The role of teacher questions in the chemistry classroom
Sofie Weiss
Dohrn
 a and
Niels Bonderup
Dohn
a and
Niels Bonderup
Dohn
 *b
*b
aDepartment of Chemistry, Aarhus University, Langelandsgade 140, DK-8000 Aarhus C, Denmark. E-mail: swdohrn@hotmail.com
bDanish School of Education, Aarhus University, Jens Chr. Skous Vej 4, Nobelparken, Building 1483, DK-8000 Aarhus C, Denmark. E-mail: dohn@edu.au.dk
First published on 4th December 2017
Abstract
The purpose of this study was to investigate how a chemistry teacher's questions influence the classroom discourse. It presents a fine-grained analysis of the rich variety of one teacher's questions and the roles they play in an upper secondary chemistry classroom. The study identifies six different functions for the teacher's questions: Student Knowledge, Request, Monologic Discourse, Clarification, Relations and Interaction of Contexts. Overall, these questions create a safe and interactive learning environment. However, the questions are predominantly closed in form. As a result, the students become highly accomplished in recalling facts but have difficulties when higher order thinking is required. The findings suggest that an interactive classroom can be created by using many engaging teacher questions. The six different categories of questions promote the students’ learning process as it gives them authority and entitles them to speak and learn.
Introduction
The language of science is of crucial importance at all levels of science education. When students acquire knowledge in the science classroom, they are in reality learning to understand the language of the field. Much of this meaning-making occurs through classroom discourse as part of teacher talk and teacher–student interactions. As stated by Lemke (1990), teaching and learning in science education are discursive activities.In classroom discourse, positions emerge which are available for the students to take up (Gresalfi, 2009). This positioning is based on the macrostructures of social interaction, which can be described as participant frameworks. Such frameworks shape the ways students are expected, obligated and entitled to interact with content and with their teachers and peers. The participants’ positions are based on the distribution of agency and authority (Vauras et al., 2012). The distribution of agency refers to the type of agency to which a student is entitled. A student may either be positioned for disciplinary agency or conceptual agency. The former applies to the recalling of content knowledge and following procedures to solve problems correctly. The latter refers to taking initiative in constructing meaning or in understanding methods and concepts. Ideally, students would work on tasks that require them to oscillate between using their conceptual tools (conceptual agency) to reach a solution to the problem and checking the correctness of this solution (disciplinary agency) (Vauras et al., 2012). However, most classrooms only offer students the opportunity to exercise disciplinary agency, as they are primarily assigned problems that require them to practise a known procedure. Student activities are therefore not stimulating and do not represent high cognitive demand tasks. Moreover, students’ learning trajectories do not progress toward full participation in activities that require authoritative use of concepts and principles.
The distribution of authority regulates who is in charge within a group, who is supposed to initiate and elaborate on task-related activities, and who performs evaluation. Authority may be distributed to only one participant. Research has shown that the most typical structure in classroom discourse is the initiation–response–evaluation (IRE) pattern (Edwards and Mercer, 1987; Lemke, 1990). In general, initiation is a teacher question. This is typically followed by a student response, which in turn is evaluated by the teacher. IRE-patterns represent a strictly unilateral distribution of authority, since only the teacher is expected to initiate and evaluate an activity (Vauras et al., 2012). Alternatively, authority may be distributed to several participants, in collaborative student groups for example, which implies that initiation, elaboration, and evaluation are expected equally from all participants, and that all contributions will be taken equally seriously (Vauras et al., 2012).
The distribution of authority is also taken into account in Mortimer and Scott's communicative approach (2003). This communicative approach focuses on whether the teacher interacts with students (either taking turns in the discourse or simply presenting material), and whether the students’ ideas are taken into account. The framework consists of four categories generated by combining two dimensions: authoritative–dialogic and interactive–noninteractive. With regard to the first dimension, authoritative discourse does not accommodate students’ points of view as the teacher predetermines a focus on the scientific point of view. If students raise ideas or questions, which are not aligned with this specific point of view, they are likely to be ignored by the teacher. Alternatively, if a student's idea is perceived by the teacher as conducive to the development of the scientific point of view, it is likely to be used. In these ways, authoritative discourse offers few opportunities for genuine dialogue and often involves instructional questions to guide students through a particular argument or explanation (Scott et al., 2006).
By way of contrast, dialogic discourse is open to different perspectives. There is always an attempt to acknowledge the views of others, and through dialogic discourse, the teacher attends to the students’ points of view as well as to the scientific view. Here, the teacher's interventions encourage thought and debate, often involving open questions (Scott, 1998; Scott et al., 2006).
The interactive–noninteractive dimension refers to the degree of involvement of other people. Classroom discourse can be interactive in the sense of allowing the participation of students or non-interactive in the sense of excluding them (Scott et al., 2006).
Combining the two dimensions, classroom discourse can be identified as either interactive or non-interactive on the one hand, and dialogic or authoritative on the other. Thus, for the interactive/authoritative communicative approach, the teacher focuses solely on the scientific idea and leads students through a question and answer routine with the aim of establishing and consolidating that point of view. In contrast, for the interactive/dialogic approach, the teacher explores students’ ideas and takes them into account, even though they may differ from the scientific one point of view. The non-interactive/authoritative approach is best represented by the formal lecture, where the teacher presents a specific point of view in a monologue. In the non-interactive/dialogic approach, the teacher summarizes different points of view, either simply listing them or exploring similarities and differences without student involvement.
Teacher questioning
Teacher questioning is a prominent feature of classroom discourse. Questions can stimulate student thinking and provide the teacher with feedback regarding students’ knowledge and understanding. Wellington and Osborne (2001) identify three different forms of question with respect to the type of answer expected: closed, open and pseudo-open. Closed questions have only one acceptable answer – for example, a name, a piece of information or an argument. ‘What is the name of a hydrocarbon that has one or more double bonds?’ is an example of a closed question, with the specific answer ‘Alkene’. Here, the teacher focuses on one specific point of view and leads students through a question and answer routine with the aim of establishing and consolidating that point of view, corresponding to the interactive/authoritative communicative approach in Mortimer and Scott's (2003) framework. Blosser (2000) has created a question classification system based on Bloom's taxonomy. Answering closed questions mainly requires thinking skills at the lowest level in Bloom's taxonomy. Since the lowest level primarily concerns factual knowledge, closed questions generally have a limited number of correct answers. They are therefore considered to belong to lower order questions (Blosser, 2000; Chin, 2004). Furthermore, research has shown that most science teachers ask closed questions that merely require students to recall facts (Childs and Mcnicholl, 2007; Lord and Baviskar, 2007; Lee and Kinzie, 2012; Biggers, 2017; Eliasson et al., 2017). This means that the students only construct superficial memo-techniques and do not engage in an in-depth meaning-making of science.Open questions, on the other hand, can have multiple acceptable answers and belong to the interactive/dialogic dimension of classroom discourse (cf.Scott et al., 2006). An example could be ‘What do you know about amino acids from your everyday life?’ This would potentially elicit a whole range of student answers that require thinking from one or more of the higher levels in Bloom's taxonomy. These types of questions draw on students’ abilities to formulate hypotheses and to identify implications. Open questions are considered to demand higher order thinking skills of the students and are therefore denoted as higher order questions (Blosser, 2000; Yip, 2004). The inclusion of higher order questions as part of classroom discourse may promote deeper and more reflective thinking.
