Using student-generated animations: the challenge of dynamic chemical models in states of matter and the invisibility of the particles
Zeynep
Yaseen

Faculty of Art and Social Sciences, School of Education, University of Technology, Sydney, Australia. E-mail: Zeynep.Yaseen@uts.edu.au
First published on 22nd June 2018
Abstract
This research investigates the use of student-generated animations in the teaching and learning of chemistry. Previous research has identified the potential for animations to contribute to student learning in science. In particular, animations have the capacity to represent the dynamic process and motions that may be inherent in some chemical concepts. This study focuses on animations that students produced with the support of their teacher and fellow students. The participants in the study were Year 11 science students and their science teacher. The teaching intervention included training the students in the use of animation software, followed by the students working in groups to create animations representing their conceptions of solid, liquid and gaseous states of matter, watching expert animations and classroom discussions. Students were supported by their teacher and encouraged to discuss ideas as they constructed their animations. Data collection included pre- and post-tests, classroom observation, video recording of lessons, collection of artefacts (the students’ animations, expert animations) and interviews with the teacher and students. Use of the student-generated animations created an opportunity to represent and discuss conceptions of the states of matter, including dynamic elements of their conceptualization. The teacher's scaffolding of the groups during the creation of their animations helped students to accurately represent their conceptions. In their analysis of the various animations, students identified differences and similarities among their animations. Data from pre-/post-tests, observations and interviews indicate that the students improved their understanding of states of matter through the teaching/learning process that occurred during the intervention.
Introduction
In chemistry education, students need to understand the dynamism of chemical changes and the structures of molecules (Ben-Zvi et al., 1986; Harrison and Treagust, 2000). Animation software programs incorporating chemical concepts involving dynamic processes and motion may play a role in helping to develop this understanding. Research focusing on the effectiveness of computer-based representations has found that such representations help students in their conceptual understanding of chemistry (Ainsworth, 1999; Tytler et al., 2006; Hoffler and Leutner, 2007; Prain and Waldrip, 2008; Rutten et al., 2012; Taber, 2013; Yakmaci-Güzel and Adadan, 2013). Other researchers have focused on animations developed by teachers themselves, or other experts, and their use to visually demonstrate scientifically acceptable concepts to students (Williamson and Abraham, 1995; Barnea and Dori, 1996; Dori et al., 2003; Hübscher-younger and Narayanan, 2007; Tytler et al., 2007; Danish and Phelps, 2010; Barak et al., 2011; Yakmaci-Güzel and Adadan, 2013).This article reports on an investigation of learning and teaching when pairs of students generated, analysed and discussed animations with their teacher in a senior chemistry class. The exploratory study aimed to investigate the components of active learning that supported students’ learning of the states of matter, including student-generated animations and viewing expert animations. Previous researchers have shown the effects of student-generated animations on conceptual understanding and have offered pedagogical reasons for creating animations, such as enjoyment, motivation, interest and engagement (Vermaat et al., 2003; Chang and Quintana, 2006). Other studies have explored how student animations influence effective learning (Barnea and Dori, 1996; Kozma and Russell, 1997; Wu et al., 2001; Carolan et al., 2008). While these studies are valuable, there is still little research examining students creating their own animations to support their science learning (Chang and Quintana, 2006; Danish and Enyedy, 2007; Hoban et al., 2009; Udo and Etiubon, 2011; Akaygun and Jones, 2014; Hoban and Nielsen, 2014).
The way in which students generate animations, the level of teacher support, and discussions with peer collaborators, in combination, can help students’ learning and thinking processes; however this has rarely been reported in the literature. When student-generated animations are used in science classes as learning tools, meaningful learning is enhanced by students explaining and commenting on them (Chang and Quintana, 2006; Hoban et al., 2009; Hoban and Nielsen, 2010, 2014; Udo and Etiubon, 2011) because students need help in their visual learning, especially student-generated animations, and it will all take place in the zone of proximal development (Vygotsky, 1978). Students are not always aware of what they should attend to and what they should ignore (Barlex and Carré, 1985). This research addresses the gap in the knowledge about how student-generated animations along with viewing expert animations, associated with peer collaborations and teacher's scaffolding, may have an effect on student learning of states of matter. Misconceptions related to students’ understanding of matter are quite extensive in the literature (Kind, 2004; Hadenfeldt et al., 2014) and specifically states of matter (Osborne and Cosgrove, 1983; Jones, 1984; Tsai, 1999; Çalık and Ayas, 2005; Tatar, 2011).
Theoretical framework
In this research context, the main aim of phenomenography is to identify how students experience, interpret, and conceptualize the concept of states of matter. Phenomenography does not claim that the results of research represent “correct” conceptions, only that the results of the research are “useful” (Orgil and Sutherlad, 2008). The results of this phenomenographic study can be useful to science educators and teachers who want to develop students’ understanding of states of matter. The researcher also looks for the underlying meaning of the concepts and the relationship between concepts – uncovering basic concepts and layers of concepts and how this impacts a perception of the world (Marton, 1994).
The design and collection of data in phenomenography is based on the students and their relation to a phenomenon (states of matter in this research). Analysis of data obtained from students during the intervention describes the experiences of states of matter in terms of the essential meaning of the qualitative variations. According to phenomenography, learning is relational – an interaction occurs between the students, intervention, and the content of the lesson. Knowledge is a product of the process of thinking and is based on the surrounding world. In the view of phenomenography, there is not a real or objective world; instead, there are numerous unreal subjective worlds (Marton, 1981; Saljo, 1994). Students are required to reflect on their meaning of experience rather than only to describe their experience in the intervention process. They represent the product of their thinking in this process.
Most phenomenographic studies in education drive their data from interviews. In this study, the interviews which provide the data are designed to encourage students to reflect on their own learning experience and to describe their actions. Interview extracts are used to show different perspectives among the students. In this study, regarding the question about states of matter, it was found that students answer this question in different ways. The alternative way of thinking is illustrated in the extracts from interviews and discussions. A large component of the analysis involved interviewing students’ thinking about how they can represent water when it undergoes changes of states of matter as it is heated and subsequently interviewing a teacher about his or her instructional experiences. Under phenomenography, the interviewer has the participants reflect on their experiences and then relate the experiences to the interviewer in such a way that the two come to a mutual understanding about the meaning of the experience.
Learning theory
This study involved a pedagogical intervention with student-generated animation, drawing on the principles from sociocultural theory (Vygotsky, 1978).A feature of the socioconstructivist theory and Vygotsky's sociocultural theory is collaboration and learning with peers. The critical point here is creating a social environment for learners. Vygotsky (1981) asserted that children learn through their interactions with others, so students should work not only with a teacher (MKO) but also with other students; they can exchange and test many different ideas in collaboration with others. Vygotskian emphasis on peer collaboration in learning is broadly consistent with the principles of cooperative learning. Cooperative learning has been described as a kind of small group instruction in a social setting in which students solve the given tasks (Slavin, 1990). In cooperative learning, students work not only with their teacher but also with other students with whom they exchange many different ideas. When engaged in cooperative learning, students have discussions with each other, exchange their ideas during group work, and are also supported by their teachers when necessary. When students work together on activities or tasks in a group, each member of the group may have a different internalization of knowledge, depending on their existing knowledge, because knowledge is constructed on learners’ previous knowledge, experience and background (Sharan, 1990; Doolittle, 1995; Powell and Kalina, 2009). In this research, students created their animations in a social environment; they exchanged their ideas, while at the same time providing support to each other, which guides each person to clarify his or her views of scientific concepts. In the methodology of this research, the choice of group work in constructing an animation was influenced by socioconstructivist theory and Vygotsky's (1962) sociocultural theory.
Literature review
How molecules and atoms behave during physical and chemical changes is not easily shown and illustrated in chemistry. This submicroscopic world of chemistry is visually and conceptually challenging. A sound understanding of chemistry requires agreement about the three levels of representation and the ability to learn chemistry with symbolic representations, but students often use symbolic representations without understanding the meaning of the chemical concepts associated with these representations (Nurrenbern and Pickering, 1987). Chemistry educators (Gabel et al., 1984) used a static drawing test to determine prospective elementary teachers’ views of the particulate nature of matter before giving instruction about the topic. In a test, they were asked to draw what happens to atoms and molecules after physical and chemical changes occurred. Most students are unable to visualise the macroscopic and symbolic representations and lack understanding about concepts in chemistry especially about the particulate nature of matter. They discussed that the results could be because of the static drawings because particles exist in a three-dimensional dynamic world. According to Johnstone (1993), transitions between these levels are easy for chemistry experts but students have difficulty in understanding the connections across all three levels at the same time, in part, because of high cognitive load involved in doing so. A number of researchers (Williamson and Abraham, 1995; Dori et al., 2003; Barak et al., 2011) have argued that working with animations that articulate transitions between these levels contributes to higher-order and critical thinking that enhances learning of science.In recent years, animations have become invaluable visualization tools, enabling the learning of science concepts involving dynamic aspects of chemistry. They can help students to move between the submicroscopic chemical level (molecular world) and the macroscopic chemical level (observable world). Animations have been used to represent the behaviour of molecules (submicroscopic level) during phase change (macroscopic level) and to assist students with conceptualizing the transition between levels (macroscopic, submicroscopic, and symbolic).
