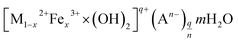Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A critical review on arsenic removal from water using iron-based adsorbents
Linlin Hao ab,
Mengzhu Liua,
Nannan Wang
ab,
Mengzhu Liua,
Nannan Wang c and
Guiju Li*a
c and
Guiju Li*a
aCollege of Marine and Environmental Sciences, Tianjin University of Science & Technology, Tianjin 300457, P. R. China. E-mail: liguij@tust.edu.cn
bDepartment of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore 117543
cSchool of Mechanical Engineering, Beijing Institute of Petrochemical Technology, Beijing Key Laboratory of Pipeline Critical Technology and Equipment for Deepwater Oil & Gas Development, Beijing 102617, P.R. China
First published on 27th November 2018
Abstract
Intensive research efforts have been pursued to remove arsenic (As) contamination from water with an intention to provide potable water to millions of people living in different countries. Recent studies have revealed that iron-based adsorbents, which are non-toxic, low cost, and easily accessible in large quantities, offer promising results for arsenic removal from water. This review is focused on the removal of arsenic from water using iron-based materials such as iron-based nanoparticles, iron-based layered double hydroxides (LDHs), zero-valent iron (ZVI), iron-doped activated carbon, iron-doped polymer/biomass materials, iron-doped inorganic minerals, and iron-containing combined metal oxides. This review also discusses readily available low-cost adsorbents such as natural cellulose materials, bio-wastes, and soils enriched with iron. Details on mathematical models dealing with adsorption, including thermodynamics, kinetics, and mass transfer process, are also discussed. For elucidating the adsorption mechanisms of specific adsorption of arsenic on the iron-based adsorbent, X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) are frequently used. Overall, iron-based adsorbents offer significant potential towards developing adsorbents for arsenic removal from water.
1. Introduction
1.1. Arsenic element in Nature
Arsenic element exists as oxides in the soil, sediments and water in many parts of the world and originates from both natural and anthropogenic activities. There are four chemical oxidation states for arsenic (−3, +3, 0, and +5) in Nature.1 The most common arsenic compounds that naturally occur are arsenite (H3AsO3 – As(III)) and arsenate (HAsO42− – As(V)). As(V) is the predominant species present under oxidizing conditions and exists as oxyanions of arsenic acid (H3AsO4, H2AsO4−, HAsO42−and AsO43−), while As(III) exists as arsenious acid (H3AsO3, H2AsO3−, HAsO32−) under mildly reducing conditions.2 Arsenic compounds have been recognized as group 1 carcinogens by the International Agency for Research on Cancer (IARC).3 The current standard for the maximum contaminant level (MCL) of arsenic in drinking water recommended by the World Health Organization (WHO) is 10 μg L−1.Arsenic species are always pH dependent.4 As(III) exists mostly as neutral H3AsO3 when the solution pH is lower than 9.2 (the pKa1 of H3AsO3 is 9.2), while the dominant species of As(V) are H2AsO4− and HAsO42− (the pKa1 of H3AsO4 is 2.3; the pKa2 of H3AsO4 is 6.8; the pKa3 of H3AsO4 is 11.6). As(III) is about 60 times more toxic than As(V),5 and the mobility of As(III) is more than that of As(V) because the probability of adsorption of neutral As(III) to a mineral surface is less than As(V).6 Therefore, chemical oxidants such as chlorine, hydrogen peroxide (H2O2), ozone (O3), permanganate, and persulfate-based systems were frequently employed to oxidize As(III) to As(V).7 For example, Zhou et al. (2017)8 and Hussain et al. (2017)9 has identified that the capacity of sodium persulfate (PS), activated by zero-valent iron (ZVI) to remove arsenic from water is much greater than that of PS alone due to the production of sulfate radicals. L. Zhou et al. (2013)10 also demonstrated that Fe(II)/persulfate oxidation could be an effective method to oxidize As(III) for the remediation of arsenic contaminated groundwater.
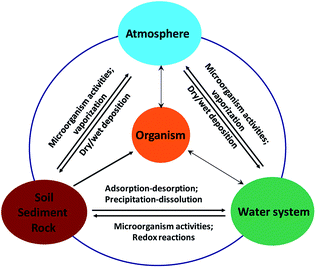
Arsenic can release into the aquatic environments by natural processes such as dissolution of minerals by weathering, microbial activity, and complexation with natural organic materials.11 On the other hand, anthropogenic activities, including industrial mining and metallurgical industries, combustion of fossil fuels, use of arsenic pesticides, herbicides, and crop desiccants, can result in arsenic contamination in soils and surface water.12 The microorganism plays an important role in transformation of minerals or weathering of rocks in the geo-aqueous solution. Furthermore, it is reported that biogeochemical activities of microorganism can control arsenic contamination in groundwater by forming arsenical biominerals, such as loellingite (FeAs2) and symplesite (Fe3(AsO4)2·8H2O).13 Spratlen et al.14 and Oremland and Stolz15 proposed that even though arsenic is highly poisonous, certain prokaryotes use arsenic oxidation for energy generation either by oxidizing arsenite or by respiring arsenate. However there is limited research on arsenic mineral dissolution with considering both of chemical and microbial process in natural environment. Arsenic circulation in Nature is schematically shown in Fig. 1.
1.2. The distribution of arsenic in natural waters
1.3. Harmful effect of arsenic pollution
Drinking water is the main source of arsenic exposure to the living organisms. Continuous exposure to arsenic pollution has been shown to cause damage to the central nervous system, kidney, skin, liver and lungs in humans.25 Arsenic can also increase glutathione peroxidase and mitochondrial superoxide dismutase (MSOD) activities in liver and lungs. In addition, chronic arsenic toxicity can cause cardiovascular diseases, hypertension and affects vascular system.26 Long-term contact with arsenic contaminated water can lead to pigmentation of the skin and development of hard patches on the palm of humans.27 To mitigate this situation, World Health Organization has reduced the maximum contaminant limit (MCL) of arsenic in drinking water from 50 to 10 μg L−1.28 Therefore, the development of more effective water treatment is required to satisfy the new regulations.1.4. Methodologies for arsenic removal from water
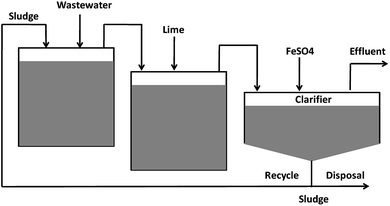
Arsenic removal methods include chemical precipitation/flocculation,29 adsorption,30 ion exchange,31 reverse osmosis32 and electro-dialysis.33 Arsenic can be removed by precipitation as ferric arsenate, calcium arsenate or arsenic sulfide. It was established that As precipitation with ferric salts is more efficient than aluminium salts.34 However, the concentration of arsenic below 10 μg L−1 is usually difficult to attain via chemical precipitation. Moreover, removal of As(III) during precipitation is considerably less effective than As(V) anions under similar conditions, and pre-oxidation is required to convert As(III) to As(V) ions in water.35 For water containing high arsenic concentrations, lime softening was an effective way to lower the arsenic concentration, followed by a use of other techniques.36 The typical techniques for the removal of high concentrations of arsenic from wastewater are shown in Fig. 2. Klerk et al. (2015)37 conducted a continuous circuit co-precipitation of As(V) with ferric ions by lime neutralization. Two-stage continuous experiments (operating at pH 4 and 8, respectively) produced the lowest residual arsenic concentration when Fe/As molar ratio was kept at 4.Ion exchange technology was also considered as another effective method to remove arsenic from water by using anion exchange resins.38 However, it is only efficient for As(V) removal, not good for the uncharged As(III) species in water. Moreover, developing ion exchange resin and the high-tech water purification systems are usually expensive. The adsorption capacity was limited because of the interference from competitive adsorption of other co-existing anions. The adsorbent regeneration process also created a sludge disposal problem.
In recent years, membrane techniques, including nanofiltration and reverse osmosis, are increasingly reported for arsenic removal from water.39 Such techniques have advantages of high-removal efficiency, easy operation and minimum toxic sludge generated during the process.40 But the initial investment and running cost are relatively high; in addition, high pressure is usually needed to force the contaminated water through the membranes. Moreover, the discharge of the concentrate, membrane fouling and flux decline are usually inevitable in the membrane process.41 The electro-dialysis was capable of removing both arsenic and other contaminants, but large amounts of insoluble coagulants were also deposited on the cathode.42
Among many techniques currently available for arsenic removal from water, the adsorption process is considered one of the most promising techniques because of low cost, high efficiency, and ease of operation.43 Iron-based adsorbents have been extensively developed and showed good removal efficiency for arsenic species from water.44 Some adsorbents such as granular ferric hydroxide (GFH) and zero-valent iron have been produced on an industrial scale as commercial adsorbents.45 However, most of the reported adsorbents seldom make the practical field applications despite their proven high efficiency of arsenic removal, owing to the interfering ions present in the water. For the iron-based adsorbents, the common anions such as Cl−, NO3−, SO42−, CO32− were not observed to have a significant influence on arsenic adsorption due to the specific chemical reaction between arsenic and iron.46 It was reported that phosphate can strongly compete with arsenic for the adsorption sites, thus decreasing the arsenic adsorption capacity.47 The presence of organic matter, such as humic acid and fulvic acid, also showed negative effects on arsenic adsorption in terms of delaying the adsorption equilibrium.48
2. Development of iron-based adsorbents for arsenic removal
Iron-based adsorbents attracted interest owing to their high efficiency in arsenic remediation, environmental friendliness and abundance on earth. In this review, attention is given towards exploring new iron-based adsorbents with high adsorption capacities for As species and a summary of the relevant mechanism of adsorption. We have referred most of the valuable published literature on arsenic remediation by adsorption. Arsenic adsorption using iron compounds, zero-valent iron, iron-based bimetal oxides, iron-doped composite adsorbents are critically reviewed and their adsorption efficiencies are compared. Besides the adsorption capacities, the characteristics of adsorption processes, including thermodynamics, kinetics and mass transfer mechanisms are also examined. The specific binding between arsenic and iron was deeply investigated by various spectral technologies. Moreover, some iron-based adsorbents are magnetic, allowing for an easy separation of the saturated materials from water in an external magnetic field.2.1. Iron oxy-hydroxides
Many different materials have been reported to have a good affinity towards arsenic, but iron oxy-hydroxides are the most widely studied because of their easy accessibility. The commonly used iron oxy-hydroxides such as, akaganèite (β-FeOOH), goethite (α-FeOOH), lepidocrocite (γ-FeOOH), ferrihydrites (Fe10O14(OH)2), green rusts can be chemically synthesized by the precipitation of Fe(III) or Fe(II) salts through the hydrolysis and oxidation processes.49–51 A scale-up method for the preparation of iron oxy-hydroxide in large amounts with a high production yield using FeSO4·H2O and FeCl2·4H2O was reported.52 The synthetic route included a sequence of oxidation, hydrolysis and precipitation using a continuous flow reactor (as shown in Fig. 3), the synthesized adsorbents presented better performance for adsorption of As(III) species as compared to commercial granular ferric hydroxide (GFH) and granular ferric hydroxide (GFO).53 Abiogenic iron oxy-hydroxide is reported to be more efficient to remove As(V) than biogenetic iron oxy-hydroxide.54 It is explained that nitrate-reducing Fe(II)-oxidizing bacteria can use nitrate as an electron acceptor to oxidize Fe(II) to precipitate the biogenic iron oxy-hydroxides in anoxic groundwater aquifers.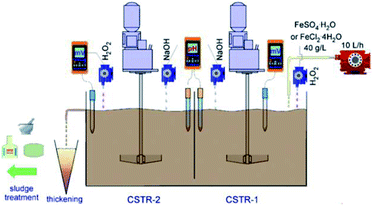 | ||
| Fig. 3 Scheme of the laboratory iron oxy-hydroxides' production.52 | ||
The photochemistry of As(III) adsorption on ferrihydrite was investigated by using the attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) and X-ray absorption near edge structure (XANES).55 The stable As(III) oxidation state in the dark is gradually transformed to As(V) on ferrihydrite in presence of light at pH 5. At the same time, Fe(III) ions were reduced to Fe(II) species during the As(III) oxidation.56 It was observed the adsorption rate (12.4 × 10−5 M s−1 m−2) on goethite was significantly faster than that of ferrihydrite (6.73 × 10−5 M s−1 m−2) at pH 5.57 The similar photochemical reaction was also observed for As(III) adsorption on goethite in the presence of dissolved oxygen.58 Laterite, a natural iron oxide mineral containing 91% of goethite, is another promising and cost-effective material for arsenic adsorption.59 The specific adsorption of arsenic is demonstrated by a strong evidence of the shift of isoelectric point. Iron-rich laterite was more effective than goethite (α-FeOOH), magnetite (Fe3O4) and hematite (Fe2O3), because of the higher specific surface area (81.2 m2 g−1).60 Natural siderite has been widely studied to remove both As(III) and As(V) species from water,61 but the adsorption rate and capacity were relatively low. For example, arsenic adsorption on natural siderite with the particle size of 0.10–0.25 mm, reached equilibrium in 3 days, and the estimated maximum adsorption capacity was only 1.04 and 0.52 mg g−1 for As(III) and As(V), respectively.62 However, As(III) adsorption on the synthetic siderite is fast and the adsorption equilibrium can be reached in 20 min.63 As the percentage of oxidized As(III) increased, the siderite was converted to lepidocrocite and goethite. Moreover, when the natural siderite was modified with polyanionic cellulose, the adsorption capacity and adsorption rate can be greatly increased.64
In most of the cases, arsenic adsorption on iron compounds could fit the Langmuir model better than Freundlich model.65 For example, As(III) adsorption on hematite,66 As(III) and As(V) adsorption on magnetite,67 goethite,68 amorphous iron hydroxide69 and magnetite–maghemite nanoparticles70 could be well described by Langmuir model, indicating the monolayer adsorption on energetically equivalent sites. However, As(V) adsorption on granular ferric hydroxide (GFH) fitted Freundlich model better with a high correlation coefficient (R2 > 0.99),71 indicating the heterogeneous active sites distributed on GFH.