Pseudo-open questions are questions that appear to be open, but in reality involve students in a ‘guess-what-I-am-thinking’ game with the teacher. An example could be ‘What can you tell me about proteins?’ This is structured as an open question, but in reality the teacher has a clear expectation that the student's answer will reflect the content covered in class. The teacher will therefore reject otherwise acceptable answers for the sake of making a specific point in the lesson. Thus, pseudo-open questions belong to the interactive/authoritative communicative approach. Students are usually so familiar with the ground rules of the classroom that they are very good at this game.
Several frameworks for teacher questioning have been proposed. Early studies on teacher questioning primarily focused on the IRE pattern of discourse (Edwards and Mercer, 1987; Lemke, 1990). Some examples are the studies performed on the importance of waiting time for students’ thinking (Rowe, 1974; Tobin, 1987). Several authors have looked at the characteristics of teacher questions that facilitate science classroom discourse. For instance, Chin (2007) describes four discourse approaches (Socratic questioning, verbal jigsaw, semantic tapestry and framing) and several strategies within these approaches that encourage student responses. Becker et al. (2015) studied how an instructor made extensive use of questioning strategies to initiate and sustain classroom discourse in an undergraduate physical chemistry course. Based on the Inquiry-Oriented Discursive Move (IO-DM) framework (Rasmussen et al., 2008), which contains four subcategories of questioning (evaluating, clarifying, explaining, justifying), they found that evaluating questions dominated the class discussion. The same framework was used by Stanford et al. (2016) in a chemistry classroom setting where it served as an analytical tool to characterize how the instructor engaged students in classroom discourse. Kawalkar and Vijapurkar (2013) describe a sequential typology of teachers’ questions and their roles in grade 7 science classes (exploring pre-requisites/setting the stage, generating ideas, probing further, refining conceptions, guiding the entire class, classroom management). Criswell (2012) analysed the features of discourse structure which emerged as teachers facilitated discussions in introductory high school chemistry classes. Based on Bereiter's (1994) notion of science as progressive discourse, Criswell analysed discourse moves associated with the realization of commitments: (1) a commitment to work toward common understanding satisfactory to all (mutual understanding); (2) a commitment to frame questions and propositions in ways that allow evidence to be brought to bear on them (empirical testability); (3) a commitment to expand the body of collectively valid propositions (expansion); and (4) a commitment to allow any belief to be subjected to criticism if it will advance the discourse (openness). Based on Blosser's (2000) framework, Eliasson et al. (2017) distinguished between four types of teacher questions: closed memory questions, closed convergent questions, open divergent questions and open evaluative questions. In Warfa et al.'s study (2014), teacher questions were categorized as either monologic or dialogic.
Although some overlap exists between the reviewed discursive frameworks, it is obvious that teacher questions are analysed with different purposes. Based on our experiences with chemistry teaching in upper secondary school, we believe that a more nuanced perspective on the types of questions asked in a typical chemistry class and how these questions influence the classroom discourse would be beneficial. The purpose of this study was therefore to investigate the function of a chemistry teacher's questions. Focus was on the questions’ function in the grade 12 chemistry classroom.
Method
Teacher questioning can be studied from diverse theoretical positions. Much of the early work on the topic was grounded in the process–product paradigm; i.e. focused on the relationship between teacher questioning practices and student outcomes in terms of academic achievement. More recently, sociolinguistics has offered an alternative paradigm for the study of questioning in classrooms. Sociolinguistics includes a range of research traditions (discourse analysis, for example) and is concerned with the interdependency of language and context with a focus on the role of social context in the interpretation of spoken language (Carlsen, 1991).In this study, the investigation of how teachers ask questions and how these questions influence the discourse in the chemistry classroom is based on video recordings in a grade 12 chemistry classroom. The teacher questions are seen as embedded in the socio-communicative interaction between teacher and students in science classroom activities. Therefore, the methodology of interactional analysis (Jordan and Henderson, 1995) has been followed, enabling the teacher's questions to be analysed in the context of the chemistry classroom. Interactional analysis is an interdisciplinary method with roots in ethnography and sociolinguistics, among other disciplines. One of its basic assumptions is that verifiable observations provide the best foundation for analysis of discourse. The data used for this study were therefore collected with video cameras. This allows for a close interrogation of the data and, most importantly, the ability to replay interesting sequences of interaction again and again throughout the analysis. Video has the ability to record social events as they take place and with a high level of detail. It can capture the complexity of interaction data which is difficult for a single researcher to keep track of as the activities take place. Thus, the use of video recordings allows the dialogue between teacher and students and their non-verbal interactions in the chemistry classroom to be scrutinized in the calm of the researcher's office. This in turn allows the identification of the facilitating role of the teacher's questions in the students’ construction of meaning.
The questions and responses studied here are seen as possible signs of ongoing thinking operations. However, as stated by Eliasson et al. (2017), we cannot claim to know what students think. What we can observe are the questions actually posed and their responses in the ongoing interpersonal visual communication, including gestures and interaction with artefacts such as drawings and pictures. It should be noted that our study is based on a sociolinguistic paradigm focused on the function of the teacher's questions and how these questions influence the discourse in the chemistry classroom. We have not investigated students’ actual learning outcomes.
