relations that they constructed. Our findings support the necessity for clear communication of and stronger emphasis on the fundamental basis of elementary steps in organic chemistry. Implications for teaching the structure of mechanistic reasoning in organic chemistry and for the design of mechanism tasks are discussed.
Introduction
The questions of how and why processes occur are core questions of the natural sciences. Thus, teaching students to reason mechanistically, i.e. the type of reasoning necessary to answer these questions, is a central goal of science education. A focus on mechanistic reasoning in teaching chemistry is highlighted in novel, research-based curricula for general chemistry like the Chemical Thinking curriculum (Talanquer and Pollard, 2010; Sevian and Talanquer, 2014) and Chemistry, Life, the Universe, and Everything (Cooper and Klymkowsky, 2013).In organic chemistry, the electron-pushing formalism symbolizes electron flow that shows how organic reactions might occur and is thus an explanatory and predictive tool of mechanistic reasoning (Bhattacharyya, 2013). Hence, for students, a first step of learning how to reason mechanistically could be learning to interpret the electron-pushing formalism as a symbol of electron flow (Flynn and Ogilvie, 2015; Flynn and Featherstone, 2017; Galloway et al., 2017). This understanding is not self-evident for students; they often use the electron-pushing formalism as a mere symbol to connect atoms of reactants in order to produce products that they try to recall from memory (Bhattacharyya and Bodner, 2005; Ferguson and Bodner, 2008; Grove et al., 2012b). To support student understanding, current curriculum development and research efforts focus on teaching strategies that foster a meaningful application of the symbolism for single mechanistic steps before students learn to reason about why the electron flow occurs (Flynn and Ogilvie, 2015; Flynn and Featherstone, 2017; Galloway et al., 2017).
In addition to the mere interpretation of the symbolism, making a hypothesis about electron flow based on established chemical principles is central to mechanistic reasoning in organic chemistry (Bhattacharyya, 2013). Building on the aforementioned approaches to teaching a deeper understanding of the formalism, a next stepping stone for students toward complex mechanistic reasoning could be learning to judge feasibility of electron flow in single mechanistic steps. Focusing on reasoning about this smallest unit of organic mechanisms is suggested by several research studies (DeFever et al., 2015; Flynn and Ogilvie, 2015) including our previous study (Caspari et al., 2018), in which we identified that students have various difficulties reasoning about multiple steps, alternative steps, and directions of mechanisms. If we want to teach students how to engage in reasoning about why single mechanistic steps occur, we need a detailed understanding of which types of knowledge pieces and connections between them are required by the discipline and utilized by students.
Theoretical framework
An analysis of mechanistic reasoning in organic chemistry must take into account domain-specific characteristics. Every type of reasoning is shaped by the subject under consideration and by the modes of disciplinary discourse used to gain and represent knowledge about the subject (Airey and Linder, 2009). Thus, mechanistic reasoning in organic chemistry is shaped by the nature of organic mechanisms, i.e. the subject, and by typical questions asked about the subject. Investigating the complexity of student mechanistic reasoning in organic chemistry, we therefore must consider what characterizes an organic mechanism and typical mechanistic questions. This information can be obtained from philosophy of science, which we use to develop stepwise the definition of mechanistic reasoning on which this publication is based.In philosophy of organic chemistry, Goodwin (2003) formulates typical questions requiring a mechanistic answer: “(1) Why do reactions of type A occur faster than reactions of type B (rather than the other way around)? (2) Why do reactions of type A have the products B, C, etc. (rather than products E, F, etc.)? (3) Why do reactions of type A have product distribution D (rather than product distributions E, F, or G, etc.)? (4) Why does reaction of type A proceed by pathway M (rather than pathways P, Q, or R, etc.)?”† (p. 142). A specific question of this type that requires reasoning about one mechanistic step—the reasoning we are interested in—could be why is the leaving group ability of bromide higher than that of chloride?
These questions have in common that they include contrast classes (Goodwin, 2003). To answer those questions, one compares at least two cases. Thus, our study is based on a definition of mechanistic reasoning as a comparative process.
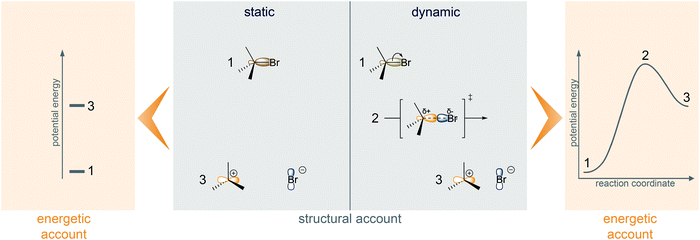
The questions further shape their respective answers and the reasoning used to reach these answers. The direct answers to the typical questions have in common that they are claims about energy (Goodwin, 2003, 2008). As explained by Goodwin (2003), a direct answer to the question about the leaving group ability of halides is that the leaving group departure of bromide has lower activation energy than the leaving group departure of chloride. This energetic answer is sufficient to answer the question, but it is not directly accessible. Instead, a student solving a problem or a chemist working in the field often has access to structural formulas, which can be used to develop a structural account from which one can infer the energetic account (Goodwin, 2003, 2008). The different atomic symbols of chlorine and bromine in the structural formulas of haloalkanes, i.e. explicit structural differences, are the starting point of the following structural account: Due to its nuclear and electronic structure, bromide is larger than chloride and therefore negative charge is better accommodated. This structural account can be used to infer that the relative product energy is lower for bromide than for chloride. The negative charge is formed in the process of the leaving group departure and is thus already partially formed in the transition state. Hence, the structural argument that larger atoms are able to better accommodate negative charge can also be used to infer a claim about the activation energy of the leaving group activity (Goodwin, 2003). The division of mechanistic reasoning in organic chemistry into two accounts, i.e. a structural and an energetic account, is shown in Fig. 1. Our study is based on a definition of mechanistic reasoning as reasoning that includes connections between structural and energetic accounts.
In addition to the dualism of structure and energy, philosophy of science has investigated the dualism of statics and dynamics (Fig. 1) in mechanistic reasoning across sciences (Machamer et al., 2000; Glennan, 2002; Bechtel and Abrahamsen, 2005; Illari and Williamson, 2011). This owes to the fact that “mechanisms are composed of both entities and activities” (Machamer et al., 2000, p. 3). According to Machamer et al. (2000) activities “are the producers of change” and entities are the “things that engage in activities” (p. 3). The division of mechanistic reasoning into reasoning about entities and reasoning about activities has already successfully been used in education research in molecular biology and physics (Russ et al., 2008; Bolger et al., 2012; van Mil et al., 2013; Southard et al., 2016; van Mil et al., 2016) as well as in organic chemistry (Caspari et al., 2018).
Typical entities in organic chemistry are molecules or atoms. These entities are characterized by their properties, e.g. charges and orbital configuration (Fig. 1). The transformation of a molecule in a mechanistic step is a typical activity. Activities can be characterized by properties like rate or reversibility. They can also be characterized by changes of properties of entities, e.g. the formation of charges in the transition state and the change of orbital configuration (Fig. 1).
Goodwin's (2003) analysis shows that mechanistic reasoning in organic chemistry includes an approach to change. For example, accumulation of negative charge on a halide leaving group is a change that occurs in a leaving group departure from a haloalkane. A static approach to this change, i.e. reasoning about the negative charge in the product of the leaving group departure step, can be used to infer a claim about the relative potential energies of the entities, i.e. the reactant and product of the step. To make a claim about the activation energy, however, a dynamic approach to the change is needed. One uses additional information about the process, namely that the negative charge is already partially formed in the transition state (Fig. 1). Hence, our study is based on a definition of mechanistic reasoning as reasoning that involves either a static or a dynamic approach to change.
Building on Goodwin's work (2003), we considered how knowledge pieces are connected in the structural account of comparative mechanistic reasoning about single reaction steps. A reasoning structure that is applicable to compare any entities undergoing the same type of mechanistic step is to construct cause–effect relations between differences of the entities and a change that is occurring in the type of mechanistic step. For example, one constructs a relation between the different sizes of the chloride and bromide leaving groups and the formation of negative charge, i.e. a change occurring in every leaving group departure from a neutral reactant. As mentioned before, the cause–effect relationship could be that, due to the larger size of bromide compared to chloride, the negative charge forming at bromide is better accommodated. To consider typical changes that occur within entire classes of mechanistic steps, e.g. leaving group departure or protonation steps, one utilizes mechanism schemata, i.e. abstract descriptions of types of mechanisms (Machamer et al., 2000). The same reasoning structure can be found when considering different molecules and different mechanistic steps. For example, another mechanistic question of the type described by Goodwin could be which proton, A or B (Fig. 2), is abstracted faster in an E2 elimination? A direct answer to this question is that the abstraction of proton A has lower activation energy (Goodwin, 2003, 2008). To construct the structural account of the answer (Goodwin, 2003, 2008), one conveys a relation between an explicit structural difference of the carbons to which the two protons are bound and a change that occurs in every E2 elimination. The explicit structural difference is that the carbon bonded to proton A has more alkyl substituents than the carbon bonded to proton B. A change that occurs in every E2 elimination is the formation of the π system of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond in the transition state. A relation between having more alkyl substituents adjacent to the site of elimination and the formation of the C
C double bond in the transition state. A relation between having more alkyl substituents adjacent to the site of elimination and the formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond can be that more alkyl substituents donate more σ electron density to mix in a bonding sense with the π* orbital forming in the transition state (Goodwin, 2003; Fleming, 2010).
C double bond can be that more alkyl substituents donate more σ electron density to mix in a bonding sense with the π* orbital forming in the transition state (Goodwin, 2003; Fleming, 2010).
This reasoning structure leads us to define mechanistic reasoning investigated in this study as comparative reasoning about cause–effect relations between explicit structural differences and structural and energetic changes occurring in a mechanistic step.
A further characteristic of this comparative single-step mechanistic reasoning is that, in many cases, several arguments have to be considered and weighed. For example, to decide which proton is abstracted faster in an E2 elimination, in addition to hyperconjugation one has to reason about steric hindrance between the base and the haloalkane and weigh these arguments (Goodwin, 2003). Each of these arguments has the structure described before and can therefore be analyzed separately based on the aforementioned definition.
Student mechanistic reasoning described in the chemistry education literature
Research studies in chemistry education have previously focused on student causal mechanistic reasoning. Core characteristics of mechanistic reasoning are that it is causal and involves reasoning about interactions (Sevian and Talanquer, 2014; Becker et al., 2016; Weinrich and Talanquer, 2016). For example, the following excerpt from a student in Weinrich and Talanquer's (2016) study is described to be a linear causal mode of reasoning used to design and evaluate a strategy to synthesize an amide:The electrons from the nitrogen would attack the carbonyl carbon displacing the electrons on the carbon oxygen bond and then the um…electrons from the oxygen would kick back down, and the chlorine would leave um leaving this NH 3 amide species and then the chlorine atom comes in and takes the extra hydrogen off of the nitrogen and you’re left with just the amide (p. 400).
In this example, the student described an activity A (i.e. nitrogen attacks the carbonyl carbon) to cause another activity B (i.e. displacing electrons on the carbon oxygen bond), which the student described to cause another activity C (i.e. electrons from the oxygen would kick back down) and so on. As in our definition of mechanistic reasoning, the mechanistic causality in the student example relies on cause–effect relations and dynamics. This commonality is also shared with the following example of a student in Becker et al.'s (2016) study that is described as a complete causal mechanistic account to explain the occurrence of London dispersion forces between two helium atoms approaching each other:
Madelyn: Um, because…an instantaneous dipole is when it just so happens that the electrons are on this side, so there's a slightly negative and slightly positive (drawing) side. And then, because this is slightly positive, it attracts the electrons of this density more towards this side so then it would turn into this [draws figure that demonstrates instantaneous and induced dipole and attractive forces] (p. 1718).
Becker et al. (2016) explain that Madelyn's statement is a complete causal mechanistic account because it includes reasoning about causes at the subatomic level and reasoning about the underlying process. The cause of London dispersion forces is given to be “electrons and protons of each atom arranged so as to create a dipole” (Becker et al., 2016, p. 1717). The underlying process is the spontaneous and induced shift of electron density. Thus, the framework of Becker et al. (2016) focuses on the completeness of students’ explanations to why and how questions about the occurrence of London dispersion forces. Although it was not a main focus of Becker et al. (2016) to analyze the pattern of causes and effects (such as A causes B causes C), they describe a complete causal mechanistic answer as one that “provide[s] a complete and logical sequence of events” (p. 1718).
