Open Access Article
Open Access ArticleCharge-induced electromechanical actuation of Mo- and W-dichalcogenide monolayers
Vuong Van Thanh *a,
Nguyen Tuan Hung
*a,
Nguyen Tuan Hung *b and
Do Van Truongcd
*b and
Do Van Truongcd
aDepartment of Design of Machinery and Robot, School of Mechanical Engineering, Hanoi University of Science and Technology, Hanoi, Vietnam. E-mail: thanh.vuongvan@hust.edu.vn
bDepartment of Physics, Tohoku University, Sendai 980-8578, Japan. E-mail: nguyen@flex.phys.tohoku.ac.jp
cDepartment of Mechatronics, School of Mechanical Engineering, Hanoi University of Science and Technology, Hanoi, Vietnam
dInternational Institute for Computational Science and Engineering, Hanoi University of Science and Technology, Hanoi, Vietnam
First published on 16th November 2018
Abstract
Using first-principle density functional calculations, we investigate electromechanical properties of two-dimensional MX2 (M = Mo, W; X = S, Se, Te) monolayers with the 1H and 1T structures as a function of charge doping for both electron and hole doping. We find that by increasing the atomic number, ZX, of X atoms (ZS < ZSe < ZTe), the work density per cycle of the MX2 monolayers are increased and decreased for the 1H and 1T structures, respectively. On the other hand, the work density per cycle of the WX2 monolayers are higher than that of the MoX2 monolayers for both the 1H and 1T structures. Therefore, WTe2 and WS2 monolayers for the 1H and 1T structures, respectively, have the best electromechanical performances in the MX2 compounds. In addition, the MX2 monolayers show a reversible strain up to 3%, which is higher than that of graphene (∼1%). Our results provide an important insight into the electromechanical properties of the MX2 monolayers, which are useful for artificial muscles applications.
1 Introduction
Artificial muscles or electromechanical actuation based on two-dimensional (2D) materials have become attractive recently because of its excellent properties such as larger surface doping, mechanical flexibility and thermal/chemical stability.1–5 For example, Xie et al.6 reported the superior strain response of a 2D graphene actuator, which has a strain approaching to 0.85%, exceeding that of the best-known CNT-based actuators. On the other hand, Ge et al.7 showed that an actuator based on the graphene oxide film is able to lift more than eight times its own weight. Very recently, Lu et al.8 reported a 2D graphdiyne-based electromechanical actuator with a high strain of up to 6.03%, and its energy density (11.5 kJ m−3) is comparable to that of mammalian skeletal muscle (∼8 kJ m−3). However, the 2D artificial muscles based on graphene, graphene oxide, and graphdiyne have still been limited in the large-scale applications such as biomedicine and rehabilitation devices because these actuator materials are expensive and difficult to synthesize. Therefore, finding new 2D actuator materials with low manufacturing cost and large strain amplitude for the artificial muscles should be necessary.Transition-metal dichalcogenides (TMDs) such as MX2 (M = Mo, W; X = S, Se, Te) have similar layered structures as graphene.9,10 The chemically exfoliated MoS2 nanosheets are synthesized by organo-lithium chemistry. By characteristics such as large-area two-dimensional flakes, thermodynamically stable and high quality, the 2D MoS2 nanosheets have very attractived for artificial muscles on a large scale applications. Recently, Acerce et al.11 showed a significant performance on the electromechanical actuation of the 2D MoS2 nanosheets by using the intercalation reactions. They reported that the mechanical stress, strain, and work density of the MoS2 nanosheets reach about 17 MPa, 0.8%, and 81 kJ m−3, respectively. However, their study has limited to the MoS2 nanosheets with only 1T structure. By using the density functional theory (DFT) calculations, Hung et al.12 investigated the actuator performance of the MoS2 monolayer with the 1H, 1T and 1T′ structures. They pointed out that the actuator performance of the MoS2 monolayers with the 1T and 1T′ structures are better than that of the 1H structure. This because the interplay between the actuation strain and Young's modulus of the MoS2 monolayer with difference structures. Both experiment and theory11,12 only focused of the MoS2 monolayer actuator, while the actuator properties of other TMDs are not fully explored yet, except for some limited experiments.13 In this sense, a systematic theoretical design of many TMDs materials could be the first step to suggest possible structures suitable as highest electromechanical actuators. Moreover, TMDs have been demonstrated that the 1H structure is more dominant than the 1T and 1T′ structure in many experiments14–16 due to their low energy formation. Therefore, it is an important to find which TMD among MX2 compounds has a high actuator performance with the 1H structure.