Interpretive framework
In this study, focus was on how teacher's questions influence the discourse in the chemistry classroom. Thus, a discourse analysis of the teacher questions was performed. To ensure a complete analysis of the discursive context, attention was also paid to contextualization cues, such as non-verbal contributions from participating students and teacher. A situative approach was adopted for this purpose, drawing on a number of social theories that frame discourse as arising from individuals’ interactions in social systems. Specifically, the theories of positioning (Gresalfi, 2009; Vauras et al., 2012) and situated learning (Lave and Wenger, 1991) are used. The analysis comprises both the teacher's ways of posing questions and the class’ joint practice in the classroom setting (i.e. the context of questions). For example, when a teacher asks a question and then immediately answers it, the students will probably recognize that verbal participation is not expected, and perhaps not desired. This methodological approach is consistent with Lave's (1988) attempt “to incorporate the active character of experience into the unit of analysis” (p. 180), and builds upon Wenger's (1998) discussion of communities of practice, insofar as the approach is designed to identify the negotiated social structures and norms that emerge through students’ engagement. This framework is similar to interactional sociolinguistic micro-analysis of the classroom discourse surrounding teachers’ questions (Bleicher, 1994), as the context includes the description of speakers, their relationships to one another, and the rules that govern their speech and enable them to make sense of what is being said (Carlsen, 1991).Setting and participants
The setting for the study presented in this article was a public upper secondary school located in a major town in an urban area of Jutland, Denmark. The school has 110 teachers and an enrolment of approximately 800 students divided between 29 classes (grades 10, 11 and 12). The participants were Mr Johnson, a veteran teacher, and his grade 12 chemistry class comprising 13 students (5 females and 8 males, aged 18–20). Besides teaching chemistry, Mr Johnson also teaches physics and maths at the school. He also teaches maths at a nearby university. On average, the class had two weekly chemistry lessons, each lasting 90 minutes. Traditional instructional methods such as teacher talk, problem solving and the use of a white board and textbooks, dominated classroom activities. The most typical classroom activities throughout the lessons were lectures, problem solving, teacher/student dialogue and student presentations. Other classroom activities comprised a demonstration experiment performed by Mr Johnson and students’ work on an experimental report.Ethical precautions
Danish rules are somewhat less restrictive than in many other countries with regard to the ethical norms for researcher involvement – and for researchers’ access to personal information about their subjects. Nevertheless, we have ensured the teacher and students’ full anonymity. All names used in this paper are pseudonyms to protect the anonymity of the participants. They have all voluntarily consented to our investigation after having been informed of its purpose and of the possibility of declining. All students were over 18; parental consent was therefore not required. We have abided by all requirements in the Danish Act on Processing of Personal Data under the authority of the Danish Data Protection Agency (http://www.datatilsynet.dk).Data collection
The first author visited the school over a period of four weeks to observe one classroom during a module on biochemistry. This totalled five observed lessons. Each lesson was videotaped using two cameras. The primary camera (Camera 1) was connected to an external microphone worn by Mr Johnson which allowed his speech to be recorded clearly. Additionally, an attempt was made to keep Mr Johnson in the frame at all times so as to record his interactions with the students in the best possible way. The secondary camera (Camera 2) recorded the sound from the classroom and framed the students’ activities. Both cameras were placed on stands in corners of the classroom to avoid disturbing the class. Both the teacher and the students seemed to act normally, an assumption supported by Mr Johnson in an informal conversation with the first author. A sketch of the set-up can be seen in Fig. 1.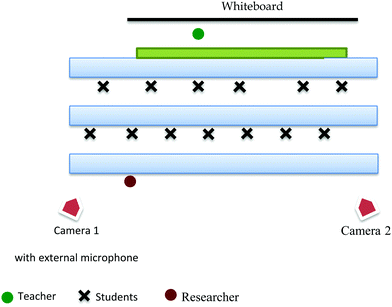 | ||
| Fig. 1 Sketch of classroom layout with the positions of the two cameras indicated. The approximate positions of the teacher, students and researcher are also marked. | ||
Data were derived from ethnographic field notes and video recordings of lessons. Content logs for each lesson were created from these two data sources within a week after each lesson. Relevant sections were transcribed in full with a focus on verbal utterances. A few non-verbal actions were included where relevant. These are indicated in parenthesis in italics in the transcripts. Interruptions or overlapping speech is marked with the use of square brackets. The aim was to present the sequences of talk as accurately as possible. All perceptible verbal utterances were therefore transcribed. To ensure readability and comprehensibility, standard orthography and punctuation were used. All transcripts are translated from the original Danish by the first author.
Data from video recordings and transcripts
The primary object of interest in the performed analysis is the influence of the teacher's questions on the discourse in the chemistry classroom. The aim was to analyse this as authentically as possible. There are, however, some limitations due to the reductive nature of all analysis (Lemke, 1998). Firstly, the data were collected with a video camera. As described above, this method has many advantages, but it also has certain constraints. The production of video recordings can be thought of as a transformation of reality into data (Jordan and Henderson, 1995). It is not an objective, faithful representation of the real world. It is limited by its positioning, zooming etc., as well as its restricted capacity for information processing. Video can only record audio and visual data. It does not record smell or heat radiation, which can be perceived by the human sensory apparatus. There is also the question of the effect of the presence of a camera on the people who are filmed. In this study, the effect was deemed negligible.From the recorded video data, transcripts were produced. The process of transcription is problematic as it changes the living spoken language into a frozen written account (Lemke, 1998). The change of medium from speech to writing changes our expectations and perceptions of language. Normal speech is filled with repetitions, hesitations, false starts etc. that are hardly noticeable in their original context, but are magnified in transcripts. Furthermore, information on, for example, intonation, humour and surprise cannot be reproduced fully in transcripts. In this study, we have attempted to reproduce this information through the use of punctuation and by describing some of the participants’ non-verbal actions.
Data analysis
A general inductive approach was used for the data analysis (Tobin, 2000; Erickson, 2012). Hypotheses about how questions asked by the teacher influence classroom discourse were developed and tested. In particular, the impact of preceding utterances on later ones was examined. By examining student utterances before and after a teacher's question, we traced how the question influenced what students said. These conjectures were developed through continuous cycles of analysis of the recorded video. First, the data were reviewed chronologically to produce content logs. These logs contain a rough summary of events in the classroom. At this stage, questions and tentative assertions were generated based on the collected data, and all incidents of teacher questions were noted. The researcher identified teacher questions by a detected rise in intonation. It should be noted that questions such as ‘Excuse me?’ have been left out as they were considered not to influence the classroom discourse.The discursive context of each question was carefully considered to enable it to be coded in terms of function and content. In the performed analysis, special attention was given to the 60 seconds after each question. The following two questions were used to guide the analysis:
• What is the function and content of the teacher question?
• How does the teacher's question influence the classroom discourse?