Unpacking the structure of Madelyn's reasoning with the framework of Sevian and Talanquer (2014) is not as clear as describing the structure of the student excerpt given before from Weinrich and Talanquer's (2016) study. One possible interpretation is that Madelyn's reasoning structure describes an activity A (i.e. spontaneous charge separation in one helium atom) as the cause of an activity B (i.e. induced charge separation in the other helium atom). One could also interpret Madelyn's response to include a description of an activity (i.e. spontaneous shift of electron density) as the cause of a new property of an entity (i.e. uneven charge distribution in one helium atom), a property that she then relied on as the cause for a second activity (i.e. attraction of the electron density of the second helium atom), which she subsequently used as the cause for a third activity (i.e. induced shift of electron density in the other helium atom). Comparing these two possible interpretations of the structure of Madelyn's reasoning, one can see that they differ in grain size (i.e. finer grain for the second interpretation), in the number of cause–effect relations they contain (i.e. more for the second interpretation), and in how they define the nature of pieces that are connected (i.e. solely activities in the first interpretation compared to activities and properties of entities in the second interpretation). Moon et al. (2016) applied the framework of Sevian and Talanquer (2014) to student reasoning in physical chemistry courses in such a way that causes and effects could also be steps of solving a mathematical equation. Thus, the nature of the task and the grain size with which one analyzes student reasoning determines what counts as sufficient causes and effects to comprise a logical sequence.
In contrast, in the reasoning structure of comparative single-step mechanistic reasoning that we developed based on Goodwin's work (2003, 2008), we define more concretely the starting points, end points, and connections between these points for cause–effect relations in which we were interested. The starting point of the structural account is an explicit structural difference of the compared molecules. The end point of a cause–effect relation within the structural account is a change that occurs in a type of mechanistic step. The connection is formed by using the explicit structural difference to reason about a cause and its effect on the change. The effect on structural change is then causally connected to a claim about energy. Fig. 3 demonstrates that, compared to the general structure of causal mechanistic reasoning (Fig. 3, I and II) in existing frameworks (Sevian and Talanquer, 2014; Becker et al., 2016), the specific structure of comparative single-step mechanistic reasoning (Fig. 3, III and IV) uses cause–effect relations to bridge the starting and the end point of the structural account (Fig. 3, i). This structure also separates reasoning about change from reasoning about other information (Fig. 3, ii). Furthermore, connections of structural and energetic accounts are part of the defined structure of comparative single-step mechanistic reasoning (Fig. 3, iii). Thus, we can investigate relations that students construct between explicit structural differences and changes (Fig. 3, i), students’ approaches to change (Fig. 3, ii), and connections between structural and energetic accounts (Fig. 3, iii) on a finer grain and add detail to the existing studies in the context of single steps of organic reactions.
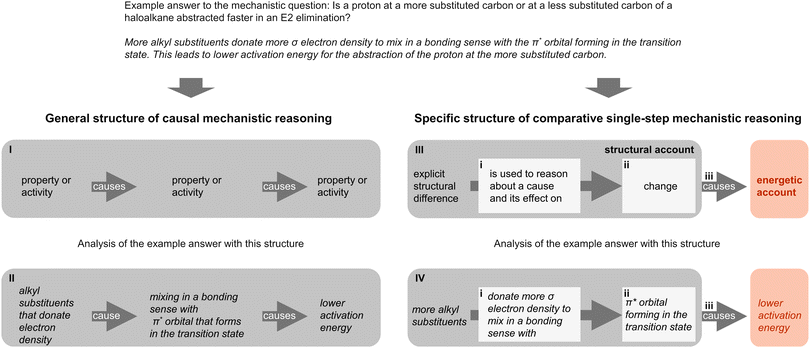 | ||
| Fig. 3 Comparison of the general structure of causal mechanistic reasoning described in previous studies in chemistry education (Sevian and Talanquer, 2014; Becker et al., 2016) and the specific structure of comparative single-step mechanistic reasoning used in our study. The general structure of causal mechanistic reasoning (I) and the specific structure of comparative single-step mechanistic reasoning (III) are applied (II and IV) to an example answer to a typical mechanistic question in organic chemistry (top). | ||
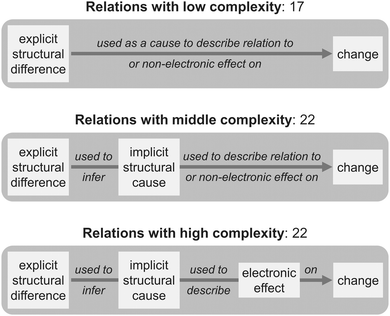
The existing frameworks for analyzing student mechanistic reasoning propose a range of reasoning levels from low to high complexity. Based on earlier educational approaches (Biggs and Collis, 1982; Grotzer, 2003; Brown et al., 2010; Bernholt and Parchmann, 2011), Sevian and Talanquer (2014) developed four modes of reasoning with increasing complexity: descriptive, relational, linear causal, and multicomponent. In a descriptive mode, only explicit features of a system are used to re-describe what is already presented in the problem context, while both explicit and implicit features are used to establish correlations in a relational mode. The identification of implicit properties also indicates a higher level of abstractness in mechanistic reasoning in organic chemistry (Weinrich and Sevian, 2017). In a linear causal mode, cause–effect relationships and interactions are included, and in a multicomponent mode, the highest in complexity, effects of several variables are considered and weighed. These modes of reasoning have been empirically validated in the context of chemical reactions used to make a desired product (Weinrich and Talanquer, 2016).
Although the levels proposed by Becker et al. (2016) additionally include an increase in conceptual sophistication, both frameworks have in common that they consist of levels with increasing complexity. Becker et al. (2016) identified five levels of reasoning about London dispersion forces in interviews with general chemistry students. In the highest level, students reason about the how and the why of the occurrence of London dispersion forces as shown in the example of Madelyn. One level lower, students’ explanations have gaps or inconsistencies in the description of the process of how London dispersion forces occur. In the lower three levels, reasoning about the process is very limited or not present, and the levels are differentiated by whether the cause is canonical and electrostatic, non-canonical and electrostatic, or non-canonical and non-electrostatic.
For comparative single-step mechanistic reasoning that connects explicit structural differences with changes, it is currently unknown whether students construct relations of differing complexity between these starting and end points and, if they do, how different levels of complexity can be characterized. However, we can assume that some of the characteristics that were found in the previous studies on mechanistic reasoning might also occur in this type of mechanistic reasoning.
In our study, we were interested not only in the complexity of relations that students construct between explicit structural differences and changes but also in how they approach change. We know from philosophy of organic chemistry that change can be approached statically or dynamically (Goodwin, 2003). A static approach to change can be used to compare the relative potential energy of reactants and products of mechanistic steps. Only with a dynamic approach to change, i.e. reasoning about changes that form in the transition state, can one make causal statements about activation energy. Research on student reasoning about predict-the-product exercises has shown that students often only consider the static structure of the products without thinking about the process, even though the latter would lead to greater success for tasks that involve transfer of knowledge (Grove et al., 2012a). However, there remains much to be learned about students’ approaches to change occurring in single mechanistic steps.
Based on Goodwin's analysis, we further know that reasoning about effects on structural changes needs to be connected to an energetic account that constitutes the direct answer to a typical mechanistic question. Popova and Bretz (2018) investigated students’ difficulties connecting reasoning about energy diagrams with reasoning about symbolic representations of substitution and elimination reactions, i.e. representations of the structural account. They found that students often match reactions with the wrong energy diagram because students focus primarily on explicit properties represented in the structural formulas. For example, students match a symbolic representation of a reaction with an energy diagram based on the idea that species with formal charges always have high potential energy. Student focus on explicit features of symbolic representations, specifically on formal charges, has previously been described in other studies (Anzovino and Bretz, 2015; Galloway et al., 2017; Graulich and Bhattacharyya, 2017). Popova and Bretz's (2018) study mainly focused on the mistakes students make when connecting the structural and the energetic account. A closer investigation of how students change from reasoning about structure to reasoning about energy can add further detail to this topic. In the context of atomic-molecular interactions (Becker and Cooper, 2014) and ionization energy (Taber, 2003, 2009), some notion of stability seems to be important to students at the intersection of reasoning about structure and energy. Students sometimes relate potential energy to stability without a causal connection to atomic-molecular interactions—the structural account (Becker and Cooper, 2014). Moreover students often relate octet structure to stability, which can sometimes lead to wrong predictions about energy changes (Taber, 2003, 2009). Since reasoning is context-dependent, we set out to learn more about students’ connections between structural and energetic accounts in the context of single steps of organic reactions.
Goals and research questions
Based on the main constituents of comparative single-step mechanistic reasoning and insight from the chemistry education literature, our goal was to investigate students’ approaches to change, the complexity of the relations they construct, and their connections between structural and energetic accounts. Specifically, our study was guided by the following research questions:(1) How do students approach structural change?
(2) How can one characterize the complexity of relations that students construct between explicit structural differences and changes?
(3) How do students connect structural and energetic accounts?
To answer the research questions, we performed a qualitative interview study with Organic Chemistry II students solving mechanism problems regarding a single leaving group departure step, a simple but representative mechanistic scenario. The in-depth analysis of students' reasoning demonstrated the applicability of the theoretical framework, allowed further development of the framework based on the data, and yielded insight into student productive resources and difficulties.
Methods
Context and participants
The research study was conducted at a German university in February and the beginning of March 2017. Students were recruited on a voluntary basis from the Organic Chemistry II course. At this German university, teaching of organic chemistry is based on a traditional curriculum. The title of the Organic Chemistry II course was Reaction Mechanisms. A leaving group departure step was introduced to students in the previous Organic Chemistry I course (in the semester before the Organic Chemistry II course) in the context of SN1 and E1 reactions. Energy diagrams of these reaction types and Hammond's postulate were covered in the lecture. In the Organic Chemistry II course, leaving group departure steps were part of multistep mechanisms. For selected topics, the lecture included discussion of energetic accounts when considering the Curtin–Hammett principle and thermodynamic vs. kinetic products of reactions. The students who took part in the study were chemistry and food chemistry undergraduates. Participants were between 19 and 27 years old. The Organic Chemistry I grades of the participants ranged from 5 to 15, with 5 being the grade necessary to pass the exam and 15 being the best possible grade in the German grading system. The average of the participants’ course grades was 11. They were asked to take part in the study via announcement in lecture and via email. 20 students took part in the study. They are given pseudonyms in this publication. All students signed a document giving their consent to (1) the transcription of their interviews by the research team or a professional transcription service, (2) the analysis of their data by the research team, and (3) the use of their data including their drawings for publication.‡ The interviews were conducted in German. Quotes were translated for this publication.Data collection
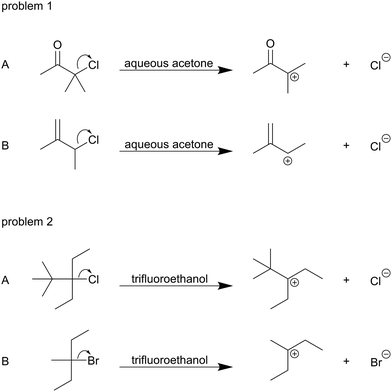
The students were interviewed and asked to think aloud while working on two mechanism problems about leaving group departure steps (Fig. 4). A LiveScribe pen was used to simultaneously record students’ drawings and verbalized reasoning in the semi-structured interviews. The first author interviewed the students over six weeks around the time of their final exam in Organic Chemistry II. This relatively wide timeframe was necessitated by scheduling considerations, but it was suitable for our study because the mechanism problems used in the interviews did not require knowledge about specific mechanisms taught in the course. With their signature on the consent form, the students agreed to not talk to other students about the interview. Thereby, we sought to minimize the probability that students found out information about the problems before they were interviewed. Furthermore, the interviewer could not observe anything during the interviews that made her think that the students had information about the problems. The interviews were divided into two parts. In the first part of the interviews, students were asked to think aloud while solving the two mechanism problems. This part of the interview lasted between 10 and 40 minutes. After the first part of the interview, students received a scaffold for their mechanistic reasoning and were asked to solve the same mechanism problems again. Only the data from the first part of the interview is covered in this publication.Problem design
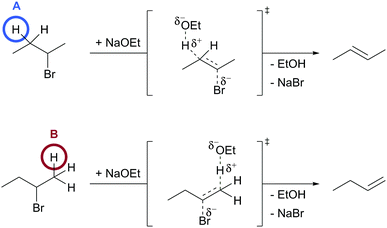
To elicit students’ mechanistic reasoning, we used a question of the type described by Goodwin (2003) to be a typical mechanistic question in organic chemistry. Students were asked to predict for which of two reactants the represented mechanistic step (Fig. 4) has lower activation energy. Thus, a complete causal answer to this question fulfills all characteristics of comparative single-step mechanistic reasoning. The problem design in a contrasting case format (Bransford and Schwartz, 1999) uses the type of case comparisons proposed for mechanistic reasoning in organic chemistry by Graulich and Schween (2018). Outside the field of organic chemistry, it has been shown that learning with contrasting cases induces relational conceptual understanding to a greater extent than learning with single cases (cf.Alfieri et al., 2013). Hence, contrasting cases seemed very appropriate to elicit students’ productive resources regarding the relations they construct between differences in the molecules and the changes the molecules have in common during the step. For example, students could construct relations between the carbonyl group that exists only in 1A (and not in 1B) and the positive charge that forms in A and B (Fig. 5).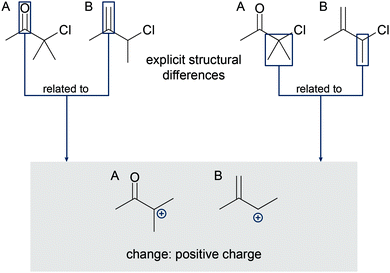 | ||
| Fig. 5 Possible relations between explicit structural differences and the positive charge in problem 1. | ||
For designing the contrasting cases, a leaving group departure step was chosen as a mechanistic example for its simplicity and representative nature. We consider a leaving group departure a relatively simple example mechanistic step because it involves only one reactant and the movement of one electron pair. It is a representative step because the changes in the reaction system during a leaving group departure are fundamental changes in many steps of organic reactions, i.e. change in molecular geometry, bonding, charge distribution, and degrees of freedom.