Studies indicate that the use of dynamic animations in chemistry helps students to develop their conceptual understanding on those specific topics associated with dynamic processes and on specific problems on tests using particle diagrams to assess conceptual understanding (Chang and Quintana, 2006; Hübscher-Younger and Narayanan, 2007; Williamson, 2008; Udo and Etiubon, 2011; Akaygun, 2016). At the submicroscopic level of chemistry, showing the dynamic nature of molecules with static pictures is difficult. Animations can show the dynamic features of a situation “directly and explicitly” (Hübscher-Younger and Narayanan, 2007, p. 235) and help students to visualize dynamic chemical processes (Chang and Quintana, 2006).
Although Udo and Etiubon (2011) report that student-generated models and representations are common features of science teaching, the inclusion of student-generated animations on a regular basis is rarely done, if at all, at the secondary school level and college level. There is little research on the ways in which student-generated animations are integrated into overarching teaching approaches. Animations promote an active learning environment, visualization of chemical processes, interpretation and reasoning (Papert, 1991; Akaygun, 2016) if combined with an appropriate instructional activity. In addition, the construction of software animations helps to make learning more enjoyable, practical, motivational and encouraging (Chang and Quintana, 2006).
In a study conducted by Hoban et al. (2009), using the Slowmation (abbreviated from ‘Slow Animation’, which is a simplified way for students to design and make a narrated stop-motion animation to explain a concept or tell a story) software program, students created a Slowmation on a chosen topic that had been assigned at the beginning of the course. The results showed that the majority of students had a significant increase in scientific content knowledge. This finding is consistent with the argument that creating animations improves students’ content knowledge in learning, because students come to understand the concept as they create an animation. This result also supports the view that when students create their own animations, they will learn more effectively, through sharing their understanding of a concept with others (Hoban and Nielsen, 2010, 2014). Hoban and Nielsen (2010) also studied Slowmation as the animation software program used by pre-service teachers and found similar results.
A study of student-generated animations was conducted by Chang and Quintana (2006). This study used Chemation, a simple 2-D modeling and animation tool for handheld devices, which allows students to create their own animations about science concepts and evaluate their visualizing, interpreting and reasoning skills. The results of the pre- and post-tests showed that student-generated animations, as an instructional aid, have an encouraging effect on the learning environment of middle-school students, and the authors argued that animations facilitated the visualization of dynamic and abstract concepts.
Research questions
The research questions guiding this study were:• How do interactions between a teacher and students and peer-to-peer interactions influence learning of states of matter when students’ attention is focused on representing and discussing dynamic processes related to the particulate nature of matter?
• What components of an instructional lesson on states of matter influence the learning of states of matter and fosters process skills?
Methodology
This study explored an intervention based on a teaching sequence that included training in the software program, creating animations and an analytical discussion of animations. In this intervention, students created their own animations to illustrate their views of science concepts.Design
The research design employed a mixed methodology, incorporating both qualitative and quantitative data gathering methods (Creswell and Plano Clark, 2007). An advantage of this mixed method approach is that it has potential to provide ‘a more comprehensive picture of what is being studied’ (McMillan and Schumacher, 2006, p. 401). Qualitative data collection included in-depth interviews, observations and video recordings. The study also drew on quantitative methods to gather evidence of learning outcomes by employing statistical analysis of pre- and post-tests. Thus, the nature of data collected in this research is not confined to either qualitative or quantitative research alone in seeking answers to the research questions (Creswell and Plano Clark, 2007).This research has also been informed by a case study methodology, in that it investigates a bounded system (Stake, 2003). The research is bounded (a) by location; (b) by time, a period of four weeks was available to refine and conduct the intervention with the teacher; and (c) by the curriculum context, namely, states of matter. This research reports on the analysis of an active learning instructional unit design to teach students how to represent three states of matter: solids, liquids, and gases at the particulate level of matter using static particle diagrams, student-generated animations, expert animations, and discussions of these with peers and the teacher. This topic is fundamental to the nature of chemistry itself, and it is therefore important to explain and illustrate dynamic phenomena in chemistry. The topic was suggested by the research partner/teacher. Multiple data sources were used in this study, including interviews, focus groups, video records and artefacts.
Participants
A preparatory pilot study for this research was completed in Australia two months before the research was undertaken. The research was conducted in two senior chemistry classes in Turkey. Teachers and students were invited to participate in the research and were provided with information about the research including advice that participation was voluntary. The participants were 28 Year 11 (16–17 years old) science students taught by the same teacher. There were eleven groups (pairs or groups of three) in this research. None of the students had previously used the animation program in a science class.The intervention
There were four phases (see Table 1), in total, of this research project, and this article reports on the intervention used in the first two phases (Phases 1 and 2) of the project. Phase 1 (2 lesson periods) and Phase 2 (2 lesson periods) were implemented in four lessons of 40 minutes with each class. The researchers discussed the theoretical basis for the lesson design with the teacher and the teacher performed all other activities, after discussion with the researcher.| Period | Time | Interventions | Instrument | Analysis methods |
|---|---|---|---|---|
| Phase 1 | 2 class hours | Pre-test | ESSA with added drawing question | SOLO taxonomy, statistical quantitative analysis |
| Introduction to K-Sketch animation | K-Sketch software program | |||
| Phase 2 | 2 class hours | Producing science animation in groups, student group discussion, teacher-facilitated active learning | K-Sketch software program, observations, audio and video recordings | Coding the students’ videotaped and audiotaped dialogues with partner and the teacher, qualitative data |
| Phase 3 | 2 class hours | Watching group animations and expert animations, discussions, teacher-facilitated active learning | Observations and video recordings | Coding the students’ videotaped dialogues, qualitative analysis |
| Phase 4 | 1 class hour | Discussions of the teaching and learning from Phase 1 to Phase 3 | ||
| After Phase 4 | Post-test | ESSA with added drawing question | SOLO taxonomy, statistical quantitative analysis | |
| Interviews with students and the teacher | Semi-structured stimulated recall interview questions | Analysis of transcript data, qualitative analysis | ||
| Students’ K-Sketch animations | ||||
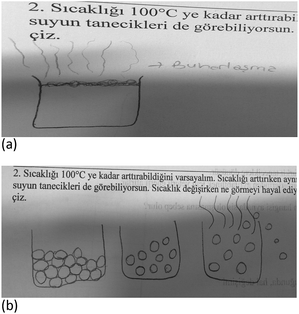
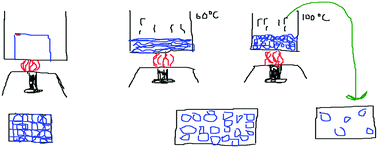
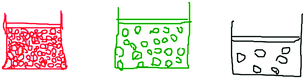
Phase 1 (80 minutes) was used to conduct the pre-test and then the K-Sketch (URL-1) animation program was taught to each class. In Phase 2 (80 minutes), the students attempted to create science-related animations, using the K-Sketch animation program, to illustrate their views of states of matter. They were asked to draw the three states of matter: solid, liquid and gas. In particular, they were asked to imagine that they could see into matter with an impossibly powerful microscope, to see what matter was made up of and what it was doing. They were then asked to draw ‘what they imagine as an animation’.
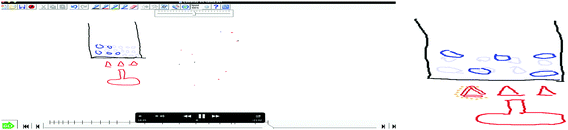
Finally, the teacher guided a reflective classroom discussion of the animations in Phases 3 and 4 (Yaseen and Aubusson, 2018). In Phase 3 (80 minutes), students had interactive discussions by presenting their animations on the board. After each group's animation had been shown and the discussion completed, the students watched expert animations created with K-Sketch (Fig. 1) as well as animations created by other software programs. All these expert animations illustrated the concepts integral to explaining ‘states of matter.’ The teacher led a discussion that provided an opportunity for students to think more about the changes of state and to see the similarities and differences between their animations and the ‘expert’ animations. In Phase 4 (40 minutes), students commented on and critiqued the teaching and learning that they experienced in all phases. A post-test assessment and student interviews were conducted after the lesson sequence. Finally, the teacher was interviewed for an hour, about her perception of the effectiveness of student-generated animations in supporting students’ learning.
Version 1.0 of K-Sketch software (2013) was used by students to construct the animations in the students’ representation creation process. It is a ‘free-hand’ animation program and not a science-tools program with predetermined scientific representations. So, it provides maximum flexibility to students. K-Sketch was also suggested by Akaygun (2016) because the students produce the animations faster compared to other animation software, such as Chemsense, with a significantly lower cognitive load. Conversations between the teacher and students and interactions among the students were videotaped and audiotaped during the students’ animation-creating process. Semi-structured (see Appendix B) stimulated recall interviews (McMillan and Schumacher, 2006) were held with all student groups and also with the teacher. Audiotapes of the interviews were transcribed.
