Open Access Article
Open Access ArticleA copper(II)-catalyzed annulative formylation of o-alkynylanilines with DMF: a single-step strategy for 3-formyl indoles†
Balaji Ganesan a,
Gopal Chandru Senadi
a,
Gopal Chandru Senadi ab,
Bing-Chun Guoa,
Min-Yuan Hungc and
Wei-Yu Lin
ab,
Bing-Chun Guoa,
Min-Yuan Hungc and
Wei-Yu Lin *a
*a
aDepartment of Medicinal and Applied Chemistry, Kaohsiung Medical University, No. 100 Shi-chuan, 1st Road, Kaohsiung 807, Taiwan. E-mail: wylin@kmu.edu.tw
bDepartment of Chemistry, SRM Institute of Science and Technology, Kattankulathur, Chennai–603203, India
cCenter for Research Resources and Development, Kaohsiung Medical University, No. 100 Shi-chuan, 1st Road, Kaohsiung 807, Taiwan
First published on 7th December 2018
Abstract
In this paper, a copper(II)-catalyzed reaction of o-alkynylanilines with dimethylformamide (DMF) in the presence of oxygen has been developed for synthesizing multisubstituted 3-formyl indole scaffolds. This one-pot reaction proceeds through a cascade 5-endo-dig cyclization followed by formylation to construct 1,2-disubstituted 3-formyl indoles. The key aspects of this synthesis method are the broad substrate scope (with 38 examples), and well tolerating various functional groups. In addition, a detailed mechanism has been proposed, where DMF may serve as a carbon source for the in situ C3 formylation of the obtained indole derivatives.
Introduction
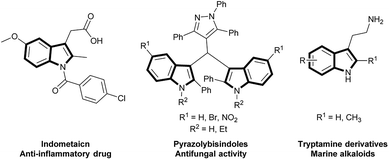
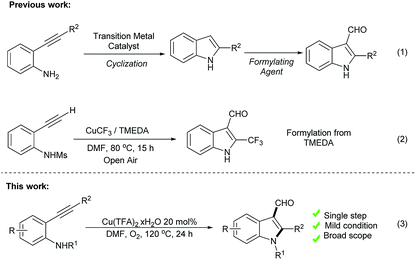
2,3-Disubstituted and 1,2,3-multi-substituted indoles are widely available in various alkaloids and natural products possessing potential biological activities. Several essential examples include indometacin, pyrazolybisindoles and substituted tryptamine derivatives, which exhibit significant anticancer activities (Scheme 1).1 Among these crucial molecules, intermediates with a formyl functional group at the C3 position can serve as a primary building block to construct substituted indoles through their broad and specific reactivity for various chemical transformations. Therefore, 1,2,3-tri-substituted indole preparation has received considerable interest in previous decades.According to a literature survey, several pioneering methods have been reported for the construction of 1,2 di-substituted 3-formyl indoles through two-step reactions. The first step involves the use of the easily accessible starting materials of o-alknylaniline derivatives and is mediated by transition metals (Pt,2 Pd,3 Ag,4 Au,5 Ir,6 In,7 Fe,8 and Cu9), the molecular halogen10 catalyzed annulation of o-alknylaniline derivatives, or free radical assisted indole construction through either C–C or C–N bond-forming reactions.11 The second step involves direct formylation by an existing indole using the Vilsmeier–Haack reaction,12 or other methods13 (Scheme 2, eqn (1)). Tsui and coworkers14 recently reported an elegant method for synthesizing 2-(trifluoromethyl)indoles from N-mesylated-2-alkynylanilines through domino cyclization and formylation by using TMEDA as a carbon synthon (Scheme 2, eqn (2)).14 Although these methods are potentially useful, some have certain limitations, such as desulfonylation, post-functionalization is necessary for the N-substitution of the indole derivatives, expensive metal-catalysts2–5 and strong acidic conditions.12 Herein, we wish to report a complementary approach by employing o-phenylethynyl N-substituted aniline (1, 3, and 5) as a starting material for the one-pot domino synthesis of multisubstituted-3-formyl indole (2, 4, and 6) using DMF in the presence of oxygen (Scheme 2, eqn (3)). Moreover, detailed mechanistic studies of the Cu(II) and O2-mediated formylation were conducted.