The reactants, constituting the contrasting pair in both problems, differed in more than one explicit structural difference. The following information about familiarity and unfamiliarity of these explicit structural differences in the context of a leaving group departure stems from consultation with the professors teaching Organic Chemistry I and II: For problem 1, relations between the additional methyl group in 1A or the alkenyl group in 1B and carbocation formation were familiar to students. Relations between the carbonyl group and carbocation formation were unfamiliar to students. The electron-withdrawing effect of the carbonyl group was covered in the Organic Chemistry II course but not in the context of a leaving group departure because infeasible mechanistic steps are usually not discussed in traditional courses. This means that, solely based on their coursework, students could not have known about repulsion between the partial positive charge at the carbonyl carbon and the positive charge forming in the transition state leading to the carbocation. For problem 2, relations between different halides and leaving group ability were familiar to students, as were relations between different alkyl chains and carbocation formation. However, the tert-butyl group compared to the methyl group also leads to greater relief of B-strain (relief of the electronic repulsion between substituents) due to the change of the bond angle from 109.5° in the reactant to 120° in the product of the leaving group departure step (Liu et al., 1998; Liu et al., 2009; Smith, 2013). While the students were taught bond angles of carbon atoms with sp3 and sp2 hybridization, the relief of B-strain was unfamiliar to them.
We included unfamiliar, advanced concepts and multiple competing factors that influence the transition states in our problem design. The first reason for doing this was that, in addition to our second research question, we wanted to probe whether students construct both familiar and unfamiliar relations. Particularly, we also wanted to compare the correspondence between success and complexity of the relations in familiar and unfamiliar contexts. This was of special interest in light of Grove et al.'s (2012a) finding that mechanistic reasoning only improves student success for mechanistic tasks that involve transfer of knowledge. The second reason was that having to consider more than one factor is a typical characteristic of mechanistic reasoning in organic chemistry (Goodwin, 2003; Kraft et al., 2010). Because of this characteristic, we wanted to probe whether the theoretical framework we developed could be used to analyze every relation a student constructed separately in an answer that contains multiple relations. The third reason was that we wanted to provide the students with tasks that would require them to form new connections between pieces of knowledge rather than to simply apply memorized rules and facts. Thus, we intended to create an interview environment in which students would walk us through their ideas rather than try to replicate what they thought the desired answer was, i.e. we intended for them to frame the interview as inquiry instead of as an examination (Russ et al., 2012). To support this framing, the students were told at the beginning of the interview that there are multiple possible answers to the problems and that we were interested in their reasoning processes and their predictions but not in the correctness of their answers.
Information about the interview process, including information about follow-up questions, is given in Appendix 1.
Data analysis
After the transcription of the interviews, our qualitative analysis was performed using the coding software MAXQDA. In a first round of coding, we addressed the first research question, how do students approach structural change? Based upon the theoretical framework we developed, we coded whether the students approached change in the mechanistic steps statically or dynamically. For example, a static approach to change could be reasoning about the positive charge of the carbocation and a dynamic approach could be reasoning about the formation of the positive charge. Using constant comparison (Corbin and Strauss, 2015), we further identified the nature of the changes that students were reasoning about among their static and dynamic approaches. More information about how approaches to change were coded is given in Appendix 2. Because we found instances in which students who approached change statically expressed doubt that their arguments supported a valid claim about activation energy, we decided to systematically analyze whether these instances of uncertainty corresponded to students' static approaches to change.The next round of coding was used to qualitatively address our second research question, how can one characterize the complexity of relations that students construct between explicit structural differences and changes? Student references to the explicit structural differences between the reactants in problems 1 and 2 began the relations, and changes coded in the first round of coding ended the relations that we analyzed. All starting and end points of the relations we found in the data are shown in Appendix 3 alongside additional explanations of how we determined starting and end points. The complexity of each relation that a student constructed was analyzed. We used constant comparison (Corbin and Strauss, 2015) to identify three levels of complexity of the relations. While the data was put first over any literature, constant comparison of the data and existing literature revealed that integration of the work of Sevian and Talanquer (2014) productively informed the description of the data (Ramalho et al., 2015). This means we did not use the modes of reasoning developed by Sevian and Talanquer (2014) as a coding scheme for our data, but aspects that differentiate between their modes of reasoning were suitable to differentiate between the levels of complexity we found in our data. These aspects were the usage of explicit properties, implicit properties, and cause–effect relationships. When coding levels of complexity, no code was given that would attribute meaning to terminology a student used, such as stability or inductive effect, unless the student explained the meaning, which they were always asked to do by the interviewer (see follow-up questions in Appendix 1). For example, if a student explained that a tertiary carbocation is more stable than a secondary, stable was treated only as a way of comparing the carbocations and no further meaning was attributed to it. This was important in light of Ferguson and Bodner's (2008) findings that students tend to use verbalism—using words they learned without necessarily implying meaning. We analyzed the highest complexity reached by a student for each relation. The coding procedure leading to the identification of the highest complexity is explained in Appendix 4. Additionally, we analyzed whether students’ success corresponded to the complexity of relations that students constructed.
A third round of coding was used to qualitatively address our third research question, how do students connect structural and energetic accounts? For our analysis we used Hypothesis Coding (Saldaña, 2016). Building on findings in the chemistry education literature mentioned earlier (Taber, 2003; Taber, 2009; Becker and Cooper, 2014; Popova and Bretz, 2018), we hypothesized that the connection between structural and energetic accounts would be weak for some students. Thus, we coded instances in which utterances or drawings in students’ structural accounts were not consistent with utterances or drawings in their energetic accounts. Based on the same findings, we hypothesized that students’ understanding of stability might make a contribution to structural or energetic accounts. Hence, we coded instances where this was the case.
In all rounds of coding, the first author (PhD candidate with a master's degree in chemistry education) coded the entire data set. During this process, constant discussion and reflection with the other two authors (professor and master student in chemistry education) were used to ensure that coding decisions faithfully represent the data. For interrater reliability, all three authors independently coded 20 percent of the data. Results were discussed until complete agreement was reached before the first author went through the entire data set again to ensure that the mutual decisions were applied consistently. After the first review process of this publication, slight changes to the coding system were made. The changes were discussed between the three researchers, and the first author coded the entire data set again. Thus, we could ensure that changes were applied faithfully to the data. There were six months between when the first author finished her previous coding and this recoding. A comparison of these separate codings—with the previous codes converted directly to the new coding system without looking back into the data—was also used to ensure the stability of the first author's coding. The second author coded 20 percent of the data with the final coding system, permitting us to calculate a Cohen's kappa coefficient of 0.95.
Results and discussion
All participants constructed relations between explicit structural differences of the contrasted molecules and changes that occur in every leaving group departure step. Due to the fact that students were asked to predict the activation energy, they all connected their structural accounts to energetic accounts. The results section is organized according to our three research questions. In the first part, we focus on students’ approaches to change. In the second part, we focus on how students described relations between explicit structural differences and these changes. The third part deals with students’ connections between their structural and energetic accounts. Our results are qualitative in nature; quantities are used to demonstrate the prevalence in the data but do not imply statistical meaning.Approaches to change
Students reasoned about several changes that occur in every leaving group departure, e.g. electron flow and formation of positive charge. The theoretical framework we developed provided the lens for analyzing two ways that students reasoned about changes, i.e. static and dynamic approaches. In the following subsections, we go into detail about students’ static and dynamic approaches to change. Appendix 5 shows all approaches to change that each student used when solving problems 1 and 2. In the last subsection, we describe a correspondence between students’ approaches to change and their certainty about the relevance of their arguments for a claim about activation energy.Jan: Then, this positive charge is distributed over this entire molecule, and this is considerably better.
It is important to note that reasoning about charge distribution might be considered reasoning about dynamics. However, it is not a dynamic approach to change because it is not reasoning about changes occurring during the leaving group departure step. One can reason about distribution of positive charge by only considering the structure of the product of the step, like Jan did. However, one needs to consider the formation of the charge during the step to form a complete causal answer to a question about activation energy.
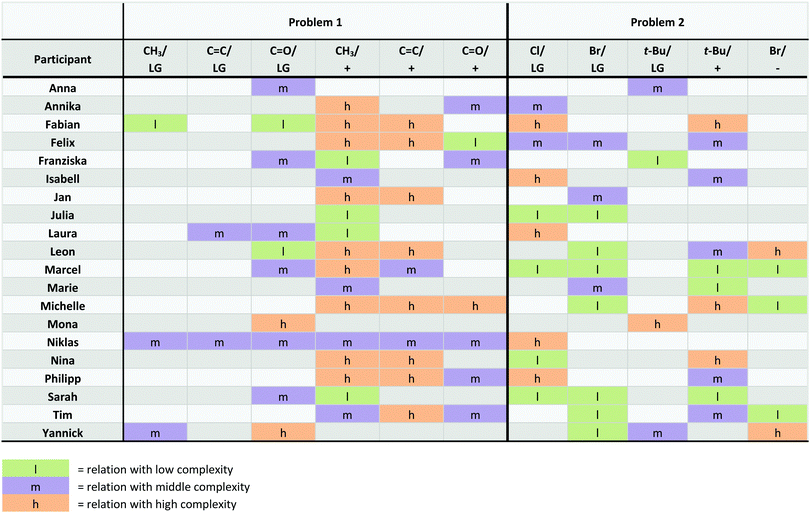
Students’ static approaches to change were grouped into categories based on the data (Table 1, left column). Table 1 shows a student example for each category and provides information about how many times each category was found in the problem solutions (40 problem solutions in total, from 20 students solving 2 problems). Students reasoned about the carbocation in 28 problem solutions. In 8 of these solutions, students did not verbalize reasoning about the positive charge of the carbocation; in the other 20, they did. Only 5 problem solutions contained reasoning about the anion, and only 3 of these contained verbalized reasoning about the negative charge.
| Static approaches to change | Dynamic approaches to change |
|---|---|
| a Numbers given in the format S (U) represent numbers of problem solutions (of 40 problem solutions in total, from 20 students solving 2 problems), where S is the number of problem solutions in which students used the respective category and U is the subset of these problem solutions in which students expressed uncertainty about the relevance of their arguments for a claim about activation energy. | |
| Carbocation: 28 (13)a | Formation of the carbocation: 8 (1)a |
| – without explicitly referring to positive charge: 8 (3)a | – without explicitly referring to formation of positive charge: 5 (1)a |
| Sarah: If I look at the substituents, A has larger substituents than B, so the cation would be more stable. | Julia: Well, it's more likely to happen because the carbocation is tertiary so that it's more stable. It's more likely to be formed than the secondary there. |
| – with explicitly referring to positive charge: 20 (10)a | – with explicitly referring to formation of positive charge: 3 (0)a |
| Leon: Well, right now I’m thinking about how we stabilize the charge at the end here, what comes out in the end. Well, I’m thinking about that. Well, at the top [1A, Fig. 4] we have two methyl groups. Each methyl group has a +I effect. That means the positive charge is stabilized here. To start, that indicates that the product at the end here [1A] is more stable than this product, the one at the end here [1B]. | Michelle: Yeah. Well, I kind of want to form the positive charge here in this step. Well, I form it from here to there. And if it's really super unstable now, then it's also more difficult to form than if it's stabilized well and when it's not quite so unstable. |
| Anion: 5 (3)a | Departure of the leaving group: 31 (4)a |
| – without explicitly referring to negative charge: 2 (1)a | – without explicitly referring to bond cleavage: 11 (3)a |
| Marcel: …the bromide has a more energetically favorable potential if it is, as an anion, free in the solution than the chloride ion if it is, as an anion, free in the solution. | Marcel: So we kind of have electron pulling in the direction of the carbonyl. And so the taking-off of the chloride ion is favored. |
| – with explicitly referring to negative charge: 3 (2)a | – with explicitly referring to bond cleavage: 20 (1)a |
| Yannick: And it's just […] more favorable that this could exist then […] as Br minus than as Cl minus. Because Cl is smaller. And there the charge is, well, rather concentrated and this isn’t as favorable. | Laura: Chlorine pulls the electrons at this position more strongly and takes them along as a leaving group more easily if it's more electronegative [than bromine]. |
Yannick: And now the activation energy. That means, well, in the end, which one happens, or which one happens faster and which one slower, or if it happens at all. And then next I would say that the oxygen atom has higher electronegativity. So the electrons, which are distributed in the molecule, in the orbitals, are pulled toward there [toward the oxygen] rather than having the Cl […] come off with an electron pair. […] Well, so this has the higher activation energy, A.