The dissolved O2 and Fe(II) ions have a significant impact on the adsorption of As(III) and As(V) species on lepidocrocite (γ-FeOOH).72 Lepidocrocite can release Fe(III) ions into the water during the adsorption and oxidation of As(III) species. It was reported the adsorbed As can be incorporated into the lattice of γ-Fe2O3 nanoparticles,73 the in-field 57Fe MÖssbauer spectra and TEM results confirmed that the incorporated As(V) ions inhibited the nanoparticle growth resulting in a low average size of the formed γ-Fe2O3 nanoparticles (as shown in Fig. 4). The δ-FeOOH with a surface area of 135 m2 g−1 exhibited an As(V) adsorption capacity of 37.3 mg g−1 at a pH 7.0.74 The kinetics data were best fitted with a pseudo-second-order, thus suggesting the formation of inner-sphere complexes between As(V) and δ-FeOOH nanoparticles.
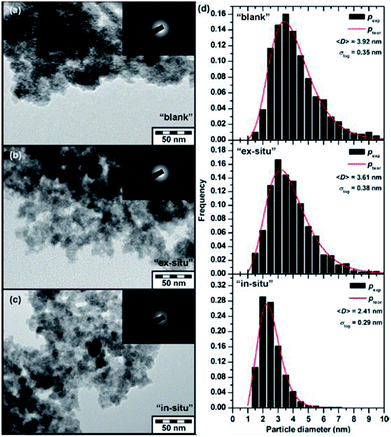 | ||
| Fig. 4 TEM images of the (a) “blank”, (b) “ex situ”, and (c) “in situ” samples and (d) particle size distribution of all three studied systems derived from the statistical processing of TEM images. Note: “Blank” sample formed after addition of ferrate(VI) only to deionized water, “in situ” sample formed after simultaneous addition of ferrate(VI) and an As(V)-containing compound to deionized water, and (iii) “ex situ” sample formed after addition of ferrate(VI) to deionized water and followed by an addition of As(V)-containing compound.83 | ||
Among the polymorphs of FeOOH such as goethite (α-FeOOH), lepidocrocite (γ-FeOOH) and akaganèite (β-FeOOH), akaganèite showed the highest adsorption capacity for arsenic.75 Akaganèite with a surface area of 330 m2 g−1 showed adsorption capacity as high as 120 mg g−1 at pH 7.5.76 The main composition of commercial granular ferric hydroxide (GFH) is β-FeOOH. Table 1 shows the adsorption capacities of arsenic on different iron compounds.
| Adsorbents | Surface area (m2 g−1) | Initial conc. (mg L−1) | pH | Adsorption capacity (mg g−1) | Ref. | |
|---|---|---|---|---|---|---|
| As(III) | As(V) | |||||
| Granular ferric hydroxide (GFH) | 240–300 | As(V): 0.1 | 6.5–7.5 | — | 1.1 | 53 |
| α-FeOOH nanoparticles | 167.8 | As(V): 100 | 3.0 | — | 76 | 68 |
| Ultrafine α-Fe2O3 nanoparticles | 162 | — | 47 | 95 | 73 | |
| Ultrafine δ-FeOOH | 135 | As(V): 20 | 7.0 | — | 37.3 | 74 |
| β-FeOOH nanoparticles | 330 | As(V): 20 | 7.5 | — | 120 | 76 |
| Magnetite–maghemite nanoparticles | 49 | As(III): 1.5 | 2.0 | 3.69 | 3.71 | 77 |
| As(V): 1.5 | ||||||
| α-Fe2O3 | As(V): 1 | 3–10 | — | 0.2 | 78 | |
| Fe3O4 nanoparticles | 179 | As(III): 70 | 5.0 | 16.56 | 46.06 | 79 |
| As(V): 25 | ||||||
| γ-Fe2O3 nanoparticles | 41–49 | As(V): 1 | 7.0 | — | 2.9 | 80 |
| Fe3O4–γ-Fe2O3 nanoparticles | 60 | As(III): 1.5 | 2.0 | 3.69 | 3.71 | 81 |
| As(V): 1.5 | ||||||
2.2. Iron-based layered double hydroxides (LDHs) as adsorbents
Iron-based LDHs, incorporating other metals, such as Mg, Ni, Zn, Mn and Co ions into iron oxides, have gained much more interest for arsenic adsorption due to the synergistic effect and the considerable higher adsorption capacities.82 The chemical compositions of their layer cations and their interlayer anions can be greatly varied, and the interlayer space can be explored for arsenic removal from water.83 The general formula of iron-based bimetal oxides is:where M2+ represents metallic divalent cations such as Mg(II), Co(II), Zn(II) and Mn(II); An− is the interlayer anion of charge n.
The most popular method of LDHs preparation is the direct co-precipitation, which is based on hydrolysis of two metal cations (e.g., Mg and Fe) by strong bases in the presence of another precursor that contains potential interlayer anions, such as carbonate (CO32−).84 Moreover, the combination of co-precipitation with other treatments such as ultrasound- and sono-assistances was frequently used to enhance adsorptive properties. The solgel method has been proven to be an effective strategy to produce high-quality LDHs.85 A nanostructured Fe–Ni-LDHs with a specific surface area of 245 m2 g−1 was synthesized as shown in eqn (1), using a co-precipitation/calcination techniques.82 The chemical transformation is given:
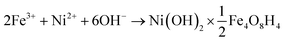 | (1) |
This Fe–Ni-LDHs exhibited very high adsorption capacity of 168.6 mg g−1 and 90.1 mg g−1 for As(III) and As(V), respectively, which are higher than most of the reported iron-containing adsorbents. The addition of Ni contributed to the porous structure, high specific surface area and increased surface functional groups (such as Ni–OH, Fe–OH), thus greatly enhancing the arsenic adsorption efficiency.86 Micro-sized Fe–Cu-LDHs exhibited much higher arsenic adsorption capacity than those of the single iron oxide and copper oxide.87 Fe–Al-LDHs also showed higher arsenic adsorption capacity than single iron oxide.88 Fe–Mn-LDHs was effective for As removal from water because the positively charged manganese oxides surface attract and oxidize As(III) to As(V) and allow increased adsorption of As(V) on iron oxides. A mesoporous Fe–Mn-LDHs was synthesized by using a hard template method.89 The maximum adsorption capacities of Fe–Mn-LDHs for As(III) and As(V), calculated by Freundlich model, were 68 and 94 mg g−1, respectively. A porous nanobimetallic Fe–Mn cubes was synthesized by Zhang et al. (2017).90 The adsorbent showed a higher specific surface area of 450 m2 g−1 than that of 138 m2 g−1 reported by Zhang et al. (2010)91 and 197 m2 g−1 reported by Hu et al. (2017).92 The adsorption capacities of porous nanobimetallic Fe–Mn cubes for As(III) calculated by Langmuir model, were 460 mg g−1. The removal mechanisms involved electrostatic attraction, surface complexation, and oxidation/adsorption due to the presence of MnO2 in the bimetal oxides. Lu et al. (2015)93 reported a Zn–Fe-LDHs to achieve the efficient removal of As(V) in aqueous solutions. The experimental result of simulated water samples showed that the adsorption of As(V) on Zn–Fe-LDH material can be well described by the Sips isotherm model with the maximum adsorption capacity of 151.37 mg g−1.
As(V) adsorption on mono-(Fe or Al) and Fe–Al-LDHs supported zeolite agreed with the Redlich–Peterson model with the correlation coefficient above 0.99.94 Similarly, As(III) and As(V) adsorption on magnetic nanoscale Fe–Mn-LDHs loaded zeolite (MFM) could be well described by Redlich–Peterson model with the correlation coefficient above 0.98.91 When the atomic ratio of Mn/Fe was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, the specific surface area of the Fe–Mn-LDHs nanoparticles reached 340 m2 g−1, which is higher than most of the reported absorbents and showed high As removal efficiency of 99.0% at pH 7.0.95 A list of arsenic adsorption on different iron-based LDHs are shown in Table 2.