Results and discussion
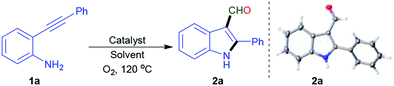
To optimize reaction parameters, preliminary screening was conducted using o-phenylethynyl aniline 1a as a model substrate (Table 1). The reaction was initially carried out using 1a in DMF (0.25 M) with 20 mol% of Cu(OAc)2·H2O as a catalyst, and TFA as an additive (2.0 equiv.) under an O2 atmosphere at 120 °C for 24 h. The desired product 2a was obtained in 31% yield (Table 1, entry 1). To optimize the catalyst, TFA was added as an additive with various copper catalysts such as anhyd. Cu(OAc)2, Cu(OTf)2, CuSO4·H2O, Cu(I), and Cu. The desired product 2a was obtained in 45% yield when Cu powder was used as a catalyst (Table 1, entries 2–6). The reaction yield did not improve when different Brønsted acids were used, including TfOH and TsOH·H2O additives (Table 1, entries 7–8). 2a was not obtained, when only Cu was added as a catalyst (Table 1, entry 9). To our delighted, Cu(TFA)2·xH2O was used as a catalyst instead of Cu/TFA, and the reaction yield increased to 52% (Table 1, entry 10). The reaction yield was reduced to 32% when the reaction was performed in air (Table 1, entry 11). Furthermore, no product was formed under a N2 atmosphere (Table 1, entry 12). Other oxidants such as DDQ, and AgOAc under the N2 atmosphere, resulted in low yield (Table 1, entries 13–14). These results indicate that the oxygen supply is crucial for the reaction. The use of alternative solvents such as, dioxane, DMSO, and a binary solvent system (THF/DMF 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) generated trace yield (Table 1, entries 15–17). When the catalyst was reduced to 5 mol% or 10 mol%, the reaction yield was reduced (Table 1, entries 18–19). The addition of another 20 mol% of Cu(TFA)2·xH2O catalyst after 12 h, did not substantially improve the reaction yield (Table 1, entry 20).
1) generated trace yield (Table 1, entries 15–17). When the catalyst was reduced to 5 mol% or 10 mol%, the reaction yield was reduced (Table 1, entries 18–19). The addition of another 20 mol% of Cu(TFA)2·xH2O catalyst after 12 h, did not substantially improve the reaction yield (Table 1, entry 20).
| Entry | Catalyst (20 mol%) | Additive (2.0 equiv.) | Oxidant | Solvent/time (h) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions 1a (1.0 equiv.), catalyst (20 mol%), solvent (3.0 mL), O2 balloon, 120 °C, 24 h at indicated time unless otherwise noted.b Isolated yield.c Reaction was performed under open air.d Reaction was performed under nitrogen atmosphere.e 5 mol% of Cu(TFA)2·xH2O was used.f 10 mol% of Cu(TFA)2·xH2O was used.g Another 20 mol% of Cu(TFA)2·xH2O was added after 12 h. | |||||
| 1 | Cu(OAc)2·H2O | TFA | O2 | DMF/20 | 31 |
| 2 | Cu(OAc)2 | TFA | O2 | DMF/14 | 37 |
