Open Access Article
Open Access ArticleDevelopment and use of a construct map framework to support teaching and assessment of noncovalent interactions in a biochemical context
Jennifer
Loertscher
*a,
Jennifer E.
Lewis
 b,
Allison M.
Mercer
b,
Allison M.
Mercer
 b and
Vicky
Minderhout
a
b and
Vicky
Minderhout
a
aDepartment of Chemistry, Seattle University, 901 12th Ave, Seattle, WA 98122, USA. E-mail: loertscher@seattleu.edu; vicky@seattleu.edu
bDepartment of Chemistry, Center for Improvement of Teaching and Research on Undergraduate STEM, University of South Florida, Tampa, FL 33620, USA. E-mail: jennifer@usf.edu; allisonm@mail.usf.edu
First published on 2nd July 2018
Abstract
Most chemistry educators agree that deep understanding of the nature of noncovalent interactions is essential for learning in chemistry. Yet decades of research have shown that students have persistent incorrect ideas about these interactions. We have worked in collaboration with a community of chemistry, biology, and biochemistry educators to develop a construct map to guide development of instructional and assessment resources related to the physical basis of noncovalent interactions in a biochemical context. This map was devised using data about student learning and expert perspectives on noncovalent interactions, resulting in a framework that provides a detailed roadmap for teaching and learning related to this essential concept. Here we describe the development of the construct map and our use of it to reform our biochemistry teaching practice. Because biochemistry relies on application of concepts learned in prerequisite courses, this construct map could be useful for wide range of courses including general chemistry, introductory biology, organic chemistry, and biochemistry.
Introduction
As an advanced interdisciplinary course, learning in biochemistry demands that students integrate knowledge from previous coursework and apply it in a biological context. Some ideas, like noncovalent interactions, recur throughout the post-secondary curriculum and are therefore, especially important and challenging for biochemistry educators to consider. Furthermore, meaningful integration of noncovalent interactions into biochemistry teaching and learning is complicated by the students’ incorrect and incomplete ideas. In an attempt to address these challenges, we engaged in a community-based process using educator knowledge to design a tool to support improved instruction of noncovalent interactions in a biochemical context.Educator knowledge
Educators have a specialized knowledge base that is rooted in the discipline being taught, but also incorporates knowledge of instructional practices and student learning development. This is known as pedagogical content knowledge (PCK) or, more recently, topic-specific professional knowledge (TSPK) (Gess-Newsome, 2015). Researchers who study this specialized knowledge believe that disciplinary knowledge does not have an inherent structure, but rather “its structures [depend] upon how one [organizes] the discipline for both inquiry and instruction.” (Shulman, 2015). Teaching of disciplinary content is context-specific and instructional planning depends on many factors including student level, student prerequisite knowledge, and intended learning outcomes. Although PCK was historically considered to be personal knowledge developed by individual practitioners, TSPK is codified by communities of experts and is accessible to the broader teaching community (Gess-Newsome, 2015). TSPK can be developed by educators working together to identify big ideas associated with a topic and subsequently developing instructional practices to support students’ understanding at a given level. Post-secondary educators often receive little formal teacher training and have limited access to teacher professional development. Given these circumstances, it is critical to generate resources for post-secondary educators that define topic-specific structures intended for use in particular instructional contexts.Threshold concepts as a means to improve teaching and learning
Threshold concepts research is a well-characterized method to engage disciplinary experts, educators, and students in conversations that can result in deepening a shared understanding of teaching and learning within a discipline (Cousin, 2008). Threshold concepts are ideas or skills within a discipline that are particularly important for learning. Distinct from core concepts, which can be seen as ‘building blocks’ of a discipline, mastery of threshold concepts shifts the perspective of the learner and leads to a transformed and integrative understanding of a discipline. Meyer and Land, the originators of this educational framework, posit that threshold concepts can be identified for any discipline using their methods (Meyer and Land, 2003). In fact, the physical basis of noncovalent interactions was recently identified as a threshold concept for biochemistry (Loertscher et al., 2014). The primary purpose in identifying threshold concepts is to provide a starting point for focused curricular redesign, since an intentional approach to teaching these ideas is likely to result in the greatest improvement in student learning (Entwistle, 2008; Perkins, 2008). The process of working collectively to identify threshold concepts empowers educators to draw on both their disciplinary expert knowledge and their experience of teaching, with the ultimate goal of improving student learning (Cousin, 2009). As a result, educators become action researchers in their own classrooms and many begin to develop a mindset of continual improvement in their teaching practice (Cousin, 2008).Student understanding of noncovalent interactions across the chemistry curriculum
Students' understandings (and misunderstandings) of noncovalent interactions are well documented in the education literature and common incorrect ideas have been characterized. For example, it is known that some students believe that hydrogen bonds can be induced, that noncovalent interactions lead to reactions, and that boiling breaks covalent bonds (Henderleiter et al., 2001; Schmidt et al., 2009). Noncovalent interactions and covalent bonds are commonly confused by beginning students (Peterson et al., 1989). When students' understanding is probed more deeply through analysis of drawings, the extent of their confusion can be more fully grasped. In a multimodal study, students’ written descriptions of intermolecular forces (hydrogen bonding, London dispersion forces, and dipole–dipole interactions) were often correct memorized definitions, but the majority (55%) of the same students pictorially represented noncovalent interactions as being within molecules. Fully 59% were inconsistent in their representation of noncovalent interactions being within/between molecules. Specifically, in the case of hydrogen bonds, 72% of the students’ drawings depicted a single molecule of ethanol and indicated the “O–H” as the hydrogen bond (Cooper et al., 2015). These incorrect understandings appear far more prevalent than previously reported, likely due to richer information gathered through drawings.Similarly, while a majority of organic chemistry students interviewed about hydrogen bonding gave a reasonable definition, further probing revealed a reliance on memorized patterns and an inability to explain how they know hydrogen bonding occurs (Henderleiter et al., 2001). In another study, incoming biochemistry students were given a pretest before instruction and consistently scored under 31% on all hydrogen bonding items, suggesting students do not have a full grasp on the concept from prerequisite courses (Xu et al., 2017). In a study focusing on student understanding of London dispersion forces, students were prompted to explain why two helium atoms would attract, but the majority of responses did not provide “causal mechanistic” reasoning in either written explanation or drawings (Becker et al., 2016). Further, more students depicted atom–dipole interactions in drawings than in written explanations, which may be due to a familiarity with the representation but an inability to understand or explain it (Becker et al., 2016). Yet another study revealed that the processes students use to predict physical properties based on molecular structure are complex and different for each individual (Cooper et al., 2013). Although an exhaustive catalog of alternative conceptions exists, it has so far not been helpful to improve student understanding (Pfundt and Duit, 1988). Overall, continued findings of student confusion regarding bonding and noncovalent interactions suggest a need to move beyond simply identifying misconceptions in order to support the multifaceted, developmental reality of learning.
Taken together, these studies show that students tend to rely on memorized definitions of noncovalent interactions as opposed to understanding the physical phenomena that underlie all of these interactions. This reliance could be due in part to the representations and language used by books and educators. For example, some books refer to hydrogen bonds and other noncovalent interactions as “just forces” that are distinct from “chemical bonds” (Taber, 2002). Given such statements, it is unsurprising that students fail to recognize common features in atomic/molecular interactions, including the electrostatic force described by Coulomb's law. In response to these difficulties, innovative secondary and post-secondary chemistry curricula have been designed to strengthen students’ understanding of the fundamental principles that underlie chemical bonding. One group developed a “bottom-up” approach in which chemical bonding is taught using a unified progression, which starts with atoms and culminates with chemical properties. In this model, noncovalent interactions are depicted as being on a continuum with other bonds, which range in strength from ionic bonds to van der Waals bonds (Kronik et al., 2008). Another group designed a curriculum based on the relationship among structure, energy, and properties in chemistry. In this case, intermolecular forces are taught in the context of energy and put into context with molecular structure and properties (Cooper and Klymkowsky, 2013).