This is a dynamic approach to change because Yannick took into account the electron flow during the mechanistic step.
Students’ dynamic approaches to change were grouped into categories based on the data (Table 1, right column). In 31 problem solutions, students reasoned about the departure of the leaving group. In 11 of these solutions, they did not go into more detail about this change. In 20 problem solutions, students included bond cleavage when reasoning about leaving group departure, like Yannick in the aforementioned example. Only 8 problem solutions contained reasoning about the formation of the carbocation. In 5 of these solutions, students did not go into more detail about this change. In 3 problem solutions, students referenced the formation of positive charge.
Even in these 3 problem solutions, i.e. the only problem solutions in which students approached the formation of the positive charge dynamically, students initially approached the carbocation statically. Take the beginning of Michelle's solution for problem 2 for example:
Michelle: According to stabilization, A is favored, that's what I would say. Because the two ethyl groups are the same [in A and B] and in A there's also the larger substituent [tert-butyl], where here [in B] there's only the methyl substituent. So the cation here in A is stabilized better. […] So I would say… A is stabilized better and therefore A also has the lower activation energy.
Only after the interviewer's question about what her statement about stabilization of the carbocation has to do with activation energy did Michelle switch to a dynamic approach:
Michelle: Then, well, if I look at this transition state again, then I also see that here the positive charge would be kind of formed. And this, well, is the one that I’m mainly looking at in the product, how it's stabilized. And it's also here [transition state], where it's still forming, it's already stabilized by the substituents. And thus the transition state is also stabilized like the product. Because the transition state is the forming carbocation, which has to be stabilized. And in the real…I mean in the product, it's just the real positive charge, which also has to be stabilized. And regarding stabilization, I'm talking about the same effects.
In this dynamic approach, Michelle reasoned about stabilization effects on the positive charge that forms in the transition states of the reaction steps. Like all students who approached the formation of the carbocation dynamically, Michelle did this after first approaching the change statically. Appendix 5 demonstrates this pattern and provides information about which students used this pattern. While Michelle integrated the formation of the positive charge in her reasoning after being prompted by the interviewer, in 20 problem solutions, students remained in a static approach (28 problem solutions in which students approached the carbocation statically minus 8 problem solutions in which they also approached the carbocation dynamically, Table 1).
Many students who approached change statically realized that reasoning about the process was required, however, they could not transform their static approach into a dynamic one. This often led the students to doubt that the effect on the change they were proposing was useful for solving the problem.
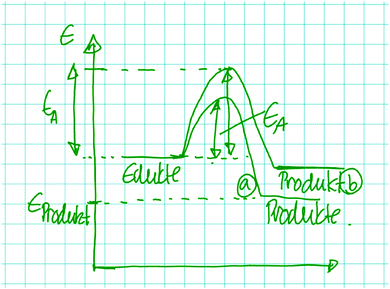
When Jan, whose static approach was introduced earlier, was asked by the interviewer what his reasoning about the positive charge in the product of the mechanistic step has to do with activation energy, he decided that his reasoning is not relevant for a claim about activation energy, as it only describes the potential energy of the product. This resulted in his drawing of energy diagrams with the carbocation 1B having a lower potential energy than that of the carbocation 1A but with both steps having the same activation energy (Fig. 6). Jan considered the positive charge to be a property of the product of the mechanistic step, but he did not consider charge formation during the step. The only change that he considered dynamically is the departure of the leaving group as is demonstrated by the following excerpt:
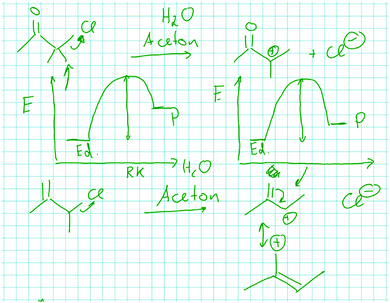 | ||
| Fig. 6 Jan visualized energy diagrams with different relative potential energies of the products but identical activation energies for the leaving group departure in problem 1A and B. | ||
Jan: Well, what I think now is that chlorine coming off probably requires a lot of energy. But this is the same for the one in the top [1A] and in the bottom [1B]. Well, the amount [of energy], this is what we need to put into it. Actually, this is already the activation energy if I'm not wrong. To go from here [reactants] to there [products of the steps], the only thing that needs to happen is that the electrons…that the chlorine comes off. So I think it [the activation energy for 1A and B] is about the same, yeah…Yeah. Yes, of course.
Although Jan, like many other students, realized the requirement to reason about the process, he did not combine dynamic reasoning about the departure of the leaving group with reasoning about charge formation. In 17 problem solutions, students approached the carbocation statically, not considering dynamics, while also reasoning about leaving group departure (Appendix 5). The 5 students who reasoned about the anion in problem 2 all combined this with a dynamic approach to the leaving group departure while remaining in a static approach to the negative charge. Appendix 5 demonstrates these patterns and provides information about which students used these patterns. The following excerpts of Yannick and Leon contain these patterns and demonstrate the students’ doubt about the usefulness of static approaches to change for solving the problems.
When solving problem 2, Yannick claimed that bromide is a better leaving group than chloride. His approach to change was dynamic on this superficial level, however, he explained the better leaving group ability by referring to the negative charge of the product instead of to the formation of negative charge:
Yannick: Because with the period…toward the bottom [of the periodic table], the molecules [means atoms] become larger. And then the charge isn’t centered as much anymore but […] is a bit more delocalized because of the size of the atom. And it's just […] more favorable that this could exist then […] as Br minus than as Cl minus. Because Cl is smaller. And there the charge is, well, rather concentrated and this isn’t as favorable.
As explained in the case of the positive charge of the carbocation, this is a static approach to the negative charge because only the negative charge in the product is considered. When asked by the interviewer what his reasoning has to do with activation energy, Yannick started doubting the relevance of his statement for a claim about activation energy. Yannick expressed his doubt by explaining that he wonders why information about an effect, i.e. the static outcome of a process, can be used to make a claim about the cause, i.e. the process:
Yannick: It always seems like…I sometimes have…You explain now…Well, I explain now in this case that the bromine has the lower activation energy because it leaves better. So I explain the cause, which I have here, why the bromine leaves better, with the effect. Well, the bromine in the reactant you know ultimately doesn’t know that it will exist as a Br minus later.
Because Yannick did not incorporate into his reasoning that the negative charge is already forming during the leaving group departure, he wondered how a reactant could “know” about an influence that, in his reasoning, only affects the product of the mechanistic step. Yannick did not transfer his static reasoning about negative charge into dynamic reasoning about formation of negative charge, although he used it to explain greater leaving group ability.
It is remarkable how Leon's reasoning developed when solving problem 2. While his excerpt is unique for the various changes in his problem solving process, the factors that induced these changes are exemplary of the types of productive resources and difficulties of many students in our study. Leon demonstrated two main productive resources: He referenced several changes that occur in all leaving group departure steps, and he identified a missing causal link when he used a static approach to change to make a claim about activation energy. At the same time, he experienced difficulty combining reasoning about the different changes and transferring a static approach to change into a dynamic approach.
Leon started his reasoning by evaluating the relative stability of the positive charges of the carbocations 2A and B.
Leon: In A there's a tert-butyl, in B there's a methyl group there. This kind of helps me again for evaluating the stability of the product at the end here, the carbocation.
In this static approach to change, he focused on the product of the mechanistic step. Then, without any intervention of the interviewer, he decided that his reasoning about the positive charge of the carbocation is irrelevant for a claim about activation energy:
Leon: But it doesn’t matter again.
After his decision that reasoning about the structure of the product does not matter for a claim about activation energy, Leon searched for a change he could approach dynamically. He stated that bromide is a better leaving group because of better charge accommodation:
Leon: And the bromide is a better leaving group than the chloride because of its size and because, on the bromide ion, the charge could be better stabilized, I mean distributed, than on the chloride ion.
Leon switched to this argument because he realized the necessity to reason about the process instead of reasoning about the structure of the product. He thought judging leaving group ability is reasoning about the process, and it is at a superficial level. But then he realized that his actual explanation for the leaving group ability was not a dynamic approach to change. Reasoning about the negative charge of the anion was a static approach like reasoning about the positive charge of the carbocation. Leon had contradicted his own argumentation: He decided that static reasoning about the carbocation cannot be used to make a claim about activation energy but proceeded to use the same type of static argument for the negative charge of the leaving group.
To express his thoughts, he used a very vivid German metaphor for which we found no English translation that covers the entire meaning. Thus, in the following quote, we use a word-for-word translation leading to an expression that does not seem to exist in English but demonstrates the frustration he expressed:
Leon: The problem is I kind of bit myself in the tail just now (in the German original: Ich beiße mir quasi gerade in den Schwanz) saying that bromide is the better leaving group because of better stabilization of the charge in the end while I disregard the argument for the reactant here [underlines the carbocation], I mean for these two [carbocations 2A and B]. Because the products… I just realized that I bit myself in the tail there (in the German original: Das merke ich selbst gerade, dass ich mir da selbst in den Schwanz beiße). That is a bit stupid. … Yeah. … Well, I haven’t found another… no, I don't know what, no, sorry, I give up on that one.
Leon gave up on the problem because he realized that the mechanistic question about activation energy requires a dynamic approach to change, but all his effort to find a dynamic way to approach the problem resulted in only static approaches to change. He did not realize that charge formation and separation are happening during the process. His difficulties might originate in use of a static approach to charges and a dynamic approach to the departure of the leaving group as isolated categories. It might have helped him if he had considered electron flow as well as charge formation and separation as interconnected changes during the activity.
Adding to Anzovino and Bretz's (2016) findings about students’ fragmented ideas about electrophiles and nucleophiles, this is another example of fragmentation of student ideas in organic chemistry. By reasoning in isolated categories, most of the students could not transfer static reasoning about charges into dynamic reasoning about charge formation during the process. In nearly half of the problem solutions (Appendix 5), students reasoned about charges and about the departure of the leaving group—several of them including electron flow in their considerations—but none of the students connected a dynamic approach to electron flow with charge formation.
Results thus far have focused on static and dynamic approaches to change. A correspondence was observed between static approaches and students’ uncertainty about whether their reasoning is relevant to predict the activation energy. In the following section, the focus shifts to the relations students constructed between differences of the contrasted molecules and changes.
Complexity of relations
Students constructed relations with three levels of complexity. To differentiate between the levels, we only coded the complexity of the relations and disregarded conceptual sophistication. As shown by Weinrich and Talanquer (2015, 2016), it is possible to analyze the complexity of reasoning independently from conceptual sophistication. In the following subsections, we describe the three levels of complexity by using examples of problem 1. Two student examples of each level of complexity, drawn from problem 2, are provided in Appendix 6. Appendix 7 shows all relations constructed by each student and their levels of complexity. In the last subsection, we describe a correspondence between the complexity of relations and success.For example, Felix asked himself about the influence of the oxygen in 1A on the positive charge of the carbocation:
Felix: I'm just thinking about what influence the oxygen in the system has on the positive charge. But I haven’t reached an answer yet. Well, right now I’m thinking about when we’ve had something like this before, where I could get a kind of analogy from. But nothing is coming to mind yet. So, well, I'm still thinking.