| 3 | Cu(OTf)2 | TFA | O2 | DMF/28 | 20 |
| 4 | CuSO4·H2O | TFA | O2 | DMF/25 | 27 |
| 5 | CuI | TFA | O2 | DMF/22 | 27 |
| 6 | Cu | TFA | O2 | DMF/20 | 45 |
| 7 | Cu | TfOH | O2 | DMF/24 | 15 |
| 8 | Cu | TsOH·H2O | O2 | DMF/24 | 18 |
| 9 | Cu | — | O2 | DMF/20 | N.R |
| 10 | Cu(TFA)2·xH2O | — | O2 | DMF/24 | 52 |
| 11c | Cu(TFA)2·xH2O | — | Open air | DMF/24 | 32 |
| 12d | Cu(TFA)2·xH2O | — | — | DMF/24 | N.R |
| 13 | Cu(TFA)2·xH2O | — | DDQ | DMF/24 | 16 |
| 14 | Cu(TFA)2·xH2O | — | AgOAc | DMF/24 | 11 |
| 15 | Cu(TFA)2·xH2O | — | O2 | Dioxane/24 | Trace |
| 16 | Cu(TFA)2·xH2O | — | O2 | DMSO/24 | Trace |
| 17 | Cu(TFA)2·xH2O | — | O2 | THF/DMF (1/1)/24 | Trace |
| 18e | Cu(TFA)2·xH2O | — | O2 | DMF/24 | 12 |
| 19f | Cu(TFA)2·xH2O | — | O2 | DMF/24 | 30 |
| 20g | Cu(TFA)2·xH2O | — | O2 | DMF/24 | 54 |
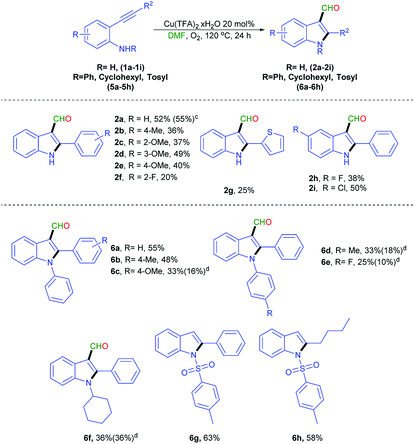
With the optimized conditions (Table 1, entry 10), the scope of this reaction was studied using o-phenylethynyl aniline derivatives [(1a–1i) and (5a–5h)], and the reaction results are summarized in Scheme 3. To evaluate the reaction scope and probable substituent effects of R2, various functional groups including substituted phenyls, and the heterocyclic group were studied (Scheme 3, 1a–1g). Compared with 1a, substrates with electron-donating groups on the phenyl ring, such as 2-Me and 2 or 4-OMe were suitable for this reaction, generating the corresponding products 2b, 2c and 2e in moderate yield. A substrate with electron-withdrawing groups, such as 2-F (1f) gave 2f in lower yield. When the substituent group was thiophene (1g), the desired was obtained in a low yield of 25% (2g). The substituent on the aryl ring (R) was subsequently studied (Scheme 3, 1h–1i). Electron-withdrawing groups, including 4-F (1l), and 4-Cl (1m) generated the corresponding products 2l and 2m in 38% and 50% yield, respectively. A scale up experiment could be performed in a 3 mmol scale of 1a, to obtain 2a in 55% yield, indicating the efficacy and scalability of the reaction. The structure of 2a was validated through X-ray crystallography analysis, and the data was presented in ESI.†18 The scope of the N-phenyl-2-phenylethynyl aniline derivatives (5a–5h) were explored and the resulting data are shown in Scheme 3. Electron-donating groups at the alkynyl position, exhibiting the desired product (6a–6c) in 33–55% yields. 4-Me (5d), and 4-F (5e) groups on the N-phenyl were formed smoothly, with 10–18% recovery of starting materials. A cyclohexyl group (5f) present on a nitrogen atom gave the desired product (6f). Surprisingly, when N-tosylate-2-phenylethynyl aniline derivatives (5g and 5h) used as substrates, only led to the formation of indole14 (Scheme 5, 6g, 6h).