In this paper, we use the first-principles calculations within DFT to calculate the electromechanical properties of the MX2 monolayers with a variety of M (M = Mo, W) and X (X = S, Se, Te) atoms as a function of charge doping for both electron and hole doping. As the main highlight of this paper, we summarizes all the periodic trends strictly obeyed by our data including the Young's modulus, work density per cycle and actuator stress for both 1H and 1T structures of the MX2 monolayers. Our results show that WTe2 and WS2 monolayers with the 1H and 1T structures, respectively, have the best electromechanical performances in the MX2 compounds at heavy electron doping.
2 Methodology
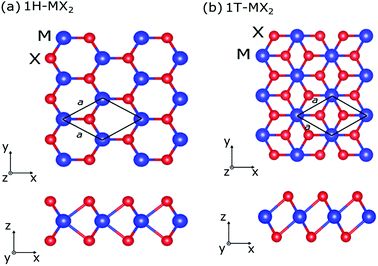
The density-functional theory are performed to calculate the electromechanical actuator of the MX2 monolayers using the Quantum ESPRESSO package.17 The pseudopotentials from the Standard Solid-State Pseudopotentials library are used.18 The exchange-correlation energy is evaluated by the general gradient approximation using the Perdew–Burke–Ernzerhof19 function. The cutoff energy of plane wave is set at 60 Ry, and 16 × 16 × 1 k-point mesh of Monkhorst–Pack scheme is used for Brillouin zone integrations.20The atomic structures of the MX2 monolayers with the 1H and 1T structures are shown in Fig. 1. The atomic positions and cell vectors of the MX2 monolayers are fully relaxed to obtain optimized atomic configurations by using the Broyden–Fletcher–Goldfarb–Shanno minimization method21–24 until all the Hellmann Feynman forces and all components of the stress are less than 0.0005 Ry/a.u. and 0.05 GPa, respectively. The periodic boundary condition is applied in all models, a vacuum space of 30 Å in the direction perpendicular to the monolayer (z direction) is used in order to avoid virtual interactions between layers.
The geometry optimization is then performed for each charge doping from −0.1 to +0.1 electron per atom (e/atom) to consider the electromechanical actuation of the MX2 monolayers. The electron (hole) doping is simulated by adding (removing) electrons to the unit cell with a uniform charge background.25,26 The elastic constants Cij of MX2 monolayers are estimated by using the Themo-pw code.27 Since the values of Cij are related to the equivalent volume of the unit cell, the calculated Cij must be rescaled by h/d0, where h is the length of the cell along z axis and d0 is the effective layer thickness of the MX2 monolayers (d0 = 6.145 Å).12 The Young's modulus, Y, and the Poisson's ratio, ν, of the MX2 monolayers can be obtained from the following equation as12
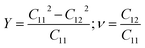 | (1) |
The power of electromechanical actuators is characterized by the stress generated, σ = Yε where actuator strain ε is defined as Δa/a0, where a0 is the lattice constant at geometry optimization for neutral case, and Δa is increment (or decrement) of a0 after the charge doping has been applied. The performance of electromechanical actuators is characterized by the work density per cycle, which is often expressed in terms of stored energy density Ws.12 The formula for the work density per cycle is given by
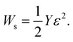 | (2) |
3 Results and discussion