9, the specific surface area of the Fe–Mn-LDHs nanoparticles reached 340 m2 g−1, which is higher than most of the reported absorbents and showed high As removal efficiency of 99.0% at pH 7.0.95 A list of arsenic adsorption on different iron-based LDHs are shown in Table 2.
| LDHs | SBET (m2 g−1) | Atomic ratio | Adsorption capacity (mg g−1) | Ref. | |
|---|---|---|---|---|---|
| As(III) | As(V) | ||||
| Fe–Ni | 245 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
168.6 | 90.1 | 86 |
| Fe–Cu | 282 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
122.3 | 82.7 | 87 |
| Fe–Al | 87.4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 1.7 |
40.6 | 37.6 | 88 |
| Fe–Mn | 154 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
68 | 94 | 89 |
| Fe–Mn | 450 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
460 | — | 90 |
| Fe–Mn | 340 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
342 | — | 95 |
| Fe–Ce | 90 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 0.8 |
— | 150 | 96 |
| Fe–Ce | 265 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
72 | 133 | 97 |
| Fe–Ti | 77.8 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65 | 15 | 98 |
2.3. Nanoscale zero-valent iron (nZVI) and nZVI supported adsorbents
In recent years, nZVI has become a hotspot of research in many fields due to its high reactivity with a standard redox potential of −0.44 V.99 NZVI can degrade the organic pollutants in presence of dissolved oxygen (DO) by transferring electrons to O2 to produce oxygen radicals (O2˙) and H2O2 in the medium. Moreover, the combination of H2O2 and Fe2+ can produce hydroxyl radicals (OH˙) which possess the strong oxidizing ability. The mechanism is shown as follows:100| Fe0 + O2 + 2H+ → Fe2+ + H2O2 | (2) |
| Fe0 + H2O2 + 2H+ → Fe2+ + 2H2O | (3) |
| Fe2+ + H2O2 → Fe3+ + OH˙ + OH− | (4) |
However, nZVI tends to agglomerate in solution, which causes a reduction in reactivity. Direct usage of nZVI in water also caused pollution because the nanoparticles themselves are considered as an emerging class of contaminants with a wide distribution in water system.101 Loading of nZVI onto appropriate supporting materials may reduce the leaching of nanoparticles into the water.
A bi-functional polystyrene resin supported nZVI was reported for the adsorption of As(III) and As(V).102 The crosslinked polystyrene with a crosslinking density of 8% was used as matrix and the maximum adsorption capacities for As(III) and As(V) reached 121 and 125 mg g−1, respectively. A nZVI-supported mesoporous carbon composite adsorbent was also reported recently and TEM images showed a homogeneous distribution of nZVI (10–20 nm) particles within the mesoporous carbon.103 The electron paramagnetic resonance (EPR) and proton binding measurements showed nZVI surface is partially oxidized to form an iron(III) oxide/hydroxide shell, which was mainly responsible for As(III) binding.103 A nZVI-supported montmorillonite was reported to show a maximum adsorption capacity of 59.9 and 45.5 mg g−1 for As(III) and As(V), respectively.104 The co-existing anions, such as chloride and carbonate slightly decreased the removal of As(III) to ∼90%, while nitrate and phosphate anions exhibited higher impact resulting in reduction of As(III) removal efficiency to ∼80%.105
A nZVI impregnated chitosan-carboxymethyl β-cyclodextrin complex was also successfully tested for arsenic removal from water.106 NZVI nanoparticles were entrapped into chitosan-carboxymethyl β-cyclodextrin complex, which enhanced the stability of Fe0 particles and the carboxymethyl β-cyclodextrin provided more active sites to interact with arsenic species. The maximum adsorption capacity was calculated by Langmuir model and found to be 18.51 and 13.51 mg g−1 for As(III) and As(V), respectively. Monodispersed nZVI particles could combine with chitosan fibres with an average fibre diameter of 195 ± 50 nm to produce functional and stable adsorbent.107 The nZVI doping on chitosan surface was typically achieved through a liquid phase reduction of FeCl3 using NaBH4. The reaction mechanism is shown as follows:
| 4Fe3+ + 3BH−4 + 9H2O → 4Fe0↓ + 3H2BO−3 + 12H+ + 6H2 | (5) |
The XPS analysis revealed that arsenic was fixed to oxy-hydroxide groups at the outer shells of nZVI surfaces, while As(III) underwent oxidation to As(V). The environmental risk of As-loaded nZVI was evaluated by Ye et al.108 The results indicated that an aerobic As(V)-reducing bacterium (Pantoea sp. IMH) preferentially reduce soluble As(V), not solid-bound As(V). Nanoscale zero-valent iron was supported onto activated carbon (NZVI/AC) for arsenic removal from drinking water. The results showed that the iron particles in the pores of carbon were needle-shaped with the size of (30–500) × (1000–2000) nm. The maximum adsorption capacity for As(III) and As(V) at pH 6.5 calculated from Langmuir model was 18.2 and 12.0 mg g−1, respectively.109 Wu et al.110 investigated the double influence mechanism of pH on arsenic removal by nZVI (with an average particle size varying from 30 to 50 nm). The results indicated that an increasing pH decreased the γ-FeOOH and increased the Fe3O4/γ-Fe2O3 content in the corrosion products of nZVI, thus enhancing the adsorption affinity of nZVI to As(V). The iron loading of fuller's earth immobilized nZVI (F-nZVI) were synthesized by borohydride reduction method,111 the maximum adsorption capacity of F-nZVI for As(III) and As(V) were observed to be 50.1 and 90.4 mg g−1, respectively. The representative studies for arsenic removal by using nZVI or supported nZVI are listed in Table 3.
| Adsorbent | pH | Initial | Adsorption capacity qmax (mg g−1) | Ref. | |
|---|---|---|---|---|---|
| Conc. (mg L−1) | As(III) | As(V) | |||
| N/S-nZVI | 6.5 | 1–100 | 121 | 125 | 102 |
| NZVI/AC | 7 | 9 | 26.8 | — | 103 |
| NZVI/mont-morillonite | 7.0 | 5 | 59.9 | 45.5 | 104 |
| NZVI | 7.0 | 0.2 | 1.8–2.0 | — | 105 |
| NZVI | 7.0 | — | 3.5 | — | 108 |
| NZVI/AC | 6.5 | 2 | 18.2 | 12.0 | 109 |
| NZVI | 6.0 | 2–100 | 1.7 | 0.7 | 110 |
| F-nZVI | 7.2 | 100 | 50.1 | 90.4 | 111 |
2.4. Iron oxy-hydroxides doped composite adsorbents
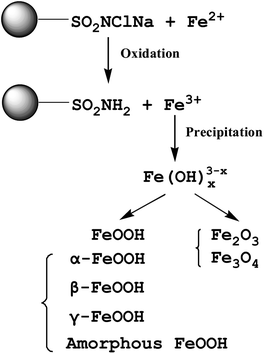
Nanomaterials received substantial attention in the area of water treatment owing to the high surface area and interesting catalytic properties.112 Iron oxy-hydroxide nanoparticles are promising adsorbents for arsenic removal because of the high reactivity, and non-toxic nature.113 However, direct addition of these nanoparticles into water is not feasible owing to the difficulty in removing them from the water after adsorption process. A few nanoparticles such as engineered silver nanoparticles and graphene oxide (GO) are known to be toxic to the living systems.114 To overcome this problem, the supporting materials such as granular activated carbons, biomass materials, polymers, zeolite, silica, clay mineral, and red mud have been extensively used to combine with iron oxide nanoparticles for arsenic adsorption from water.115In order to improve arsenic adsorption, GAC impregnation using a solution of iron salt is most frequently used to synthesize iron oxide doped GAC.119 Lee et al. (2015)120 reported iron oxides incorporated activated carbon for As(V) removal from water by hydrothermal method. It was indicated that the Redlich–Peterson model was the most suitable model for describing the equilibrium data. Experimental factors such as nature of iron salt, concentration, pH, and treatment time play key roles towards arsenic adsorption capacity. The adsorption mechanisms are associated with electrostatic attraction, ion exchange, and surface complexation.121 The arsenic adsorption was most efficient when the iron loading content on GAC was ∼6%, further increases in iron content unexpectedly decreased the arsenic adsorption capacity.122 Phosphates and silicate anions significantly decreased arsenate removal at pH > 8.5, while sulfate, chloride, and fluoride anions had minimal effects.123
Hematite and akaganèite loaded GAC was synthesized to remove As(V) and As(III) from water.124 The surface area and pore volume slightly decreased after doping the GAC with hematite and akaganèite nanoparticles due to the obstruction of micropores, but As(V) adsorption capacity was significantly enhanced after modification. Iron oxide nanoparticles decorated GAC was prepared using microwave-assisted hydrothermal technique and tested for water purification.125 Iron oxide deposited on GAC was characterized as β-FeOOH after 3 min, and β-FeOOH was gradually transformed to α-Fe2O3 after 6 min of heating. The mechanism of the synthetic route is proposed as follows:
| AC− + Fe3+ → AC + Fe2+ | (6) |
| 2Fe2+ + MnO2(s) + 2H2O → 2FeOOH(s) + Mn2+ + 2H+ | (7) |
The GAC treated with a FeCl3 solution of lower concentration (i.e. 0.05 M) was more efficient for removing arsenic than those treated with higher concentration (i.e. 0.2 mol L−1) of the FeCl3 solution.126 The use of Fe(II) is favourable for obtaining higher Fe content inside the iron-doped activated carbons. The surface oxidation of GAC by concentrated HNO3/H2SO4 or HNO3/KMnO4 could greatly increase the densities of carboxylic or other functional groups on the surface. The iron loading amount correlated well with the number of surface carboxy- and hydroxyl-functional groups.127 The effect of experimental conditions (i.e., pre-oxidation, contact time, and iron concentration) on the distribution and morphology of iron oxy-hydroxide on GAC was also examined.128 The authors indicated that the contact time and iron concentrations have no significant effect on iron loading content, the use of KMnO4 yielded teeth-like iron oxyhydroxide nanoparticles, while the absence of KMnO4 pretreatment produced spherical nanoparticles.129 Table 4 shows the comparison of arsenic adsorption on different iron modified activated carbon.