An implicit structural property of the oxygen was not part of the relation he constructed. He directly used the depicted oxygen to reason about potential influences on the positive charge.
Franziska constructed a relation between the explicit structural difference secondary vs. tertiary and the carbocation:
Franziska: Well, first of all, I look at these… ions here… these cations. And I’m sort of looking for which one is more stable. And in B it's, you know, a secondary cation, and in A it's a tertiary. And, a tertiary cation is more stable than a secondary. That's why I think this is more stable, and therefore the activation energy is maybe smaller.
It is important to note that stability is implicit, nevertheless, the relation Franziska conveyed was coded to be of low complexity. This is because she used stabilization to describe the effect on the change, i.e. on the carbocation, and not an implicit structural property like electron donation that causes this effect (Fig. 7). To probe whether Franziska would express a more complex relation, she was asked why the tertiary carbocation is more stable than the secondary. Franziska answered:
Franziska: Because of… well… I don't know right now how to explain it. …Well, you sort of have three bonds. And then… Honestly, I don't really know why it's like that. I only know… Well, we always learned that it's more stable if it has more bonds.
Her answer shows that the relation she constructed between the number of bonds and the stabilization of the carbocation remained low complexity and was only a memorized correlation.
For instance, Annika drew the resonance structure of the carbocation shown in Fig. 8 and then explained why she thought the resonance structure does not look good:
Annika: But this [ Fig. 8 , resonance structure on the right] actually looks totally stupid, so I don't think this would form like that. […] Well, between the oxygen atom and the carbon atom we have a big difference in electronegativity. Oxygen is much more electronegative than carbon. That's why […] this resonance could form at all. In other words, oxygen is more electronegative, so oxygen pulls the electrons more toward itself, so this carbon here [carbonyl carbon] is partially positively charged. […]
Interviewer: […] Why does this look stupid?
Annika: Because of the two positive charges on two carbon atoms next to each other. I can't remember ever seeing something like this.
Annika constructed a relation between an implicit structural property (i.e. the partial positive charge of the carbonyl carbon) and the positive charge of the carbocation. The relation she described is that the two positive charges are next to each other and do not look good. This shows that Annika constructed a relation but did not use the partial positive charge of the carbonyl as a cause for an electronic effect on the positive charge of the carbocation.
Marie established a relation between an implicit structural property of the additional methyl group in 1A (i.e. electron donation) and the positive charge of the carbocation:
Marie: And the methyl substituent has a positive inductive effect. And that means it's electron-donating, and that's why we can stabilize the positive charge. And, well, in A we have two of these methyl substituents and in B there's only one. That means, because of that, I would say that A has the lower activation energy.
The relation Marie constructed was coded to be of middle complexity because, for a cause–effect relationship, the electronic effect on the positive charge was missing. It might be that she thought about an electronic effect when giving the following answer to the interviewer's question of what stable means to her:
Marie: That means, well, here, we have a positive charge. And this would actually… This would prefer to be neutral.
However, Marie communicated only the “wants” of the positive charge—a teleological explanation (Talanquer, 2013, p. 1419)—and no electronic effect of the electron donation on the positive charge, e.g. weakening of the positive charge. Due to the fact that we only coded what students actually verbalized, the relation was coded to be of middle complexity.
Laura conveyed a relation with middle complexity that was not coded to have high complexity because the causal chain was not closed. While employing this reasoning she drew Fig. 9:
Laura: In B we have the alkenyl substituent, a sort of electron-donating group. And that means that this position here is probably negative [draws δ- at the lower C of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/i_char_e001.gif) C bond, Fig. 9]. And at this positon then it's rather delta positive [draws δ+ at the C attached to the Cl, Fig. 9]. And of course here a large delta minus [draws larger δ- at the Cl, Fig. 9]. Here, right, delta plus and here delta minus. That means, I would say now that the activation energy in B is lower. And that's also my answer now.
C bond, Fig. 9]. And at this positon then it's rather delta positive [draws δ+ at the C attached to the Cl, Fig. 9]. And of course here a large delta minus [draws larger δ- at the Cl, Fig. 9]. Here, right, delta plus and here delta minus. That means, I would say now that the activation energy in B is lower. And that's also my answer now.
Interviewer: Can you explain to me again what exactly you do with delta plus and minus […] and how this helps you?
Laura: The chloride comes off as a leaving group and takes the electrons along. And this means if I have, at this position, where it leaves, that means here [pointing to the C attached to the leaving group], rather delta positive, that means fewer electrons at this position… I mean the electron density is lower there, then it's easier for the chloride to take the electrons along. And because of the alkenyl group down here, well over there, I have a slightly +I effect, which then produces delta minus again at the next position [δ- at the lower C of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/i_char_e001.gif) C bond, Fig. 9]. And this means, here at this position, I have a greater delta plus [δ+ at the C attached to the Cl, Fig. 9]. Then the chloride comes off more easily. Yeah.
C bond, Fig. 9]. And this means, here at this position, I have a greater delta plus [δ+ at the C attached to the Cl, Fig. 9]. Then the chloride comes off more easily. Yeah.
She identified partial charges (Fig. 9) as implicit structural properties and related them to the departure of the leaving group by describing how easily the chloride can take the electrons along. Because Laura used a heuristic of alternating partial charges, her statement did not reflect a closed causal chain. She did not explain why partial charges alternate or why a delta positive charge at the reacting carbon leads to easier departure of chloride taking the electron pair along.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vs. C
O vs. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and a change (i.e. electron movement) with high complexity:
C) and a change (i.e. electron movement) with high complexity:
Mona: And I think actually that B should be faster because in A we have the oxygen, which pulls the electrons toward itself. And so this electron pair, with which the chlorine is attached to the molecule, is pulled away from the chlorine. So it's more difficult for it to leave as a chloride ion because it's more difficult for it to kind of take the electron pair along in A.
The relation Mona constructed between the carbonyl group and the electron movement has high complexity because Mona inferred an implicit structural property (i.e. electron pulling) and used this as a cause for an electronic effect on the change (i.e. difficult to take the electron pair along).
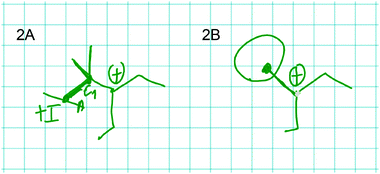
Felix established a relation between the alkenyl group and the positive charge of the carbocation that has the same complexity as the relation Mona constructed. While doing so, Felix drew the resonance structure shown in Fig. 10:
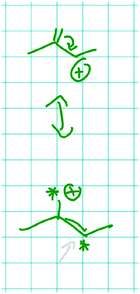 | ||
| Fig. 10 Flipping of the double bond and resonance structure of the carbocation in problem 1B drawn by Felix. | ||
Felix: Let the double bond flip over here and then make something like that [draws resonance structure of the product of the step 1B, Fig. 10]. […] And this would be resonance stabilized, which generally speaks for… and kind of says this is energetically more favorable because resonance is always good. I can't do this in A. […]
Interviewer: […] You were talking about resonance stabilization. Can you explain to me again what this means?
Felix: Yes. I can kind of shift the charge in a molecule without really changing the molecule's properties. And because the charge is basically delocalized and can flip in the molecule from here to there, it's stabilized. Because it's not localized in one place.
Felix used an explicit structural difference between the represented molecules (i.e. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vs. C
C vs. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) to infer an implicit structural property (i.e. flipping of the bond). He used this implicit structural property as a cause for an electronic effect on the change he was reasoning about (i.e. delocalization of positive charge of the carbocation).
O) to infer an implicit structural property (i.e. flipping of the bond). He used this implicit structural property as a cause for an electronic effect on the change he was reasoning about (i.e. delocalization of positive charge of the carbocation).
Nina's reasoning starting with the additional methyl group in 1A initially did not have such high complexity:
Nina: And the one is tertiary and the other is secondary. And basically tertiary is better stabilized because then the +I effects come from both of the CH 3 groups.
But when the interviewer asked Nina to explain the term +I effect, she added complexity to the relation she formed:
Nina: Well, +I effect is basically… I mean describes that the C atom here [C atom of one methyl substituent in 1A] would push electrons over there [to the positive charge] and then the… I mean the bonding electrons between the two would then be localized more on the side of the positively charged C. And so the charge here becomes a bit smaller.
Nina used an implicit structural property of the additional methyl group in 1A (i.e. the donation of electrons) as a cause for an electronic effect on the positive charge of the carbocation (i.e. weakening of the positive charge).
Students’ formation of relations with high complexity often involved follow-up questions of the interviewer asking for the meaning of specific terminology, like inductive effects or stability. In these cases, students first constructed relations with lower complexity and then the complexity increased after follow-up questions. For example, Franziska and Nina both stated that the tertiary carbocation is more stable than the secondary. While the complexity of Franziska's relation did not increase after the follow-up question, as shown in the section about low complexity, the complexity of Nina's relation increased to high complexity. This demonstrates that a researcher or educator can only determine the complexity of a relation upon which a verbalized shortcut is based if students are asked to further explain their reasoning.
All three levels of complexity have the same starting and end points, i.e. explicit structural differences and changes. While the lowest level of complexity is characterized by a direct connection of starting and end points, students used more information when constructing a relation with middle complexity and developed a full cause–effect relationship when the complexity of the relation was high (Fig. 7). A more complex relation is not necessarily any better (or worse) than a less complex one. The advantage of a less complex relation is that it can be more easily communicated than a complex relation and enables faster decision making. We often observed that students used a less complex relation as a shortcut for a more complex relation that we could then elicit using follow-up questions. Our data from students who could not further explain relations with low complexity demonstrate that they often had to rely on memorized facts and rules. This is a disadvantage of relations with low complexity because inability to recall is a main barrier for mechanistic reasoning in organic chemistry (Ferguson and Bodner, 2008). Furthermore, in an unfamiliar context, more complex relations were more successful than less complex relations as is shown in the following section.
Relations starting with a familiar, explicit structural difference were those that began with the additional methyl group in 1A, the alkenyl group in 1B, the tert-butyl group in 2A, or one of the halides in 2A or 2B. Relations starting with an explicit structural difference unfamiliar to students in the context of leaving group departure were those that began with the carbonyl group in 1A. The B-strain effect in problem 2 would also have been unfamiliar to students but they did not reason about it.
Nearly all relations that started with the familiar additional methyl group in 1A, the familiar alkenyl group in 1B, or the familiar tert-butyl group in 2A were successful (Table 2). Most of the relations that started with the familiar bromide were successful, while most of the relations that started with the familiar chloride were unsuccessful (Table 2). While no considerable correspondence between the level of complexity and success was observable for relations that started with all of these familiar, explicit structural differences, more complex relations that started with the carbonyl group—unfamiliar in the context of a leaving group departure step—were more successful than less complex relations (Table 2).
| Starting points of relations | ||||||
|---|---|---|---|---|---|---|
| CH3 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
t-Bu | Cl | Br | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
|
| Ratio of successful to unsuccessful relations with low complexity | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| Ratio of successful to unsuccessful relations with middle complexity | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| Ratio of successful to unsuccessful relations with high complexity | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
Because the carbonyl group was the only unfamiliar, explicit structural difference that students used to construct relations and because the methyl group was the most used familiar, explicit structural difference, these two differences are best suited to demonstrate these findings in more detail. A relation starting with the additional methyl group was coded to be successful if the student described that the effect on a change is positive, e.g. stabilization of the positive charge or easier departure of the leaving group. A relation starting with the carbonyl group was coded to be successful if the student described that the effect on a change is negative, e.g. destabilization of the positive charge or more difficult departure of the leaving group.
All relations that students constructed starting with the additional methyl group in problem 1A were successful (Table 2). This was independent of the complexity of the relations.
Students constructed 5 relations with low complexity, and they were all successful (Table 2). For example, Julia stated that the carbocation is more stable because of the additional methyl group:
Julia: Lower activation energy, well, so that it happens, right. Well, immediately, I would say it's, well, lower in A. Well, it's more likely to happen because the carbocation is tertiary so that it's more stable. It's more likely to be formed than the secondary there. That's what immediately comes to my mind.
By using a rule, i.e. tertiary carbocations are more stable than secondary carbocations, Julia successfully predicted the effect of the additional methyl group on the positive charge.
Students conveyed 6 relations with middle complexity, and, like the relations with low complexity, they were all successful (Table 2). This is demonstrated for example by Yannick, who explained that the electron donation of the additional methyl group increases the capacity of the chloride to undergo the leaving group departure process:
Yannick: What I also just thought about the methyl group is that it actually also has a +I effect. Where […] electrons are pushed toward this carbon [carbon attached to the leaving group]. So the chlorine might actually come off more easily.