Comparisons were made between codes emerging from different situations in different lessons, and similar codes were combined. These inductively derived codes were then refined through an iterative process and compared throughout the entire body of collected data. This improved the alignment between data and codes due to greater specificity. The emergent codes were refined by adding to, deleting from, or modifying the existing list, resulting in a number of subcodes which were subsumed under six major codes. The subcodes depicted specific questioning strategies. Thus, six code domains were identified with respect to function: Student Knowledge, Request, Monologic Discourse, Clarification, Relations, and Interaction of Contexts. These domains indicate the different functions of the teacher questions. In some instances, there is a one-to-one relation between the function and the content, while in others, more than one code falls into the same functional domain. The final and complete list of codes can be seen in Table 1. The codes will be described in detail in the following section.
| Code (content) | Definition | Total no. questions | Frequency in percentage | |
|---|---|---|---|---|
| Student knowledge | Academic | Academic content of the course | 103 | 22.9 |
| Diagnostic | Check of student knowledge | 75 | 16.7 | |
| Request | Organisational | Organisational issues related to the academic content | 92 | 20.5 |
| Practical | Practical difficulties | 56 | 12.5 | |
| Monologic discourse | Academic | Highlight the thought process of the teacher | 51 | 11.4 |
| Clarification | Academic | Clarification of student contributions | 47 | 10.5 |
| Relations | Social | Personal or social content | 14 | 3.1 |
| Interaction of contexts | Academic | Academic content of the course related to everyday life and society | 11 | 2.4 |
| In total | 449 | 100 |
Results
The results showed that the teacher used questions in the chemistry classroom for varying purposes. Six different functions were identified. In two of these domains, two different codes were identified with respect to content. These will be presented in the following sections with regard to their influence on the classroom discourse, and how they facilitate students’ learning processes.Student knowledge
This category refers to the questions that the teacher uses to gain information about the students’ knowledge. These questions can be divided into two subcategories based on the content of the questions.| 1 | Mr Johnson | First of all, we are going to take a look at amino acids |
| 2 | Mr Johnson | How is an amino acid built? There is a general structure that has to be present for it to be an amino acid. John? (nods at John) |
| 3 | John | Isn’t it that amino group? |
| 4 | Mr Johnson | An amino group needs to be present. That's right. (draws an amino group) |
| 5 | John | On the left side of |
| 6 | Noah | Of the side chain |
| 7 | Mr Johnson | On the left side of? (points at Olivia) |
| 8 | Olivia | A carboxylic acid |
| 9 | Mr Johnson | Eh, directly? |
| 10 | Olivia | No |
| 11 | [John | No] |
| 12 | Olivia | On a C (Mr Johnson draws a C-atom next to the amino group and connects it to a carboxylic acid) |
| 13 | [John | On a side chain, side chain] |
| 14 | [Ava | Shut up! (directed towards John)] |
| 15 | Olivia | Next to the carboxyl |
| 16 | Mr Johnson | And a carboxyl group. Yes, what else? (points at Ava) |
| 17 | Ava | Yes, and then there is a, there is also an H group [as well as] |
| 18 | [Mr Johnson | Yes], there is an H-atom. That is quite right (draws an H-atom on the middle C-atom). And then something down here which can be a lot of different things, right? (draws an R which represents the side group). It can easily be an extra carboxylic acid. It can be something with sulphur. It can be an an, eh, amine more in the same molecule. That wouldn’t be a problem. But this here is the general, this is the general structure. That means that this up here (Mr Johnson indicates a part of the structure on the whiteboard) has to be present. |
The homework for the lesson had been a textbook passage on the subject. Therefore, the episode can be regarded as a quick check of the students’ knowledge of the read material. The teacher performs this check by asking closed questions, which all have one correct answer. This is obvious, for example, in his follow-up question in line 9 after Olivia's imprecise answer in line 8. When academic questions are asked during lectures, they have the additional benefit of activating the students in a situation where they are otherwise passive listeners.
This type of questioning is typical in the observed classroom, and such questions elicit quick responses from the students, which helps the teacher move the lesson along. This appears to be part of the teacher's overall questioning strategy. Another objective is to maintain control of the flow of talk in the classroom. Non-verbal gestures are used to nominate and evaluate the speakers. Students are nominated with nods or by being pointed at, and a correct answer elicits an addition being made to the drawing of the general structure of an amino acid on the whiteboard. The example contains one instance where Mr Johnson interrupts the current speaker (line 18). Ava breaks the ongoing rhythm with interchanging turns of teacher questions and brief student answers by attempting to expand on her answer with the next logical addition. Mr Johnson cuts her off, effectively ending the teacher/student dialogue and beginning a teacher monologue. This move serves the purpose of moving the lesson along and avoiding confusion with a half-finished or incorrect student answer.
| 1 | Mr Johnson | It must have received the H+-ion from somewhere. It can’t simply be from water; then the pH wouldn’t be equal to 7 anymore, right? There must be something else delivering it. |
| 2 | Multiple students | Mmm |
During student presentations, there is a higher degree of interaction. Here, the dialogue largely depends on the students' answers, as they dictate whether repetition or additional information is required or whether the explanation can continue. Here the students' knowledge is thus checked.
| 1 | Mr Johnson | You don’t know how to measure it, right? Or what? Do you know? |
| 2 | Emily | We haven’t read about it in the book yet. |
| 3 | Mr Johnson | No, but it's possibly something, if you know it, then you’ve read it on your own or you’ve learned it in biology |
The discourse also evolves differently when Mr Johnson uses these questions to help students’ problem solving. This often takes place in one-on-one situations or with a small group of students. In this context, the students often give a direct answer, either verbally or non-verbally. This elicits more direct information about their knowledge. The teacher's role as a facilitator is therefore improved with this information.
| 1 | Mr Johnson | Normally, you would have some data with a vertical scale on it, right? |
| 2 | Chloe | Yes. But like here, for example, here this is like the smallest, because it is 1, right? |
| 3 | Mr Johnson | Yes |
| 4 | Chloe | Do you take the smallest and assign 1 to that or what? |
| 5 | Mr Johnson | No, in this case we know how many H's it has … |
The addition of ‘right?’ at the end of Mr Johnson's utterances can be seen as a discourse marker. Discourse markers are expressions such as right, well, so, however, and then which signal a sequential relationship between the current basic message and the previous discourse (Schiffrin, 1988; Fraser, 1990). Discourse markers are useful guides for clarifying a speaker's communicative intention. Many expressions that function as a discourse marker are ambiguous, and function as a different syntactic unit on other occasions. In this regard, ‘right’ probably marks a challenge of the students’ ideas or a request of the students to further substantiate what they have said.