| AC type | SBET (m2 g−1) | SBET (after iron loading) | Iron loading content (mg g−1) | Iron phase | Adsorption capacity (mg g−1) | Ref. | |
|---|---|---|---|---|---|---|---|
| As(III) | As(V) | ||||||
| Bituminous based Filtrasorb 400 | 929 | 863 | 0.95% | HFO | — | 2.45 | 120 |
| Coconut shell | 667 | 388 | 1.5% | HFO | — | 1.25 | 121 |
| Commercial NC-100 | 2100 | — | 4.56% | Fe(II) | 0.035 (initial total As conc. is 0.31 mg L−1) | 122 | |
| Commercial NC-100 | 2100 | 1575 | 2.2% | HFO | 0.035 (initial total As conc. is 0.31 mg L−1) | 123 | |
| ACF cloth | 1720 | 3.56% | Fe3O4 | — | 4.16 | 124 | |
| Lignite-based AC | 11.4% | Amorphous FeOOH | — | 0.26 (initial As conc. is 0.12 mg L−1) | 125 | ||
| Wood-based BAX-1500 | 2143 | 918 | 8.5% | Amorphous FeOOH | 32.96 | 126 | |
| Starbon300 | 213 | 141 | 5.6% | nZVI | 26.8 | — | 127 |
| F400 AC | 896 | 1.31% | Amorphous HFO | — | 4.56 | 128 | |
| Straw activated carbon | 723 | 11.7% | Amorphous HFO | 51.3 | 33.8 | 129 | |
| Darco 20 × 50 | 650 | — | 4.22% | β-FeOOH, amorphous HFO | — | 1.95 (initial As conc. is 40 mg L−1) | 130 |
| Sawdust-based AC | — | 349 | 39% | Fe3O4 | — | 204 (initial As conc. is 40 mg L−1) | 131 |
| Fe2+ + 2Fe3+ + 8OH− → Fe3O4↓ + 4H2O | (8) |
The thermodynamic results indicated the adsorption of As(V) on MGO is an endothermic process and the kinetic data were fitted with the pseudo-second-order model. At low pH values, the co-existing anions showed an inhibiting effect while an enhancing effect was observed on As(V) adsorption at high pH values.136
Biochar is another material that can be obtained from pyrolysis of agricultural waste.140 Because of its easy availability and low cost, biochar has been considered as an alternative adsorbent for polluted water treatment.141 Biochar loaded with iron oxy-hydroxides particles act as a good adsorbent for the removal of arsenic pollutants from water.142 In a recent study, a walnut based biochar loaded with α-FeOOH was formed through direct hydrolysis of an iron salt and showed good adsorptive performance for arsenic from water.143 The iron impregnated biochar showed much better adsorption ability with maximum adsorption capacity of 2.16 mg g−1 than the pristine biochar with no As adsorption capacity. Also, the authors proposed the chemisorptions mechanism based on the evidence of large shifts in the binding energy of Fe2p, As3d, O1s and C1s region after As adsorption. The results indicated a change in chemical speciation of As(V) ions getting reduced to As(III) species, and Fe(II) was oxidized to Fe(III) during the adsorption process.144 Another Fe-loaded biochar was derived from sugar beet pulp (BP) agricultural residues.145 The authors found that GAC, preoxidized by hydrogen peroxide (H2O2) or potassium permanganate (KMnO4), could significantly increase the iron loading amount from 5% to 10–32%. Apricot stone was activated by treating it with phosphoric acid (H3PO4) and carbonized under nitrogen flow led to the formation of biochars, which was modified with iron oxyhydroxides to prepare the hybrid adsorbents.146 The comparison of Fe(II) loaded GAC (GAC-Fe(II)) and Fe(III) loaded GAC (GAC-Fe(III)) for As(V) adsorption revealed that GAC-Fe(III) has a better adsorptive performance for arsenic extraction than GAC-Fe(II) adsorbent. More interestingly, the authors indicated that As(V) adsorption on GAC-Fe(II) is an endothermic process, while As(V) adsorption on GAC-Fe(III) is an exothermic process according to the values of enthalpy change (ΔHΘ).147
Chitin and chitosan are the most abundant biopolymers in Nature. Chitosan is derived from chitin, which is the main constituent of the exoskeleton of crustaceans.148 Chitosan has a strong affinity towards ferric ions, which further uptake arsenic species from aqueous mediums. Chitosan complexed with Fe(III) ions showed the highest As(V) adsorption efficiency as compared to Cu(II), La(III), Mo(VI) and Zr(IV) complexed chitosan. The As(V) ion adsorption capacity of self-supported Fe(III)-chitosan membrane reached 109 mg g−1.149
Yamani et al. (2014)150 reported a Fe3O4@Zr(OH)4 impregnated chitosan beads (MICB) for arsenic removal. The maximum adsorption capacity of the MICB was calculated to be 35.7 mg g−1 for As(V), and 35.3 mg g−1 for As(III) at pH 6.8. When the groundwater was used to examine the arsenic removal ability of MICB, the initial arsenic concentration of 0.103 mg L−1 in water could be reduced to less than 0.01 mg L−1 after 5 h with an adsorbent dosage of 1 g L−1.150
Cellulose is regarded as one of the most affordable raw materials available for the preparation of various functional materials.151 The abundant hydroxyl groups on the cellulose can be used directly or modified with other functional groups to extract toxic metal ions from water. In recent years, there is a growing interest in the utilization of natural lignocellulose materials as cheap and environment-friendly adsorbents.152 Agricultural residue materials such as straws, corn stalks, sugarcane bagasse and sawdust are abundant and readily available as natural resources for potential applications.153 For most of the natural cellulose materials, pretreatment by NaOH solution is a good way of increasing the specific surface area and to make the hydroxyl group more easily accessible for modification.154 The wheat straw was used to prepare a magnetic adsorbent with different Fe3O4 contents on the surface and used for arsenic extraction.155 Interestingly, the authors observed that Fe3O4 loading onto the wheat straw exhibited much higher adsorption capacity of As(V) (24–30 mg As/g Fe3O4) than the bare Fe3O4 (6–7 mg As/g Fe3O4). A novel Fe2O3 impregnated cross-linked cellulose was prepared by precipitation method for As(V) removal from water.156 The results indicated that Temkin model agreed for the adsorbate–adsorbent system, revealing the process of adsorption is a physicochemical process involving the hydroxyl (–OH) groups of the adsorbent surface. A clear relationship was found between the surface acidic groups and iron content. After modifying the jute fibre surface with succinic anhydride to incorporate carboxyl groups, the maximum iron loading was increased from 102 mg g−1 to 208 mg g−1.157 A list of iron modified biocomposite materials for arsenic adsorption is shown in Table 5.
| Adsorbents | pH | Initial conc. of As (mg L−1) | Iron loading content (mass, %) | Capacity (mg g−1) | Ref. | |
|---|---|---|---|---|---|---|
| As(III) | As(V) | |||||
| Iron modified jute fibre | 7.0 | 100 | 18.1 | 12.7 | 156 | |
| Fe3O4 coated wheat straw | 6–8 | As(III): 28 | — | 3.9 | 8.1 | 157 |
| As(V): 28 | ||||||
| Nano-iron/oyster shell | 6.8 | As(III): 1.8 | 50.2 | 0.9 | — | 158 |
| Iron oxide coated fungal biomass | 7.2 | As(III): 1.3 | — | 5.4 | 10.3 | 159 |
| As(V): 0.9 | ||||||
| ZVI nanoparticles modified starch | 5.0 | As(III): 2 | — | 12.2 | 14 | 160 |
| As(V): 2 | ||||||
| Iron-loaded orange peel | 3.0, 10.0 | — | 5.6 | 68.2 | 68.6 | 161 |
| FeCl3 treated chestnut shell | 9.0 | As(III): 100 | — | 0.9 | — | 162 |
Recent studies on arsenic removal using anion exchange resins and fibres showed interesting results.165,166 Ociński et al. (2014)167 synthesized a hybrid polymer by dispersing iron oxides into a poly(styrene-divinylbenzene) (St/DVB) matrix. The supporting polymer contained sulfonamide groups (–SO2NH2 2.3 mmol g−1), and sulfonic acid groups (–SO3− 0.3 mmol g−1), which led to the incorporation of 12% Fe content in the matrix. The maximum adsorption capacity for this adsorbent calculated by Sips model168 reached 26.14 and 10.88 mg g−1 for As(III) and As(V), respectively. The presence of interfering ions such as chlorides, sulfates, bicarbonates and carbonate ions did not show any influence on both As(III) and As(V) adsorption, but a low concentration of phosphate anions caused an essential drop in As(V) removal efficiency. A schematic diagram of the simple two-stage synthetic process is shown in Fig. 5.
 | ||
| Fig. 5 The schematic diagram of iron oxides deposited on poly(styrene-divinylbenzene) (St/DVB) matrix. | ||
The arsenic removal capacity was not always proportional to iron loading content. For example, Hu et al. (2017)169 prepared hydrated ferric oxide (HFO) loaded polymer, and observed that the adsorption capacity of As(V) increased with an increase of Fe mass percentage from 3 to 15%, but a further increase of Fe content resulted in a significant decline of the adsorption capacity. Similar results were also found using a HFO loaded strong base anion (SBA) exchange resin with a total Fe content of 318 mg Fe/g dry adsorbent.170 The comparison of SBA support and HFO/SBA adsorbent by microscopy was shown in Fig. 6, the HFO/SBA adsorbent developed a deep brown colour due to the dispersion of HFO particles. In order to overcome the drawback of nanoparticles used alone, superparamagnetic Fe2O3 nanoparticles dispersed cellulosic sponges were prepared and tested.171 The adsorption capacities of cellulose–Fe2O3 adsorbent were 2.11 mmol g−1 and 12.09 mmol g−1 for As(III) and As(V), respectively, which are higher than that of iron nanoparticles in suspension.171
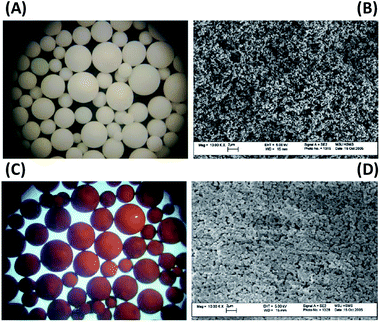 | ||
| Fig. 6 (A) Light microscope photograph of SBA (45×); (B) SEM images of SBA and (C) light microscope photograph of HFO/SBA adsorbent (45×); (D) SEM images of HFO/SBA adsorbent.169 | ||
Kumar et al. (2016)172 reported an iron–aluminium hydroxides coated macroporous polyacrylamide for arsenic adsorption. The in situ chemical co-precipitation method was used for preparing iron–aluminium hydroxides particles by adding 25% NH4OH to iron and aluminium salts solution. The synthesized adsorbent showed an experimental maximum adsorption capacity of 82.3 and 49.6 mg g−1 for As(III) and As(V), respectively. Anirudhan et al. (2013)173 observed that the incorporation of Fe(III) ions could enhance the porous structure and increase the specific area of Fe(III)-coordinated cellulose adsorbent from 21.7 to 31.6 m2 g−1. The maximum adsorption capacity for As(V) calculated by Langmuir isotherm equation was 105.47 mg g−1. The synthesized adsorbent has been tested using a simulated groundwater sample with no significant decrease in adsorption capacity.
An in situ remediation for arsenic from groundwater by using an aquifer iron coating method was considered as an effective and simple way for arsenic remediation in rural and remote areas where groundwater is used as the main water resource for drinking.182 A continuous injection of FeSO4 and NaClO solutions for 96 h led to the formation of a uniform α-FeOOH (30–50 nm) coating on the surface of the sand. During this process, ferrous iron can also be adsorbed and subsequently oxidized to form new ferric hydroxide particles, which can be used to adsorb arsenic from water. The process of adsorption/co-precipitation with fine goethite particles resulted in arsenic immobilization.182 Titanium dioxide (TiO2) is a famous photocatalyst that offers a relatively inexpensive and environmentally safe way to achieve oxidation of As(III) to As(V).183 TiO2 nanoparticles doped with 10% Fe adsorbent could effectively oxidize As(III) to As(V).184 The maximal adsorption capacities calculated by Langmuir isotherm model were 8.61 and 17.35 mg g−1 for As(III) and As(V), respectively. The presence of SO42− anion hindered the adsorption of only As(III), while PO43− anion decreased the adsorption capacities of both As(III) and As(V) species from water.184
In the case of Fe doped materials, X-ray mapping, EDX and XPS methods are frequently used to determine the surface concentration of Fe ions. For example, Fan et al. (2018)185 used X-ray mapping to investigate the distribution of Fe in the carbon matrix. Gallios et al. (2017)186 employed X-ray mapping to demonstrate that the impregnated iron was uniformly distributed on the internal surface of the granular activated carbon. Li et al. (2013)187 used XPS to create the elemental map of magnetic nanoparticles impregnated with N-doped porous carbon.