Biochemistry educators may believe that students enter their courses with a firm grasp of noncovalent interactions because this concept is covered in prerequisite courses including secondary level chemistry as well as general chemistry, introductory biology, and organic chemistry at the post-secondary level. Yet, the literature summarized above clearly shows that this is not the case. Understanding this knowledge gap is paramount for teaching biochemistry, as noncovalent interactions direct a wide range of important biochemical phenomena including ligand binding (Sears et al., 2007), enzyme–substrate interactions (Bretz and Linenberger, 2012), and macromolecular structure formation (Tansey et al., 2013). Therefore, it was not surprising that the physical basis of noncovalent interactions emerged as a threshold concept for biochemistry (Table 1) (Loertscher et al., 2014). Interviews of post-secondary students, conducted as part of the process to identify threshold concepts, revealed a superficial understanding of noncovalent interactions, which was in stark contrast to the way experts understand this concept (Loertscher et al., 2014). Whereas many students rely on memorized rules and view the interaction types as distinct, experts possess deep tacit knowledge of the electrostatic force, described by Coulomb's law, which underpins all noncovalent interactions (Hunter, 2004; Bissantz et al., 2010). In addition, experts have a nuanced understanding of the ways in which molecular characteristics and local environment influence the strength of noncovalent interactions. Therefore, although intermolecular forces are introduced in courses that precede biochemistry, in order to make predictions about noncovalent interactions in the complex macromolecular environment of the cell, students must move beyond a memorized notion of noncovalent interactions to a deep understanding of the underlying electrostatic force and its components of charge and distance. Furthermore, through interviews conducted to identify threshold concepts, we discovered that educators and students have a false sense that students understand noncovalent interactions and that commonly used terms like “intermolecular forces” can mask incorrect or incomplete ideas (data not shown). Consequently, we chose the deliberately unfamiliar term “physical basis of interactions” to refer to this threshold concept, in an attempt to push students and educators to critically evaluate their learning and teaching of this concept (Green et al., 2017).
| Name | Knowledge statement | Biochemical ideas that are unlocked once this concept is understood | Connections that were invisible prior to deep understanding of the concept |
|---|---|---|---|
| The physical basis of interactions | Interactions occur because of the electrostatic properties of molecules. These properties can involve full, partial, and/or momentary charges. | Once this concept is understood, similarities between different types of interactions become clear. Although interactions are given different names, they are all based on the same electrostatic principles. | A core biochemical principle is that structure governs function. Correct understanding of noncovalent interactions is essential in integrating structure and function. |
Construct maps
In order to improve learning and teaching related to noncovalent interactions in undergraduate biochemistry courses, we sought an instructional and assessment tool to provide biochemistry educators insight into those aspects of the concept that students do not understand. Evidence suggests that students' understanding of noncovalent interactions develops throughout the post-secondary biology, chemistry, and biochemistry curriculum. Therefore, construct maps, which outline learning as a developmental process, provide a good starting point for development of formative and diagnostic assessments, reevaluation of curriculum, and design of targeted instructional interventions to enhance student learning. Construct maps are commonly used in primary and secondary education, for example as the first building block in the Berkeley Evaluation and Assessment Research (BEAR) Assessment System (Wilson, 2009). Construct maps contain levels with descriptive statements corresponding to distinct developmental learning stages related to a given concept. Evidence for construct maps is based primarily on topic-specific pedagogical content knowledge (educators' experience of how students learn a given concept) and empirical evidence of student performance (National Research Council, 2001; Wilson, 2009). A useful construct map is specific enough to direct development of relevant assessment and instructional tools, yet general enough to be useful in diverse curricular contexts.Much of the literature surrounding construct maps is closely linked to learning progressions. Often, learning progressions are seen as either descriptive (observed progression of student learning) or prescriptive (idealized model for teachers to follow) (Taber, 2017). Like a construct map, a learning progression organizes the content of a discipline into increasing order of complexity. As students advance along the progression, their understanding of the subject becomes more like expert understanding. One important caveat is that a truly complete learning progression is not necessarily linear; it can be composed of constellations of ideas, culminating in a comprehensive understanding of the discipline. A typical learning progression contains one or several construct maps at its core, but goes beyond the map to include explicit connections among concepts to promote knowledge integration as well as explicit links from content to models and examples to support targeted instruction. Therefore, a fully developed learning progression contains a collection of potential instructional strategies to move students from one level to the next (Stevens et al., 2009). Indeed, it is possible to develop a construct map, or a set of construct maps, into a full-fledged learning progression, for example, the construct map “Melting, Evaporation, Boiling, and Sublimation” is one dimension of the learning progression “Changes in State” (Wilson, 2009; Black et al., 2011).
While the learning progressions literature has influenced our work, our tool is a construct map at this time because connections between the provided levels are sometimes implied and the map is limited to noncovalent interactions, with no guides to help integrate with other related topics such as acid/base equilibria. The physical basis of noncovalent interactions construct map organizes and integrates interdisciplinary learning milestones generally and linearly to coincide with a common biochemistry teaching trajectory. As such, contributions from physical chemistry are not included, but contributions from organic chemistry are, such as the interpretation and utility of pKa. Our construct map is the necessary foundation for developing a learning progression that integrates the physical basis of interactions into the broader context of biochemistry.
Using a construct map to inform teaching practice in biochemistry
In this work, we addressed the research question: can a community of educators use their collective knowledge and experience of teaching noncovalent interactions to develop a tool that can be used to guide teaching and assessment in post-secondary biochemistry? As a result of this investigation we present a detailed construct map that was designed and refined by a working group of biology, chemistry, and biochemistry educators and therefore contributes to the professional knowledge base of the biochemistry education community. The literature reviewed above shows that post-secondary students have a poor understanding of the underlying principles that govern noncovalent interactions, yet biochemistry educators may be unaware of these gaps in prerequisite knowledge. The construct map was designed with the recognition that effective teaching practices are context-specific and that biochemistry educators could benefit from a framework that summarizes expected prerequisite knowledge related to noncovalent interactions and defines aspects of learning about this concept that are uniquely biochemical. Specifically, the construct map could be a useful tool to uncover student knowledge gaps using formative or summative assessments, guide the biochemistry-specific applications of noncovalent interactions, and support critical reflection on biochemistry teaching practice. In order to demonstrate how the construct map can inform instruction, we describe examples of how it illuminated and changed our teaching practice in an undergraduate biochemistry course. Our intent is that the existence of the construct map will empower educators to develop and share curricular materials that are useful for promoting student growth in understanding of noncovalent interactions in different curricular contexts in the biochemistry sequence.Methods
Project context and faculty participants
This work is part of a National Science Foundation funded project to improve student understanding of threshold concepts in biochemistry. Since 2007, we have worked to cultivate a community of over 100 biology, chemistry, and biochemistry college and university educators who are engaged in improving teaching and learning in the molecular life sciences. From this community, a total of 39 people attended one or both of two, three-day workshops to develop instructional and assessment materials related to threshold concepts held in summer 2015 and 2016. As part of the workshops, participants learned about threshold concepts and construct maps, and participated in a process to critique and refine a draft construct map describing the physical basis of noncovalent interactions (process described in detail below). Workshop participants included 35 professors, two graduate students (including co-author Mercer), and two postdoctoral fellows. In addition to workshop participants, an additional biochemistry education expert, two biology education experts, three of the authors (Lewis, Loertscher, and Minderhout), and two undergraduate student researchers contributed to the process of writing and revising the construct map described here. In total, those involved in this process represent diverse institutional contexts including two-year colleges (7%), small four-year colleges (24%), master's-level universities (27%), and large research universities (42%). Gender distribution was reasonable with 42% of participants being men and 58% being women.Construct map refinement process
A construct map describing students’ understanding of the physical basis of noncovalent interactions was drafted using the process for designing construct maps described by Briggs et al. (2006). Several data sources were used to define targeted learning outcomes at different points in chemistry/biochemistry curricula. First, participants at the 2015 summer workshop were asked, “What are the targeted student learning outcomes related to this concept in this course?” Responses, summarized in Appendix 1, were collected for six general chemistry courses and 17 biochemistry courses, and were used to draft the initial construct map. Statements included in the draft construct map were further refined based on the authors’ personal experience teaching general chemistry and biochemistry and on published research and curricular guidelines (Holme and Murphy, 2012; Raker et al., 2013; Tansey et al., 2013; Loertscher et al., 2014).The draft construct map was refined by participants of the 2016 summer workshop. Over half of the participants had attended previous workshops and were familiar with the physical basis of noncovalent interactions as a threshold concept. Prior to beginning the revision process, participants learned about construct maps through reading, presentations, and a small-group activity prompting exploration of an existing construct map. Participants were then divided into groups of four to complete an activity aimed at evaluating and refining the draft construct map that had been prepared by the authors prior to the workshop (complete activity can be found in Appendix 2). Twelve participants worked intensively on this process and the remaining 13 provided feedback on their work.