Data collection and recording procedures
Both qualitative and quantitative methods were incorporated into the research methodology. The data sources included the Essential Secondary Science Assessment (ESSA) extract questionnaire (pre- and post-tests), the K-Sketch animations produced by students, videotaped and audiotaped recordings of student interactions during the animation creation process, and interviews with students and the teacher. Quantitative pre- and post-test data were used to explore the possible changes in students’ understanding with descriptive statistical analysis (McMillan and Schumacher, 2006). The following sections describe each data source.Essential Secondary Science Assessment (ESSA) questions were chosen as pre- and post-tests. ESSA is a science assessment program in NSW, Australia. It is based on the Years 7–10 science syllabus. It helps students ‘to see the relevance of science and to make meaning of scientific knowledge, understanding, skills, values and attitudes’ (NSW Public Schools, 2014). This test was chosen for the pre- and post-tests because it subjected student respondents to extensive expert scrutiny and analysis based on a well-established framework (SOLO) that allowed the quality of responses to be validly differentiated.
In ESSA, experts assess students’ answers using the SOLO (Structure of the Observed Learning Outcome) model over several years. The SOLO taxonomy has been identified as a recognizable progression in the research literature (Biggs, 1995). Therefore, it helps the researcher to identify features of students’ responses that are described in the marking guidelines (see Appendix A) and apply them for accurate and consistent marking. Six questions assessing student understanding of particular theory of matter were taken from the ESSA test. The students were asked to choose the correct answer from multiple-choice box-ticking questions concerning science experiments, including some about heating ice, or for some questions, to provide a written response. The researchers included a final question, inviting students to use their imaginations to explain the ice-heating experiment. In this last question, students drew the molecules as temperature changes, rather than explaining in words.
Taken together, the answers to these questions provided information about the students’ scientific knowledge about states of matter, before and after the instructional unit.
Data analysis
All audio and video recordings and transcriptions were in Turkish and then translated into English. The data transcripts were analysed and reported in English. A Turkish–English bilingual speaker back-translated them into Turkish to check the translation.Data analysis started with coding the students’ videotaped and audiotaped dialogues. In this coding process, the researcher used the transcribed student–student (partner) and teacher–student interactions during the animation creation process. The coding was checked by two researchers and disagreements in coding were resolved by negotiation. Both researchers were chemistry educators and had experience in ESSA marking and coding.
Student's drawings were categorized according to adherence to simplified elements of The Particle Theory of Matter: The Kinetic Molecular Theory (KMT). Each student's drawing was categorised according to whether or not it included representations of the KMT postulates, and then the number of postulates in the pre-test and post-test were calculated for comparison purposes. Specifically, students’ drawings were coded according to their emphasis on the movement of particles, based on the following five postulates (NSW Department of Education and Communities, 2011):
(1) All matter consists of extremely small particles.
(2) All particles of one substance are identical.
(3) The spaces between particles are very large compared with the size of particles themselves (in gases).
(4) The particles in matter have both attractive and negative forces between atom and molecules.
(5) Gas and liquid particles of matter are constantly in motion. Particles in a solid vibrate (jiggle) but do not generally move from place to place with no net translational motion.
Open-ended questions in the ESSA Questionnaire (see Appendix A) were scored according to the SOLO taxonomy levels (see Appendix C). Students’ responses for both pre- and post-tests were analysed in each of the six levels of the marking rubric. The Wilcoxon signed-rank test was used to compare the number of postulates in the drawings of pre- and post-test answers. Each postulate was also compared for pre- and post-tests in terms of percentages.
Nonparametric statistics were used to analyse the numerical data. The Wilcoxon signed-rank test was selected for evaluating the students’ answers both prior to and following the students’ representation creation process, in terms of understanding the key aspects of states of matter. The Wilcoxon signed-rank test was employed to identify any statistically significant difference between the pre- and post-test results of students’ responses in the ESSA questionnaire. The results were then statistically analysed in a paired sample t-test and the p-value was calculated to test for a statistically significant difference between the students’ pre- and post-test results for the multiple-choice answers and for their drawings. For the descriptive analysis, the sum of the students’ tests scores and mean values were reported. In addition, for the descriptive analysis of Q2.1 and Q2.3, the inter-quartile range (IQR) was also reported and used to measure the spread of the data points in the data set. The generated plot shows the complete picture of the tendency of the data set, based on the pre- and post-test results.
The concept of trustworthiness is used in this study, rather than the concept of reliability, because the qualitative section is extremely thorough and the quantitative component was assessed using a reliable and confidential assessment criteria, the SOLO taxonomy (Biggs, 1995).
Results
This section draws together the research findings of this study. The students’ pre- and post-test results are reported, as are the audiotaped and videotaped data from the intervention. Following the pre- and post-test results, peer-to-peer interactions, teacher–student interactions and expert animations, the student and teacher interviews will be given in this section.Pre- and post-tests
The Wilcoxon signed-rank test and the paired sample t-test were used to compare the students’ pre- and post-test results. For the total of the students’ scores in Q1.1, 15 students (54%) chose the right answer in the pre-test and 26 students (93%) chose the right answer in the post-test for this question. The results indicate that there was a statistically significant difference between the students’ pre- and post-test results for Q1.1 (Wilcoxon signed-rank test, p < 0.05). However, the test results for Q1.2 show that there was no statistically significant difference between the students’ pre- and post-test results for those questions related to concepts that were not taught using student-generated animations (paired sample t-test, p = 0.157, Q1.3 Wilcoxon signed-rank test, p = 0.317, Q1.4 Wilcoxon signed-rank test, p = 1.000) (Table 2).
| Questions | Statistically significant difference between pre- and post-tests |
|---|---|
| Q1.1 | YES |
| Q1.2 | NO |
| Q2.1 | YES |
| Q2.2 | YES |
| Q2.3 | YES |
For the descriptive analysis of open-ended questions, the IQR was used to measure the spread of data points in the data set. This shows the central tendency of the data. The IQR was 3 in the pre-test results and 2 in the post-test results. While the 95% confidence interval for the mean was 2.06, i.e. in the lower bound, and increased to 3.38 for the post-test results, it was 3.42, in the upper bound, and increased to 4.45 for the post-test results. While the median was 3 in the pre-test results, it increased in the interquartile range to 4 for the post-test results. This shows the difference between the IQR of the pre- and post-tests. This indicates a statistically significant improvement in student understanding of states of matter from pre to post the intervention.
The Wilcoxon signed-rank test was used to compare the number of postulates in the drawings of the pre- and post-test answers to Q2.2. The results indicate a statistically significant difference between students’ pre- and post-test results based on the number of postulates included (Wilcoxon signed-rank test, p < 0.05). An example from the pre- and post-test drawings of one student is described and shown below.
In addition to the number of all postulates, each postulate was also compared for the pre- and post-test in terms of percentages (see Table 3). The pre-/post-test results indicated that students’ understanding of states of matter improved with the implementation of active learning, which included the construction of student-generated animations and the viewing of expert animations, combined with discussion and analysis of the views underpinning these animations, with peers and the teacher.
| KMT postulates | Pre-tests (%) | Post tests (%) |
|---|---|---|
| 1 | 84.2 | 94.7 |
| 2 | 36.8 | 73.7 |
| 3 | 21.1 | 57.9 |
| 4 | 0 | 26.3 |
| 5 | 15.8 | 68.4 |
Even though students had ideas about what they were drawing, they needed to ask their partner about concepts about which they were unclear. Sometimes they needed advice or confirmation, but they also needed to learn concepts about which they were unsure. Students discussed both the representation and the underlying concept to which the representation was giving meaning.
Partner interactions had different shapes in each dialogue. In some groups, giving instructions or orders to the partner took place, rather than offering conceptual explanations. Some of them gave directions to their partners without any explanations. They tried to complete animations according to their thinking or understanding and did not ask for their partners’ ideas while drawing the animation. In addition, the students in groups often agreed with each other and respected the opinions of their partners. They also understood that each partner could freely contribute to the group.
Some partner interactions helped the students to become aware of deficiencies in their conceptions of the states of matter, particularly about the motion of particles. They used the word “should” and then they added the new information (see Table 4). Some group partners offered advice to each other while working towards a common aim. They tried to advise their partner on exactly what they had planned. For example, S24 gave advice to S12 about the movement of particles using sentences containing ‘should’ and continuing with ‘because’. His suggestions were about both the submicroscopic and macroscopic interpretive levels of the phenomenon. He mentioned that the movement of gas particles was at the submicroscopic level and steam above the liquid was macroscopic (see Table 4). In some cases, the other partner had not thought about the ‘motion of particles’ until he heard it from his partner. He may not be aware of it. When he heard the word ‘motion’ he/she was silent for seconds and tried to delete the animation and start again but his partner did not want to do this. After failing to show motion on the initial animation, they deleted it and created a new animation.
Students created their animations using a software program (K-Sketch), which meant that they had discussed with their partners not only conceptual ideas but also technical issues, such as drawing pens, saving buttons, the speed of animations and the colours of molecules (see Table 4).
The next section explains and gives examples of the teacher's guidance of the students’ representation creation processes. The teacher generally led the conversations about phenomena to clarify the students’ understanding.