3. Adsorption mechanisms
Many studies demonstrated that arsenic adsorption on iron-based materials occurs through formation of inner-sphere complexes such as monodentate, bidentate, or tridentate bonds.188,189 X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS) techniques are commonly used to investigate the mechanism including the formation of different types of complexes and the redox transformation of adsorbed As on adsorbents.Liu et al. (2015)190 demonstrated that arsenic adsorption on magnetite nanoparticles (MNPs) is an endothermic process. The X-ray absorption fine structure (EXAFS) spectra suggested that As(V) adsorption on MNPs mainly through the formation of bidentate binuclear corner-sharing complexes (2C) with the typical interatomic Fe–As distance of 3.35–3.39 Å. As(III) adsorption on MNPs occurs through tridentate hexanuclear corner-sharing (3C) complexes with the typical inter-atomic Fe–As distance of 3.49–3.67 Å. The typical inter-atomic Fe–As distance of the bidentate binuclear corner-sharing complexes (2C) is ∼3.3–3.4 Å, and that of the monodentate mononuclear corner-sharing complexes (1V) is ∼3.5–3.6 Å.190 For the arsenic loaded MNPs exposed to air, XANES and XPS results revealed the complex redox transformation of the adsorbed arsenic, which was shown in Fig. 7. XPS spectra were used to investigate the mechanism of As(V) adsorption on MNPs.191 It was revealed that the surface oxygen and iron atoms act as Lewis acids, while arsenate anions, act as a Lewis base. The specific adsorption reaction was formed through the formation of inner-sphere complexes. The monoprotonated bidentate complexes were dominant and no reduction of As(V) was observed on the surface of the MNPs. Extended X-ray Absorption Fine Structure (EXAFS) spectra suggested the predominant formation of bidentate binuclear corner-sharing complexes (2C) for As(V), and tridentate hexanuclear corner-sharing (3C) complexes for As(III) on MNP surfaces.191 Also, As(V) can be reduced to As(III) because of the role played by the reactive Fe(II). Thi et al. (2015)192 compared the adsorptive performance of Fe3O4 and Mn, Cu doped Fe3O4 nanoparticles for As(III) wastewater treatment. The paper reported that Cu doped Fe3O4 nanoparticles have higher adsorption capacity towards arsenic than Fe3O4 and Mn-doped Fe3O4 nanoparticles because the substitution of Cu2+ ions for smaller radii of Fe2+ could increase the porosity and specific surface area of Cu doped Fe3O4 nanoparticles. The saturation magnetic moments of the adsorbent decreased from 65.9 emu g−1 to 53.2 emu g−1 after doping with Cu ions.192
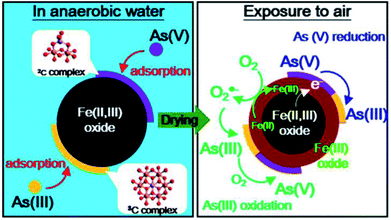 | ||
| Fig. 7 Mechanism of arsenic adsorption on magnetite nanoparticles in anaerobic water and air-enriched water.190 | ||
The arsenic adsorption on synthetic siderite was greatly enhanced from ∼10 mg g−1 to around 120 mg g−1 under oxidizing conditions, while 75% of the siderite was transformed to goethite during arsenic adsorption.193 The EXAFS spectra indicated the bidentate binuclear corner-sharing complexes (2C) and mononuclear corner-sharing complexes (1V) are involved during As(III)/As(V) adsorption on siderite. In aerobic conditions, Fe(II) was gradually oxidized to Fe(III), the As(III) oxidation and complexes between Fe(II)–Fe(III) minerals greatly improved arsenic adsorption.193 As(V) adsorption on hematite and goethite through the mechanism of ligand exchange.194
Besides of surface complexation in arsenic adsorption, electrostatic attraction and ion exchange are also contributed to the arsenic removal. The isoelectric point (pHiso) is an important factor influencing the adsorption capacity and rates. For example, Fe2O3 crystalline structures exhibited pHiso of around 7.2, while the synthetically derived Fe2O3 typically exhibits an pHiso between 8.1 and 8.8.195 The pHiso of typical iron (oxy)hydroxide such as goethite (α-FeOOH) and magnetite was reported to be 6.9 and 6.4, respectively.195 β-FeOOH, which was demonstrated an excellent binding capacity for As(III) and As(V), the pHiso was determined to be 8–9. It is understandable that adsorbents with high isoelectric points could decrease the electrostatic repulsion forces between the adsorbent surface and negatively charged arsenic species in pH environments greater than pHiso.196
Ion exchange is also an important mechanism for arsenic adsorption,197 especially of the iron-based layered double hydroxides (LDHs) which are theoretically the best anion exchangers due to their potential to host arsenic anions in their interlayer space, which considerably increase their anion removal performance. This ability of the interlayer space to host arsenic anions makes LDHs superior to a majority of anion exchangers.197 The schematic mechanism of H2AsO4− adsorption on typical Mg–Fe-LDHs was shown in Fig. 9. Zhu et al. (2015)198 prepared an iron-manganese binary oxide (FeMnOx) for arsenic adsorption with adsorption capacities of 47.05 and 49 mg g−1 for As(III) and As(V) ions, respectively. The XPS spectra indicated that a portion of As(III) was converted to As(V) in presence of MnO2. Wang et al. (2014)199 reviewed the mechanism of arsenic on Fe–Al binary metal oxides. The authors indicated that the presence of Fe2+ ions in FeO could reduce As(V) to As(III) while Fe2+ is oxidized to Fe3+ in the form of Fe2O3 (eqn (9)).
| Fe2+ + H3AsO4 → Fe3+ + H3AsO3 + OH− | (9) |
Yan et al. (2012)200 investigated the mechanism of As(III) adsorption on nZVI nanoparticles using XAS method, the authors revealed that As(III) species underwent two stages of transformations after As adsorption on the nZVI surface. The As–O bonds are broken and the arsenic species are further reduced and diffused across the thin iron oxide layer, which resulted in the formation of As–Fe bonds. Different arsenic valence states of As(0), As(III), and As(V) were observed in nZVI after As(III) adsorption. As(III) was distributed throughout the oxide shell, As(0) was embedded at the interface of Fe(0) core and iron oxide shell, and As(V) existed primarily in the iron oxide layer.200 The mechanism of As(III) adsorption on nZVI nanoparticles was shown in Fig. 8. The inner-sphere complexation between arsenic and iron compounds was demonstrated by many researchers and summarized in Table 6.
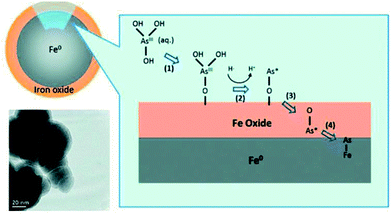 | ||
| Fig. 8 The schematic diagram of the adsorption of As(III) species on nZVI particles.200 | ||
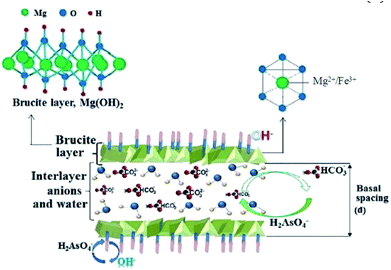 | ||
| Fig. 9 Schematic mechanism of As(V) adsorption on Mg–Fe–CO32−-LDHs.197 | ||
| Adsorption reaction | Complexation constants | |||
|---|---|---|---|---|
| α-FeOOH | Fe3O4 | Fe(OH)3 | HFO | |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeOH + H+ → FeOH + H+ → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeOH+2 FeOH+2 |
7.47 | 4.60 | — | 7.29 |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeOH → FeOH → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeO− + H+ FeO− + H+ |
−9.51 | −8.20 | — | −8.93 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| As(III) adsorption constants | ||||
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe–OH + H3AsO3 → Fe–OH + H3AsO3 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe − H2AsO3 + H2O Fe − H2AsO3 + H2O |
39.93 | 38.41 | 40.20 | 38.76 |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe–OH + H2AsO3− → Fe–OH + H2AsO3− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe − HAsO3− + H2O Fe − HAsO3− + H2O |
32.4 | 33.02 | — | 31.87 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| As(V) adsorption constants | ||||
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe–OH + H3AsO4 → Fe–OH + H3AsO4 → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe − H2AsO4 + H2O Fe − H2AsO4 + H2O |
31.00 | 29.31 | 29.88 | |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe–OH + H2AsO4− → Fe–OH + H2AsO4− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe − HAsO4− + H2O Fe − HAsO4− + H2O |
26.81 | 23.51 | 24.43 | |
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe–OH + HAsO24− → Fe–OH + HAsO24− → ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Fe − AsO34− + H2O Fe − AsO34− + H2O |
20.22 | 10.58 | 18.10 | |
4. Separation of iron-based magnetic adsorbents from water
Separation of the pollutant-laden adsorbents from water was always considered as a major challenge for drinking water treatment processes.205 It is important to separate the pollutant saturated adsorbents out of reactor and distribution systems to avoid causing the secondary environmental pollutions through the disposal of these materials.Magnetite nanoparticles (MNPs) are promising adsorbents for As removal because of their high adsorption capacity and easy separation from water under of a low external magnetic field.206 Sepúlveda et al. (2018)207 compared the magnetic property of Fe3O4 and Cu doped Fe3O4 nanoparticles. The saturation magnetic moments of the adsorbent decreased from 65.9 emu g−1 to 53.2 emu g−1 after doping with Cu ions. Magnetic nanocomposite (MNC) was synthesized by modifying Fe3O4 with 3-amino propyltrimethoxysilane for toxic metals removal from water.208 A hybrid Fe3O4–chitosan adsorbent showed higher affinity toward As(III) compared to As(V), and the adsorption behaviour was well described using both Langmuir and Freundlich isotherms.209 γ-Fe2O3 embedded biochar could be easily separated from the solution by a magnet. The saturation magnetization of the γ-Fe2O3/biochar composite was determined to be 69.2 emu g−1, which was very close to that of pure γ-Fe2O3 materials (76.0 emu g−1).210 A Fe3O4 loaded wheat straw showed typically superparamagnetic behaviour with the loop area being zero.211 The saturated magnetizations reached 6.18, 9.12 and 11.87 emu g−1, respectively, depending on the Fe3O4 content.
5. Future research needs on technology development
Development of simple and inexpensive water treatment systems is very important for providing potable water to millions of people. So far, most research efforts were focused on developing novel adsorbent materials with high adsorption capacities. Fewer efforts have focused on regeneration or disposal of arsenic-bearing sludge. Among the mentioned iron-based adsorbents, iron oxide, the iron doped activated carbon, and iron modified chelating resin were most widely used in practice for arsenic removal from drinking water. Two commercial inorganic adsorbents – Activated Alumina (AA) and Granular Ferric Hydroxide (GFH) are most widely used for arsenic adsorption. These materials currently allow the maximum allowable concentration (MAC) of arsenic (10 μg L−1) to be achieved. The pilot system showed adsorption based on GFH exhibited good performance in removing arsenic from groundwater. The initial concentration of 400 μg L−1 was reduced to less than 20 μg L−1 in most of the tube wells.212 Natural hematite, magnetite, and goethite, which are suitable for both As(III) and As(V) removal, were also expected to be used in practice due to abundant presence. The major disadvantages are the comparatively low adsorption capacity and very long time requirement (∼2 days) to reach the equilibrium.213 Iron(III)-loaded chelating resin were used for As(III) removal from drinking water, and up to 98% removal of As(III) was achieved at pH 6.0.214 Notably, the range of costs for these adsorbents varied widely while few adsorbents can be produced at the relatively lower costs. Therefore, the “low-cost” and “easy-to-use” technologies for arsenic removal in the low- and lower medium-income countries are very important for future development of iron-based adsorbents.Moreover, use of nanomaterials for arsenic adsorption has been explored in recent years, but the nanostructured adsorbents tend to agglomerate together, which decrease the adsorption and removal efficiency. Therefore, loading nanoparticles onto appropriate supporting materials is becoming a feasible strategy with the advantages of high reactivity and easy separation from water. The research has to continue for developing such adsorbents based technologies to be applied in the field in a sustainable manner.