With the successful outcome of 2,3 di-substituted indole derivatives, this method was extended to synthesize of 1,2,3 tri-substituted indole derivatives by using the N-benzylated 2-phenylethynyl aniline derivatives (3a–3u) under the optimized conditions (Scheme 4). When 3a was tested, the desired product 4a was obtained in 34% yield. Substituents at the R2 position with a phenyl ring having electron-donating groups, such as 2-Me (3b), 4-Me (3c), 2-OMe (3d), and 4-OMe (3f) had appropriate reactivity. By contrast, the electron-withdrawing groups, including 3-Cl (3g), 3-NO2 (3h), and 3-CF3 (3i), produced the corresponding products in moderate yields (32–44%). Thiophene group (3j) smoothly proceeded in 60% yield. Aliphatic groups, such as hexyl (3k) and cyclopropane (3l), were examined, the desired products were obtained in 30% and 33% yields, respectively. The substitution of a phenyl ring bearing an electron donating groups such as 4-Me (3m), 4-OMe (3n), and t-Bu (3o), on R1 provided moderate yield (44–54%). The presence of electron withdrawing groups (3p–3r) on a benzyl ring generated a similar moderate yield. An indole phenyl ring bearing with electron withdrawing groups such as F (3s), Cl (3t), and Br (3u) provided in good yield. During this reaction, the formation of debenzylation products was observed (ESI†). This formation might be a side reaction of substrate with molecular oxygen under high temperature.14,15
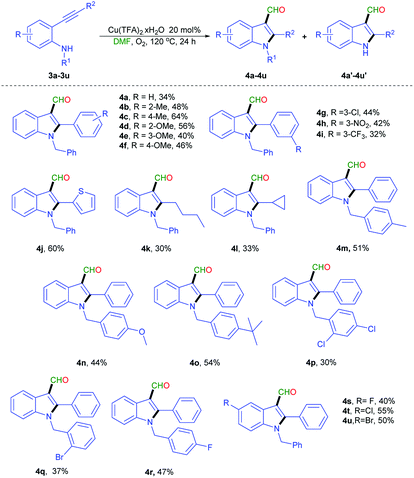 | ||
| Scheme 4 Scope N-benzylated-2-phenylethynyl aniline derivativesa,b,c. aReaction conditions: 3a–3v (0.3 mmol), Cu(TFA)2·xH2O (20 mol%), DMF (3 mL), 120 °C, 24 h, O2. bIsolated yield. cPlease see Table S1 in ESI† for isolated yield of 4a′–4u′. | ||
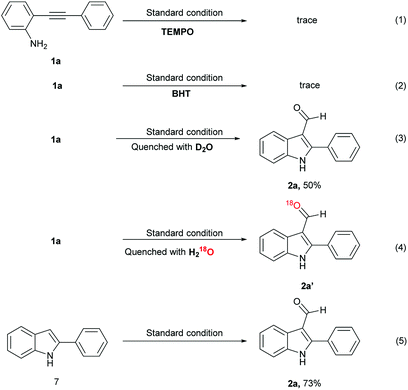
To understand the formation of the 3-formyl indoles derivatives, several control experiments were performed (Scheme 5). Table 1 (vide supra) indicates that when oxygen was replaced by air or nitrogen, the reaction yield was reduced to 32% and 0%, respectively (Table 1, entries 11 and 12). These results revealed that sufficient supply of oxygen is necessary for this reaction. When a 2.5 equivalent of common radical scavengers, including TEMPO or BHT was added, a trace amount (<5%) of 2a was detected through GC-MS and the starting material remained. The results suggested that this reaction may occur through a radical process (Scheme 5, eqn (1) and (2)). The addition of D2O after the completion of the reaction could not afford the deuterium-labeled aldehyde in 50% yield (Scheme 5, eqn (3)). The addition of 18O-labeled water to the reaction mixture under a standard condition afforded 100% of 18O-labeling product 2a′ (Scheme 5, eqn (4), and ESI†), where the oxygen atom of the formyl group is originated from water. The reaction with the 3-H-indole substrate 7 generated 2a in good yield (73%) under the standard condition (Scheme 5, eqn (5)). These results indicate that the product formation was through a pathway involving radical intermediates that utilized molecular oxygen as an oxidant.