Revisions and comments generated at the workshop were compiled by the authors and an updated construct map draft was generated. This revision was sent to workshop participants with the following questions:
Would you have time to look at the construct map and provide feedback especially with regard to the following?
• Regardless of levels, are statements correct and relevant? If not, please explain.
• Do the levels and characteristics (not the descriptions) make sense and seem appropriate? If not, please explain.
• Are all of the statements in the description distinct from all others? If not, please highlight overlap.
• Do the descriptions align with the levels? If not, what do you suggest?
• Any other feedback?
Detailed responses were received from four workshop participants and the construct map was further revised. Feedback from these experts helped sharpen language and determine whether concepts assigned to given levels were appropriately placed and distinct. Experts also verified that concepts described were taught in the courses identified. Finally, feedback from one of the experts provided language that distinguished expert from student understanding.
The revised construct map was sent to one additional biochemistry expert who had not participated in the workshop and to two biologists who teach introductory biology, since the first four respondents were chemists or biochemists. Feedback from these experts was used to inform another set of changes. Specifically, biology experts verified that noncovalent interactions are taught in introductory biology courses and that many of the statements in the first two levels of the construct map align with concepts covered as part of biology instruction. This step verified that, although noncovalent interactions comprise a fundamental chemistry concept, the concept is also taught in biology courses.
The final step in developing the construct map was to check the utility of the map for guidance regarding the development of curricular materials. Two of the authors (Loertscher and Minderhout), who teach both general chemistry and biochemistry, used the construct map to evaluate several of their existing learning activities and assessments. This field-testing uncovered the need for additional clarifications within some of the levels. Specifically, we realized that several statements were either too general or too complex to be used to generate appropriately targeted assessment questions. For example, additional statements were added to level 4 to concretely describe how students consider the distance term of Coulomb's law within the context of biological macromolecules. The final version is shown in Table 2.
| Level | Characteristics | Description |
|---|---|---|
| 5 | Recognition that although generalizations are often useful, high level distinctions between interaction types also play a role | Experts have an internalized understanding of Coulomb's law. It may be so internalized that they do not realize how and when they use it. Experts know: |
| (a) There is only one electrostatic force, described by Coulomb's law, but there are many specific ways in which interactions can occur within and between molecules, atoms, and ions. | ||
| (b) That relative strengths of noncovalent interactions lie on a continuum and that the strengths of named interactions (ion–ion, dipole–dipole, etc.) may overlap. | ||
| (c) The charge term in Coulomb's law can involve full, partial, and/or momentary charges due to changes in electron distribution and the local environment surrounding charges. | ||
| (d) The distance and charge terms in Coulomb's law are always changing due to the dynamic nature of molecule structure and the fluctuating nature of charge. | ||
| (e) Fluctuations in charge and distance result in fluctuating electrostatic forces and affect electrostatic interactions. | ||
| (f) That quantum mechanical models may be invoked to explain some phenomena related to structure and catalysis. | ||
| 4 | Specific environment in folded proteins, active sites, or binding sites influences noncovalent interactions | Students know |
| (a) that noncovalent interactions influence the folding and conformational stability of proteins. | ||
| (b) that charged/polar amino acids in folded proteins interact with charged/polar entities within or outside a protein. | ||
| (c) that noncovalent interactions influence the binding of substrates and other molecules with enzymes. | ||
| (d) that noncovalent interactions influence the effectiveness of enzyme catalysis. | ||
| Students are able to | ||
| (e) use charge and distance (both terms in Coulomb's law) to predict strength of noncovalent interactions. | ||
| (f) consider 3D spatial arrangement and microenvironment when evaluating strength of noncovalent interactions between biological molecules. | ||
| (g) Consider the 3D special arrangement in enzyme active sties and how that might affect substrate binding and catalysis. | ||
| 3 | Aqueous environment influences noncovalent interactions | Students know |
| (a) that pKa determines the charge of a functional group at a given pH and solvent environment. | ||
| Students are able to | ||
| (b) use magnitude of charge to predict strength of noncovalent interactions. | ||
| (c) predict how a change in amino acid sequence may affect noncovalent interactions. | ||
| (d) predict how a change in pH will produce a change in the charge of an ionizable group. | ||
| (e) consider the equilibrium distribution of the charges of an ionizable group at or around the pKa (i.e. Some molecules are charged and others are not) in making predictions about the effects of charge. | ||
| 2 | Beginning to make generalizations about noncovalent interactions | Students know |
| (a) that noncovalent interactions occur because of the electrostatic properties of molecules. These properties can involve full, partial, and/or momentary charges. | ||
| (b) that noncovalent interactions dictate states of matter depending on the temperature and pressure. | ||
| (c) that pKa is a property of the acid and conjugate base in water and is an indicator of acid strength. | ||
| (d) that solvent interacts with solutes and that alteration of the solvent environment can influence the strength of noncovalent interactions. | ||
| (e) that steric effects influence molecular interactions. | ||
| Students are able to | ||
| (f) define noncovalent interactions, distinguish among them, and rank their relative strength. | ||
| 1 | Memorized understanding of noncovalent interactions | Students know |
| (a) the names of the noncovalent interactions. | ||
| (b) memorized definitions of noncovalent interactions and view them as distinct. | ||
| (c) that hydrogen bonding is very important in biological systems. | ||
| (d) a memorized relationship between boiling point and molecular weight. | ||
| Students are able to | ||
| (e) identify hydrogen bonding as a stronger interaction than other dipole–dipole interactions or London dispersion forces in molecules of comparable size. | ||
Course context for instructional innovations derived from construct map
The completed construct map was used by two of the authors (Loertscher and Minderhout) to evaluate their teaching practice in a one-term (10 weeks) biochemistry course. These authors have a combined 40 years of experience teaching biochemistry at the post-secondary level. The course is taught 2–3 times each academic year at a medium-sized, master's granting university in the United States. Students are typically in their junior or senior year and are most are pursuing majors in biochemistry or cell and molecular biology. Typical enrollment is 20–35 students and the course is taught using process-oriented guided inquiry learning (POGIL) (Minderhout and Loertscher, 2007). General chemistry, organic chemistry, and introductory biology are required prerequisites. To undertake this part of the project, we systematically examined whether the existing instructional and assessment practices in our biochemistry course aligned with the construct map at the appropriate levels. We began with a comparison of our existing learning objectives to the map, seeking to uncover unexpressed assumptions about knowledge and use of the physical basis of interactions and to make our objectives more explicit in this regard. Subsequently, we examined our teaching practices in light of the revised objectives and planned modifications for specific lessons. Finally, we mapped existing assessment items onto specific portions of the construct map.Results and discussion
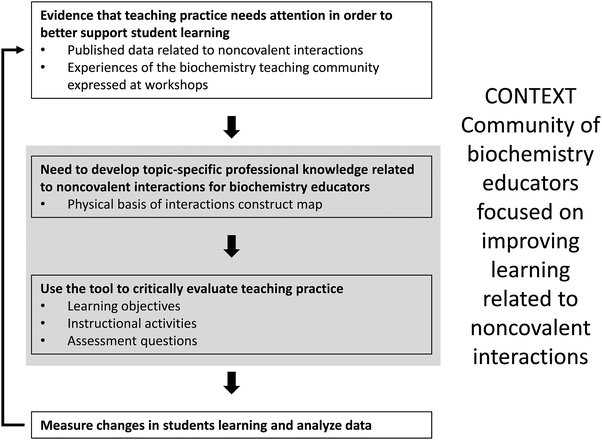
In collaboration with a community of chemistry, biology, and biochemistry educators, we have developed a construct map of student understanding to guide development of instructional and assessment resources related to the physical basis of noncovalent interactions in a biochemical context (Table 2). Fig. 1 summarizes how engaging in a community conversation about teaching noncovalent interactions in biochemistry led to the development of the construct map and subsequent use of it to critically reflect on our own teaching. The information in the gray box is the focus of the work described here, but this work, as shown in the figure, fits into a larger process, the ultimate goal of which is to improve student learning of noncovalent interactions. Development of the construct map was motivated by a desire in the biochemistry teaching community to critically evaluate and improve teaching practice related to a biochemical threshold concept – the physical basis of interactions.Physical basis of noncovalent interactions: levels of understanding
The levels (1–5) of the construct map describe targeted student learning outcomes at different points in chemistry, biology, and biochemistry curricula. As described below, the construct map levels may align with different instructional levels (for example upper secondary versus introductory post-secondary) depending on institutional context. In making this construct map, it was assumed that students exhibiting a given level of understanding are also proficient with stated outcomes at lower levels. Level 5 is considered expert understanding and may not be attained by undergraduate students. Therefore levels 1–4 should be the primary focus when using the construct map to inform instructional or assessment design for secondary and post-secondary courses. A detailed description of each level is given below.Level 1 describes a basic understanding of noncovalent interactions. Some students may attain this level of understanding in upper secondary level chemistry, but many, especially in the United States, will first attain this level in post-secondary general chemistry and introductory biology. Some educators attempt to frame their instruction on noncovalent interactions within the context of the electrostatic force described by Coulomb's law (Kronik et al., 2008). Yet students’ understanding at this level is often characterized by memorized definitions of noncovalent interactions, including dipole–dipole interactions, hydrogen bonding, London dispersion interactions, and ion–dipole interactions. Students exhibiting this level of understanding are able to correctly identify noncovalent interactions and make some correct statements about relative strengths of interactions based on memorized definitions. Such students would not be able to explain why interactions occur and would struggle to correctly identify noncovalent interactions in unfamiliar molecules, such as those encountered in a biochemistry course.