In Table 1, we show the optimized lattice parameters of the MX2 monolayers, in which the lattice parameters of the MoX2 and WX2 monolayers have quite similar values. The lattice constants of the MX2 monolayers increase with increasing of atomic number of M, ZM (ZMo < ZW), and X, ZX (ZS < ZSe < ZTe), atoms. Our calculated results are in good agreement with previous theoretical results,9,28 indicating that the present calculations are reasonable and reliable. In addition the total energy of the MX2 monolayers with the 1H structure (1H-MX2) are smaller than that of the 1T structure (1T-MX2). Therefore, the MX2 monolayers with 1H structure are more stable than 1T structure. In many experiments, the 1H structure also was found to be abundantly in sample.29,30 We also note that MoTe2 and WTe2 monolayers might not have ideal 1T phase. They form the distorted 1T structure, namely 1T′ structure.31 Since the MX2 monolayers with 1T′ structure exhibit an anisotropic behavior,12,31 in present study, we only focus on the isotropic 1H- and 1T-MX2 monolayers.| MX2 | a0 | Etot | C11 | C22 | C12 | C66 | Y | ν |
|---|---|---|---|---|---|---|---|---|
| 1H-MoS2 | 3.19 | −190.798 | 207 | 207 | 42 | 83 | 197 | 0.200 |
| 1H-MoSe2 | 3.31 | −224.510 | 173 | 173 | 32 | 71 | 167 | 0.180 |
| 1H-MoTe2 | 3.55 | −190.204 | 129 | 129 | 41 | 44 | 116 | 0.320 |
| 1T-MoS2 | 3.18 | −190.739 | 169 | 169 | −4.0 | 86 | 167 | −0.020 |
| 1T-MoSe2 | 3.28 | −224.462 | 166 | 166 | −3.2 | 85 | 166 | −0.019 |
| 1T-MoTe2 | 3.53 | −190.169 | 140 | 140 | −0.39 | 70 | 140 | −0.003 |
| 1H-WS2 | 3.19 | −597.594 | 230 | 230 | 43 | 94 | 222 | 0.185 |
| 1H-WSe2 | 3.32 | −631.292 | 193 | 193 | 31 | 81 | 188 | 0.160 |
| 1H-WTe2 | 3.56 | −596.969 | 140 | 140 | 27 | 57 | 135 | 0.190 |
| 1T-WS2 | 3.20 | −597.530 | 171 | 171 | −2.2 | 87 | 171 | −0.013 |
| 1T-WSe2 | 3.29 | −631.238 | 159 | 159 | −9.3 | 84 | 159 | −0.060 |
| 1T-WTe2 | 3.54 | −596.931 | 136 | 136 | −12 | 73 | 135 | −0.086 |
To calculate the actuator response of the MX2 monolayers, we firstly check the mechanical moduli at the neutral condition case (q = 0). The values of the elastic constants Cij, Young's modulus Y and Poisson's ratio ν of the MX2 monolayers are listed in Table 1. Y of the MX2 monolayers decreases with increasing ZX. On the other hand, by increasing ZM, Y is increased and decreased for the 1H and 1T structures, respectively, expect that Y of the 1T-MoS2 monolayer is smaller than that of the 1T-WS2 monolayer. Therefore, the WS2 monolayer shows the stiffest materials in the MX2 compounds with Y = 222 GPa and Y = 171 GPa for the 1H and 1T structures, respectively. In addition, the Poisson's ratio is also an important mechanical properties. Our results show that ν of the 1H structures is positive, while ν of the 1T structures is negative. It is known that the negative Poisson's ratio are also found in other 2D materials such as in black phosphorus (ν = −0.5),32 single-layer graphene ribbons(ν = −1.51),33 and δ-phosphorene (ν = −0.267).34 The 2D materials with native Poisson's ratio could have useful applications, for example, as vanes for aircraft gas turbine engines, sponges, and fasteners.35
Although the atomic structures of the MX2 monolayers has been optimized, they do not guarantee a dynamical stability under the charge doping. Therefore, we analyze the generic elastic stability conditions for the MX2 structures at each charge doping level. In particular, in the 2D hexagonal structure, the necessary and sufficient conditions of stability are C11 > |C12| > 0 and C66 > 0.36 In Fig. 2(a) and (b), we show the elastic constants (C11, C12 and C66) of the MX2 monolayers as a function of charge doping q ranging from −0.1 to 0.1 e/atom for the 1H and 1T structures, respectively. Our results reveal that C11 > |C12| > 0 and C66 > 0 for both all the 1H and 1T structures. Therefore, the MX2 monolayers show a stable structure under both electron and hole doping cases. After revealing the stable configurations, the electromechanical properties of the MX2 monolayers are investigated.