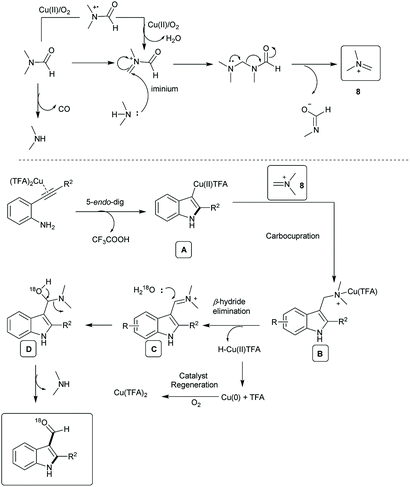
The proposed mechanism is based on the control experiments and previous reports13e,14,16,17 (Scheme 6). DMF was activated by Cu(II) and oxygen to generate the iminium ion 8.13e,14,16 The simultaneous electrophilic activation of alkyne by Cu(II) and intramolecular 5-endo-dig cyclization generated the vinyl Cu(II) intermediate A.14 The carbocupration of the intermediate A and the reaction with an iminium ion led to formation of the N–Cu(II) intermediate B.13e,14,16,17 The β-hydride elimination of the B gave the primary iminium species C along with the catalytic regeneration of Cu(0) in the presence of oxygen. The nucleophilic addition of 18O-labeled water to the C generated the hemiketal intermediate D. Finally, the elimination of dimethylamine from the D generated the desired 3-formyl indoles derivatives.
Experimental
Experimental procedure,11a crystallographic data and spectroscopic data of new starting materials and final products were given in ESI.†18In sealed tube, o-alkynylaniline derivatives (1a–1i, 5a–5h & 3a–3u) (0.3 mmol) was taken in DMF (3 mL). To the stirred solution, Cu(TFA)2·xH2O (20 mol%) was added and allowed to stir under O2 source at 120 °C, until the completion of starting material (∼24 h). The reaction mixture was quenched with ice cold water and extracted with ethyl acetate. Combined organic layer washed with brine, dried over MgSO4 and concentrated in vacuum. Then the crude material was purified by column chromatography using 20% ethyl acetate in hexane as eluent to afford the desired product (2a–2i, 6a–6h & 4a–4u).
Conclusions
In summary, in this paper, a new synthetic method was developed for the direct synthesis of 1,2-disubstituted 3-formyl indole or 2-substituted 3-formyl indole from the common starting materials of o-alkynylaniline through 5-endo-dig cyclization followed by an iminium ion attack at the C3 position. Compared with other methods, this one-pot reaction was using the inexpensive Cu-catalyst and DMF as a formyl carbon source, without any additives. This method can be used with a wide range of functional groups and the desired products can be obtain up to moderate yield. In the near future, this method can be adapted as a potential route for the direct synthesis of 3-formyl indole. Further investigation of this reaction in continuous flow system are currently ongoing in our laboratories.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge funding from the Ministry of Science and Technology (MOST 106-2113-M-037-009-), Taiwan, and the Center for Research Resources and Development (CRRD) of Kaohsiung Medical University for Mass and 400 MHz NMR analyses.Notes and references
- T. V. Sravanthi and S. L. Manju, Eur. J. Pharm. Sci., 2016, 91, 1–10 CrossRef CAS PubMed.
- (a) P. Rubio-Marques, M. A. Rivero-Crespo, A. Leyva-Perez and A. Corma, J. Am. Chem. Soc., 2015, 137, 11832–11837 CrossRef CAS PubMed; (b) N. Chaisan, W. Kaewsri, C. Thongsornkleeb, J. Tummatorn and S. Ruchirawat, Tetrahedron Lett., 2018, 59, 675–680 CrossRef CAS.
- (a) C. Xu, V. K. Murugan and S. A. Pullarkat, Org. Biomol. Chem., 2012, 10, 3875–3881 RSC; (b) J. Kwon, J. Chung, S. Byun and B. M. Kim, Asian J. Org. Chem., 2016, 5, 470–476 CrossRef CAS; (c) E. Tyrrell, L. Whiteman and N. Williams, Synthesis, 2009, 829–835 CrossRef CAS.