Level 2 describes understanding that could be exhibited by students across a range of educational levels including advanced, upper secondary level chemistry, or post-secondary general chemistry, introductory biology, and organic chemistry courses. At this level, students move beyond rote memorization and begin to make generalizations about noncovalent interactions. Students know that noncovalent interactions are based on attraction between opposite charges and that these atomic-scale interactions influence macroscopic properties like boiling point. Because students at this level more critically analyze molecular properties and macroscopic data, such as boiling points, they are able to rank the relative strengths of interactions between small molecules. Finally, students at this level are beginning to realize that molecular charge characteristics are not fixed, but rather depend on pH, solvent environment, and the movement of electrons.
Level 3 describes the ways in which understanding of noncovalent interactions develops after students have studied amino acid and protein structures in the aqueous environment of the cell, usually in the context of a biochemistry course. Students at this level know that the strength of interactions depends on the magnitude of interacting charges and that many important biological molecules exhibit charge characteristics that depend on pH. Specifically, students have studied how some amino acid side chains can be either polar or charged depending on pH and that the strength of expected noncovalent interactions therefore depends on pH and pKa, which together determine the ionization state of a molecule. Furthermore, students know that changes in the amino acid sequence of a protein can result in changes in noncovalent interactions and affect protein structure.
Level 4 shows enhanced awareness of how the three-dimensional structure of biological macromolecules affects the strength of noncovalent interactions. This understanding comes from the study of enzyme catalysis and protein–ligand interactions, both of which depend on specific noncovalent interactions within a defined three-dimensional space. This may be the first time that the relevance of the distance term in Coulomb's law becomes apparent to students. For example, they may encounter examples of how the one-carbon difference in side chain length between the amino acids aspartate and glutamate can change enzyme–substrate binding affinity or enzymatic activity.
Level 5 provides examples of expert-level understanding of noncovalent interactions. Some undergraduate students may attain this level of understanding, but development of real expert understanding is an ongoing process that continues in graduate school and throughout an individual's career. We would argue that expert understanding never ceases to deepen and therefore the expert characteristics included in the construct map are intended to be examples as opposed to a thorough description of expert knowledge. Level 5 is characterized by a nuanced engagement with the complexity of chemical, physical, and biological systems and by the fact that experts often apply this tacit knowledge without being aware of it.
Application of the construct map to instructional design and assessment
In recent decades, the conversation among science educators has shifted from a focus on what we teach to a focus on what students learn. The rise of pedagogies of engagement have enabled college and university faculty to step away from the lectern and to engage more deeply with students on a daily basis (Eberlein et al., 2008). Through these personal interactions and an increasing body of discipline-based education research, many of us teaching in the molecular life sciences have become more aware of the limitations in students’ understanding (Villafañe et al., 2011; National Research Council, 2012; Xu et al., 2017). Although these realizations can be disheartening, they can also motivate educators to reevaluate their instructional practices.Some ideas can be considered to be uniquely “biochemical”, yet biochemistry is concerned largely with the application of fundamental chemical and physical principles to complex biological systems. Therefore, effective learning in biochemistry relies heavily on prerequisite knowledge, and students’ thinking can easily become tangled as they encounter larger molecules and more complicated systems than they had previously seen. It can be challenging for teachers to determine whether student difficulties they observe in biochemistry classes arise from gaps in prior knowledge, incorrect prior knowledge, difficulty understanding newly-introduced biochemical information, difficulty integrating information from multiple sources, or a combination of these factors. In our experience facilitating faculty development workshops aimed at improving teaching and learning in biochemistry, it became apparent that biochemists find teaching about noncovalent interactions particularly challenging because of the variation in students' prerequisite knowledge and the complexity of the molecules and systems being studied. Therefore, biochemistry educators can benefit from a tool that organizes topic-specific professional knowledge related to noncovalent interactions into a framework that may be used to design, evaluate, and improve instructional and assessment materials.
The newly developed construct map provided two of us the opportunity to evaluate our own teaching practice in biochemistry with respect to the role of noncovalent interactions. The examples provided below illustrate that deep engagement with the construct map can prompt critical reflection and changes in instructional practices.
Alignment of course learning objectives with the construct map
Two of the authors (Loertscher and Minderhout) had previously defined detailed learning objectives for a one-term biochemistry course covering macromolecular structure and function and an introduction to metabolism. We reviewed the learning objectives related to macromolecular structure and function and identified with an asterisk all objectives that required an understanding of noncovalent interactions (Appendix 3). Of the 70 total objectives, 29 of the statements related directly to noncovalent interactions. This emphasis is not surprising given the prevalence and importance of noncovalent interactions in macromolecular structures. Stated learning objectives spanned the range of student learning levels (1–4) defined in the construct map (see Table 3 for examples).| Level 1 | Describe and give examples of noncovalent interactions that are common in biological systems including hydrogen bonds, ion pair interactions, dipole–dipole interactions, and dispersion forces. Explain what all of them have in common. State at least one defining characteristic of each. |
| Level 2 | Compare and contrast the solvation of nonpolar compounds and solvation of polar/charged compounds in water. |
| Level 2 | Given a lipid structure, be able to identify regions of the molecule as polar, nonpolar, amphipathic, or charged, and use this information to suggest a function for the molecule as a whole. |
| Level 3 | Describe how changes in solution pH influence the net charge of a protein, protein solubility, and the interactions between proteins and other macromolecules in solution. |
| Level 3 | Predict the charge on any amino acid in a polypeptide given information on environment and pKa. Explain how pKa values can change based on neighboring functional groups. |
| Level 4 | Name the interactions that stabilize 3D protein structure and predict which of these interactions might be relevant in a given peptide sequence. |
| Level 4 | Given the structure of a ligand, propose the identity and placement of amino acids that would form the ligand binding pocket of a protein that is optimized to selectively bind that ligand. |
As biochemists, we understand the importance of noncovalent interactions in enzyme-mediated catalysis and our learning objectives related to enzymes reflect this understanding. For example, noncovalent interactions (1) define enzyme specificity for substrate, (2) influence binding affinity and therefore Km, (3) stabilize transition state thereby affecting catalytic rate, and (4) determine which molecules will act as inhibitors. In concordance with the relative importance of noncovalent interactions, we identified seven of the eighteen learning objectives for enzymes as depending on understanding of noncovalent interactions. However, closer examination of the individual learning objectives revealed that many of the highlighted objectives associated with enzymes did not include explicit language about noncovalent interactions. We realized that our expert, tacit knowledge enabled us to know that noncovalent interactions are important, yet we had neglected to highlight that connection for students. As a result, we revised a number of the objectives to more explicitly map to Level 4 of the construct map (Table 4).