6. Conclusions
A brief review of the removal of arsenic ions from water using iron-based adsorbents has been presented. A few adsorbents discussed in this review include iron compounds such as iron oxides, oxy-hydroxides such as amorphous hydrous ferric oxide (FeOOH), goethite (α-FeOOH), hematite (α-Fe2O3), iron-based LDHs, zero-valent iron nanoparticles, iron-doped activated carbon, biocomposite materials, iron-doped polymers and iron-doped mineral oxides. Relative advantages and disadvantages of the iron-based adsorbents used for the removal of arsenic from water have been mentioned. The mechanism of arsenic adsorption on iron-containing adsorbents was summarized as the formation of inner-sphere complexes such as monodentate, bidentate and tridentate complexes. Roles of Fe(0), Fe(II) and Fe(III) in the oxidation of As(III) to As(V) and its extraction efficiency are also included.Iron-oxyhydroxide doped biosorbents yielded interesting results, considering their abundance and low cost. For the iron modified adsorbents, a thorough investigation on leaching of ions from the adsorbents has to be conducted to understand the stability of the adsorbent under different experimental conditions. Iron compounds and iron-based LDHs have higher arsenic adsorption efficiencies, but they are usually difficult to remove from water owing to the nano- to micrometer size of the particles. Overall, there exist significant progress and benefit on using iron loaded biomass or polymers for removing arsenic species from groundwater in a practical way to make potable water accessible for the rural population.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the technical support from the department of chemistry and National University of Singapore. H. L. thanks, China Scholarship Council for financial support for the joint PhD programme.References
- S. I. Siddiqui and S. A. Chaudhry, Process Saf. Environ. Prot., 2017, 111, 592–626 CrossRef CAS.
- S. I. Siddiqui and S. A. Chaudhry, Curr. Environ. Eng., 2017, 4, 81–102 CrossRef CAS.
- K. Jomova, Z. Jenisova and M. Feszterova, et al., J. Appl. Toxicol., 2011, 31, 95–107 CAS.
- Y. Yang, Y. Ling and C.-H. Wang, et al., J. Colloid Interface Sci., 2018, 530, 658–666 CrossRef PubMed.
- W. W. Wong, H. Y. Wong and A. B. M. Badruzzaman, et al., Nanotechnology, 2017, 28, 31–49 CrossRef PubMed.
- B. J. Pan, B. C. Pan and W. M. Zhang, et al., Chem. Eng. J., 2009, 151, 19–29 CrossRef CAS.
- V. K. Sharma, R. Zboril and R. S. Varma, Acc. Chem. Res., 2015, 48, 182–191 CrossRef CAS PubMed.
- Z. M. Zhou, W. N. Deng and Y. L. Yang, et al., Huanjing Kexue, 2017, 38, 4623–4631 Search PubMed.
- I. Hussain, M. Y. Li and Y. Q. Zhang, et al., J. Environ. Chem. Eng., 2017, 5, 3983–3990 CrossRef CAS.
- L. Zhou, W. Zheng and Y. F. Ji, et al., J. Hazard. Mater., 2013, 263, 422–430 CrossRef CAS PubMed.
- L. Fang, X. Y. Min and R. F. Kang, et al., Sci. Total Environ., 2018, 639, 110–117 CrossRef CAS PubMed.
- J. G. Hering, I. A. Katsoyiannis and G. A. Theoduloz, et al., J. Environ. Eng., 2017, 143, 117–122 CrossRef.
- K. Tazaki, I. A. Rafiqul and K. Nagai, et al., Can. J. Earth Sci., 2003, 40, 1725–1738 CrossRef CAS.
- M. J. Spratlen, M. V. Gamble and M. Grauperez, et al., Food Chem. Toxicol., 2017, 105, 387–396 CrossRef CAS PubMed.
- R. S. Oremland and J. F. Stolz, Science, 2003, 300, 939–944 CrossRef CAS PubMed.
- M. A. Hoque, W. G. Burgess and K. M. Ahmed, Hydrol. Processes, 2017, 31, 2095–2109 CrossRef.
- D. Postma, N. T. H. Mai and V. M. Lan, et al., Environ. Sci. Technol., 2017, 51, 838–845 CrossRef CAS PubMed.
- S. V. Jadhav, E. Bringas and G. D. Yadav, et al., J. Environ. Manage., 2015, 162, 306–325 CrossRef CAS PubMed.
- M. Singh, A. K. Singh and N. Srivastava, et al., Environ. Earth Sci., 2010, 59, 1703–1715 CrossRef CAS.
- W. Baeyens, A. de Brauwere and N. Brion, et al., Sci. Total Environ., 2007, 384, 409–419 CrossRef CAS PubMed.
- A. Barats, G. Féraud and C. Potot, et al., Sci. Total Environ., 2014, 473, 422–436 CrossRef PubMed.
- V. J. Ritchie, A. G. Ilgen and S. H. Mueller, et al., Chem. Geol., 2013, 335, 172–188 CrossRef CAS.
- M. Arain, T. Kazi and J. Baig, et al., Food Chem. Toxicol., 2009, 47, 242–248 CrossRef CAS PubMed.
- Q. Hu, D.-W. Gao and H. Pan, et al., RSC Adv., 2014, 4, 40071–40077 RSC.
- M. Monrad, A. K. Ersbøll and M. Sørensen, et al., Environ. Res., 2017, 154, 318–324 CrossRef CAS PubMed.
- T. G. Kazi, K. D. Brahman and J. A. Baig, et al., J. Hazard. Mater., 2018, 357, 159–167 CrossRef CAS PubMed.
- M. R. Awual, M. Shenashen and T. Yaita, et al., Water Res., 2012, 46, 5541–5550 CrossRef CAS PubMed.
- I. Andjelkovic, S. Azari and M. Erkelens, et al., RSC Adv., 2017, 7, 3941–3948 RSC.
- V. Pallier, G. Feuillade-Cathalifaud and B. Serpaud, et al., J. Colloid Interface Sci., 2010, 342, 26–32 CrossRef CAS PubMed.
- G. Ungureanu, S. Santos and R. Boaventura, et al., J. Environ. Manage., 2015, 151, 326–342 CrossRef CAS PubMed.
- C. G. Lee, P. J. J. Alvarez and A. Nam, et al., J. Hazard. Mater., 2017, 325, 223–229 CrossRef CAS PubMed.
- J. Yoon, G. Amy and J. Chung, et al., Chemosphere, 2009, 77, 228–235 CrossRef CAS PubMed.
- S. Vasudevan and J. Lakshmi, Can. J. Chem. Eng., 2012, 90, 1017–1026 CrossRef CAS.
- D. Mohan and C. U. Pittman, J. Hazard. Mater., 2007, 142, 1–53 CrossRef CAS PubMed.
- T. Sun, Z. Zhao and Z. Liang, et al., J. Colloid Interface Sci., 2017, 495, 168–177 CrossRef CAS PubMed.
- N. K. Asmel, A. R. M. Yusoff and L. S. Krishna, et al., Chem. Eng. J., 2017, 317, 343–355 CrossRef CAS.
- R. J. De Klerk, T. Feldmann and R. Daenzer, et al., Hydrometallurgy, 2015, 151, 42–50 CrossRef CAS.
- M. Laatikainen, M. Sillanpää and T. Sainio, Desalin. Water Treat., 2016, 57, 13770–13781 CrossRef CAS.
- P. Bahmani, A. Maleki and H. Daraei, et al., J. Colloid Interface Sci., 2017, 506, 564–571 CrossRef CAS PubMed.
- R. Mezzenga, S. Bolisetty and N. Reinhold, et al., Chem. Commun., 2017, 53, 142–151 RSC.
- L. Hao, N. Wang and G. J. Li, Chemosphere, 2018, 202, 768–776 CrossRef CAS PubMed.
- P. Song, Z. Yang and G. Zeng, et al., Chem. Eng. J., 2017, 317, 707–725 CrossRef CAS.
- R. Chowdhury, Chem. Ecol., 2017, 33, 560–571 CrossRef CAS.
- W. Driehaus and F. Dupont, Eur. J. Water Qual., 2010, 36, 138–147 Search PubMed.
- A. M. A. Pintor, B. R. C. Vieira and S. C. R. Santos, et al., Sci. Total Environ., 2018, 642, 1075–1089 CrossRef CAS PubMed.
- G. Yang, Y. Liu and S. Song, Chem. Eng. J., 2015, 3, 1497–1503 CAS.
- J. Lalley, C. Han and X. Li, et al., Chem. Eng. J., 2016, 284, 1386–1396 CrossRef CAS.
- H. Fakour, Y. F. Pan and T. F. Lin, Water, Air, Soil Pollut., 2015, 226, 1–16 CrossRef CAS.
- P. Qi and T. Pichler, Chemosphere, 2016, 145, 55–60 CrossRef CAS PubMed.
- J. Cantu, L. E. Gonzalez and J. Goodship, et al., Chem. Eng. J., 2016, 290, 428–437 CrossRef CAS PubMed.
- M. Podder and C. Majumder, Spectrochim. Acta, Part A, 2016, 153, 298–314 CrossRef CAS PubMed.
- S. Tresintsi, K. Simeonidis and G. Vourlias, et al., Water Res., 2012, 46, 5255–5267 CrossRef CAS PubMed.
- M. C. Collivignarelli, M. Canato and S. Sorlini, et al., Water Practice & Technology, 2016, 11, 115–126 Search PubMed.
- S. Kleinert, E. M. Muehe and N. R. Posth, et al., Environ. Sci. Technol., 2011, 45, 7533–7541 CrossRef CAS PubMed.
- S. L. Nicholas, M. L. Erickson and L. G. Woodruff, et al., Geochim. Cosmochim. Acta, 2017, 211, 228–255 CrossRef CAS.
- N. Bhandari, R. J. Reeder and D. R. Strongin, Environ. Sci. Technol., 2011, 45, 2783–2789 CrossRef CAS PubMed.
- N. Bhandari, R. J. Reeder and D. R. Strongin, Environ. Sci. Technol., 2012, 46, 8044–8051 CrossRef CAS PubMed.
- X. Liu, H. F. Feng and J. W. Fu, et al., Environ. Pollut., 2018, 241, 240–246 CrossRef CAS PubMed.
- A. Maiti, H. Sharma and J. K. Basu, et al., J. Hazard. Mater., 2009, 172, 928–934 CrossRef CAS PubMed.
- S. Aredes, B. Klein and M. Pawlik, J. Cleaner Prod., 2013, 60, 71–76 CrossRef CAS.
- H. Guo, Y. Li and K. Zhao, J. Hazard. Mater., 2010, 176, 174–180 CrossRef CAS PubMed.