Students constructed 9 relations with high complexity, and they also were successful (Table 2), as demonstrated by Leon:
Leon: Well, okay, alkane substituents stabilize the positive charge here in the middle by partially shifting the electrons in these bonds toward the positive charge. So this charge becomes weakened, I mean just… well, not really distributed, well, it becomes weakened or stabilized by these substituents. And here [1B] we have only one group, one alkane substituent, so a greater stabilization takes place in the top [1A] than in the bottom [1B].
Students learned already in Organic Chemistry I or even earlier that tertiary carbocations are more stable than secondary carbocations and that, in the case of the tertiary, the departure of the leaving group requires less activation energy. Whether students just remembered the rule or whether their reasoning was based on causal considerations was not the determining factor of their success. In this familiar context, it was sufficient to use the rule to predict the effect of the additional methyl group on the positive charge or the departure of the leaving group.
Our analysis revealed different results when the explicit structural difference was unfamiliar. More complex relations starting with the carbonyl group in 1A, i.e. an unfamiliar property in the context of a leaving group departure, were more successful. Less complex relations were less successful (Table 2).
Students constructed 3 relations with low complexity, and they were all unsuccessful. For instance, Leon stated that the carbonyl group influences the chloride to leave more easily:
Leon: And I say […] that because of the reactivity of the carbonyl group at the top, I mean in the example at the top [1A], that the whole thing is more reactive, that chloride comes off more easily than in the bottom one [1B].
For his reasoning, Leon tried to use an analogy:
Leon: I’m trying to remember how it was with the enols. Well, the H is more acidic anyway. And this is also… I'm pretty sure that it‘s a more reactive chlorine atom than those in other positions. It's just more reactive at the α-C. And, well, that's why I say that the activation energy is lower in the top [1A].
In the unfamiliar context, memorizing a rule (i.e. that enols are reactive at the α-carbon) without specifying for which context this rule applies (i.e. deprotonation) was not sufficient to make a successful prediction. In an unfamiliar context, causal reasoning is necessary to appropriately transfer knowledge.
Compared to relations with low complexity, more of the relations with middle complexity were successful. In particular, students constructed 11 relations with middle complexity, and 6 of them were unsuccessful while 5 of them were successful (Table 2).
For example, Marcel made the unsuccessful prediction that the electron-withdrawing effect of the carbonyl group positively influences the departure of chloride:
Marcel: So we kind of have electron pulling in the direction of the carbonyl. And so the taking-off of the chloride ion is favored.
In contrast to Marcel, Tim made a successful prediction that the partial positive charge at the carbonyl carbon destabilizes the carbocation:
Tim: Right, for example in the case of the ketone […] they have partial charges because of the electronegativity, the oxygen, partial negative, the carbon, partial positive. That means, partial positive charges, they pull electrons toward themselves. That means, they would again pull the electrons from this C to themselves. This destabilizes the positive charge again.
Compared to relations with middle complexity, more of the relations with high complexity were successful. In particular, students conveyed 3 relations with high complexity, and they were all successful, as shown by Michelle:
Michelle: Well, in A, the thing is of course that, at the top, the O also has a negative inductive effect so that it pulls electrons. This isn’t so bad in B. […]
Interviewer: You used the term… I mean the thing with the negative inductive effect, with the O, can you explain that to me again, why this [1A] is disadvantaged by it?
Michelle: The O is more electronegative than the C's around it. So it has a certain drive to pull the electrons toward itself. And, well, this destabilizes the positive charge even more. […]
Interviewer: You said this […] destabilizes the charge. What does this mean for you when you say this?
Michelle: Well, of course formally this is a single positive charge and it also remains a single positive charge. But […] if I destabilize this more, then it becomes even more unstable, then it's kind of even more positive.
In summary, more complex relations were only more successful than less complex relations in the case of an unfamiliar, explicit structural difference. Using rules without considering the underlying causality was only successful in a familiar context. In an unfamiliar context, which requires transfer of knowledge, only more complex reasoning can differentiate between appropriate and inappropriate rules and analogies.
So far, we have focused on students’ structural accounts. In the following section our focus shifts to students’ connections between structural and energetic accounts.
Connection of the structural and the energetic account
Connecting the structural and the energetic account was challenging for students when predicting the relative activation energies of the mechanistic steps. Specifically, students experienced difficulty in clarifying whether their description referred to structure or energy and in considering both accounts when using the representation of only one of them (i.e. structural formulas or energy diagrams).Students often used some notion of stability to connect their structural and energetic accounts. For example, Marie's structural account included that the additional methyl group in problem 1A donates electrons toward the positive charge of the carbocation. Her energetic account was that the activation energy in 1A is lower. Marie claimed that her structural account supports relative stability of the positive charge in 1A compared to that in B and used this to develop her energetic account of the relative activation energies of 1A and B.
Marie: And the methyl substituent has a positive inductive effect. And that means it's electron-donating, and that's why we can stabilize the positive charge. And, well, in A we have two of these methyl substituents and in B there's only one. That means, because of that, I would say that A has the lower activation energy.
When students were asked what stability meant to them, some answered with a purely energetic account while others answered with a structural account or some combination of the two.
4 students answered with an energetic account of stability. For example, Niklas mentioned that destabilization refers to entering a state that is energetically disfavored:
Interviewer: You said the carbocation is destabilized. What do you mean with… What does the term mean for you in this context?
Niklas: Well, I’m thinking… Right, a carbocation is an electron deficient molecule… I mean a molecule where electrons are missing. So it has a positive charge. And if then there is a group attached that pulls electrons… Right, destabilized, this means that it's energetically disfavored, well, then the activation energy is higher.
It is probable that, in Niklas' explanation, stability referred to the potential energy of the carbocation. It is also possible that he did not differentiate between potential energy of the carbocation intermediate and the activation energy of its formation. Yet his statement clearly showcases that, for him, stability referred to some type of energetic consideration.
3 students answered with a structural account of stability. These students explained that stability implies an electronic effect on the positive charge. For instance, when Felix said that the positive charge is stabilized, he implied the electronic effect of delocalization of the positive charge:
Interviewer: You were talking about resonance stabilization. Can you explain to me again what this means?
Felix: Yes. I can kind of shift the charge in a molecule without really changing the molecule's properties. And because the charge is basically delocalized and can flip in the molecule from here to there, it's stabilized. Because it's not localized in one place.
4 students answered by combining structural and energetic accounts. For example, for Fabian, stabilizing the carbocation implied weakening the positive charge and making the carbocation more energetically favorable:
Interviewer: You said that, by this [what Fabian had described previously, i.e. electron donation of the additional methyl group in 1A], the structure at the end here [pointing to the carbocation] is stabilized. What does stabilized mean in this case, the term?
Fabian: A positive charge is basically less favorable than a neutral. And, because I can push a bit of partial charge into it, it's energetically favorable. Because I don’t have such a strong, localized charge then.
When Michelle was first asked what stability meant for her, she answered with a structural account:
Michelle: For the carbocation, to me, stable means the smallest possible positive charge at this point.
In addition to this structural account of stability, she used stability to make statements about the energy diagram that she drew. Thus, the interviewer wanted to know whether, for her, stability implied a claim about energy or whether energy was something that she inferred by considering stability to mean smallest possible positive charge in this case:
Interviewer: Okay. Well, do I understand it right, that the term stability in this case refers to the state of this positive charge […] and the energy again is something I could infer from that? Or does the term stability already describe something about the energy at the same time? If you use it like that. That wasn’t clear to me.
Michelle: Well, by definition it's, like, more about the energy, so I say stable is what is low in energy. But if I look at this substance now, then I don't see the energy diagram in front of me, but I see the positive charge, which I’ve drawn there. And so if I look at the substance, I mean the molecule, then I think about the positive charge, how could the stability be? And I infer the energy. […] In reality, of course, this all correlates. But if you would start with the definition, you would finally say it's stable because it's low in energy. And then you would ask, why is it low in energy? Because the positive charge is stabilized here.
With her utterance, Michelle showcased that she used stability to refer to structure and energy. Thus, stability did not have a clearly defined meaning. While, by Michelle's definition, stability implied information about energy, Michelle claimed that she was only thinking about a structural meaning of stability when looking at the structural representation given in the interview. This indicates that Michelle's structural account, which used typical structural representations of mechanisms, seemed to be disconnected from her energetic account, which used energy diagrams as the representation.
Further evidence of a relatively weak connection between students’ structural and energetic accounts, using the representations from those accounts, was found in the interviews of 8 students. In their structural accounts, these students referred to the positive charge of the carbocation; however, they drew an exothermic energy diagram. Thus, in their energetic accounts, students neglected that a carbocation is a high energy intermediate, while, in sound chemical reasoning, this is the reason to refer to this change. Nina, when reasoning about the structural account, even mentioned that she thinks that a positive charge is always disfavored:
Nina: Actually, this is always unfavorable if… I mean the molecules always seek to not be charged because this is more favorable.
However, later in the interview, when reasoning about the energetic account, she drew the exothermic energy diagram shown in Fig. 11. This observation shows that, when Nina drew the energy diagram, she did not incorporate her reasoning about positive charge, i.e. reasoning about the structural account. This finding adds further detail to the findings of Popova and Bretz (2018) who asked students explicitly to connect structural representations and energy diagrams. Many students in their study connected the occurrence of formal charges with high energy intermediates. By contrast, we did not explicitly ask students to connect the two kinds of representations. The students in our study also reasoned about the positive charge of a carbocation as something unfavorable, nevertheless, many students drew exothermic energy diagrams. This demonstrates that when students were not explicitly asked to connect reasoning about representations of the structural and energetic accounts the connections they formed between the accounts were even weaker.
In summary, students often used stability to connect their structural and energetic accounts. The meaning of stability—a transition word from the structural to the energetic account—was not clearly defined for many students. Some students implied a structural meaning, some an energetic meaning, and some both meanings. Moreover, we found hints for a relatively weak connection between reasoning about the representations of the structural and energetic accounts, i.e. the structural formulas and energy diagrams.
Limitations of the study
We could only analyze what students verbalized, which is not necessarily everything they were reasoning about. For example, a student reasoning about the stabilization of positive charge may or may not have implied an electronic effect. Similarly, a student who verbalized reasoning about effects on the carbocation could have either reasoned about effects on the positive charge or only reasoned about the entity carrying positive charge. To be consistent, we only coded what students actually said.A further limitation is that students’ uncertainty about the relevance of static approaches to change for claims about activation energy could have been provoked by the interviewer's questions about what their statement has to do with activation energy. Some students started doubting the relevance of their thinking before any intervention of the interviewer, but many did so afterward. Regardless of when the students expressed doubt, the fact that they explained it with reference to some sort of dynamics missing in their reasoning demonstrates that the students had a cause for doubt independent of the intervention.
All relations between explicit structural differences and changes that students constructed in our study can be described sufficiently with the three levels of complexity, low, middle, and high. These levels share some differentiating characteristics (explicit vs. implicit and relational vs. cause–effect) with the modes of reasoning Sevian and Talanquer (2014) developed in a broader context. Due to their general structure (Fig. 7), we also argue that the levels are suitable for describing relations between explicit structural differences of molecules and changes occurring in other single mechanistic steps. However, while relations of low complexity found in the present study were always mere correlations between explicit structural differences and changes, we can think of other relations that do not include implicit structural properties but are causal on an explicit level. For example, if one compares a non-bulky and a bulky base in an E2 elimination of a haloalkane, one could claim without reasoning about implicit properties that steric hindrance is higher in the case of the bulky base. The relation between the bulky base and higher steric hindrance is not a cause–effect relation on the electronic level because nothing is said about electronic repulsion, but explanations on the explicit level can serve as a “sort of analogy […] supporting (roughly) causal explanations” (Goodwin, 2008, p. 126). Further research needs to be done including diverse mechanistic steps in order to develop a comprehensive model describing levels of complexity of relations.
Conclusions and implications
By investigating student reasoning in a relatively simple but representative scenario, i.e. a leaving group departure step, we have demonstrated that the mechanistic framework we developed can productively guide analysis of student comparative mechanistic reasoning about single steps of organic reactions. We could use the analysis in this study to extend the mechanistic framework by adding levels of complexity of relations that students constructed.When asked about the activation energy, students who approached change statically seemed to realize a missing causal link. However, only a few students transferred their static approach to charges into dynamic reasoning about charge formation. Many students reasoned about charges in the product of the step and about the departure of the leaving group from the carbon skeleton, but they treated these changes as isolated categories. A static approach to change can be a way to communicate a complex topic more easily. But students can only understand the causal connection between activation energy and effects on static properties, e.g. positive charge of a carbocation, if their reasoning is based on an awareness that these properties form and are affected already during the process of the mechanistic step. Students’ approaches to change found in this study can be combined productively by emphasizing continuous electron flow including charge separation when teaching the process of bond cleavage. This description of the process might help students to reason about positive charge more dynamically, to include formation of negative charge in their reasoning more often, and to connect electron flow with forces occurring between charges.