| Original learning objective | Revised learning objective |
|---|---|
| Describe the factors that allow enzymes to function as catalysts and discuss the factors that influences enzymatic rates. | Describe the factors that allow enzymes to function as catalysts and discuss the factors that influences enzymatic rates, especially the role of noncovalent interactions in stabilizing the transition state. |
| Define the constants Km, kcat and Vmax. Interpret each in terms of enzyme active site structure and function. | Define the constants Km, kcat and Vmax. Interpret each in terms of enzyme active site structure/function and the molecular level interactions between the enzyme and the substrate or transition state. |
| Assess catalytic efficiency of an enzyme using the kcat/Km ratio. | Assess catalytic efficiency of an enzyme using the kcat/Km ratio. Use your understanding of the molecular interactions of the enzyme with both the substrate and the transition state to discuss large and small kcat/Km ratios. |
| Apply Ki data to propose active site substrate specificity. | Apply Ki data to propose active site substrate specificity. Propose possible molecular interactions and further inhibition studies to investigate substrate specificity. |
| Describe allosteric binding in the context of enzymes. | Describe allosteric binding in the context of enzymes and consider the physical basis for all binding interactions. |
Changes in instructional practice
In addition to evaluating learning objectives using the construct map we also modified aspects of daily instruction related to macromolecular structure and function. One of us (Loertscher), wrote Coulomb's law on the blackboard nearly every day and referred to either the charge or distance component, as appropriate. Because we realized that we had not emphasized the role of noncovalent interactions in enzyme-mediated catalysis, we decided to introduce a guided inquiry activity that asked students to evaluate the role of charge and distance in determining the strength of a binding interaction immediately prior to the part of the course focusing on enzymes. The guided inquiry activity we used was previously published (Werth, 2017) and focused on the binding of ligands to the serotonin receptor. The binding pocket for the serotonin receptor resembles an enzyme active site, but the lack of catalytic activity means that students can focus on the binding interaction without the more complicated consideration of catalytic parameters. The activity guides students through a short review of levels 1 and 2 understanding and continues to develop levels 3 and 4. The level 3 understanding explored relates to the charge magnitude term in Coulomb's law, while the level 4 understanding relates to the distance component of Coulomb's law. As a preview to enzyme active sites, the activity also prompts students to consider three-dimensional spatial arrangement by examining binding strength of serotonin in different orientations in the binding site as well as the binding of other common cellular molecules.Using the construct map to improve assessment
Similar to its application in instructional design, the construct map can support assessment by defining clear learning targets. For example, educators could improve the quality of existing test or quiz questions by identifying the targeted level of student achievement for a given assessment and then critically evaluating whether items within that assessment relate to statements in the targeted level of the map. When writing new assessment questions, educators could use statements within given levels as a starting point. Some educators may choose to share the construct map with students as a study tool to monitor their learning progress.Box 1: Example of an exam question that elicits student responses related to stated levels of the construct mapCertain proton binding sites in hemoglobin are of higher affinity in the deoxy form than the oxy form. The difference in proton affinity means that the pKa values change depending on chemical environment around an ionizable group and interactions it makes with neighboring amino acids. For example, His-146 at the C-terminus of each beta chain forms a salt bridge with Asp-94 in the same chain, but only if the His-146 is protonated. The salt bridge stabilizes the proton against dissociation.(a) Given the information above, what do you expect for the pKa value of His-146 in the deoxy form of hemoglobin? Explain your reasoning. (b) Based on your answer in (a), what is the predominant form of His-146 at pH 7.4? (c) The pH of the lungs is around 7.4, while the pH of the tissues is often lower. Propose a reasonable hypothesis for how the presence or absence of the salt bridge between His and Asp affects the ability of hemoglobin to transport oxygen. A high-quality answer will consider the effect of the equilibria between different conformations of the quaternary structure of hemoglobin. |
In considering our biochemistry course, our target is to assess students’ understanding at levels 3 and 4 of the construct map. As a first step, we analyzed our existing midterm exam questions related to macromolecular structure and function for evidence that we had been asking students to demonstrate appropriate understanding. We were able to identify several questions that had the potential to elicit student responses at the appropriate level. In other words, questions that were intended to provoke discussion of noncovalent interactions and their effects on the system of interest. A sample analysis of a question related to protonation state and protein structure in hemoglobin (Box 1) is described here. We found that different parts of the question aligned with different levels of the construct map. In order to interpret the information in the stem of the question about the structure/affinity relationship in hemoglobin, students could draw on Level 3 understandings:
Students know that pK a determines the charge of a functional group at a given pH and solvent environment.
Parts a and b of the question ask about the relationship among pH, pKa, and charge on amino acid sidechains in hemoglobin. To address these questions, students could draw on Level 2 and Level 3 understandings:
Level 2: Students know that noncovalent interactions occur because of the electrostatic properties of molecules. These properties can involve full, partial and/or momentary charges.
Level 3: Students are able to predict how a change in pH will produce a change in the charge of an ionizable group.
To integrate this information and make a hypothesis relating pH and hemoglobin function, as is requested in part c of the question, students could draw on Level 4 understandings:
Level 4: Students know that charged/polar amino acids in folded proteins interact with charged/polar entities within or outside a protein.
Level 4: Students know that these noncovalent interactions influence the folding and conformational stability of proteins.
Level 4: Students are able to consider 3D spatial arrangement and microenvironment when evaluating strength of noncovalent interactions between biological molecules.
Although the target was to assess level 3 and 4 understanding, it is not surprising that students would need to draw on level 2 understanding to interpret the stem of the question. When writing this question, we had intended to scaffold the third, most difficult part of the problem, by prompting students to think about simpler ideas in the first two parts.
The exercise of aligning the exam question with the construct map, assured us that in this case, we had written a question that maps to levels 2 and 3 for the first two parts and level 4 for the third part. We would need to analyze student responses in order to determine whether the question functioned as intended, but the construct map provided us at least one way to evaluate validity of a midterm exam question. Although we have previously collected validity evidence for questions we developed as part of our educational research, the process of using the construct map to evaluate our teaching practice prompted us to be more critical of assessment in our own classroom. This analysis also prompted consideration of Bloom's taxonomy, which organizes learning tasks from simple (lower-order cognitive skills) to more complex (higher-order cognitive skills (Bloom, 1956)). The construct map presents a similar progression from simple to complex thinking as students are asked to consider layers of complexity and situate their learning in a biochemical context. Therefore, as educators develop assessment questions related to noncovalent interactions, it may be helpful to use the construct map in tandem with a resource, such as the Blooming Biology Tool (Crowe et al., 2008). Together these tools could prompt development of questions that target appropriate content (described in the construct map) and cognitive level of difficulty (described by the Blooming Biology Tool).