- F. Li, H. Guo and X. Zhou, et al., Chemosphere, 2017, 168, 777–785 CrossRef CAS PubMed.
- H. Guo, Y. Li and K. Zhao, et al., J. Hazard. Mater., 2011, 186, 1847–1854 CrossRef CAS PubMed.
- K. Zhao, H. Guo and X. Zhou, Appl. Geochem., 2014, 48, 184–192 CrossRef CAS.
- B. Dousova, M. Lhotka and J. Filip, et al., J. Hazard. Mater., 2018, 357, 440–448 CrossRef CAS PubMed.
- J. Tomaszewska, S. Jakubiak and J. Michalski, et al., Appl. Surf. Sci., 2016, 366, 529–534 CrossRef CAS.
- H. J. Shipley, S. Yean and A. T. Kan, et al., Environ. Toxicol. Chem., 2009, 28, 509–515 CrossRef CAS PubMed.
- M. K. Ghosh, G. E. J. Poinern and T. B. Issa, et al., Korean J. Chem. Eng., 2012, 29, 95–102 CrossRef CAS.
- J. C. Lee, E. J. Kim and H. W. Kim, et al., Geoderma, 2016, 270, 76–82 CrossRef CAS.
- T. Türk, İ. Alp and H. Deveci, J. Environ. Eng., 2009, 136, 399–404 CrossRef.
- K. Banerjee, G. L. Amy and M. Prevost, et al., Water Res., 2008, 42, 3371–3378 CrossRef CAS PubMed.
- L. Wang and D. E. Giammar, J. Colloid Interface Sci., 2015, 448, 331–338 CrossRef CAS PubMed.
- R. Prucek, J. í. Tuček and J. Kolařík, et al., Environ. Sci. Technol., 2013, 47, 3283–3292 CrossRef CAS PubMed.
- M. C. Faria, R. S. Rosemberg and C. A. Bomfeti, et al., Chem. Eng. J., 2014, 237, 47–54 CrossRef CAS.
- A. Samanta, S. Das and S. Jana, ChemistrySelect, 2018, 3, 2467–2473 CrossRef CAS.
- X. Sun, C. Hu and X. Hu, et al., J. Chem. Technol. Biotechnol., 2013, 88, 629–635 CrossRef CAS.
- S. R. Chowdhury and E. K. Yanful, J. Environ. Manage., 2010, 91, 2238–2247 CrossRef CAS PubMed.
- W. Tang, Q. Li and S. Gao, et al., J. Hazard. Mater., 2011, 192, 131–138 CAS.
- L. Feng, M. Cao and X. Ma, et al., J. Hazard. Mater., 2012, 217, 439–446 CrossRef PubMed.
- H. Park, N. V. Myung and H. Jung, et al., J. Nanopart. Res., 2009, 11, 1981–1989 CrossRef CAS.
- S. R. Chowdhury, E. K. Yanful and A. R. Pratt, Environ. Earth Sci., 2011, 64, 411–423 CrossRef CAS.
- L. Yu, H. Liu and C. Liu, et al., Part. Part. Syst. Charact., 2016, 33, 323–331 CrossRef CAS.
- N. Candu, D. Paul and I. C. Marcu, Catal. Today, 2018, 306, 154–165 CrossRef CAS.
- X.-Z. Guo, P.-A. Yin and H. Yang, Microporous Mesoporous Mater., 2018, 259, 123–133 CrossRef CAS.
- N. Chubar and M. Szlachta, Chem. Eng. J., 2015, 279, 885–896 CrossRef CAS.
- S. Liu, S. Kang and G. Wang, et al., J. Colloid Interface Sci., 2015, 458, 94–102 CrossRef CAS PubMed.
- G. Zhang, Z. Ren and X. Zhang, et al., Water Res., 2013, 47, 4022–4031 CrossRef CAS PubMed.
- H.-J. Hong, W. Farooq and J.-S. Yang, et al., Sep. Sci. Technol., 2010, 45, 1975–1981 CrossRef CAS.
- Z. P. Zhang, Y. L. Zhang and C. M. Dai, et al., Microporous Mesoporous Mater., 2014, 200, 235–244 CrossRef.
- G. Zhang, X. F. Xu and Q. H. Ji, et al., ACS Appl. Mater. Interfaces, 2017, 9, 14868–14877 CrossRef CAS PubMed.
- S. Zhang, H. Niu and Y. Cai, et al., Chem. Eng. J., 2010, 158, 599–607 CrossRef CAS.
- Q. Hu, Y. Liu and X. Gu, et al., Chemosphere, 2017, 181, 328–336 CrossRef CAS PubMed.
- H. Lu, Z. Zhu and H. Zhang, et al., Chem. Eng. J., 2015, 276, 365–375 CrossRef CAS.
- E. B. Simsek and U. Beker, Korean J. Chem. Eng., 2014, 31, 2057–2069 CrossRef CAS.
- S. Kong, Y. Wang and Q. Hu, et al., Colloids Surf., A, 2014, 457, 220–227 CrossRef CAS.
- B. Chen, Z. Zhu and Y. Guo, et al., J. Colloid Interface Sci., 2013, 398, 142–151 CrossRef CAS PubMed.
- C. Bo, Z. Zhu and S. Liu, et al., ACS Appl. Mater. Interfaces, 2014, 6, 14016–14025 CrossRef PubMed.
- K. Gupta and U. C. Ghosh, J. Hazard. Mater., 2009, 161, 884–892 CrossRef CAS PubMed.
- M. Gil-Díaz, J. Alonso and E. Rodríguez-Valdés, et al., Sci. Total Environ., 2017, 584, 1324–1332 CrossRef PubMed.
- F. Fu, D. D. Dionysiou and H. Liu, J. Hazard. Mater., 2014, 267, 194–205 CrossRef CAS PubMed.
- P. Asharani, Y. Lianwu and Z. Gong, et al., Nanotoxicology, 2011, 5, 43–54 CrossRef CAS PubMed.
- Q. Du, S. Zhang and B. Pan, et al., Water Res., 2013, 47, 6064–6074 CrossRef CAS PubMed.
- M. Baikousi, Y. Georgiou and C. Daikopoulos, et al., Carbon, 2015, 93, 636–647 CrossRef CAS.
- S. Bhowmick, S. Chakraborty and P. Mondal, et al., Chem. Eng. J., 2014, 243, 14–23 CrossRef CAS.
- M. Biterna, L. Antonoglou and E. Lazou, et al., Chemosphere, 2010, 78, 7–12 CrossRef CAS PubMed.
- M. T. Sikder, S. Tanaka and T. Saito, et al., J. Environ. Chem. Eng., 2014, 2, 370–376 CrossRef.
- N. Horzum, M. M. Demir and M. Nairat, et al., RSC Adv., 2013, 3, 7828–7837 RSC.
- L. Ye, W. Liu and Q. Shi, et al., Environ. Pollut., 2017, 230, 1081–1089 CrossRef CAS PubMed.
- H. Zhu, Y. Jia and X. Wu, et al., J. Hazard. Mater., 2009, 172, 1591–1596 CrossRef CAS PubMed.
- C. Wu, J. Tu and W. Liu, et al., Environ. Sci.: Nano, 2017, 4, 1544–1552 RSC.
- R. Yadav, A. K. Sharma and J. N. Babu, J. Environ. Chem. Eng., 2016, 4, 681–694 CrossRef CAS.
- X. Zhang, Y. Wang and X. Chang, et al., Environ. Sci.: Nano, 2017, 4, 679–688 RSC.
- Y. Babaee, C. N. Mulligan and M. S. Rahaman, J. Chem. Technol. Biotechnol., 2017, 92, 112–120 Search PubMed.
- D. Fu, Z. He and S. Su, et al., J. Colloid Interface Sci., 2017, 505, 105–114 CrossRef CAS PubMed.
- D. Mohan, A. Sarswat and Y. S. Ok, et al., Bioresour. Technol., 2014, 160, 191–202 CrossRef CAS PubMed.
- U. Balasubramani, R. Venkatesh and S. Subramaniam, et al., J. Hazard. Mater., 2017, 340, 241–252 CrossRef CAS PubMed.
- A. M. Cooper, K. D. Hristovski and T. Möller, et al., J. Hazard. Mater., 2010, 183, 381–388 CrossRef CAS PubMed.
- G. Muñiz, V. Fierro and A. Celzard, et al., J. Hazard. Mater., 2009, 165, 893–902 CrossRef PubMed.
- P. T. Almazán-Sánchez, M. Castañeda-Juárez and V. Martínez-Miranda, et al., Water, Air, Soil Pollut., 2015, 226, 1–13 CrossRef.
- S.-J. Lee, J.-H. Park and Y. T. Ahn, Water, Air, Soil Pollut., 2015, 226, 9 CrossRef.
- M. E. Sigrist, L. Brusa and H. R. Beldomenico, et al., J. Environ. Chem. Eng., 2014, 2, 927–934 CrossRef CAS.
- A. V. Vitela-Rodriguez and J. R. Rangel-Mendez, J. Environ. Manage., 2013, 114, 225–231 CrossRef CAS PubMed.
- A. Zach-Maor, R. Semiat and H. Shemer, J. Colloid Interface Sci., 2011, 357, 440–446 CrossRef CAS PubMed.
- A. V. Vitelarodriguez and J. R. Rangelmendez, J. Environ. Manage., 2013, 114, 225–231 CrossRef CAS PubMed.
- K. D. Hristovski, P. K. Westerhoff and T. Möller, et al., Chem. Eng. J., 2009, 146, 237–243 CrossRef CAS.
- E. Deliyanni, T. J. Bandosz and K. A. Matis, J. Chem. Technol. Biotechnol., 2013, 88, 1058–1066 CrossRef CAS.
- C. Nieto-Delgado and J. R. Rangel-Mendez, Water Res., 2012, 46, 2973–2982 CrossRef CAS PubMed.
- V. Fierro, G. Muniz and G. Gonzalez-Sánchez, et al., J. Hazard. Mater., 2009, 168, 430–437 CrossRef CAS PubMed.
- Y. Xiong, Q. Tong and W. Shan, et al., Appl. Surf. Sci., 2017, 416, 618–627 CrossRef CAS.
- Q. Chang, W. Lin and W.-c. Ying, J. Hazard. Mater., 2010, 184, 515–522 CrossRef CAS PubMed.
- Z. Liu, F.-S. Zhang and R. Sasai, Chem. Eng. J., 2010, 160, 57–62 CrossRef CAS.
- M.-L. Chen, Y. Sun and C.-B. Huo, et al., Chemosphere, 2015, 130, 52–58 CrossRef CAS PubMed.
- L. Guo, P. Ye and J. Wang, et al., J. Hazard. Mater., 2015, 298, 28–35 CrossRef CAS PubMed.
- S. Vadahanambi, S.-H. Lee and W.-J. Kim, et al., Environ. Sci. Technol., 2013, 47, 10510–10517 CAS.
- V. Chandra, J. Park and Y. Chun, et al., ACS Nano, 2010, 4, 3979–3986 CrossRef CAS PubMed.
- G. Sheng, Y. Li and X. Yang, et al., RSC Adv., 2012, 2, 12400–12407 RSC.
- M. N. Sahmoune and A. R. Yeddou, Desalin. Water Treat., 2016, 57, 1–16 CrossRef.
- Z. Du, T. Zheng and P. Wang, et al., Bioresour. Technol., 2016, 201, 41–49 CrossRef CAS PubMed.