Teacher's guidance during the animation creation process
The teacher actively guided the students and provided scaffolding to improve and organize their understanding through questioning, shaping and assessing their representations. The analysis of the teacher's scaffolding was categorized into the following categories (see Table 5): (1) repeating a student's previous sentence and asking further questions; (2) reminding students of what they are attempting; (3) clarifying the student's claims; (4) summarizing their drawings and eliciting information; (5) encouraging them; (6) providing additional direct information; and (7) highlighting significant omissions and recommending changes to address them.| Teacher–student interaction | |
|---|---|
| Categories | Dialogue with coding words |
| Repeating a student's previous sentence and asking further questions | S7: The most irregular pattern will be in the gas phase. |
| T: Highly irregular pattern? And what happens? | |
| S4: The capacity of movement increases. | |
| S7: In other words, its kinetic energy increases. | |
| T: Simply, its energy increases. | |
| Reminding students of what they are attempting | S24: Do we need to show the particles’ molecular structure? |
| T: Yes, you are imagining that you are looking through the microscope at solid, liquid and gas. | |
| T: How do you want to draw it, you should draw it as you wish. You will show solid and also molecules of a solid. In other words, you will show their microscopic views, and what the solid molecules are doing inside their solid or liquid and gas states. | |
| Clarifying the student's claims | T: What are you trying to show? |
| S1: There are strong interactions between solid particles; therefore, I am drawing the bonds. | |
| Summarizing their drawings and eliciting information | T: No, no, I mean that in the solid phase, are these molecules of solid or solid matter by itself? |
| S20: They are solid matter; we have not drawn solid molecules yet. | |
| Encouraging them | T: Oooo! Looks like you have pretty much improved your animation. Explain to me what happened, what has changed? |
| T: What did you do? | |
| S5: We were drawing liquid. It was solid before, then turned to liquid and it is going to be gas now. | |
| Providing additional direct information | S9: Is something missing? |
| T: What can we add? Your other classmates tried to add motion. | |
| S9: Motion? | |
| T: Vibration to solids. | |
| S9: Really? (very surprised). | |
| S6: That is ridiculous, because matter is always in vibration. | |
| T: In the solid phase? | |
| Highlighting significant omissions and recommending changes to address them | T: Your visualization in the animation should be good. I thought that you would draw like this, because you mentioned vibration. If these ones had vibration, we would have good results. (After a while the teacher goes near the students again, to check whether they drew vibration or not) |
| S4 and S7: (Silent) | |
| T: Did you give it motion? | |
The teacher sought to support students during the animation creation process. While the students were creating their animations, she gave them different kinds of guidance.
First, she focused on the students’ explanations. While students were talking about their animations, she interrupted them by repeating their last sentence to confirm their understanding (see Table 5).
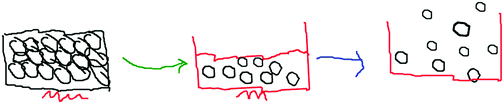
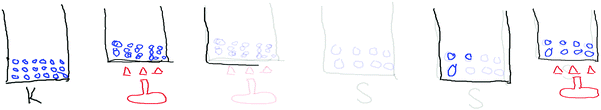
The teacher observed the students while they created their animations and as they discussed them with their partners. She reminded them that they were not only creating animations at the macroscopic level, but also at the submicroscopic level. Therefore, they should not forget to look through an immensely powerful imaginary microscope, while creating the solid, liquid and gas animations. She wanted to make sure all the students understood that they were creating submicro-level drawings of particles. She came to each group and reminded them of this (see Table 5). After the teacher prompted them for the submicroscopic view of particles, they added bonds to their particles (S1 and S8 animations) in Fig. 3.
The teacher also tried to interact with the groups by asking what they were doing or what they were creating. She encouraged the groups to make claims, by asking them what they were doing at that moment. S1 answered “There are strong interactions between solid particles, therefore, I’m drawing the bonds.” The pair were drawing the distribution of solid particles and explained that they had strong interactions (see Fig. 4).
The teacher also summarized what she understood from the groups’ animations. Her aim was to compare the ideas the students understood from the animations, and what they had tried to draw and claimed. She did not seek to tell students the right answers while she was summarizing their animations. Rather, she sought to understand their current thinking about each state of matter. Students were expected to make a reasoned claim about the concepts they were drawing. The teacher began a discussion during the animation creation process, by questioning students about the rationale of their drawings.
The teacher gave positive feedback that focused on students’ improving generally rather than on specific conceptual outcomes. In the above example, even though the teacher encouraged S3 and S5, they did not want to talk about their animations.
The students’ engagement with their animations clarified their own ideas about states of matter. Therefore, the teacher did not usually provide conceptual information to students during the animation process. However, in a few student–teacher interactions, the teacher did provide conceptual information to the groups (see Table 5). She explained the vibration of solid particles and how to add motion to them, such as spinning.
The teacher used scaffolding and teacher–student discussions to guide students during the animation creation process. However, a few times she influenced the students to change the way they imagined states of matter to be. The teacher reported, “Some students got the idea very quickly; some students had difficulty in understanding what to do.”
Expert animations
The intervention in this research has approached the topic differently, with the students watching the expert animations only after they produced their own animations. They critiqued the expert animations, highlighting the similarities and differences between the ‘expert’ animations and their own animations. They distinguished not only the different parts of the animations but also when their ideas about the concepts were unclear or inconsistent with the expert animations. Even though students were initially unsure about how to critique their classmates’ animations, they gained confidence during this process and learned how to critique even the expert animations.When they watched the expert animations, they realised that the changes of state as represented in their own animations were different from those of the expert animations. They identified those attributes of their animations and their ideas about changes of state that were unclear or inconsistent with the expert representations. As they later explained in the interviews, they learned about states of matter by watching the expert animations and comparing their represented views with those represented by experts.
They indicated that watching the experts’ animations was an indispensable part of learning through the animation creation process. Many expressed surprise that they still lacked understanding of these concepts despite having studied them many times at school. For some, the experts’ animations allowed them to recognise deficiencies depicted in their own animations and their misconceptions about the arrangement of particles in liquids. Many had represented their ideas accurately, only to realise that these ideas were wrong. Others became able to distinguish between ideas that were conceptually correct but representationally inaccurate due to difficulties in drawing them.
Students also revealed that it was helpful to see the expert animations after they had critiqued their classmates’ animations. Some groups changed their ideas after watching the expert animations even though they had been satisfied with their animations after the classmate discussions. For example, even though some students claimed they understood the motion of particles on each state, they became aware of the correct arrangement of particles within the liquid phase after watching the expert animations.
In summary, the students claimed they could not have learned the correct concepts about states of matter without having participated in the discussions that followed the creation of their animations. Without their own student-generated animations, it would have been impossible to imagine or think about the concepts in the same way. The pedagogic value came from the combination of both creating the animations and having the follow-up discussions.
Students’ interviews
S8: because we exchanged our ideas, our thinking…For example, I asked S1 about molecules. She explained that oxygen will be in the center, others will be hydrogen.
Then S1 asked me whether the drawing of solid molecules is round or rectangular.
S12 and S24 indicated that they perceived learning to create their animation was useful. In particular, they commented that animating with their peer helped them to identify misconceptions. S24 explained:
S24: We had an opportunity to see our mistakes and we learned how to make (animations) correctly. We made connections between visual and oral and brought them together. …when we visualized them, we thought about how they are moving, how they are. We transferred our thinking to there (the animation).
S24 also explained that drawing the animation helped them to visualize submicroscopic conceptions of states of matter. He commented on water changing states at the macrolevel. He explained that the peer process of creating the animation enabled him to go beyond merely recognizing that water changes from liquid to gas (or steam) to think more deeply at the submicroscopic level. It helped them to show their views and exchange ideas with others, which contributed to students’ science understanding.
When asked about partner interaction in the interview, S7 claimed:
S7: I mean movement not only in one particle, the whole kinaesthetic movement idea. I learned the idea of the whole movement of sub-molecular particles from S4.
This shows that S4 contributed new ideas to S7's conceptualization of states of matter. S4 stated:
S4: Actually, the idea of the movement of molecules is from S7.
S4 explained that she learned about the movement of particles from her partner S7. They both agreed that without partner interaction, their animation would have been totally different.
In another example, S20 mentioned that he and his partner tried to convince each other about the arrangement of particles. S20 stated:
S20: I said let's draw this (without spaces) as a liquid but my partner said no we should show the spaces between molecules. I said No and I won.
Shortly during partner interactions, each partner asked questions and requested advice about unclear concepts. The learning and knowledge were thus constructed through social interactions in groups. Except for two students who had passive partners, all acknowledged that the group work would have been less enjoyable without their partners’ contributions.
S8: For example, I asked her how we could draw molecules. My teacher explained to us but we still couldn’t draw them.
S5: We had drawn them like this, she came over to us and told us to be more explanatory, explain more, but we didn’t listen to her. She told us to redraw it, but we continued as it was.
S7: She (the teacher) gave us some ideas. For example, she guided us on the motion of particles. She said you can vibrate them, or you can give different movements to the particles. If our teacher hadn’t guided us, we would have given direct motion to the molecules not vibration.
In the interview with S7, it is evident that the teacher contributed critical scientific information to the group, that is, ‘the vibration of solid molecules’.
These responses show that the teacher intended not to give additional information to students, but some of her questions and statements provided direct information to extend students’ dynamic representations.