Limitations of the study
The construct map was developed in the United States in collaboration with post-secondary educators. Although we have made an effort to generalize our work, we recognize that there are some aspects that may feel less accessible to those outside this context. For example, noncovalent interactions are covered extensively in secondary level chemistry curricula in some countries, but we were unable to include secondary level educators in the workshops described herein. Our hope is that the descriptive detail included in the construct map will enable educators at the secondary and post-secondary levels to align the map appropriately with their specific contexts. Furthermore, while, we believe that the construct map could be useful to those teaching general chemistry, organic chemistry, or other courses, biochemistry education is the lens through which we view the construct map at this time. We look forward to expanding collaboration with colleagues in these other areas.Conclusions
We developed a construct map on the physical basis of interactions to assist us, and the biochemistry community at large, in designing targeted curricular activities and detailed assessments to monitor the progress of our students’ learning in this area. The construct map details what students are to know and to be able to do related to the physical basis of noncovalent interactions. The degree of detail arose from the collaborative process we describe in which the breadth and depth of teaching experiences of a community of biochemistry educators were utilized to deepen and refine each idea and statement. Importantly, this process helped us to make our collective tacit knowledge about noncovalent interactions visible to ourselves as educators. The highly specific statements contained in the construct allow for high-quality assessment and improvement of teaching practice related to noncovalent interactions. Using the construct map helped guide our selection of what to teach and how to teach aspects of biochemistry and to evaluate assessments designed to monitor student progress in understanding. The exercise of reviewing our own course materials revealed the value of the construct map as a tool for improving teaching practice. The examples we provide from our own teaching context are intended as exemplars for ways in which the construct map could be useful for other educators to improve their own teaching practice. We imagine that other educators will have additional ideas that will broaden the scope and utility of the construct map tool and we look forward to those discussions.Conflicts of interest
There are no conflicts to declare.Appendix 1: learning outcomes related to the physical basis of noncovalent interactions defined by 2015 workshop participants
| General Chemistry (N = 6) |
| • Define the different types of noncovalent interactions |
| • Apply knowledge of noncovalent interactions to explain bulk properties like boiling point, melting point, and solubility |
| • Rank relative strengths of noncovalent interactions |
| • Compare and contrast noncovalent interactions and covalent bonds |
| • Recognize that noncovalent interactions occur between molecules, not within molecules |
| • Identify which types of interactions are possible for given molecules |
| • Draw noncovalent interactions |
| Biochemistry (N = 17) |
| Prerequisite concepts from general chemistry: |
| • Define the different types of noncovalent interactions |
| • Rank relative strengths of noncovalent interactions |
| • Identify which types of noncovalent interactions are possible for given molecules |
| • Apply knowledge of noncovalent interactions to explain bulk properties like boiling point, melting point, and solubility |
| • Draw noncovalent interactions |
| New concepts for biochemistry: |
| • Describe relationships between distance, angle, and strength of noncovalent interactions |
| • Apply knowledge of noncovalent interactions in a wide range of contexts |
| • Apply knowledge of noncovalent interactions to explain macromolecular structure including protein folding and interactions that stabilize macromolecules |
| • Apply knowledge of noncovalent interactions to make predictions about binding events including enzyme–substrate binding, binding of small molecules to transporters, protein–ligand interactions, and binding of regulatory molecules to macromolecules |
| • Recognize that formation of noncovalent interactions releases energy |
| • Understand that noncovalent interactions are reversible and that interacting and non-interacting forms of molecules can be in equilibrium |
| • Predict where in a protein or other macromolecular structure one would expect to find polar and nonpolar amino acids/molecules |
| • Explain enzymatic reaction mechanisms based on interactions among enzymes, substrates, intermediates, and products |
| • Recognize that both noncovalent interactions and entropic factors contribute to macromolecular structure formation |
Appendix 2: activity used by 2016 workshop participants to explore construct maps
WhyConstruct maps are the starting point to designing ordered multiple choice (OMC) questions. While we have devised construct maps for our threshold concepts (TCs), we are interested in your ideas based on your experiences with your students. We continue to examine our construct maps with the physical basis of interactions and steady state.
Plan
1. Assign roles
a. Manager – keep track of time and keep the group on task
b. Recorder – document group's work as instructed
c. Spokesperson – be prepared to share group's ideas
2. Use the TC table as a resource, it is a yellow sheet found in your workshop folder
3. Be prepared to turn in all notes at the end of activity.
Tasks
1. Individually think about an “A” student leaving your biochemistry course (Level 2). Complete the following two statements: 5 minutes to write statements
a. After completing biochemistry “A” students know that:
b. After completing biochemistry “A” students are able to:
2. Individually think about an “A” student entering your biochemistry course (Level 1). Complete the following two statements: 5 minutes to write statements
a. An “A” student entering biochemistry knows that:
b. An “A” student entering biochemistry is able to:
3. Manager, after the 10 minutes of individual time, pass out the construct map for the TC Thermodynamics of macromolecular structure formation.
4. Individually skim/read over the construct map (5 minutes to skim/read)
5. As a group begin with the Level 2 statements and errors. Recorder, keep notes about the discussion for the spokesperson to read. Discuss the following topics in whatever order makes sense to your group: (20 minutes to discuss and revise, Spokesperson keep running notes for group discussion)
a. Do any of the statements seem misplaced at this level? Which ones? Should they be at Level 3 or Level 1?
b. Are any statements incorrect? Fix or indicate the problem.
c. Are any statements unclear? Fix or indicate the problem.
d. Are there redundant items? If so which ones should be combined and how?
e. Can you think of additional items? Please record
i. Statements
ii. Common errors
iii. Incomplete ideas or simplified ideas
f. Move onto 6. If finished before 20 minutes
6. As a group begin with the Level 1 statements and errors. Recorder keep notes about the discussion for the spokesperson to read. Discuss the following topics in whatever order makes sense to your group: (15 minutes to discuss and revise, Spokesperson keep running notes for group discussion)
a. Do any of the statements seem misplaced at this level? Which ones? Should they be at Level 3 or Level 2?
b. Are any statements incorrect? Fix or indicate the problem.
c. Are any statements unclear? Fix or indicate the problem.
d. Are there redundant items? If so which ones should be combined and how?
e. Can you think of additional items? Please record
i. Statements
ii. Common errors
iii. Incomplete ideas or simplified ideas
7. Review, discuss and refine Expert statements (Level 3) (5 minutes to discuss and revise)
8. Prepare your recorder and spokesperson to share ideas. 5 minutes
| Level | Description |
|---|---|
| 3 | Experts have an internalized understanding of Coulomb's Law. It may be so internalized that they do not realize how and when they use it. Their internalized sense allows them to explain how the issues of distance, charge and pKa apply to macromolecular phenomena such as catalytic rate and ligand binding. |
| Expert biochemist | (a) The charges in Coulombs law can involve full, partial, and/or momentary charges due to the movement of electrons and the shielding of the nuclear charge. The values in Coulombs Law are always changing due to the fluctuating values of the charge |
| (b) The distances in Coulombs law are dependent on the distance that separates the charges, which are measured in angstroms. The distances are always changing due to the continuous motion of all particles, which results in fluctuating value of force. | |
| (c) The values of pKa determine the protonation state of functional groups | |
| (d) Experts recognize that quantum mechanical models may be invoked to explain some phenomena | |
| 2 | At the end of biochemistry students know |
| Finish biochemistry “A” student | (a) that interactions occur because of the electrostatic properties of molecules. These properties can involve full, partial, and/or momentary charges. |
| (b) that the magnitude of these charges matter. | |
| (c) that the strength of these interactions are dependent on the distance that separates them. (only some A students invoke this) | |
| At the end of biochemistry students are able to | |
| (d) use their knowledge of charge to explain data related to molecular interactions | |
| (e) use their knowledge of distance to make predictions about strength of interactions | |
| (f) use their knowledge of pKa to make predictions about molecular charge | |
| Common errors and omissions: | |
| • While students understand the electrostatic properties of molecules they may not refer to the fundamental issues of charge and distance unless specifically prompted to do so. They have not internalized these ideas | |
| • Students exhibit limited understanding of the magnitude of charges, especially partial charges. | |
| • Students exhibit limited understanding of the equilibrium nature of the charges at or around the pKa (i.e. Some molecules are charged and others are not) | |
| • Students exhibit very limited understanding of the effect of distance on the magnitude of charged interactions. | |
| • There is a limited sense of the special arrangements in the active site or in binding sites. | |
| • Students exhibit an over reliance on memory, using memorized words/phrases in responses that do not reveal if a fundamental understanding of the interactions has been achieved. | |
| 1 | Upon entering biochemistry, students know |
| Enter biochemistry “A” student | (a) about IMFs and can name them |
| (b) that IMFs occur because of the electrostatic properties of molecules. These properties can involve full, partial, and/or momentary charges. | |
| (c) that IMFs cause molecules to exist in condensed phases, (liquid and solid) | |
| (d) pKa is involved with acids and bases | |
| Common errors and omissions: | |
| • Students exhibit very little understanding of the equilibrium nature of the charges at or around the pKa (i.e. Some molecules are charged and others are not) | |
| • Students exhibit limited to no understanding of the magnitude of charges, especially partial charges. | |
| • Students exhibit no understanding of the effect of distance on the magnitude of charged interactions. | |
| • There is no sense of the special arrangements in the active site or in binding sites. | |
| • Students exhibit an over reliance on memory, using memorized words/phrases in responses that do not reveal if a fundamental understanding of the interactions has been achieved. | |
Appendix 3: learning objectives distributed to students in the biochemistry course described in this study
Biochemical foundations(1) List the major structures of eukaryotic and prokaryotic cells and describe their function.