While a great deal of research in organic chemistry education reveals that student reasoning relies heavily on rote memorization (cf.Graulich, 2015), in our study, we could elicit students’ productive resources regarding the construction of relations. It is important to note that constructing relations with high complexity often involved follow-up questions from the interviewer. If interviews required follow-up questions to differentiate between students who employed relations with high complexity and students who used relations with low complexity, then it is also necessary for educators to require extensive explanations from their students to make this differentiation. Like Stowe and Cooper (2017), who found that most traditional organic chemistry exam questions do not ask students to provide their reasoning for an exam answer, we suggest—based on our findings—that asking for the underlying causal connections of an answer should be a main component of exam questions. In our interviews, follow-up questions used to probe whether students are able to use more complex reasoning could have elicited verbalization of preexisting reasoning that would have otherwise remained hidden during analysis. It is also likely that further reasoning processes were induced by the strategy of the follow-up questions. If students are taught how cause–effect relations are constructed and if they practice identifying and filling in missing causal links, i.e. reasoning that was supported by the interviewer in our study, then students might be able to increase the complexity of their reasoning.
Students' relatively weak connections of their structural and energetic accounts suggest that they need to engage more often in converting every part of their reasoning about the structural account into representations of the energetic account. Students can only connect their structural and energetic accounts if they know which statement implies information about structure and which statement implies information about energy. However, for students in our study, stability implied information about either structure or energy, or both. This indicates that, when teaching mechanistic reasoning in organic chemistry, it might help students to replace stability with the concrete meaning that is intended. Instead of teaching that the positive charge is stabilized, students could be taught that, for a cause–effect relation, the electronic effect on a change must be described within the structural account before a connection to the energetic account can be made.
Our findings have further implications for designing mechanistic tasks. Problems involving contrasting cases were appropriate to elicit student mechanistic reasoning for the purpose of our study. Hence, using contrasting cases in teaching organic chemistry, as proposed by Graulich and Schween (2018), might help elicit students' productive resources and difficulties and foster their mechanistic reasoning. Similar to the findings of Grove et al. (2012a), we found that more complex relations were only more successful than less complex relations if transfer of knowledge was required to solve a mechanistic task. Unfamiliarity in the problem, which necessitated transfer of knowledge, was achieved by using a reactant that does not undergo the represented mechanistic step. If a more complex relation leads to higher success in solving a problem, this could motivate students to construct complex relations more often. Hence, using infeasible mechanistic steps as one option of contrasting cases might foster more complex reasoning. While for advocates of traditional curricula this idea of creating a task might be disconcerting, it could help students to use cause–effect reasoning to differentiate between feasible and infeasible mechanistic steps—a key task in solving traditional and nontraditional mechanism problems.
Because the framework integrates information about the components of a mechanism and about the structure used in mechanistic reasoning to connect the components, it might also be helpful to communicate the framework in an adapted format to students to help them structure their reasoning. For example, the following structure might help students to engage in comparative single-step mechanistic reasoning: (1) compare explicit structural features of the represented molecules, (2) infer implicit structural properties from explicit differences, (3) use implicit structural properties as a cause to reason about an electronic effect on identical changes in the mechanistic steps, and (4) connect this structural account to an energetic account. If students approach change dynamically, they can make a claim about relative activation energies and thus about the feasibility of a molecule undergoing the transformation. Hence, after learning how to use the electron-pushing formalism, learning to use the structure of comparative single-step mechanistic reasoning can be a next step for students to engage in more complex mechanistic reasoning in organic chemistry.
Conflicts of interest
There are no conflicts to declare.Appendix 1. Interview process including follow-up questions
– Warm-up exercise:Depict the electron movement that leads from the reactant to the products in the reaction steps with a curved arrow. Explain the meaning of the arrow.
Purposes of the warm-up exercise:
• Students become familiar with the mechanistic step.
• Students start talking about the step in terms of a less complex task than answering the actual mechanistic question.
• We probed whether all students could apply the electron-pushing formalism and explain that it represents electron movement.
• Students’ answers to the task were not part of our analysis.
– Mechanistic question:
Form a hypothesis about which reaction has lower activation energy for the represented step. Explain your thoughts during the entire process of forming the hypothesis. (It could be the case that, for one of the reaction steps, the activation energy is so high that the reaction step does not happen.)
– Follow-up questions:
(1) Can you demonstrate what you said using a representation?
• Purpose: We wanted to probe students’ connections between their verbalized reasoning and representations.
(2) You said… What does the term… mean in this context?
• Purpose: We wanted to know which meaning students implied when they used specific terms like stability or inductive effect.
(3) You said… leads to… Why is that?
• Purpose: We wanted to examine whether students could construct a more complex relation.
(4) You said… What does this statement have to do with activation energy?
• Purpose: We used this question when students utilized a static approach to change to probe whether they could transform it into a dynamic approach.
Appendix 2. Coding of approaches to change
An approach to change in a student solution was coded in the event that the student used a change for a comparison of the two leaving group departure steps in problems 1 and 2. First, approaches to change were coded to be either static or dynamic. Based on the data, all static approaches to change were grouped into categories and all dynamic approaches were grouped into categories. For this categorization two criteria were used. The first criteria to categorize changes was the nature of the changes. This led to two categories of static approaches to change, i.e. carbocation and anion. These were further divided into subcategories depending on whether the students explicitly referred to the properties positive and negative charge or whether they remained on a more superficial level only verbalizing reasoning about the entities carrying these properties, i.e. carbocation and anion. We also obtained two categories of dynamic approaches to change, i.e. formation of the carbocation and departure of the leaving group. When students compared the leaving group capacity of chloride and bromide, this approach to change was also coded as leaving group departure because it implies consideration of the process rather than consideration of static structure. The dynamic approaches were also further divided into subcategories depending on whether the students included the change of properties in their verbalized reasoning, i.e. formation of positive charge or bond cleavage, or whether they remained on a more superficial level.In the following, we outline examples that show what this coding system meant for our data. Student answers that contained verbalized reasoning about stabilization of positive charge received a code for a static approach to the positive charge. Meanwhile, student answers that contained verbalized reasoning about both stabilization of positive charge and stabilization of positive charge during its formation received codes for a static and a dynamic approach to the positive charge. Giving separate codes for a static and a dynamic approach to change of the same property, i.e. positive charge, faithfully described the data because students always explained effects on the positive charge of the structure of the product before they approached the change dynamically in a second step. Student explanations that bromide is a better leaving group because of better stabilization of the negative charge received two codes, one for the static approach to negative charge and another for the dynamic approach to leaving group departure.
An additive coding procedure was used to analyze whether students included properties of molecules, e.g. charges, in their reasoning or whether they remained on a more superficial level. Therefore “working codes” and “final codes” were given. In the event that a student reasoned about the carbocation in one utterance, the student received the code “static—carbocation without explicitly referring to positive charge” as a “working code.” If the student did not verbalize reasoning about the positive charge of the carbocation later in the transcript, the answer received the code “static—carbocation without explicitly referring to positive charge” as a “final code.” In the event that the student reasoned about the positive charge of the carbocation later in the transcript, the student received the code “static—carbocation with explicitly referring to positive charge” as a “final code.”
Appendix 3. Starting and end points of relations
The following list shows starting and end points of all relations that were found in the data:Problem 1:
– CH3vs. H/carbocation (i.e. starting point/end point)
– C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vs. C
C vs. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/carbocation
O/carbocation
– C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vs. C
O vs. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/carbocation
C/carbocation
– CH3vs. H/leaving group departure
– C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vs. C
C vs. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/leaving group departure
O/leaving group departure
– C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vs. C
O vs. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/leaving group departure
C/leaving group departure
Problem 2:
– t-Bu vs. CH3/carbocation
– t-Bu vs. CH3/leaving group departure
– Cl vs. Br/leaving group departure
– Br vs. Cl/leaving group departure
– Br vs. Cl/anion
Additional explanation for starting points
The difference between carbonyl and alkenyl and the difference between chloride and bromide appear twice in combination with the same end point because they could comprise starting points of two different relations. For example, a student could explain that the delocalization of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond stabilizes the positive charge of the carbocation and that the electron-withdrawing effect of C
C double bond stabilizes the positive charge of the carbocation and that the electron-withdrawing effect of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O destabilizes the positive charge. Similarly, a student could explain that the size of bromide would account for higher leaving group ability of bromide and that the electronegativity of chloride would account for higher leaving group ability of chloride.
O destabilizes the positive charge. Similarly, a student could explain that the size of bromide would account for higher leaving group ability of bromide and that the electronegativity of chloride would account for higher leaving group ability of chloride.
Additional explanation for end points
The changes that were used as end points were coded in the round of coding before coding relations (Appendix 2). Based on the data, the end point “carbocation” included reasoning about the carbocation with and without explicit reference to the positive charge and formation of the carbocation with and without explicit reference to the positive charge. The end point “leaving group departure” included reasoning about the leaving group departure with and without explicit reference to bond cleavage. The end point “anion” included reasoning about the anion with and without explicit reference to the negative charge.Appendix 4. Additive coding procedure leading to the identification of the highest complexity of relations
We wanted to analyze the highest complexity a student was able to achieve for each relation they established between a specific, explicit structural difference and a specific change (Appendix 3). To code the highest complexity, we used an additive coding procedure employing “working codes” and “final codes” as explained in the following. If, in one student utterance, a relation between an explicit structural difference and change was established, we coded this utterance with the respective complexity using a “working code.” Then we moved on to the next utterance and decided whether the next utterance made any contribution to the aforementioned relation so that higher complexity could be coded. This procedure was carried out separately for each relation between an explicit structural difference and a change (Appendix 3) until the end of a student answer to a problem was reached. At the end, the highest complexity for each relation was given as a “final code” for the entire answer to a problem. This additive coding procedure avoided the overwhelming cognitive load otherwise caused by keeping in mind all of the important details of a student's response when coding the highest complexity of each relation in an entire problem solution.Appendix 5. Patterns of students’ approaches to change
Table 3 shows all approaches to change that each student used when solving problems 1 and 2. In it, one can observe several combinations of approaches to change. Some students only approached one change per problem solution, i.e. they utilized a static approach to the carbocation or a dynamic approach to the leaving group departure. As noted in the paper, in the event that students approached the carbocation dynamically, they always combined this with a static approach. Similarly, a static approach to the anion was always combined with a dynamic approach to the leaving group departure. This combination only appeared for problem 2 because the leaving groups differ only in this problem. Moreover, many students combined a static approach to the carbocation with a dynamic approach to the leaving group departure.| a Leon and Marcel utilized the combination of a static approach to the anion and a dynamic approach to the departure of the leaving group (red) as well as the combination of a static approach to the carbocation and a dynamic approach to the leaving group departure (orange). To be consistent in color coding, the cell for the dynamic approach to the leaving group departure should be orange and red. It is displayed in red due to graphical limitations. |
|---|
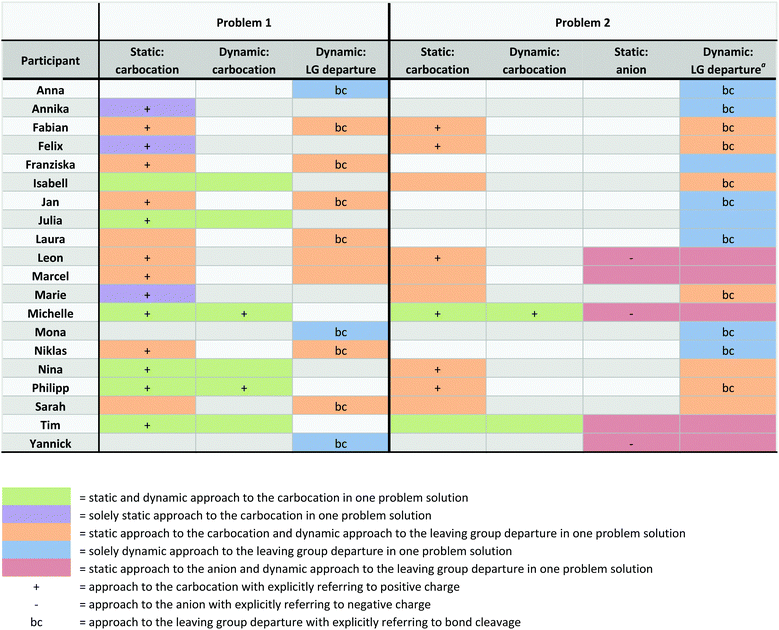
|
Appendix 6. Examples of levels of complexity of relations drawn from problem 2
Table 4 shows two student examples of each level of complexity of relations, i.e. low complexity, middle complexity, and high complexity, drawn from problem 2.Appendix 7. Complexity of relations that each student constructed
Table 5 shows the complexity of relations that each student constructed between explicit structural differences of the two reactants in problems 1 and 2 and changes that occur in the leaving group departure steps.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, Cl, Br, and t-Bu) and a change (labelled as LG for leaving group departure, + for carbocation, and – for anion). A colored cell demonstrates that a student constructed a relation between the explicit structural difference and the change labelled at the top of the column. A cell in light or medium grey shows that a student did not construct a relation between the explicit structural difference and the change labelled at the top of the column
O, Cl, Br, and t-Bu) and a change (labelled as LG for leaving group departure, + for carbocation, and – for anion). A colored cell demonstrates that a student constructed a relation between the explicit structural difference and the change labelled at the top of the column. A cell in light or medium grey shows that a student did not construct a relation between the explicit structural difference and the change labelled at the top of the column
Acknowledgements
This publication represents a component of the first author's doctoral (Dr. rer. nat.) thesis in the Faculty of Biology and Chemistry at the Justus-Liebig-University Giessen, Germany. We thank all students who participated in the study. The authors are grateful to Jessica Karch for her help with translating students’ utterances from German to English. We thank the Graulich research group, especially Leonie Lieber, for fruitful discussions about the research. The authors are grateful to Andrew Gnann for refining wording of this publication.Notes and references
- Airey J. and Linder C., (2009), A Disciplinary Discourse Perspective on University Science Learning: Achieving Fluency in a Critical Constellation of Modes, J. Res. Sci. Teach., 46, 27–49.