Request
In the classroom, Mr Johnson requests the students’ help from time to time. The first subcategory refers to questions that have an organizational content, while the second comprises requests with regard to practical difficulties.The first example is taken from a lecture on the structure of proteins. Specifically, Mr Johnson is explaining the peptide bond. He then reminds the students that they have some previous knowledge of the subject. In this context, Mr Johnson seeks confirmation from the students that they do indeed possess knowledge of peptide bonds from a previous assignment (“Didn’t you have an assignment where you solved that?”). This is confirmed by the subsequent student response. Thus, this question fulfils three purposes. It reminds the students that part of the academic content of the lesson is something they are already familiar with, offering them the opportunity to connect and integrate their knowledge on peptide bonds from different situations. Secondly, it requires them to recall previous information and reflect on it in the current situation, increasing the students’ body of content knowledge which is part of becoming scientifically literate. Lastly, it supports the development of a shared foundation of knowledge between the teacher and students.
The second example is Mr Johnson's response to a student question regarding a specific task in an assignment that he is in the middle of explaining to the class (“Were we also supposed to comment?”). Mr Johnson has overlooked that part of the task also was to comment on the result. The question therefore seeks information about the requirements of the task. It is quickly answered by another student. Subsequently, the teacher answers the student's question with regard to the task, and the lesson moves on. The question therefore functions as a simple fact-check of information.
Collectively, these questions request information from the students. As shown, this ranges from repetition to simple fact checking. Thus, the students are encouraged to reflect on their knowledge and invited to participate actively in lessons. The students accept this invitation as demonstrated by the fact that they answer most of these questions.
| 1 | Mr Johnson | It is weird. Can’t I move it up a bit? I don’t get it (changes a setting) Like that. Is that the only option? It only does this and that. |
| 2 | Emily | Um Mr Johnson? |
| 3 | Mr Johnson | I could do it anyhow. Yes? |
| 4 | Emily | You know there's a small square in the top corner. It allows you to change it to full screen. Next to the (points). Up there in the corner. |
| 5 | Mr Johnson | (Maximizes the window). That didn’t work at all. The picture is still the same size. Try and tug it up a (changes a setting). All right, it is larger now. |
In this example, Emily has to get Mr Johnson's attention before she can offer her help. This makes it clear that he is not expecting an answer from a student. He is preoccupied trying to solve the technical issue. By asking these questions, Mr Johnson shows his students that he is not flawless. This is an important element in building good personal relations with students. He admits that he has a technical difficulty and is unsure how to fix it. This opens the floor for the students to be the capable helpers. This small shift in power has the potential of giving the students self-confidence by using everyday knowledge otherwise unacknowledged in the classroom setting. However, Mr Johnson quickly reclaims the control of the classroom talk through his evaluation of Emily's answer in the typical IRE-pattern. This evaluation is initially very negative, but her help is finally acknowledged by Mr Johnson as being successful. Subsequently, he continues his lecture.
Monologic discourse
This type of question is used by Mr Johnson to illustrate his line of thought when contemplating a problem. The following is a sequence containing three examples of this type of question. It took place in the middle of a lesson while the students were either working on problems or on their experimental reports on the solubility constants for silver halides. Mr Johnson is helping two students with their report. Specifically, he is in the middle of showing them how to determine the concentration of the silver ions.| 1 | Mr Johnson | And what is it, what do we have? We have the formula (looks in papers). The equation, you see, it indicates that the voltage we measure. The voltage we measure, U0, that is equal to 58 mV times the logarithm of the concentration of the silver ions in beaker 1 and beaker 2 here (writes the equation on the whiteboard). |
| 2 | Mr Johnson | And what do we have next? We have some concentrations that show that (looks in papers). Where the hell is it? Where did they go? Oh, here they are. Okay. Eh. |
| 3 | Mr Johnson | What is it that we do? We have. In one of them. That is. The one called [Ag]1 up here, we can substitute that with 0.01 M. And the one called [Ag]2, that will vary. That is the one we are to calculate, right? |
| 4 | Olivia | Hmm |
In this example, Mr Johnson uses Nernst's equation to demonstrate how the concentration of the silver ions should be determined. Here he has substituted [Ag+]1 with the actual value used in the present experiment. This illustrates the first step in the interpretation of the equation that must be completed to calculate the concentration of the silver ions.
The teacher himself answers all the questions in example 3. This is an observed trend for this type of question. Students rarely offer an answer, and when they do, they are not acknowledged. These question can therefore also be categorized as non-interactive/dialogic cf. Scott et al. (2006). The three italicized questions in the example above are clearly used to drive the teacher's deduction forward. They are also very general in their phrasing and could be applied to a wide range of problems.
Clarification
This category covers questions that Mr Johnson uses to clarify the meaning of the students’ contributions, whether concerning students’ questions, answers, work, statements or penmanship. Thus, it is vagueness or incorrectness in the students’ contributions that triggers the questions. Unlike the other question types, these questions are almost all answered by the students. The students’ answers take the form of both questions and statements. Furthermore, this question type is not evenly distributed throughout the lessons. Only a handful of the total of 47 clarification questions occur during classroom plenaries. The majority occurs in clusters of 4–7 questions in quick succession during problem-solving activities. An example of such a cluster occurs while Mr Johnson is helping two students who are having trouble calculating the solubility constants for silver halides.| 1 | Mr Johnson | That is 10 to the minus 17th. How did you get 10 to the minus 13th 14th? It looks like you have overlooked a power of 10 somewhere. That one. |
| 2 | Mr Johnson | How much did you have of the silver? No, wait a minute. Isn’t there something wrong with these calculations? How much silver did. How much silver iodine. How much did you have of the silver solution? Did you have 40 mL of each? |
| 3 | Michael | No |
| 4 | Olivia | No |
| 5 | Mr Johnson | What did you have? |
| 6 | Michael | Let me see (looks in papers). No, we only had 40 mL in one of them. And then |
| 7 | Mr Johnson | How much did you have in the other one? You had either 30 30 mL |
| 8 | Olivia | 30 silver |
| 9 | Mr Johnson | You have to be careful in your calculations since you are using, as far as I can tell. Um, you are using the 40 mL for both one substance and the other. That's no good. You have to remember the amount of substance we’re calculating. |
Here Mr Johnson asks seven clarifying questions in the span of just one minute. The purpose of these questions is to pinpoint where the students’ calculations have gone wrong. The four questions in line 2 come in such quick succession that the pauses in between are not long enough for the students to answer. This suggests that the teacher is thinking while asking questions. It is only the last questions that seems to be intended for the students to answer. As Michael and Olivia answer Mr Johnson's questions, they seem to realize their mistakes in lines 3 and 4. In line 6, Michael realizes their specific mistake: that they only used 40 mL of one of the substances. Mr Johnson scaffolds this realization with his questions. The sequence ends with Mr Johnson quickly outlining how the changes in values affect the calculations.