(2) Describe the canonical process by which the information contained in genes leads to fully functional proteins. Name relevant molecules associated with this process and correctly use the terms replication, transcription and translation.
(3) Recognize and name common functional groups of biomolecules.
(4) Define the term polymer and discuss why some biomolecules are polymers while others are not.
(*5) Describe the special role water plays in the chemistry of life both as a solvent and a reactant.
(*6) Describe and give examples of noncovalent interactions that are common in biological systems including hydrogen bonds, ion pair interactions, dipole–dipole interactions, and dispersion forces. Explain what all of them have in common. State at least one defining characteristic of each.
(*7) Compare and contrast the solvation of nonpolar compounds and solvation of polar/charged compounds in water.
(*8) Describe the roles of enthalpy and entropy in interactions between nonpolar molecules and water.
(*9) Define the terms Ka, pKa, ionization, and buffer. Explain and produce titration curves of polyprotic acids given appropriate data including pKa.
(10) Define the terms Gibbs free energy and chemical equilibrium. Explain their relationship with each other.
(11) Define ΔG and ΔG°. Explain why free energy changes are important in biochemistry.
Protein Structure (Primary, Secondary, Tertiary, Quaternary)
(*1) Given the structure of any of the 20 amino acids in their neutral form, draw them at any other pH.
(2) Know the three-letter abbreviations for each of these amino acids.
(3) Define and characterize the four levels of protein structure.
(*4) Identify the ionizable groups of each free amino acid, and describe how the ionizable groups change when free amino acids are condensed into peptides.
(5) Recognize the chirality of amino acids.
(6) Describe the chemical reaction that generates a peptide from free amino acids.
(7) Draw a peptide bond and recognize the coplanar atoms.
(8) Estimate the pI of an amino acid or peptide given a sequence or titration curve.
(*9) Describe how changes in solution pH influence the net charge of a protein, protein solubility, and the interactions between proteins and other macromolecules in solution.
(10) Produce titration curves of peptides given appropriate data including pKa and analyze titration curves for peptides to obtain pKa values and identify amino acids.
(*11) Predict the charge on any amino acid in a polypeptide given information on environment and pKa. Explain how pKa values can change based on neighboring functional groups.
(12) Explain how mutations in DNA affect protein structure and function.
(13) Discuss how structural aspects of the peptide bond, as well as steric restrictions, determine the secondary and tertiary structure of proteins.
(*14) Diagram α-helix and β-sheet secondary structures and the specific patterns of interactions in the peptide backbone that stabilize them. Given a peptide sequence and appropriate data, predict which structure is most likely.
(*15) Given an example of a 3D protein structure, identify secondary structural elements and the interactions between secondary structure elements.
(16) Define the “hydrophobic effect” and articulate the entropy and enthalpy changes that occur when two nonpolar groups in an unfolded polypeptide chain associate in water.
(*17) Name the interactions that stabilize 3D protein structure and predict which of these interactions might be relevant in a given peptide sequence.
Biochemical methods
(1) Know what urea, SDS, and reducing agents (BME and DTT) do.
(*2) Explain the conceptual and chemical basis for the following biochemical techniques used to investigate proteins: ion exchange chromatography, affinity chromatography, size exclusion chromatography, polyacrylamide gel electrophoresis, isoelectric focusing, ELISA, and western blot.
(3) Diagram the process of each of the biochemical techniques listed above.
(4) Predict the outcome when a series of the biochemical techniques listed above is performed on a sample.
(5) Plan a biochemical separation scheme using these techniques and given information about the starting material and desired outcome. (5A)
(6) Describe the difference between analytical and preparative techniques and give two examples of each.
(7) Compare and contrast information obtained from each of these biochemical techniques.
(8) Analyze data generated from the techniques listed above to elucidate aspects of protein structure.
Ligand binding
(*1) Given a structure of a generic protein ligand, draw the possible noncovalent interactions the ligand could engage in, including relative strength and distance constraints of those interactions.
(*2) Describe what is meant by a “ligand binding pocket”. Explain the roles of 3D protein structure and specific amino acid side chains in creating a binding pocket.
(*3) Describe the role of protein structure (primary, secondary, tertiary, quaternary), including charge and distance/orientation of functional groups, in the strength and selectivity of the interactions between ligands and a binding sites.
(*4) Given the structure of a ligand, propose the identity and placement of amino acids that would form the ligand binding pocket of a protein that is optimized to selectively bind that ligand.
(5) Given a binding curve (x-axis = ligand concentration, y-axis = fraction of binding sites occupied), label the dissociation constant (Kd) and explain what it says about relative binding strength.
(*6) Relate binding strength and Kd values to molecular structure by considering interactions.
(*7) Explain what makes binding more or less favorable generally.
Enzymes (catalysis, kinetics, inhibition, regulation)
(1) Define what makes a chemical reaction spontaneous.
(2) Explain the effect of enzymes on biological reactions. What would life look like in the absence of enzymes?
(3) Distinguish between a Keq and k.
(4) Draw and interpret reaction coordinate diagrams for catalyzed and uncatalyzed reactions
(*5) Explain the statement, “the enzyme binding to the transition state is more favorable than binding to the substrate” and draw a reaction coordinate diagram that depicts the above statement visually.
(6) Apply chemical reaction rate information to enzymatic reactions.
(*7) Describe the factors that allow enzymes to function as catalysts and discuss the factors that influences enzymatic rates. There are many factors, be sure to consider interactions that affect the transition state.
(8) List the assumptions of Michaelis–Menten kinetics. Describe the conditions under which these assumptions are reasonable.
(*9) Define the constants Km, kcat and Vmax. Interpret each in terms of enzyme active site structure/function and the interactions between the enzyme and the substrate or transitions state at the molecular level.
(10) Assess catalytic efficiency of an enzyme using the kcat/Km ratio.
(11) Use your understanding of the molecular interactions of the enzyme with both the substrate and the transition state to discuss large and small kcat/Km ratios.
(*12) Apply previously-learned information on protein structure to an enzyme system.
(13) Compare and contrast Michaelis–Menten plots and Lineweaver–Burk plots. Be able to extract catalytic constants from each type of plot.
(14) Define Km,app and Vmax,app, and interpret inhibition data based on Lineweaver–Burk plots.
(15) Distinguish between competitive and noncompetitive inhibition based on inhibitor structure and inhibition plots.
(*16) Apply Ki data to propose active site substrate specificity and propose possible interactions and further inhibition studies to investigate substrate specificity.
(17) Discuss the known mechanisms for enzyme regulation.
(*18) Describe allosteric binding in the context of enzymes and consider the physical basis for all binding interactions.
Lipids and membranes
(1) Define the term lipid. Identify and list the structural features common to all lipids.
(*2) Given a lipid structure, be able to identify regions of the molecule as polar, nonpolar, amphipathic, or charged, and use this information to suggest a function for the molecule as a whole.
(3) Make predictions about membrane fluidity given lipid composition.
(4) Describe how cholesterol modulates membrane fluidity.
(5) Describe the differences between a micelle and a bilayer. Given a lipid structure, predict whether the lipid will form micelles or bilayers.
(*6) Describe the noncovalent interactions and thermodynamic driving force of lipid association and membrane resealing.
(*7) Predict the distribution of polar and nonpolar residues in the surface vs. interior of integral membrane proteins, and contrast to the distribution of residues in cytoplasmic proteins.
(*8) Describe the factors that stabilize interactions between lipids and transmembrane proteins.