Teacher's interview
Students’ interviews showed that those students who had a more comprehensive understanding of the concept claimed that the teacher supported them and that the teacher's support was important. In addition, when the researcher asked the teacher about her support, she commented that she thought that it was necessary. She explained that the teacher is a ‘power’ in the class and used her position to help students to correct their misconceptions while they were drawing. She stated:If I were not there, they might have corrected their misconceptions, but they might not, too. I helped them while they were creating their animations, I mean while they were thinking, I helped them to think differently and more conceptually. I never said anything directly about the concept, but I gave additional information to those groups of students. I tried to ask open-ended questions. For example, one of the groups was thinking that solid particles have more spaces than liquid particles. I asked open-ended questions like, ‘How do solid particles behave differently, think again, than liquid particles?’
She explained that they were always thinking when creating their animations; therefore, they learned the concept being studied more effectively and in the end, the group developed a more acceptable view of the arrangement of particles. The teacher judged her students only based on their ongoing formative assessment (what she saw and heard) during the animation creating process.
Until they create for themselves, like until they get the pen in their hands, everything is blurry.
She explained that students did not have enough conceptual knowledge, even in the basic concept of “states of matter” before the process of creating an animation. They created animations themselves, so they corrected their mistakes by themselves.
The teacher signalled her intention to use student-generated animations in future teaching:
I want to use student-generated animations in my chemistry classes, because while they were drawing, they started to think and imagine, and then to think about their imaginings.
She also wants to use this whole animation process for her other students:
I wish we had intervened with this animation creation process with my Year 9 and 10 chemistry students as well, as I saw that the students were getting deep understanding of the concepts when they created [animations]. I’ve learned the program now and have an idea of the whole learning process. I will try the same process with the Years 9 and 10 students. I really think that animations contributed to the students’ learning.
In fact, the students started the lesson with non-scientific views of the states of matter and while they did improve their understanding, the facts are clear that the students did not develop a “deep conceptual understanding of the states of matter”. The students’ understanding of the states of matter was still at a primitive level.
The teacher also commented that the partner interactions were an effective feature in the learning process. She explained that while students created their animations in groups, they “thought aloud” and their partners listened to their thinking. Therefore, they corrected their mistakes and they added new information to their partners’ ideas during the group discussions. The teacher also added that if students created the animations individually, their ideas would probably not change.
The teacher said the whole process would not have been effective if the students had created their animations without discussing and watching the animations of the other students and the experts. She also noted, ‘When students learned the concept well, they critiqued expert animations. When they really reached the conceptual understanding, they would have critical thinking about concepts.’ She said that when students watched an expert animation, ‘their thinking process started again’ as they asked how they can use the features of the expert animations in their own animations. The expert animation helped them to learn the details of the concept being studied.
In addition, she claimed that after students had drawn their own animations, they gave more attention to the expert animations as they sought the similarities and differences between their animations and those of the ‘expert’ animators. She therefore preferred using student-generated animations rather than expert animations alone because while students are drawing, they start to think and imagine, and their cognitive engagement increases.
Discussion
This section discusses the findings from a wide range of data collected in this study. This is contextualised in relation to the two research questions. The implications of the study for science teaching and learning are discussed. Potential areas for future research are then identified before the concluding statement about the research is elaborated.How do interactions between a teacher and students and peer-to-peer interactions influence the learning of states of matter when the students’ attention is focused on representing and discussing dynamic processes related to the particulate nature of matter?
The results of this study suggest that the students developed their understanding of chemical concepts from participating in this process. In particular, the pre- and post-test results showed that they developed an expanded knowledge of chemistry and states of matter, when they used the software animation program to construct their own representations.All students were actively engaged during the animation creation process, through which this intervention was applied. The students commented that they had created better animations in small groups than they would have as individuals, with small group discussions helping them to think through ideas underpinning their concepts. While creating their animations, students had friendly discussions, were smiling, and sometimes singing. When they disagreed with each other, most tried to negotiate, and eventually found common explanations and reasons on which to base agreements.
While there is evidence that individually creating or drawing chemistry images helps students to improve their visualization and education at the microlevel (Eilam and Poyas, 2010), this article suggests that the use of student-generated representations, in combination with analysis of animations by students’ own critiques and discussions, supports the learning of fundamental basic chemistry concepts, in this instance, states of matter.
When novices are relatively unfamiliar with the software they are using, the animations they generate will never be able to represent their visualizations accurately. However, errors in an animation or representation do not necessarily mean that its creator has the particular misconceptions suggested by the animation. The animation is needed as a tool for the imagination. Students’ attempts to imagine what happens as matter changes states make them more receptive to learning from others. Continuing dialogue that includes public and open acceptance appears important for exploring and developing ideas.
All of the students said they preferred using the animation software and found it more enjoyable and encouraging than traditional learning methods they had experienced. For example, one student claimed that she preferred using student-generated animations rather than expert animations alone.
These experiences are consistent with those reported by other researchers. Chang and Quintana (2006), for example, concluded that student-generated animations have an encouraging effect on the learning environment of middle-school students. Barak et al.'s (2011) also reported that students who studied science with animated movies had more motivation to learn and enjoy science than students who studied with traditional methods.
Students worked in groups while they were creating their animations. Student interviews and videotapes of their interactions show how they interacted with partners to clarify chemical concepts and gain advice, information and confirmation. Interactions appeared effective when pairs of student partners engaged in discussions and shared ideas about a concept. They learned through the interactions in their group, and they expanded their thinking to their partners, because each group member had a different internalization of knowledge, depending on their existing knowledge (Powell and Kalina, 2009). In this study, each partner asked questions and requested advice about unclear concepts. The learning was facilitated through social interactions in groups, while they were explicating their ideas via creating scientific animations. They shared their ideas and socially negotiated meanings, as they realized that there are various ways, other than their own, to solve problems or reach a conclusion.
According to Jonassen, Peck and Wilson (1999), collaboration among students helps their personal growth and contributes to high levels of student satisfaction; improves both their relationships with others and their communication skills; increases motivation, knowledge and respect of each other's opinions; and helps them to learn to listen respectively. This is consistent with Stieff et al.'s (2005) assertion that students using a visualisation tool in group work share their ideas and learn each other's alternative views about an idea or concept. It is also consistent with a socioconstructivist view on learning: students interacting in a group construct knowledge for each other and create a small culture to solve a given task collaboratively (Richard and Audrey, 2004). The learning and knowledge were thus constructed through social interactions in groups.
These results are consistent with Vygotsky's (1981) theory of learning through social interactions and the research of Barak et al. (2011), who, as mentioned earlier, found that students who studied science using animated movies increased their understanding through subsequent group discussions. They shared their ideas and socially negotiated meanings as they realised that there are various ways, other than their own, to solve problems or reach a conclusion.
The socioconstructivist perspective on student learning also highlights the critical role of interactions among peers for their cognitive development (Prawat and Floden, 1994; Gredler, 1997; Ernest, 1998). This accords with the students’ and the teacher's claims and is consistent with Jonassen et al.'s (1999) assertion that when students work together and discuss in groups rather than doing individual work, they develop their cognitive strategies and knowledge about what they are doing, along with their understanding about why they are doing it.
Of the 11 groups, 10 groups mentioned learning about spacing between particles in a liquid state. They were all satisfied that they had learnt a more correct view of the arrangement and movement of particles. They explained that prior to the animation lesson sequence, they were not aware that liquids do not have spaces between particles.
S18: There were some mistakes or misconceptions in some group's animations. For example, if we didn’t discuss the movement of gas particles that were including easier flow, we couldn’t get appropriate understanding of the concept. Our mistakes could not be corrected. I think it was good.
The teacher also reported that there was useful discussion among students, which encouraged them to think about the concepts. The teacher became aware of students’ misconceptions and otherwise hidden understanding, while they were creating their animations. This negotiation of meaning is supported by the sociocultural perspective of learning.
What components of an instructional lesson on the states of matter influence the learning of states of matter and fosters process skills?
The animation is needed as a tool for the imagination. Without engaging in the process of drawing the animation to represent their views, students tend to be concerned about the risk of being lost in their own ideas and being relatively unproductive as learners. Their attempts to imagine what happens as matter changes states makes them more receptive to learning from others, particularly the experts’ animations. This is also related to the critical initial step in constructivist learning where learners articulate their ideas (Maxim, 2013). Continuing dialogue that includes public and open acceptance appears important for exploring and developing ideas.According to the teachers and students, however, watching expert animations alone would not have been as helpful for learning had they not already constructed their own animations. This is broadly consistent with Hoban and Nielsen (2013) claim that creating animations improves students’ content knowledge of chemistry and students need to think in multiple representations of the same concept in order to reach the final representation. This study indicates that producing and critiquing a variety of student and expert animations contributed to learning and created an opportunity to see multimodal representations.
The teacher observed the students while they were using the K-Sketch animation program and discussing their animations with their partners, and reminded them of what they were attempting if they deviated from representing their personal conceptual understanding. She asked questions and summarized their drawings to help them improve and organize their interpretation of the concepts. Her scaffolding of the groups, as they were animating, helped to elicit the students’ current views of science and to correct their misconceptions. Asking them to make reasoned claims about the concepts is consistent with reports indicating that verbalizing claims when drawing helps students to synthesize concepts (Tytler et al., 2013). In this study students were required to explain and elaborate their ideas about science phenomena, the reasoning underpinning these views and the ways in which these ideas are being illustrated in their animations.