- Alfieri L., Nokes-Malach T. J. and Schunn C. D., (2013), Learning Through Case Comparisons: A Meta-Analytic Review, Educ. Psychol., 48, 87–113.
- Anzovino M. E. and Bretz S. L., (2015), Organic chemistry students' ideas about nucleophiles and electrophiles: the role of charges and mechanisms, Chem. Educ. Res. Pract., 16, 797–810.
- Anzovino M. E. and Bretz S. L., (2016), Organic chemistry students' fragmented ideas about the structure and function of nucleophiles and electrophiles: a concept map analysis, Chem. Educ. Res. Pract., 17, 1019–1029.
- Bechtel W. and Abrahamsen A., (2005), Explanation: a mechanist alternative, Stud. Hist. Philos. Biol. Biomed. Sci., 36, 421–441.
- Becker N. M. and Cooper M. M., (2014), College Chemistry Students' Understanding of Potential Energy in the Context of Atomic-Molecular Interactions, J. Res. Sci. Teach., 51, 789–808.
- Becker N., Noyes K. and Cooper M., (2016), Characterizing Students’ Mechanistic Reasoning about London Dispersion Forces, J. Chem. Educ., 93, 1713–1724.
- Bernholt S. and Parchmann I., (2011), Assessing the complexity of students' knowledge in chemistry, Chem. Educ. Res. Pract., 12, 167–173.
- Bhattacharyya G., (2013), From Source to Sink: Mechanistic Reasoning Using the Electron-Pushing Formalism, J. Chem. Educ., 90, 1282–1289.
- Bhattacharyya G. and Bodner G. M., (2005), “It Gets Me to the Product”: How Students Propose Organic Mechanisms, J. Chem. Educ., 82, 1402–1407.
- Biggs J. B. and Collis K. F., (1982), Evaluating the quality of learning: the SOLO taxonomy (Structure of the Observed Learning Outcome), New York: Academic Press.
- Bolger M. S., Kobiela M., Weinberg P. J. and Lehrer R., (2012), Children's Mechanistic Reasoning, Cognit. Instruct., 30, 170–206.
- Bransford J. D. and Schwartz D. L., (1999), Rethinking Transfer: A Simple Proposal With Multiple Implications, Rev. Res. Educ., 24, 61–100.
- Brown N. J. S., Nagashima S. O., Fu A., Timms M. and Wilson M., (2010), A Framework for Analyzing Scientific Reasoning in Assessments, Educ. Assess., 15, 142–174.
- Caspari I., Weinrich M. L., Sevian H. and Graulich N., (2018), This mechanistic step is “productive”: organic chemistry students' backward-oriented reasoning, Chem. Educ. Res. Pract., 19, 42–59.
- Cooper M. and Klymkowsky M., (2013), Chemistry, Life, the Universe, and Everything: A New Approach to General Chemistry, and a Model for Curriculum Reform, J. Chem. Educ., 90, 1116–1122.
- Corbin J. and Strauss A., (2015), Basics of Qualatative Research: Techniques and Procedures for Developing Grounded Theory, Los Angeles: Sage.
- DeFever R. S., Bruce H. and Bhattacharyya G., (2015), Mental Rolodexing: Senior Chemistry Majors’ Understanding of Chemical and Physical Properties, J. Chem. Educ., 92, 415–426.
- Ferguson R. and Bodner G. M., (2008), Making sense of the arrow-pushing formalism among chemistry majors enrolled in organic chemistry, Chem. Educ. Res. Pract., 9, 102–113.
- Fleming I., (2010), Molecular Orbitals and Organic Chemical Reactions, Chichester: John Wiley & Sons.
- Flynn A. B. and Featherstone R. B., (2017), Language of mechanisms: exam analysis reveals students' strengths, strategies, and errors when using the electron-pushing formalism (curved arrows) in new reactions, Chem. Educ. Res. Pract., 18, 64–77.
- Flynn A. B. and Ogilvie W. W., (2015), Mechanisms before Reactions: A Mechanistic Approach to the Organic Chemistry Curriculum Based on Patterns of Electron Flow, J. Chem. Educ., 92, 803–810.
- Galloway K. R., Stoyanovich C. and Flynn A. B., (2017), Students’ interpretations of mechanistic language in organic chemistry before learning reactions, Chem. Educ. Res. Pract., 18, 353–374.
- Glennan S., (2002), Rethinking Mechanistic Explanation, Philos. Sci., 69, S342–S353.
- Goodwin W. M., (2003), Explanation in Organic Chemistry, Ann. NY Acad. Sci., 988, 141–153.
- Goodwin W. M., (2008), Structural formulas and explanation in organic chemistry, Found. Chem., 10, 117–127.
- Graulich N., (2015), The tip of the iceberg in organic chemistry classes: how do students deal with the invisible? Chem. Educ. Res. Pract., 16, 9–21.
- Graulich N. and Bhattacharyya G., (2017), Investigating students' similarity judgments in organic chemistry, Chem. Educ. Res. Pract., 18, 774–784.
- Graulich N. and Schween M., (2018), Concept-Oriented Task Design: Making Purposeful Case Comparisons in Organic Chemistry, J. Chem. Educ., 95, 376–383.
- Grotzer T. A., (2003), Learning to Understand the Forms of Causality Implicit in Scientifically Accepted Explanations, Stud. Sci. Educ., 39, 1–74.
- Grove N. P., Cooper M. M. and Cox E. L., (2012a), Does Mechanistic Thinking Improve Student Success in Organic Chemistry? J. Chem. Educ., 89, 850–853.
- Grove N. P., Cooper M. M. and Rush K. M., (2012b), Decorating with Arrows: Toward the Development of Representational Competence in Organic Chemistry, J. Chem. Educ., 89, 844–849.
- Illari P. M. and Williamson J., (2011), What is a mechanism? Thinking about mechanisms across the sciences, Euro. Jnl. Phil. Sci., 2, 119–135.
- Kraft A., Strickland A. M. and Bhattacharyya G., (2010), Reasonable reasoning: multi-variate problem-solving in organic chemistry, Chem. Educ. Res. Pract., 11, 281–292.
- Liu K. T., Hou S. J. and Tsao M. L., (1998), B-Strain and Solvolytic Reactivity Revisited. Nucleophilic Solvent Participation and Abnormal Rate Ratios for Tertiary Chloroalkanes, J. Org. Chem., 63, 1360–1362.
- Liu K. T., Hou S. J. and Tsao M. L., (2009), Nucleophilic Solvent Participation in the Solvolysis of Tertiary Bromoalkanes, J. Chin. Chem. Soc., 56, 425–430.
- Machamer P., Darden L. and Craver C. F., (2000), Thinking about Mechanisms, Philos. Sci., 67, 1–25.
- Moon A., Stanford C., Cole R. and Towns M., (2016), The nature of students' chemical reasoning employed in scientific argumentation in physical chemistry, Chem. Educ. Res. Pract., 17, 353–364.
- Popova M. and Bretz S. L., (2018), Organic chemistry students’ challenges with coherence formation between reactions and reaction coordinate diagrams, Chem. Educ. Res. Pract., 10.1039/c8rp00064f.
- Ramalho R., Adams P., Huggard P. and Hoare K., (2015), Literature Review and Constructivist Grounded Theory Methodology, Forum Qualitative Sozialforschung/Forum: Qualitative Social Research, 16, 19.
- Russ R. S., Scherr R. E., Hammer D. and Mikeska J., (2008), Recognizing Mechanistic Reasoning in Student Scientific Inquiry: A Framework for Discourse Analysis Developed From Philosophy of Science, Sci. Educ., 92, 499–525.
- Russ R. S., Lee V. R. and Sherin B. L., (2012), Framing in Cognitive Clinical Interviews About Intuitive Science Knowledge: Dynamic Student Understandings of the Discourse Interaction, Sci. Educ., 96, 573–599.
- Saldaña J., (2016), The Coding Manual for Qualitative Researchers, Los Angeles: Sage.
- Sevian H. and Talanquer V., (2014), Rethinking chemistry: a learning progression on chemical thinking, Chem. Educ. Res. Pract., 15, 10–23.
- Smith M. B., (2013), March's Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, Hoboken: John Wiley & Sons.
- Southard K., Wince T., Meddleton S. and Bolger M. S., (2016), Features of Knowledge Building in Biology: Understanding Undergraduate Students' Ideas about Molecular Mechanisms, CBE Life Sci. Educ., 15, 1–16.
- Stowe R. L. and Cooper M. M., (2017), Practicing What We Preach: Assessing “Critical Thinking” in Organic Chemistry, J. Chem. Educ., 94, 1852–1859.
- Taber K. S., (2003), Understanding ionisation energy: physical, chemical and alternative conceptions, Chem. Educ. Res. Pract., 4, 149–169.
- Taber K. S., (2009), College Students’ Conceptions of Chemical Stability: the widespread adoption of a heuristic rule out of context and beyond its range of application, Int. J. Sci. Educ., 31, 1333–1358.
- Talanquer V., (2013), When Atoms Want, J. Chem. Educ., 90, 1419–1424.
- Talanquer V. and Pollard J., (2010), Let's teach how we think instead of what we know, Chem. Educ. Res. Pract., 11, 74–83.
- van Mil M. H. W., Boerwinkel D. J. and Waarlo A. J., (2013), Modelling Molecular Mechanisms: A Framework of Scientific Reasoning to Construct Molecular-Level Explanations for Cellular Behaviour, Sci. Educ., 22, 93–118.
- van Mil M. H. W., Postma P. A., Boerwinkel D. J., Klaassen K. and Waarlo A. J., (2016), Molecular Mechanistic Reasoning: Toward Bridging the Gap Between the Molecular and Cellular Levels in Life Science Education, Sci. Educ., 100, 517–585.
- Weinrich M. L. and Sevian H., (2017), Capturing students’ abstraction while solving organic reaction mechanism problems across a semester, Chem. Educ. Res. Pract., 18, 169–190.
- Weinrich M. L. and Talanquer V., (2015), Mapping students' conceptual modes when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 16, 561–577.
- Weinrich M. L. and Talanquer V., (2016), Mapping students' modes of reasoning when thinking about chemical reactions used to make a desired product, Chem. Educ. Res. Pract., 17, 394–406.
Footnotes |
| † Goodwin used unusual punctuation within these questions to showcase the function of different parts of the questions. Due to the fact that we do not mention these functions, adopting unusual punctuation would be distracting for the reader. That is why we did not exactly adopt the original punctuation. |
| ‡ At the German university where the research was conducted, no further approval, e.g. by an Institutional Review Board (IRB), was required. One student did not consent to the use of her drawings for publication. Hence, her drawings are not used. |
| This journal is © The Royal Society of Chemistry 2018 |