(9) Broadly explain the function(s) of transmembrane proteins and give examples.
Acknowledgements
The work described in this document was supported by the National Science Foundation DUE-1224868. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. Thanks to all of the educators and researchers who participated in this project: Norris Armstrong, Rodney Austin, Charvann Bailey, Adam Cassano, Hannah Chapin, Colleen Conway, Carol Dieckman, Jacqueline Drak, Erin Dolan, Shari Dunham, Stephen Dunham, Martina Ederer, Katherine Frato, Thomas Freeman, Jennifer Grant, Nicholas Grossoehme, Timothy Hayes, Bruce Heyen, Brian Hogan, Marina Holz, Nicole Iranon, Henry Jakubowski, Brett Kaiser, Kelly Keenan, Peter Kennelly, Anne Kruchten, Paula Lemons, Kent Littleton, Sunil Malapati, Amy Medlock, Tracey Murray, Jeffrey Owens, Kalyn Owens, David Parkin, Martina Rosenberg, Duane Sears, John Shabb, Regina Stevens-Truss, Heather Tienson, Carin Thomas, Mark Werth, and Xiaoying Xu. We also appreciate the insights of Alexa Dragon and Jorence Pagdanganan, undergraduate research students who provided feedback throughout the development process.References
- Becker N., Noyes K. and Cooper M., (2016), Characterizing students’ mechanistic reasoning about London dispersion forces, J. Chem. Educ., 93, 1713–1724.
- Bissantz C., Kuhn B. and Stahl M., (2010), A medicinal chemist's guid to molecular interactions, J. Med. Chem., 53, 5061–5084.
- Black P., Wilson M. and Yao S. Y., (2011), Road maps for learning: a guide to the navigation of learning progressions, Measurement: Interdisciplinary Research & Perspective, 9, 71–123.
- Bloom B. S., (1956), Taxonomy of educational objectives, Ney York: David McKay.
- Bretz S. L. and Linenberger K. J., (2012), Development of the enzyme–substrate interactions concept inventory, Biochem. Mol. Biol. Educ., 40, 229–233.
- Briggs D. C., Alonzo A. C., Schwab C. and Wilson M., (2006), Diagnostic assessment with ordered multiple-choice items, Educational Assessment, 11, 33–63.
- Cooper M. M. and Klymkowsky M. W., (2013), The trouble with chemical energy: why understanding bond energies requires an interdisciplinary systems approach, CBE Life Sci. Educ., 12, 306–312.
- Cooper M. M., Corley L. M. and Underwood S. M., (2013), An investigation of college chemistry students' understanding of structure–property relationships, J. Res. Sci. Teach., 50, 699–721.
- Cooper M. M., Williams L. C. and Underwood S. M., (2015), Student understanding of intermolecular forces: a multimodal study, J. Chem. Educ., 92, 1288–1298.
- Cousin G., (2008), Threshold concepts: old wine in new bottles or new forms of transactional curriculum inquiry, Threshold concepts within the disciplines, 261–272.
- Cousin G., (2009), Researching learning in higher education: an introduction to contemporary methods and approaches, New York: Routledge.
- Crowe A., Dirks C. and Wenderoth M. P., (2008), Biology in bloom: implementing Bloom's taxonomy to enhance student learning in biology, CBE Life Sci. Educ., 7, 368–381.
- Eberlein T., Kampmeier J., Minderhout V., Moog R. S., Platt T., Varma-Nelson P. and White H. B., (2008), Pedagogies of engagement in science, Biochem. Mol. Biol. Educ., 36, 262–273.
- Entwistle N., (2008), in Land R., Meyer J. H. F. and Smith J. (ed.), Threshold Concepts within the Disciplines, Rotterdam: Sense Publishers.
- Gess-Newsome J., (2015), in Barry A., Friedricksen P. and Loughran J. (ed.), Re-examining Pedagogical Content Knowledge in Science Education, New York: Routledge.
- Green D. A., Loertsher J., Minderhout V. and Lewis J. E., (2017), For want of a better word: unlocking threshold concepts in natural sciences with a key from the humanities? High. Educ. Res. Dev., 1–17.
- Henderleiter J., Smart R., Anderson J. and Elian O., (2001), How do organic chemistry students understand and apply hydrogen bonding? J. Chem. Educ., 78, 1126.
- Holme T. and Murphy K., (2012), The ACS Exams Institute undergraduate chemistry anchoring concepts content map I: General Chemistry, J. Chem. Educ., 89, 721–723.
- Hunter C. A., (2004), Quantifying intermolecular interactions: guidelines for the molecular recognition toolbox, Angew. Chem., Int. Ed., 43, 5310–5324.
- Kronik L., Nahum L., Mamlok-Naanam R. and Hofstein A., (2008), A new “bottom-up” framework for teaching chemical bonding, J. Chem. Educ., 85, 1680.
- Loertscher J., Green D., Lewis J. E., Lin S. and Minderhout V., (2014), Identification of threshold concepts for biochemistry, CBE Life Sci. Educ., 13, 516–528.
- Meyer J. and Land R., (2003), Threshold concepts and troublesome knowledge: Linkages to ways of thinking and practising within the disciplines, Edinburgh: University of Edinburgh.
- Minderhout V. and Loertscher J., (2007), Lecture-free biochemistry, Biochem. Mol. Biol. Educ., 35, 172–180.
- National Research Council, (2001), Knowing what students know: the science and design of educational assessment, National Academies Press.
- National Research Council, (2012), Discipline-based education research: understanding and improving learning in undergraduate science and engineering, National Academies Press.
- Perkins D., (2008), in Land R., Meyer J. H. F. and Smith J. (ed.), Threshold Concepts within the Disciplines, Rotterdam: Sense Publishers.
- Peterson R. F., Treagust D. F. and Garnett P., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students' concepts of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach., 26, 301–314.
- Pfundt H. and Duit R., (1988), Bibliography. Students' alternative frameworks and science education.
- Raker J., Holme T. and Murphy K., (2013), The ACS Exams Institute undergraduate chemistry anchoring concepts content map II: Organic Chemistry, J. Chem. Educ., 90, 1443–1445.
- Schmidt H. J., Kaufmann B. and Treagust D. F., (2009), Students' understanding of boiling points and intermolecular forces, Chemistry Education Research and Practice, 10, 265–272.
- Sears D. W., Thompson S. E. and Saxon S. R., (2007), Reversible ligand binding reactions: Why do biochemistry students have trouble connecting the dots? Biochem. Mol. Biol. Educ., 35, 105–118.
- Shulman L. S., (2015), in Barry A., Friedricksen P. and Loughran J. (ed.), Re-examining Pedagogical Content Knowledge in Science Education, New York, Routledge.
- Stevens S. Y., Shin N. and Krajcik J. S., (2009), Towards a model for the development of an empirically tested learning progression.
- Taber K., (2002), Chemical misconceptions: prevention, diagnosis and cure, Royal Society of Chemistry.
- Taber K. S., (2017), Researching moving targets: studying learning progressions and teaching sequences, Chem. Educ. Res. Pract., 18, 283–287.
- Tansey J. T., Baird T., Cox M. M., Fox K. M., Knight J., Sears D. and Bell E., (2013), Foundational concepts and underlying theories for majors in “biochemistry and molecular biology”, Biochem. Mol. Biol. Educ., 41, 289–296.
- Villafañe S. M., Bailey C. P., Loertscher J., Minderhout V. and Lewis J. E., (2011), Development and analysis of an instrument to assess student understanding of foundational concepts before biochemistry coursework, Biochem. Mol. Biol. Educ., 39, 102–109.
- Werth M. T., (2017), Serotonin in the Pocket: non-covalent interactions and neurotansmitter binding, CourseSource DOI:10.24918/cs.2017.14.
- Wilson M., (2009), Measuring progressions: assessment structures underlying a learning progression, J. Res. Sci. Teach., 46, 716–730.
- Xu X., Lewis J. E., Loertscher J., Minderhout V. and Tienson H. L., (2017), Small changes: using assessment to direct instructional practices in large-enrollment biochemistry courses, CBE Life Sci. Educ., 16, ar7.
| This journal is © The Royal Society of Chemistry 2018 |