- (a) A. Arcadi, M. Chiarini, L. Del Vecchio, F. Marinelli and V. Michelet, Chem. Commun., 2016, 52, 1458–1461 RSC; (b) S. Karmakar, P. Das, D. Ray, S. Ghosh and S. K. Chattopadhyay, Org. Lett., 2016, 18, 5200–5203 CrossRef CAS PubMed; (c) H. P. Sun, L. Xiao, W. Li, Q. Xie and L. M. Shao, Synthesis, 2017, 49, 4845–4852 CrossRef CAS.
- (a) E. Chong and S. A. Blum, J. Am. Chem. Soc., 2015, 137, 10144–10147 CrossRef CAS PubMed; (b) J. E. Perea-Buceta, T. Wirtanen, O. V. Laukkanen, M. K. Makela, M. Nieger, M. Melchionna, N. Huittinen, J. A. Lopez-Sanchez and J. Helaja, Angew. Chem., Int. Ed., 2013, 52, 11835–11839 CrossRef CAS PubMed; (c) S. Z. Liang, L. Hammond, B. Xu and G. B. Hammond, Adv. Synth. Catal., 2016, 358, 3313–3318 CrossRef CAS; (d) O. S. Morozov, A. V. Lunchev, A. A. Bush, A. A. Tukov, A. F. Asachenko, V. N. Khrustalev, S. S. Zalesskiy, V. P. Ananikov and M. S. Nechaev, Chem.–Eur. J., 2014, 20, 6162–6170 CrossRef CAS PubMed; (e) K. C. Majumdar, S. Samanta and B. Chattopadhyay, Tetrahedron Lett., 2008, 49, 7213–7216 CrossRef CAS.
- (a) E. Kumaran and W. K. Leong, Tetrahedron Lett., 2014, 55, 5495–5498 CrossRef CAS; (b) X. W. Li, A. R. Chianese, T. Vogel and R. H. Crabtree, Org. Lett., 2005, 7, 5437–5440 CrossRef CAS PubMed.
- (a) N. Sakai, K. Annaka, A. Fujita, A. Sato and T. Konakahara, J. Org. Chem., 2008, 73, 4160–4165 CrossRef CAS PubMed; (b) N. Sakai, K. Annaka and T. Konakahara, Org. Lett., 2004, 6, 1527–1530 CrossRef CAS PubMed.
- V. Terrasson, J. Michaux, A. Gaucher, J. Wehbe, S. Marque, D. Prim and J. M. Campagne, Eur. J. Org. Chem., 2007, 5332–5335 CrossRef.
- (a) Y. B. Ye, K. P. S. Cheung, L. S. He and G. C. Tsui, Org. Chem. Front., 2018, 5, 1511–1515 RSC; (b) H. F. Wang, Y. M. Li, L. L. Jiang, R. Zhang, K. Jin, D. F. Zhao and C. Y. Duan, Org. Biomol. Chem., 2011, 9, 4983–4986 RSC; (c) F. Liu and D. Ma, J. Org. Chem., 2007, 72(13), 4844–4850 CrossRef CAS PubMed; (d) S. Cacchi, G. Fabrizi and L. M. Parisi, Org. Lett., 2003, 5(21), 3843–3846 CrossRef CAS PubMed; (e) K. S. Kumar, B. Rajesham, M. S. Ramulu, B. Bhaskar, S. N. Dash, M. A. Ashfaq, R. Nagarapu, A. A. Khan, S. Lehtonen and M. Pal, RSC Adv., 2016, 6, 100487 RSC; (f) S. Cacchi, G. Fabrizi, L. M. Parisi and R. Bernini, Synlett, 2004, 2, 0287–0290 CrossRef; (g) G. A. Slough, V. Krchňák, P. Helquist and S. M. Canham, Org. Lett., 2004, 6(17), 2909–2912 CrossRef CAS PubMed.
- Y. Takeda, R. Kajihara, N. Kobayashi, K. Noguchi and A. Saito, Org. Lett., 2017, 19, 6744–6747 CrossRef CAS PubMed.