While the students were creating the animations, she encouraged them to be confident in exposing themselves to the risk of being wrong and to show their thinking freely. She explained that there would be “nothing wrong in their animations”, “there would be only different ways of expressing their ideas”. She also explained that they “could ask any question if they were unclear”, and she “would be their guide”.
For the most part, the teacher intervened gently during the student-generated animation process. She gave students the task, encouraged them to collaborate, and as she walked around she asked what they were doing. She helped students to identify, discuss, explain and map the similarities and differences between students’ and experts’ animations. With this sort of mediation, the learning was more valuable than if the students had viewed the experts’ animation alone.
The discussions about how the students saw the matches and mismatches between their own animations and those of their classmates and the experts appeared to be an essential element of the learning process. The teacher's comments and the students’ post-test results show that this may help the students to focus on the main concepts to be learned and to minimise the generation of further misconceptions.
Limitations and further research
This study was originally planned to be conducted in three different colleges, but two schools withdrew from the research at short notice. This reduced the sample size and prevented comparisons with various forms of the intervention. The sample size (N = 28) was therefore limited by the availability of the students in the remaining college and how many there were in the teacher's classes. The results drawn from the data cannot be generalised to other learning settings and grade levels or to all teachers. Rather, the reader needs to consider the context of this study and determine the confidence with which its findings might be applicable to guiding practice in other settings (Aubusson, 2002).The following suggestions are offered for further research in this area.
First, implementing distinct parts of the intervention separately, or repeating the study with selected elements of intervention being removed, could yield valuable insights into the relative importance of different phases of the intervention. Examples could include an analysis of the expert animations only or of the students’ construction of their own animations without taking the experts’ animations into account. The intended comparison with a class using student-generated animations alone would also be a useful next step. Second, research with a comparison group of students who draw but did not animate would tease out the role of dynamic representations in this group's understanding of science concepts. Third, because this research studied only one concept and this was limited to only three states of matter, it would be productive to investigate other key concepts in fields additional to chemistry. Fields involving dynamic, microscopic and macroscopic processes, such as physics, geology and biology, are difficult to visualise. The findings of such investigations should inform the extent to which students may benefit from generating animations in these fields. Fourth, it would be useful to repeat the process with the same class but with a different grade level. Further investigations such as these will add to our understanding of how to use student-generated animations combined with effective pedagogy to promote students’ learning in science.
Implications
The analysis of the teaching/learning process illustrates that combining the production of student animations may be an effective way of developing students’ understanding of states of matter. An implication of this research is that science teaching, in general, might benefit from the development of multiple ways (creating, discussing with peers and the teacher) of using student-generated animations.This study implies that when students create and analyse animations they seem more likely to be aware of the dynamic nature and motion of particles in the three states of matter, particularly vibration in the solid state. Teachers should consider the integration of animations that include motion and also enable their students to find opportunities to create them. Through such learner-generated animations, students can render their thinking ‘visible’ and help teachers take targeted action that supports students to understand relevant concepts. With regard to group work, while the students were working on the same task (generating science animations) they integrated their varied ideas and found themselves in an explanatory framework that helped them in developing their science understanding.
Conclusions
Student-generated animations and expert animations when supported by an active learning approach that included peer-to-peer and teacher-led discussions and analysis of animations, appear to have the potential to contribute to student learning about states of matter. This study identified how the interactions of students helped to clarify their understanding and misunderstanding, while they were creating animations about chemistry concepts. Because some scientific conceptions are much more difficult to illustrate in static drawings, the specific affordance of animation in this intervention is its capacity to represent their dynamic elements. Even so, it was challenging for students to show, in their animations, the molecular movement exactly as they intended, and this sometimes led to imperfections in their representations, rather than a demonstration of an inaccurate conceptualization per se. Aubusson and Fogwill (2006) have speculated that the errors and imperfections in student-generated analogies (in this study animations) create a locus for learning and that relatively poor student-generated animations may be better stimuli for learning than perfected animations. The inadequacies, inaccuracies and failings in the student-generated animations were useful, because they provided critical points for discussion and critique that enabled learning.Notes
The study was conducted according to ethical protocols approved by the Human Research Ethics Committee of the University of Technology Sydney.Some of the animations are available online at the following URL:
https://www.youtube.com/watch?v=OtozEmpeEgg
https://www.youtube.com/watch?v=IPz_4eTQIWc
Conflicts of interest
There are no conflicts to declare.Appendix A: pre–post test
Q1. At school, Alex investigated the heating of water using the following apparatus.1. What is happening to the particles of water as the water is heated?
□ They are getting bigger.
□ They are getting smaller.
□ They are moving more slowly.
□ They are moving more quickly.
2. Alex found that she could not heat the water above 100 °C. The best explanation is that, at 100 °C,
□ water particles chemically react.
□ water particles take up less space.
□ water particles turn into different particles.
□ water particles absorb enough energy to break free from one another.
3. Which process causes fog to form on a mirror?
□ melting
□ freezing
□ condensing
□ evaporating
4. When a mirror fogs up, the change of state can be shown as
□ liquid → gas
□ gas → liquid
□ liquid → solid
□ solid → liquid
Q2. A science class was doing an experiment to observe temperature changes when heating ice. Each group started the experiment with four cubes of ice and a small amount of water in a beaker. They heated the beaker, with constant stirring, over a low Bunsen burner flame as shown in the diagram below. They measured the temperature every minute and recorded the results in a table.
One group of students obtained the following results.
1. Using the information from the results table, describe what was happening in the first 9 minutes of the experiment.
2. Suppose that you are increasing the temperature until 100 °C and you can see the particles. Please draw what you imagine you would see as the temperature changes.
3. Using your knowledge of particle theory, explain why this happens.
Appendix B: interview questions
Appendix B1: interview questions for teachers
The interview will be audio recorded so that I can capture all peer discussions. However, you can ask me to turn the recorder off at any time during the interview – without giving me an explanation. I also assured that your identity and your students’ identity would not be revealed in publications or to anyone else.Students’ learning:
1. Firstly, Do you think that students exactly transferred their imaginations or thinking to their own animations?
2. Does animation creation process (including discussions before and during the animations) contribute to their previous learning or helped to learn new things about the concept?
3. Does creating animations help students’ understanding? How? Does the process of creation of an animation contribute to students’ learning? How?
Working in groups:
4. Was working as pairs useful for students’ learning? How?
5. What did students mostly discussed with their partners before starting animation? Is this discussion useful for students? Why?
6. If students create the animations alone, would it be different? What kind of differences would be in their animations? Is it not necessary?
Teacher support:
7. Did you support or only observe your students while they were creating animations? What kind of support did you provide for your students?
8. Did your participation to the animation creation process contribute to your teaching methods? How?
Implications:
9. Do you want to use student-generated animations in your teaching? How do you use animations in your classes?
10. Are animations really necessary for students to understand the…concept? What kind of other activities or teaching methods can help their thinking processes and help us to understand their thinking?
11. Can students also show their imagination without drawing animations? How?
12. Was creating animations helpful for you to understand students’ thinking?
Thank you for your contributions, is there anything else you would like to talk about before you leave or is there anything you want to ask me?
Appendix B2: student interview questions
Semi-structured interview questions (students)1. It was really interesting to watch your animation. How do you think that the animation process went?
2. What did you really mean in this part of your animation?
3. What does the space between particles mean? What is there between these particles? (empty space, air)
4. Do you really want to draw these spaces between particles? Why?
5. Why did you draw the arrangement of the particles in this way?
6. Why do the particles have different states? What makes them in different physical states? (attraction forces between the particles of matter, and the strength of these forces)
7. What does this motion show? (the particles in three states of matter are in continuous motion)
8. Why did you draw them in this shape (round, rectangular, dot, etc.) or different colour (H and O molecules)?
9. Is this drawing really your imagination? Is there anything that you would like to show better?
10. What did you learn about the states of the particles? Did creating an animation help you for this topic? How? Can you give an example of how this helped your learning?
11. Did creating an animation influence your thinking about the melting ice?
12. While you were creating your animation with your partner, did your partner influence your opinions? If yes how? Can you give an example?
13. Before starting to create an animation, what did you discuss with your partner? For example, did you discuss about the concept of matter and get agreement and start to draw or did you just talk about how to draw or did you directly start to create an animation without discussion and during the process did you talk about concepts? Did you explain to your partner about your thinking and your imagination?
14. Did you ask anything to your teacher? What did you ask your teacher? Did he/she help your learning? How? Can you give an example?