- D. Setyono and S. Valiyaveettil, ACS Sustainable Chem. Eng., 2014, 2, 2722–2729 CrossRef CAS.
- G. K. Von, M. S. Alam and M. Hubmann, et al., Bioresour. Technol., 2017, 236, 106–110 CrossRef PubMed.
- X. Zhu, B. Chen and L. Zhu, et al., Environ. Pollut., 2017, 227, 98–115 CrossRef CAS PubMed.
- X. Hu, Z. Ding and A. R. Zimmerman, et al., Water Res., 2015, 68, 206–216 CrossRef CAS PubMed.
- P. Lodeiro, S. M. Kwan and J. T. Perez, et al., Chem. Eng. J., 2013, 215, 105–112 CrossRef.
- A. Ö. A. Tuna, E. Özdemir and E. B. Şimşek, et al., Chem. Eng. J., 2013, 223, 116–128 CrossRef CAS.
- G. S. Raju, M. R. H. M. Haris and A. Azura, et al., J. Rubber Res., 2013, 16, 179–194 CAS.
- M. Jang, W. Chen and F. S. Cannon, Environ. Sci. Technol., 2008, 42, 3369–3374 CrossRef CAS PubMed.
- J.-h. Xu, N.-y. Gao and Y. Deng, et al., Chem. Eng. J., 2013, 222, 520–526 CrossRef CAS.
- R. N. Shinde, A. Pandey and R. Acharya, et al., Water Res., 2013, 47, 3497–3506 CrossRef CAS PubMed.
- A. Gupta, M. Yunus and N. Sankararamakrishnan, Ind. Eng. Chem. Res., 2013, 52, 2066–2072 CrossRef CAS.
- J. S. Yamani, S. M. Miller and M. L. Spaulding, et al., Water Res., 2012, 46, 4427–4434 CrossRef CAS PubMed.
- M. Rahim and M. R. H. M. Haris, J. Radiat. Res. Appl. Sci., 2015, 8, 255–263 CrossRef CAS.
- R. Kumar, R. K. Sharma and A. P. Singh, J. Mol. Liq., 2017, 232, 62–93 CrossRef CAS.
- H. Liimatainen, J. Sirviö and A. Haapala, et al., Carbohydr. Polym., 2011, 83, 2005–2010 CrossRef CAS.
- M. Iqbal, A. Saeed and I. Kalim, Sep. Sci. Technol., 2009, 44, 3770–3791 CrossRef CAS.
- Y. Tian, M. Wu and X. Lin, et al., J. Hazard. Mater., 2011, 193, 10–16 CrossRef CAS PubMed.
- S. Sharma, K. Balasubramanian and R. Arora, Desalin. Water Treat., 2016, 57, 1–17 CrossRef PubMed.
- L. Hao, T. Zheng and J. Jiang, et al., RSC Adv., 2015, 5, 10723–10732 RSC.
- L. Fan, S. Zhang and X. Zhang, et al., J. Environ. Manage., 2015, 156, 109–114 CrossRef CAS PubMed.
- J. F. Cárdenas-González, I. Acosta-Rodríguez and Y. Téran-Figueroa, et al., 3 Biotech, 2017, 7, 226–234 CrossRef PubMed.
- M. Mosaferi, S. Nemati and A. Khataee, et al., J. Environ. Health Sci., 2014, 12, 74–85 CrossRef PubMed.
- F. Meng, B. Yang and B. Wang, et al., ACS Sustainable Chem. Eng., 2017, 42, 723–738 Search PubMed.
- Ş. Targan and V. N. Tirtom, Desalin. Water Treat., 2014, 56, 1029–1036 CrossRef.
- D. D. Maksin, A. B. Nastasović and A. D. Milutinović-Nikolić, et al., J. Hazard. Mater., 2012, 209, 99–110 CrossRef PubMed.
- K. Taleb, J. Markovski and M. Milosavljević, et al., Chem. Eng. J., 2015, 279, 66–78 CrossRef CAS.
- M. R. Awual, S. A. El-Safty and A. Jyo, J. Environ. Sci., 2011, 23, 1947–1954 CrossRef CAS.
- M. R. Awual and A. Jyo, Water Res., 2009, 43, 1229–1236 CrossRef CAS PubMed.
- D. Ociński, I. Jacukowicz-Sobala and J. Raczyk, et al., React. Funct. Polym., 2014, 83, 24–32 CrossRef.
- K. Foo and B. Hameed, Chem. Eng. J., 2010, 156, 2–10 CrossRef CAS.
- J. L. Hu, X. S. Yang and T. Liu, et al., Water Sci. Technol., 2017, 76, 403–409 Search PubMed.
- C. M. Iesan, C. Capat and F. Ruta, et al., Water Res., 2008, 42, 4327–4333 CrossRef CAS PubMed.
- D. Morillo, G. Pérez and M. Valiente, J. Colloid Interface Sci., 2015, 453, 132–141 CrossRef CAS PubMed.
- P. S. Kumar, R. Q. Flores and C. Sjöstedt, et al., J. Hazard. Mater., 2016, 302, 166–174 CrossRef PubMed.
- T. S. Anirudhan, L. Divya and J. Parvathy, J. Chem. Technol. Biotechnol., 2013, 88, 878–886 CrossRef CAS.
- B. Hu and H. Luo, Appl. Surf. Sci., 2010, 257, 769–775 CrossRef CAS.
- L. B. de Paiva, A. R. Morales and F. R. V. Díaz, Appl. Clay Sci., 2008, 42, 8–24 CrossRef.
- K. Gupta, K. Biswas and U. C. Ghosh, Ind. Eng. Chem. Res., 2008, 47, 9903–9912 CrossRef CAS.
- W. Xu, J. Wang and L. Wang, et al., J. Hazard. Mater., 2013, 260, 498–507 CrossRef CAS PubMed.
- X. Ren, Z. Zhang and H. Luo, et al., Appl. Clay Sci., 2014, 97, 17–23 CrossRef.
- S. K. Maji, Y.-H. Kao and C.-J. Wang, et al., Chem. Eng. J., 2012, 203, 285–293 CrossRef CAS.
- D. Srivastava and R. C. Vaishya, Desalin. Water Treat., 2015, 53, 2565–2577 CrossRef CAS.
- M. Pantoja, H. Jones and H. Garelick, et al., Environ. Sci. Pollut. Res., 2014, 21, 495–506 CrossRef CAS PubMed.
- X. Xie, Y. Wang and K. Pi, et al., Sci. Total Environ., 2015, 527, 38–46 CrossRef PubMed.
- Z.-L. Shi, C. Du and S.-H. Yao, J. Taiwan Inst. Chem. Eng., 2011, 42, 652–657 CrossRef CAS.
- I. Andjelkovic, D. Stankovic and J. Nesic, et al., Ind. Eng. Chem. Res., 2014, 53, 10841–10848 CrossRef CAS.
- J. Y. Fan, Z. X. Ding and Z. W. Zhao, et al., Chem. Eng. J., 2011, 350, 453–462 CrossRef.
- G. P. Gallios, A. K. Tolkou and I. A. Katsoyiannis, et al., Sustainability, 2017, 9, 1684–1702 CrossRef.
- Y. Li, S. Zhu and Q. Liu, et al., Water Res., 2013, 47, 4188–4197 CrossRef CAS PubMed.
- Y. Liang, X. Min and L. Chai, et al., Chemosphere, 2017, 168, 1142–1151 CrossRef CAS PubMed.
- S. Zhang, X.-y. Li and J. P. Chen, J. Colloid Interface Sci., 2010, 343, 232–238 CrossRef CAS PubMed.
- C.-H. Liu, Y.-H. Chuang and T.-Y. Chen, et al., Environ. Sci. Technol., 2015, 49, 7726–7734 CrossRef CAS PubMed.
- J. B. Farideh, A. A. Najafpoor and M. Davoudi, et al., Environ. Prog. Sustainable Energy, 2018, 37, 951–960 CrossRef.
- T. M. Thi, N. T. H. Trang and N. T. Van Anh, Appl. Surf. Sci., 2015, 340, 166–172 CrossRef CAS.
- H. Guo, Y. Ren and Q. Liu, et al., Environ. Sci. Technol., 2013, 47, 1009–1016 CrossRef CAS PubMed.
- J. Tofan-Lazar and H. A. Al-Abadleh, J. Phys. Chem. A, 2012, 116, 10143–10149 CrossRef CAS PubMed.
- M. P. Yannick, Desalination, 2011, 281, 93–99 CrossRef.
- M. L. Chen, Y. Sn and C. B. Huo, et al., Chemosphere, 2015, 130, 52–58 CrossRef CAS PubMed.
- S. Mills, A. Christry and J. M. Genin, et al., Mineral. Mag., 2012, 76, 1289–1336 CrossRef CAS.
- J. Zhu, RSC Adv., 2015, 5, 67951–67961 RSC.
- J. Y. Wang, T. P. Zhang and M. Li, et al., RSC Adv., 2015, 8, 22694–22709 RSC.
- W. Yan, R. Vasic and A. I. Frenkel, et al., Environ. Sci. Technol., 2012, 46, 7018–7026 CrossRef CAS PubMed.
- F. Pinakidou, M. Katsikini and K. Simeonidis, et al., J. Hazard. Mater., 2015, 298, 203–209 CrossRef CAS PubMed.
- M. S. U. Sharif, R. K. Davis and K. F. Steele, et al., Appl. Geochem., 2011, 26, 496–504 CrossRef CAS.
- H. A. Mengistu, A. Tessema and M. B. Demlie, et al., Water SA, 2015, 41, 157–167 CrossRef CAS.
- D. H. Kiril and M. Jasmina, Sci. Total Environ., 2017, 598, 258–271 CrossRef PubMed.
- H. Jiang, Y. Li and W. Zhang, et al., Sep. Sci. Technol., 2017, 52, 634–643 CrossRef CAS.
- R. M. Kardia, P. R. Fatima and R. M. Rene, J. Mol. Liq., 2018, 264, 253–260 CrossRef.
- P. Sepúlveda, M. A. Rubio and S. E. Baltazar, et al., J. Colloid Interface Sci., 2018, 524, 177–187 CrossRef PubMed.
- A. Masoumi, M. Ghaemy and A. N. Bakht, Ind. Eng. Chem. Res., 2014, 53, 8188–8197 CrossRef CAS.
- D. Malwal and P. Gopinath, Colloid Interface Sci. Commun., 2017, 19, 14–19 CrossRef CAS.
- M. Zhang, B. Gao and S. Varnoosfaderani, et al., Bioresour. Technol., 2013, 130, 457–462 CrossRef CAS PubMed.
- A. E. Pirbazari, E. Saberikhah and S. S. H. Kozani, Water Resources and Industry, 2014, 2, 23–37 CrossRef.
- M. A. Ali, A. B. M. Badruzzaman and M. A. Jalil, et al., J. Appl. Sci., 2001, 7, 99–120 Search PubMed.
- J. Giménez, M. Martínez and J. Pablo, et al., J. Hazard. Mater., 2007, 141, 575–580 CrossRef PubMed.
- C.-N. Ji, R. J. Qu and Q.-H. Tang, et al., Water Sci. Technol.: Water Supply, 2016, 16, 1603–1613 CAS.
| This journal is © The Royal Society of Chemistry 2018 |