Relations
This category contains teacher questions that create social relations between teacher and students. It comprises questions that are not related to the academic content. Rather, they address subjects that are related to the participants’ lives outside the classroom. These questions are mostly addressed to one student at a time and usually elicit an answer. It seems that the questions initiate small breaks from their roles in the learning environment for both the teacher and the students. With one exception, the questions all occur at transition times: the beginning of class, the end of class or when moving from one classroom activity to another. At these times, the content of the talk is not strictly academic, and the class is slightly unsettled until the next activity is started. It seems that the questions improve the personal relations between Mr Johnson and the students as they share information about their lives.Interaction of contexts
This category contains teacher questions that create relations between the content of the lesson and the outside world. The questions in this category are academic in nature and related to scientific literacy as well as science, technology and society (STS) as they aid the students in learning to relate the academic content of the lesson to the world around them and to their everyday lives (Bybee, 2015; Pedretti and Nazir, 2015). Scientific literacy defines what the public should know about science in order to live more effectively with respect to the natural world, and the aim of STS is to give students knowledge about the science/society interface and the ability to make decisions about science-related social issues (DeBoer, 2000). Only 11 questions in total were observed. However, these questions reflect the time Mr Johnson devotes to the articulation of scientific literacy in the chemistry classroom. The questions all occur during lectures. An example has been chosen from a lecture on biofuel. Here Mr Johnson has just explained how biofuel can be used in both vehicles and power plants.| 1 | Mr Johnson | A power plant can produce electricity, or a combined heat and power plant can produce both heat and electricity. Do you know why it is a good idea? |
| 2 | William | Excuse me? |
| 3 | Mr Johnson | A combined heat and power plant rather than just a power plant. |
| 4 | William | Yes, because it doesn’t [emit CO2] |
| 5 | [Ava | Sustainable] |
| 6 | Mr Johnson | What? |
| 7 | Michael | Sustainable |
| 8 | Ava | Yes |
| 9 | Mr Johnson | Why is it more sustainable than a power plant? |
| 10 | William | It is because [um, the power plant releases] |
| 11 | [Ava | It is not as] |
| 12 | William | All sorts of radioactivity |
| 13 | Mr Johnson | No! |
| 14 | William | The power plant |
| 15 | Mr Johnson | No, no, it's not a bloody nuclear power plant. No, no, no. It is just a. Yes? (nods towards Michael) |
| 16 | Michael | Isn’t it because you can utilize it for both electricity and heat? |
| 17 | Mr Johnson | Yes, why is that possible? Why can you use it for heat? Why can’t you utilize all of it for electricity? |
| 18 | Michael | Hm |
| 19 | Mr Johnson | The second law of thermodynamics. Oh well, that was a bit mean. No, you can’t utilize. You can’t convert heat to work 100 percent. |
In this sequence, Mr Johnson tries to establish links both within the material taught in the class and between this material and the surrounding society. He connects the biochemistry of biofuels with its use in the production of electricity at power plants. This connection is straightforward. Building on this, Mr Johnson attempts to get the students to reflect on the advantages of a combined heat and power plant. To do this successfully, the students would have to remember what they have been taught about thermodynamics and apply it meaningfully in this real-world context. The students have difficulties with this. Even with Mr Johnson's scaffolding questions in line 17, the students cannot make the connection, and he ends up making it for them. According to Wu (2003), it is not surprising that most students are not able to apply the scientific knowledge they have learnt at school to real-life situations, because they are not given opportunities to do so at school.
Discussion
To learn chemistry, the students need to learn how to articulate scientific knowledge using the discourse of science. It is stated by Bleicher (1994) that it is crucial that students talk in the science classroom in order to develop this discourse. Learning the scientific discourse can be compared to learning a new language (Evagorou and Osborne, 2010). The science teacher is therefore fundamentally a teacher of language. Mr Johnson fulfils this role as seen in the example where he corrects Ava's contribution to “…and a carboxyl group”. This adds precision to her answer and is a classic strategy for a language teacher. In this way, the students are introduced to the discourse of science throughout the lessons.Research suggests that it is beneficial when the teacher asks open questions as they stimulate the students’ learning process (Lee and Kinzie, 2012). Such questions are typically placed at the top end of Bloom's taxonomy (Blosser, 2000; Krathwohl, 2002; Chin, 2004). However, it has also been shown that most science teachers ask questions that are predominantly closed (Childs and Mcnicholl, 2007; Lord and Baviskar, 2007; Lee and Kinzie, 2012; Biggers, 2017; Eliasson et al., 2017). Mr Johnson is one such teacher. The classroom discourse does not feature open questions with academic content, nor epistemological questions. For instance, he asks the students: “How is an amino acid built?” This is a closed question which only has one correct answer. The students only need to recall the information they have read in their textbooks to answer this question. A more open question could have been posed; for instance “What do you know about amino acids?” This could have led to an array of different student answers. However, as argued by Wellington and Osborne (2001), this would in fact constitute a pseudo-open question as the students would have to guess what answer the teacher is looking for. No open questions with academic content and only a few pseudo-open questions are found in the observed data.
Mr Johnson dominates the classroom discourse. He predominantly uses the IRE pattern, which supports the fact-oriented enactment of the curriculum he teaches the students. In addition, the students mostly solve problems that require them to use a known procedure. As such, the students primarily exercise their disciplinary agency and only very rarely their conceptual agency. Observations indicated that the students struggle to create connections within the academic content and to relate its significance to the surrounding society. The teacher presents the students with opportunities to make these connections, but the students are unable to do so. This may be due to the fact that the students mainly use their disciplinary agency and are seldom required to use their conceptual agency. It has, however, been shown by Bleicher (1994) that the teacher's control of the flow of talk in the classroom is in accordance with the expectations of both teacher and students. Thus, it is difficult to change the conversational patterns of the classroom discourse due to the power of the cultural expectations of the participants. Even a teacher who attempts to make students the dominant speakers might find the students unwilling collaborators as this role may conflict with their expectations.
Furthermore, in our observations, students’ answers to the teacher's questions are very short and not very complex in their structure. Evagorou and Osborne (2010) suggest that epistemological questions would enhance the students’ scientific reasoning. This could include questions such as: “How do you know?”, “How would you justify that view?” or “Why do you believe you are right?” This type of question is also not found in the observed classroom. Thus, based on the framework of Mortimer and Scott (2003), the classroom discourse is characterized as predominantly interactive/authoritative, as the teacher tightly controls which responses he accepts. This was especially the case when key points needed to be made, or when ideas were consolidated. This finding is hardly unexpected since the relationship between teacher and student is obviously not an equal one; the teacher has the authority (Childs and Mcnicholl, 2007).