Appendix C
Table 6.| Level | Coding | Features | Samples of student responses |
|---|---|---|---|
| NA | No attempt | ||
| 1 | 0 | Meaningless attempt | In the first 9 minutes of the experiment, it went up in ones. |
| It was starting to change a fair bit. | |||
| u | 1 | Change of state (ice melts/solid changes to liquid) | Temperature is increasing. It has increased until the temperature was 89 °C. |
| Identifies one trend in the temperature data (at first the temperature stays the same/stays the same for the first 2–3 minutes/it heats up (rapidly) after 3 minutes/temperature rises/it gets hotter) | There is not much difference in the first two minutes, but then after a period of 3–9 minutes the temperature increases | ||
| One (or more temperature/time points) | After 9 minutes, it is 89 °C. | ||
| m | 2 | At least two U elements OR | In the first 9 minutes, the temperature increased from 1 °C to 89 °C. This happened because the flame heated the water and melted the ice cubes. |
| Identifies the two trends (but misses stating the link to all the ice having melted) | The temperature stayed the same for the first two minutes. Then it quickly got much hotter in every minute. | ||
| r | 3 | Makes an explicit connection between ice melting and the temperature beginning to increase/change | This happens because the ice has to melt first before it can start heating up |
| In the first 9 minutes…the temperature increases. It stayed the same for two minutes but after three minutes the temperature increases more than 10 °C per minutes…After all the ice melts the water…becomes hotter. | |||
| U | 4 | Heat increases the movement of particles | The particles in ice are not moving but when you heat it, the ice starts to melt and particles can move around |
| Heat increase the movement of particles in a liquid | Particles separate when it gets hotter so since the temperature has increased the particles separated and become water | ||
| Heat causes particles in a solid to vibrate (faster) | The heat gives the ice particles more energy so they move around more | ||
| Particles spread apart when ice changes to water | The heat can break the energy holding the particles together in ice | ||
| Heat is absorbed by ice particles as the ice melts | |||
| M | 5 | At least two U elements (NOT if they combine the first point with either the second or third one (they have to distinguish the two kinds of motion)) | When the particles are frozen, they stick to each other but when heat is added the particles move away from each other and move around. Therefore it goes from ice to water. |
| As a solid is heated, the particles start to vibrate. They vibrate more and more vigorously until the particles break apart and start to roll around. When all the particles are rolling around, the solid has become a liquid. | |||
| R | 6 | A cause and effect explanation for the change of state and subsequent rise in temperature | When heat is first added, it is used to overcome the strong forces of attraction holding the particles in ice together. The particles vibrate faster and faster, until they have enough energy to separate out and start moving around. At this point ice melts to form water. Then as more heat is passed, the particles move even faster and the temperature of the water increases. |
Appendix D
Learning objectives for students: (science content and practice)• Students being able to relate the macroscopic properties of matter to its microscopic properties (kinetic energy of molecules, random motion of molecules).
• Students being able discuss the changes of state in terms of the energy of molecules.
• Students being able to compare macroscopic and microscopic models of matter for each state.
• Students being able to understand how changing the temperature of matter changes the macroscopic and microscopic properties.
Process skills objectives
• Students being able to create a scientific animation on states of matter.
• Students being able to discuss with their partner (or groups of three) while creating their animations.
• Students being able to explain their scientific animations created by K-sketch to the whole class.
• Students being able to compare their animations with classmate animations.
• Students being able to compare their animations with expert animations.
• Students being able to criticize peers’ animations and expert animations.
• Students being able to discuss the properties of an ideal animation in the states of matter.
• Students being able to evaluate the learning process (at the end of discussions).
Appendix E
A list of the “other animation software programs watched by students” or URLs to these animations or a brief description of these animations. https://www.chem.purdue.edu/gchelp/atoms/states.htmlhttp://www.chem.purdue.edu/gchelp/liquids/boil2.html
https://phet.colorado.edu/sims/html/states-of-matter-basics/latest/states-ofmatter-basics_en.html
Acknowledgements
Funding of this research work was supported by the University of Technology Sydney, the Faculty of Art and Social Sciences, and the STEMEd Research Centre.References
- Ainsworth S., (1999), The functions of multiple representations, Comput. Educ., 33(2–3), 131–152, DOI:10.1016/S0360-1315(99)00029-9.
- Akaygun S., (2016), Is the oxygen atom static or dynamic? The effect of generating animations on students’ mental models of atomic structure, Chem. Educ. Res. and Pract., 17, 788–807, 10.1039/c6rp00067c.
- Akaygun S. and Jones L. L., (2014), Words or Pictures: a comparison of written and pictorial explanations of physical and chemical equilibria, Int. J. Sci. Educ., 36(5), 783–807, DOI:10.1080/09500693.2013.828361.
- Aubusson P., (2002), Using metaphor to make sense and build theory in qualitative analysis, Qual. Rep., 7(4), 1–14, retrieved from http://nsuworks.nova.edu/tqr/vol7/iss4/1.
- Aubusson P. and Fogwill S., (2006), Role play as analogical modelling in science, in Aubusson P., Harrison A. G. and Ritchie S. (ed.), Metaphor and analogy in science education, Dordrecht: Springer, pp. 93–104.
- Barak M., Ashkar T. and Dori Y. J., (2011), Learning Science via animated movies: its effect on students’ thinking and motivation, Comput. Educ., 56(3), 839–846, DOI:10.1016/j.compedu.2010.10.025.
- Barlex D. and Carré C., (1985), Identifying our beliefs about learning, in Visual Communication in Science, Cambridge: Cambridge University Press, pp. 1–21.
- Barnea N. and Dori Y. J., (1996), Computerized molecular modeling as a tool to improve chemistry teaching, J. Chem. Inf. Comp. Sci., 36(4), 629–636, DOI:10.1021/ci950122o.
- Ben-Zvi R., Eylon B.-S. and Silberstein J., (1986), Is an atom of copper malleable? J. Chem. Educ., 63(1), 64–66, DOI:10.1021/ed063p64.
- Biggs J., (1995), Assessing for learning: Some dimensions underlying new approaches to educational assessment, Alberta J. Educ. Res., 41(1), 1–17, retrieved from http://eric.ed.gov/?id=EJ502051.
- Çalık M. and Ayas, A., (2005), A Comparison of Level of Understanding of Eighth-Grade Students and Science Student Teachers Related to Selected Chemistry Concepts, J. Res. Sci. Teach., 42(6), 638–667, DOI:10.1002/tea.20076.
- Carolan J., Prain V. and Waldrip B., (2008), Using representations for teaching and learning in science, Teach. Sci.: J. Aust. Sci. Teachers Assoc., 54(1), 18–23, retrieved from http://eric.ed.gov/?id=EJ904915.
- Chang H. and Quintana C. (ed.), (2006), Student-generated animations: supporting middle school students' visualization, interpretation, and reasoning of chemical phenomena, Proceedings of the 7th International Conference of the Learning Sciences, Bloomington, IN: Lawrence Erlbaum Associates, pp. 71–77.
- Creswell J. W. and Plano Clark V. L., (2007), Designing and conducting mixed methods research, Thousand Oaks, CA: SAGE Publications.
- Danish J. A. and Enyedy N., (2007), Negotiated representational mediators: how young children decide what to include in their science representations, Sci. Educ., 91(1), 1–35, DOI:10.1002/sce.20166.
- Danish J. A. and Phelps D., (2010), Representational practices by the numbers: how kindergarten and first grade students create, evaluate, and modify their science representations, Int. J. Sci. Educ., 33(15), 2069–2094, DOI:10.1080/09500693.2010.525798.
- Doolittle P. E., (1995), Understanding cooperative learning through Vygotsky's Zone of Proximal Development, in The Lilly National Conference on Excellence in College Teaching, Columbia, SC.
- Dori Y. J., Barak M. and Adir N., (2003), A web-based chemistry course as a means to foster freshmen learning, J. Chem. Educ., 80(9), 1084–1092, DOI:10.1021/ed080p1084.
- Eilam B. and Poyas Y., (2010), External visual representations in science learning: the case of relations among system components, Int. J. Sci. Educ., 32(17), 2335–2366, DOI:10.1080/09500690903503096.
- Ernest P., (1998), Social constructivism as a philosophy of mathematics, Albany, New York: State University of New York Press.
- Gabel D. L., Sherwood R. D. and Enochs L., (1984), Problem-solving skills of high school chemistry students, J. Res. Sci. Teach., 2, 221–233, DOI:10.1002/tea.3660210212.
- Gredler M. E., (1997), Learning and instruction: theory into practice, 3rd edn, Upper Saddle River, NJ: Prentice-Hall.
- Hadenfeldt J., Xiufeng L. and Neumann K., (2014), Framing students’ progression in understanding matter: a review of previous research, Stud. Sci. Educ., 50(2), 181–208, DOI:10.1080/03057267.2014.945829.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in Grade 11 chemistry, Sci. Educ., 84(3), 352–381, DOI:10.1002/(SICI)1098-237X(200005)84:3<352::AID-SCE3>3.0.CO;2-J.
- Hoban G. and Nielsen W., (2010), The 5 Rs: a new teaching approach to encourage Slowmations (student-generated animations) of science concepts, Teach. Sci.: J. Aust. Sci. Teachers Assoc., 56(3), 33–38, retrieved from http://ro.uow.edu.au/cgi/viewcontent.cgi?article=1215&context=edupapers.
- Hoban G. and Nielsen W., (2013), Learning science through creating a “Slowmation”: a case study of preservice primary teachers, Int. J. Sci. Educ., 35(1), 119–146, DOI:10.1080/09500693.2012.670286.
- Hoban G. and Nielsen W., (2014), Creating a narrated stop-motion animation to explain science: the affordances of “Slowmation” for generating discussion, Teach. Teach. Educ., 42, 68–78, DOI:10.1016/j.tate.2014.04.007.