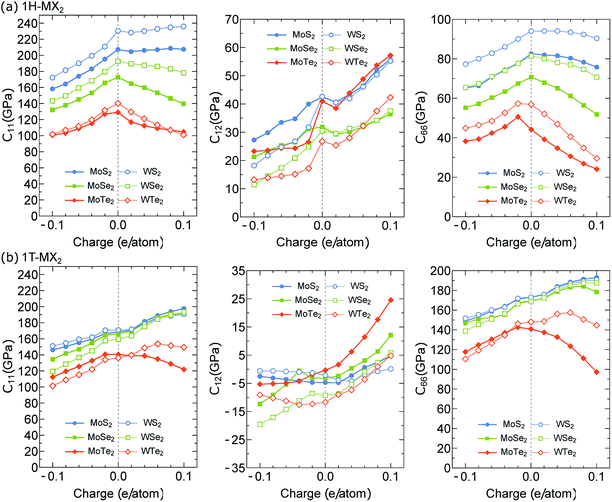 | ||
| Fig. 2 Elastic constants C11, C12 and C66 of the (a) 1H-MX2 and (b) 1T-MX2 monolayers plotted as function of charge (electron and hole) doping per atom. | ||
In Fig. 3(a) and (b), we show the Young's modulus Y of the 1H- and 1T-MX2 monolayers, respectively, as a function of the charge doping (q ≠ 0). For the 1H-MX2 monolayers, Y decreases for both the electron (q < 0) and hole (q > 0) dopings, except that Y of the WS2 monolayer is approximately constant for the hole doping case, as shown in Fig. 3(a). For the 1T-MX2 monolayers, Y decreases and increases for the electron and hole dopings, respectively, except that Y of the MoTe2 decreases for both electron and hole dopings, as shown in Fig. 3(b). We note that since C12 ≪ C11 (see Fig. 2), the Young's modulus in eqn (1) can rewritten as Y ∼ C11 for both 1H- and 1T-MX2 monolayers. Therefore, the behaviours of Y are similar to C11 under the electron and hole doping cases. The results obtained show that the maximum Y of the MX2 monolayers are 222 GPa and 197 GPa for the 1H-WS2 and 1T-MoS2 monolayers, respectively, at q = 0.1 e/atom. Y of the MX2 monolayers is thus comparable to that of the stainless steel (192 GPa).37 It notes that the materials with high Y value can generate large force per unit area.