In spite of the lack of open or epistemological questions, Mr Johnson has created an interactive classroom with his authoritative questions. Hence, the students are activated in the classroom, which is important for their learning process. Furthermore, they are often included in a dialogue where they contribute with questions, answers and statements. In fact, the students ask many questions, both solicited and unsolicited by Mr Johnson. It has not been possible to determine a pattern in which such questions occur, nor to identify any characteristic discursive features surrounding them. Questions are asked during all classroom activities: lectures, teacher/student dialogues, demonstration experiments, student presentations, classroom discussion and problem solving. These questions demonstrate the students’ curiosity, and their involvement with and reflection on the material they are taught.
It seems that the students are empowered to speak up due to shifts distributing greater authority to them. This distribution of authority is continually negotiated in the classroom, as the positioning is a moment-by-moment process (Vauras et al., 2012). Here it is of importance that Mr Johnson accepts the students’ unsolicited contributions. This shows that he gives them the authority to speak when they have questions or wish to add something to the classroom discussion. Additionally, it appears that the teacher's questions in the categories Request and Relations have a positive influence. In the classroom discourse following these questions, a slight shift of authority can be detected. If only for a short time span, the authority is distributed multilaterally between both teacher and students rather than being placed solely with Mr Johnson. Hence, these two elements create a more equity-based classroom, which allows the students to articulate their scientific knowledge. In other words, there is a clear movement toward the dialogic pole of the dialogic/authoritative dimension. Although Mortimer and Scott (2003) have presented authoritative and dialogic discourses as constituting two poles of a dimension, it is important to recognize their intimate dynamic linkage in practice. According to Scott et al. (2006), both dialogicity and authoritativeness contain the seed of their opposite pole in the dimension, and in this way we see the dimension as tensioned and dialectic, rather than as being an exclusive dichotomy.
Furthermore, it seems that the teacher's actions influence the personal relationship between him and the students, and that this relationship impacts the students’ motivation to learn. In addition to his questions as coded in the categories Request and Relations, he tells them anecdotes from his life and asks about their lives with the questions coded Social. Anecdotes and humour are triggers of interest and can improve relationships between teachers and students (Dohn et al., 2009).
In summary, the teacher has succeeded in creating an interactive and safe environment for the students to learn science. We suggest that this is due to a number of factors. Firstly, the high number of teacher questions opens the floor for the students to articulate their scientific knowledge. Secondly, the teacher gives the lessons a clear structure. He attempts to make this structure explicit and invites the students to influence it through his use of Organization questions. This ensures a clear framing of the learning environment, which contributes to the students’ sense of security. Thirdly, the students are given authority in the classroom. Especially the questions coded in the categories of Request and Relations seem to have had a significant influence in this regard. Thus, the studied classroom seems to promote the students’ learning processes as it gives them authority and entitles them to speak and learn.
While dialogic discourse allows students to argue and justify their ideas, the authoritative discourse also has its place in the classroom, particularly when already-constructed shared knowledge needs to be emphasized (Chin, 2006). Mortimer (1998) suggests that alternating between these two types of discourse is important for developing conceptual thinking on the intra-psychological plane, and Scott (1998, 2006) suggests that learning in the classroom will be enhanced by achieving some kind of balance between presenting information and allowing exploration of ideas.
Conclusion
Using an inductive approach and a detailed level of analysis, we have identified several functions of teacher questions in the chemistry classroom. Although the questions were mainly authoritative, the findings suggest that an interactive classroom can be created with the use of many, engaging teacher questions. This study provides specific examples of one teacher's questioning that may be illustrative for chemistry teachers who are interested in honing their discursive skills and in adopting approaches to classroom interaction that foster productive student responses. The findings from this study also have potential in terms of translating research insights into practical advice for chemistry teachers regarding tactical moves in classroom discourse. Any conclusions that can be drawn from this study are, of course, very limited. This is hardly surprising given that previous research has shown that the purpose of teacher questioning is to evaluate what students know. The teacher asks a closed question that is diagnostic, that requires a short answer, and that is usually pitched at the recall or lower-order cognitive level. However, what can be recognized is that the analytical framework used was able to uncover differences in the nature of the teacher questions. One limitation of this study is that, in the analysis and interpretation of video data, questions and responses as well as visual communication that includes gestures, were used as a primary marker of interactional or cognitive function, and the study is thus, at best, inferential. We have no evidence regarding how the teacher questions affected students’ thinking and understanding of the content. It must also be conceded that the analysis provides no insight into the teacher's intentions with the questions. A second limitation relates to time. In order to study social processes, the researcher should spend significant time embedded in the classroom to understand the sociocultural context. Although this study involved four weeks of observation, there may be a risk of missing something. A third limitation relates to the limited data set of this study, making generalizations problematic. It should be noted that, to the extent that the results are generalizable, they may only be true for similar populations. Expanding the data set and studying different populations would be the next step towards a more general understanding of the influence of teacher questions.Conflicts of interest
There are no conflicts to declare.References
- Becker N., Stanford C., Towns M. and Cole R., (2015), Translating across macroscopic, submicroscopic, and symbolic levels: the role of instructor facilitation in an inquiry-oriented physical chemistry class, Chem. Educ. Res. Pract., 16, 769–785.
- Bereiter C., (1994), Implications of postmodernism for science, or, science as progressive discourse, Educ. Psychol., 29, 3–12.
- Biggers M., (2017), Questioning Questions: Elementary Teachers’ Adaptations of Investigation Questions Across the Inquiry Continuum, Res. Sci. Educ, 1–28.
- Bleicher R., (1994), High school students presenting science: an interactional sociolinguistic analysis, J. Res. Sci. Teach., 31, 697–719.
- Blosser P. E., (2000), Ask the Right Questions, Arlington: NSTA Press.
- Bybee R., (2015), Scientific Literacy, in Gunstone R. (ed.), Encyclopedia of Science Education, Dordrecht: Springer Netherlands.
- Carlsen W. S., (1991), Questioning in Classrooms: A Sociolinguistic Perspective, Rev. Educ. Res., 61, 157–178.
- Childs A. and McNicholl J., (2007), Investigating the Relationship between Subject Content Knowledge and Pedagogical Practice through the Analysis of Classroom Discourse, Int. J. Sci. Educ., 29, 1629–1653.