- Hoban G., McDonald D., Ferry B. and Hoban S., (2009), Simplifying Animation to Encourage Preservice Teachers’ Science Learning and Teaching Using “Slowmation”, in Siemens G. and Fulford C. (ed.), Proceedings of EdMedia: World Conference on Educational Media and Technology 2009, Cheapasake, VA: Association for the Advancement of Computing in Education (AACE), pp. 2838–2847.
- Hoffler T. N. and Leutner D., (2007), Instructional animation versus static pictures: a meta-analysis, Learn. Instruct., 17(6), 722–738, DOI:10.1016/j.learninstruc.2007.09.013.
- Hübscher-Younger T. and Narayanan N. H., (2007), Turning the tables: investigating characteristics and efficacy of student-authored animations and multimedia representations, in Lowe R. and Schotz W. (ed.), Learning with animations: research implications for design, Cambridge, England: Cambridge University Press, pp. 235–262.
- Johnstone A. H., (1993), The development of chemistry teaching: a changing response to changing demand, J. Chem. Educ., 70(9), 701–705, DOI:10.1021/ed070p701.
- Jonassen D. H., Peck K. L. and Wilson B. G., (1999), Learning with technology: technologies for meaning making, Learning with Technology, Ohio: Prentice Hall, pp. 1–18.
- Jones B., (1984), How Solid is a Solid: Does It Matter? J. Res. Sci. Teach., 14, 104–113, DOI:10.1007/BF02356796.
- Kind V., (2004), Beyond Appearance: students' misconceptions about basic chemical ideas, London: Royal Society of Chemistry.
- Kozma R. B. and Russell J., (1997), Multimedia and understanding: expert and novice responses to different representations of chemical phenomena, J. Res. Sci. Teach., 34(9), 949–968, DOI:10.1002/(SICI)1098-2736(199711)34:9<949::AID-TEA7>3.0.CO;2-U.
- Marton F., (1981), Phenomenography: describing conceptions of the world around us, Instruct. Sci., 10, 177–200, DOI:10.1007/BF00132516.
- Marton F., (1988), Phenomenography: exploring different conceptions of reality, in Fetterman D. (ed.), Qualitative approaches to evaluation in education: the silent revolution, New York: Praeger, pp. 176–205.
- Marton F., (1994), On the structure of awareness, in Bowden J. A. and Walsh E. (ed.), Phenomenographic research: variations in method, Melbourne, Australia: Office of the Director EQARD, Royal Melbourne Institute of Technology, pp. 176–205.
- Maxim G. W., (2013), Dynamic social studies for constructivist classrooms: inspiring tomorrow's social scientists, 10th edn, Boston, MA: Pearson.
- McMillan J. H. and Schumacher S., (2006), Research in Education: evidence-based inquiry, Boston, MA: Pearson.
- NSW Department of Education and Communities [DEC], (2011), retrieved on 17 Oct 2017, from http://www.dec.nsw.gov.au/.
- NSW Public Schools (2014), Learning and Teaching, retrieved on 25 Mar. 2018, from http://www.schools.nsw.edu.au/learning/7-12assessments/essa/.
- Nurrenbern S. C. and Pickering M., (1987), Concept learning versus problem solving: is there a difference? J. Chem. Educ., 64(6), 508, DOI:10.1021/ed064p508.
- Orgil M. and Sutherlad A., (2008), Undergraduate chemistry students’ perceptions of and misconceptions about buffers and buffer problems, Chem. Educ. Res. Prac., 9, 131–143.
- Osborne R. and Cosgrove M., (1983), Children conceptions of the changes of state of water, J. R. Sci. Teach., 20(9), 825–838, DOI:10.1002/tea.3660200905.
- Papert S., (1991), Situating constructionism, in Papert I. H. S. (ed.), Constructionism, Norwood, NJ: Ablex Publishing, pp. 32–64.
- Powell K. and Kalina C., (2009), Cognitive and social constructivism: developing tools for any effective classroom, J. Educ., 130(2), 241–250, retrieved from http://eric.ed.gov/?id=EJ871658.
- Prain V. and Waldrip B., (2008), A study of teachers’ perspectives about using multimodal representations of concepts to enhance science learning, Int. J. Sci. Educ., 8(1), 5–24, DOI:10.1080/14926150802152152.
- Prawat R. S. and Floden R. W., (1994), Philosophical perspectives on constructivist views of learning, Educ. Psychol., 29, 37–48.
- Richard A. Y. and Audrey C., (2004), Introduction: constructivism and social constructivism in the career field, J. Vocational Behavior, 64(1), 373–388, DOI:10.1016/j.jvb.2003.12.005.
- Rutten N., Van Joolingen W. R. and Van der Veen J. T., (2012), The learning effects of computer simulations in science education, Comput. Educ., 58(1), 136–153, DOI:10.1016/j.compedu.2011.07.017.
- Saljo J., (1988), Learning in educational settings: methods of inquiry, in Ramsden P. (ed.), Improving learning: new perspectives, London: Kogan Page, pp. 32–48.
- Saljo R., (1994), Minding action: conceiving of the world versus participating in cultural practices, Nordisk Pedagogik, 14(2), 71–80.
- Sharan S. (ed.), (1990), Cooperative learning, theory and research, New York: Praeger Publishers.
- Slavin R. E., (1990), Cooperative learning: theory, research, and practice, Boston: Allyn and Bacon.
- Stake R. E., (2003), Case studies, in Denzin K. and Lincoln Y. (ed.), Strategies of qualitative inquiry, 2nd edn, Thousand Oaks, CA: Sage, pp. 134–166.
- Stieff M., Bateman R. C. and Uttal D. H., (2005), Teaching and learning with three-dimensional representations, in Gilbert J. K. (ed.), Visualization in science education, Netherlands: Springer, pp. 93–118.
- Taber K. S., (2013), Upper secondary students’ understanding of the basic physical interactions in analogous atomic and solar systems, Res. Sci. Educ., 43, 1377–1406.
- Tatar E., (2011), Prospective primary school teachers' misconceptions about states of matter, Educ. Res. Rev., 6(2), 197–200.
- Tsai C. C., (1999), Overcoming Junior High School Students' Misconceptions About Microscopic Views of Phase Change: A Study of an Analogy Activity, J. Sci. Educ. Technol., 8(1), 83–91, DOI:10.1023/A:1009485722628.
- Tytler R., Peterson S. and Prain V., (2006), Picturing evaporation: learning science literacy through a particle representation, Teach. Sci.: J. Aust. Sci. Teachers Assoc., 2(1), 12–17, retrieved from http://dro.deakin.edu.au/view/DU:30004071.
- Tytler R., Prain V. and Peterson S., (2007), Representational issues in students learning about evaporation, Res. Sci. Educ., 37(3), 313–331, DOI:10.1007/s11165-006-9028-3.
- Tytler R., Prain V., Hubber P. and Waldrip B., (2013), Constructing representations to learn in science, Rotterdam, The Netherlands: Sense.
- Udo M. E. and Etiubon R. U., (2011), Computer-based science simulations, guided-discovery and students’ performance in chemistry, Mod. Appl. Sci., 5(6), 211–217, DOI:10.5539/mas.v5n6p211.
- URL-1, (2013), K-Sketch, retrieved on October 25, 2013 from http://www.k-sketch.org.
- Vermaat H., Kramers-Pals H. and Schank P., (2003), The use of animations in chemical education, International Convention of the Association for Educational Communications and Technology Conference, Anaheim, CA, USA.
- Vygotsky L. S., (1962), Thought and language, Cambridge, MA: MIT Press.
- Vygotsky L. S., (1978), Mind in society: the development of higher psychological processes, Cambridge, MA: Harvard University Press.
- Vygotsky L. S., (1981), The genesis of higher mental functions, in Wertsch J. V. (ed.), The concept of activity in Soviet psychology, White Plains, NY: Sharpe, pp. 144–188.
- Williamson V., (2008), The particulate nature of matter: an example of how theory-based research can impact the field, in Bunce D. and Cole R. S. (ed.), Nuts and bolts of chemical education research, Washington, DC: American Chemical Society, pp. 67–78.
- Williamson V. M. and Abraham M. R., (1995), The effects of computer animation on the particulate mental models of college chemistry students, J. Res. Sci. Teach., 32(5), 521–534, DOI:10.1002/tea.3660320508.
- Wu H.-K., Krajcik J. S. and Soloway E., (2001), Promoting understanding of chemical representations: students’ use of a visualization tool in the classroom, J. Res. Sci. Teach., 38(7), 821–842, DOI:10.1002/tea.1033.
- Yakmaci-Güzel B. and Adadan E., (2013), Use of multiple representations in developing preservice chemistry teachers’ understanding of the structure of matter, Int. J. Environ. Sci. Educ., 8(1), 109–130, retrieved from https://eric.ed.gov/?id=EJ1008597.
- Yaseen Z. and Aubusson P., (2018), Exploring Student-Generated Animations, Combined with a Representational Pedagogy, as a Tool for Learning in Chemistry, Res. Sci. Educ., 1–20, DOI:10.1007/s11165-018-9700-4.
| This journal is © The Royal Society of Chemistry 2018 |