- (a) G. C. Senadi, J. Q. Wang, B. S. Gore and J. J. Wang, Adv. Synth. Catal., 2017, 359, 2747–2753 CrossRef CAS; (b) G. C. Senadi, B. S. Gore, W. P. Hu and J. J. Wang, Org. Lett., 2016, 18, 2890–2893 CrossRef CAS PubMed; (c) S. S. K. Boominathan, G. C. Senadi, J. K. Vandavasi, J. Y. F. Chen and J. J. Wang, Chem.–Eur. J., 2015, 21, 3193–3197 CrossRef CAS PubMed; (d) G. C. Senadi, W. P. Hu, S. S. K. Boominathan and J. J. Wang, Adv. Synth. Catal., 2013, 355, 3679–3693 CrossRef CAS.
- (a) J. Qu, N. Kumar, M. Alamgir and D. S. Black, Tetrahedron Lett., 2009, 50, 5628–5630 CrossRef CAS; (b) S. Naureen, F. Chaudhry, N. Asif, M. A. Munawar, M. Ashraf, F. H. Nasim, H. Arshad and M. A. Khan, Eur. J. Med. Chem., 2015, 102, 464–470 CrossRef CAS PubMed; (c) M. Nyerges, A. Pinter, A. Viranyi, I. Bitter and L. Toke, Tetrahedron Lett., 2005, 46, 377–380 CrossRef CAS.
- (a) J. B. Chen, B. Liu, D. F. Liu, S. Liu and J. Cheng, Adv. Synth. Catal., 2012, 354, 2438–2442 CrossRef CAS; (b) Z. P. Yin, Z. C. Wang and X. F. Wu, Org. Biomol. Chem., 2018, 16, 3707–3710 RSC; (c) Q. D. Wang, J. M. Yang, D. Fang, J. M. Ren and B. B. Zeng, Tetrahedron Lett., 2017, 58, 2877–2880 CrossRef CAS; (d) Q. D. Wang, B. Zhou, J. M. Yang, D. Fang, J. M. Ren and B. B. Zeng, Synlett, 2017, 28, 2670–2674 CrossRef CAS; (e) B. Zhang, B. Liu, J. B. Chen, J. H. Wang and M. C. Liu, Tetrahedron Lett., 2014, 55, 5618–5621 CrossRef CAS; (f) H. Y. Fei, J. T. Yu, Y. Jiang, H. Guo and J. Cheng, Org. Biomol. Chem., 2013, 11, 7092–7095 RSC; (g) X. Li, X. Y. Gu, Y. J. Li and P. X. Li, ACS Catal., 2014, 4, 1897–1900 CrossRef CAS.
- Y. B. Ye, K. P. S. Cheung, L. S. He and G. C. Tsui, Org. Lett., 2018, 20, 1676–1679 CrossRef CAS PubMed.
- (a) A. A. Haddach, A. Kelleman and M. V. Deaton-Rewolinski, Tetrahedron Lett., 2002, 43, 399–402 CrossRef CAS; (b) A. Hfaiedh, H. Ben Ammar, J. F. Soule and H. Doucet, Org. Biomol. Chem., 2016, 14, 4947–4956 RSC; (c) H. Suzuki, A. Tsukuda, M. Kondo, M. Aizawa, Y. Senoo, M. Nakajima, T. Watanabe, Y. Yokoyama and Y. Murakami, Tetrahedron Lett., 1995, 36, 1671–1672 CrossRef CAS.
- X. S. Wu, Y. Zhao and H. B. Ge, J. Am. Chem. Soc., 2015, 137, 4924–4927 CrossRef CAS PubMed.
- W. Gati, F. Couty, T. Boubaker, M. M. Rammah, M. B. Rammah and G. Evano, Org. Lett., 2013, 15, 3122–3125 CrossRef CAS PubMed.
- CCDC number 1876301 (2a)† contains the supplementary crystallographic data for this paper..
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1876301 (2a). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra09214a |
| This journal is © The Royal Society of Chemistry 2018 |