- Chin C., (2004), Questioning Students in ways that encourage thinking, Teach. Sci.: J. Aust. Sci. Teach. Assoc., 50, 16–21.
- Chin C., (2006), Classroom Interaction in Science: teacher questioning and feedback to students’ responses, Int. J. Sci. Educ., 28, 1315–1346.
- Chin C., (2007), Teacher questioning in science classrooms: approaches that stimulate productive thinking, J. Res. Sci. Teach., 44, 815–843.
- Criswell B. A., (2012), Reducing the degrees of freedom in chemistry classroom conversations, Chem. Educ. Res. Pract., 13, 17–29.
- DeBoer G. E., (2000), Scientific literacy: another look at its historical and contemporary meanings and its relationship to science education reform, J. Res. Sci. Teach., 37, 582–601.
- Dohn N. B., Madsen P. and Malte H., (2009), The situational interest of undergraduate students in zoophysiology, Adv. Physiol. Educ., 33, 196–201.
- Edwards D. and Mercer N., (1987), Common Knowledge: The Development of Understanding in the Classroom, New York: Routledge.
- Eliasson N., Karlsson K. G. and Sørensen H., (2017), The role of questions in the science classroom – how girls and boys respond to teachers’ questions, Int. J. Sci. Educ., 39, 433–452.
- Erickson F., (2012), Qualitative Research Methods for Science Education, in Fraser B. J., Tobin K. and McRobbie C. J. (ed.), Second International Handbook of Science Education, Dordrecht: Springer Netherlands.
- Evagorou M. and Osborne J., (2010), The role of language in the learning and teaching of science, in Osborne J. and Dillon J. (ed.), Good Practice in Science: What Research Has to Say, 2nd edn, Glasgow: Open University Press.
- Fraser B., (1990), An approach to discourse markers, J. Pragmat., 14, 383–398.
- Gresalfi M. S., (2009), Taking Up Opportunities to Learn: Constructing Dispositions in Mathematics Classrooms, J. Learn. Sci., 18, 327–369.
- Jordan B. and Henderson A., (1995), Interaction Analysis: Foundations and Practice, J. Learn. Sci., 4, 39–103.
- Kawalkar A. and Vijapurkar J., (2013), Scaffolding Science Talk: the role of teachers' questions in the inquiry classroom, Int. J. Sci. Educ., 35, 2004–2027.
- Krathwohl D. R., (2002), A Revision of Bloom's Taxonomy: An Overview, Theor. Pract., 41, 212–218.
- Lave J., (1988), Cognition in practice: mind, mathematics, and culture in everyday life, New York: Cambridge University Press.
- Lave J. and Wenger E., (1991), Situated learning: legitimate peripheral participation, Cambridge, England: Cambridge University Press.
- Lee, Y. and Kinzie, M. B. (2012), Teacher question and student response with regard to cognition and language use, Instruct. Sci., 40, 857–874.
- Lemke J., (1990), Talking Science: Language, Learning, and Values, New York: Alex Publishing Corporation.
- Lemke J., (1998), Analysing Verbal Data: Principles, Methods, and Problems, in Tobin K. and Fraser B. (ed.), International handbook of science education, London: Kluwer Academic Press.
- Lord T. and Baviskar S., (2007), Moving Students From Information Recitation to Information Understanding: Exploiting Bloom's Taxonomy in Creating Science Questions, J. Coll. Sci. Teach., 36, 40–44.
- Mortimer E. F., (1998), Multivoicedness and univocality in classroom discourse: an example from theory of matter, Int. J. Sci. Educ., 20, 67–82.
- Mortimer E. and Scott P., (2003), Meaning Making In Secondary Science Classrooms, McGraw-Hill Education (UK).
- Pedretti E. and Nazir J., (2015), Science, Technology and Society (STS), in Gunstone R. (ed.), Encyclopedia of Science Education, Dordrecht: Springer Netherlands.
- Rasmussen C., Kwon O. and Marrongelle K., (2008), A framework for interpreting inquiry-oriented teaching, in The Eleventh Special Interest Group of the Mathematical Association of America on Research in Undergraduate Mathematics Education, San Diego, CA.
- Rowe M. B., (1974), Relation of wait-time and rewards to the development of language, logic, and fate control: part II-Rewards, J. Res. Sci. Teach., 11, 291–308.
- Schiffrin D., (1988), Discourse markers, Cambridge: Cambridge University Press.
- Scott P., (1998), Teacher Talk and Meaning Making in Science Classrooms: A Vygotskian Analysis and Review, Stud. Sci. Educ., 32, 45–80.
- Scott P. H., Mortimer E. F. and Aguiar O. G., (2006), The tension between authoritative and dialogic discourse: a fundamental characteristic of meaning making interactions in high school science lessons, Sci. Educ., 90, 605–631.
- Stanford C., Moon A., Towns M. and Cole R., (2016), Analysis of Instructor Facilitation Strategies and Their Influences on Student Argumentation: A Case Study of a Process Oriented Guided Inquiry Learning Physical Chemistry Classroom, J. Chem. Educ., 93, 1501–1513.
- Tobin K., (1987), The Role of Wait Time in Higher Cognitive Level Learning, Rev. Educ. Res., 57, 69–95.
- Tobin K., (2000), Interpretive research in science education, in Kelly A. E. L. and Lesh R. A. (ed.), Handbook of research design in mathematics and science education, Mahwah: Lawrence Erlbaum Associates, Publishers.
- Vauras M., Kinnunen R., Kajamies A. and Lehtinen E., (2012), Interpersonal regulation in instructional interaction: a dynamic systems analysis of scaffolding, in Volet S. E. and Vauras M. (ed.), Interpersonal Regulation of Learning and Motivation: Methodological advances, New York: Routledge.
- Warfa A.-R. M., Roehrig G. H., Schneider J. L. and Nyachwaya J., (2014), Role of Teacher-Initiated Discourses in Students’ Development of Representational Fluency in Chemistry: A Case Study, J. Chem. Educ., 91, 784–792.
- Wellington J. and Osborne J., (2001), Language and literacy in science education, Berkshire, Open University Press.
- Wenger E., (1998), Communities of practice: Learning, meaning, and identity, Cambridge, England: Cambridge University Press.
- Wu H.-K., (2003), Linking the microscopic view of chemistry to real-life experiences: intertextuality in a high-school science classroom, Sci. Educ., 87, 868–891.
- Yip D. Y., (2004), Questioning skills for conceptual change in science instruction, J. Biol. Educ., 38, 76–83.
| This journal is © The Royal Society of Chemistry 2018 |