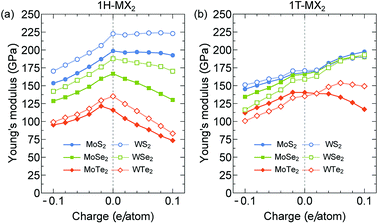 | ||
| Fig. 3 Young's modulus of the (a) 1H-MX2 and (b) 1T-MX2 monolayers plotted as a function of charge doping per atom. | ||
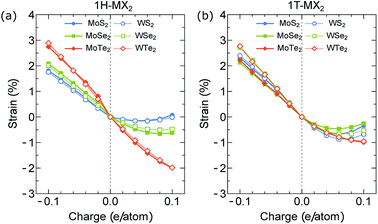
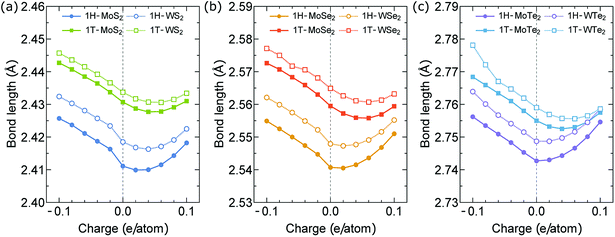
In Fig. 4(a) and (b), we show the actuator strain ε of the 1H- and 1T-MX2 monolayers as a function of charge doping q ranging from −0.1 to 0.1 e/atom. In the neutral case (q = 0), we obtain ε = 0. For the charge doping case, ε is approximately a linear function of q for electron doping (q < 0), while it is non-linear function of q for hole doping (q > 0). As shown in Fig. 4(a), the absolute values of ε of the 1H-MX2 monolayers mainly depend on X atoms, in which MTe2 > MSe2 > MS2 for both electron and hole doping cases. In contrast, the absolute values of ε of the 1T-MX2 monolayers mainly depend on M atoms (WX2 > MoX2), as shown in Fig. 4(b). For the MoS2 monolayer, the strain of the 1T structure (2.25%) is higher than that of the 1H structure (1.78%) at q = −0.1 e/atom. The MoS2 monolayer with the 1T structure is thus predicted high actuation performance, which is consistent with previous results.12 As shown in Fig. 4(a) and (b), unlike the MS2 and MSe2 monolayers, the strain of the MTe2 monolayers (MoTe2 and WTe2) with the 1H structure are higher than that with the 1T structures. The highest ε is found in the MoTe2 (2.74%) and WTe2 (2.9%) monolayers with the 1H structure at q = −0.1 e/atom, respectively, which is higher than that of carbon nanotubes and graphene (∼1%).6,25,38 Since the 1H structure is dominates in the TMDs structures, the MoTe2 and WTe2 monolayers may be the ideal choice for the artificial muscles. In order to understand these behaviors of the actuator strain, the bond lengths between M and X atoms are investigated at different charge doping level, as shown in Fig. 5. In the neutral case (q = 0), the M (Mo, W)–Te bond lengths of 1H- and 1T-MX2 are higher than the M–Se and M–S bond lengths, respectively. For the electron doping, the M–X bond lengths increase linearly by increasing q (q < 0), while they increase non-linearly for the hole doping (q > 0). As shown in Fig. 5(a)–(c), the electron doping largely change the M–X bond lengths compared with the hole doping. We note that the electron doping will “pull down” many interlayer bands in the TMD's band structure, while hole doping do not.12 Such a phenomenon might contribute to the higher strain actuators by the electron doping rather than the hole doping.
In Fig. 6(a) and (b), we show the actuator stress of the MX2 monolayers as a function of charge doping for the 1H and 1T structures, respectively. In the neutral case (q = 0), we obtain σ = 0 because ε = 0. For the electron doping (q < 0), the stress of the MX2 monolayers with both the 1H and 1T structures increases with increasing |q|. The highest stresses are found in the WS2 monolayers for both the 1H (σ = 3 GPa) and 1T structures (σ = 3.6 GPa) due to their highest Young's modulus as shown in Fig. 3. For the hole doping (q > 0), the highest stress of the MX2 monolayers with the 1H structure is found in the WTe2 monolayers with σ = −1.65 GPa at q = 0.1 e/atom, while it is found in WS2 monolayers with 1T structures (σ = −1.62 GPa at q = 0.1 e/atom).
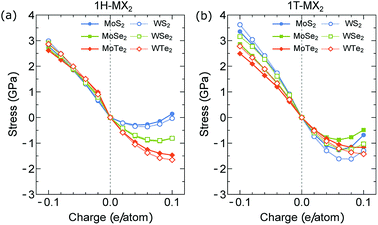 | ||
| Fig. 6 Stress generated by (a) 1H-MX2 and (b) 1T-MX2 monolayers plotted as function of charge doping per atom. | ||
Finally, we investigate the performance of the electromechanical actuators of the MX2 monolayers. In Fig. 7(a) and (b), we show the work density per cycle Ws of the 1H- and 1T-MX2 monolayers as a function of charge doping, respectively, in which the Ws is determined by eqn (2). For the electron doping (q < 0), Ws of the MX2 monolayer with both 1H and 1T structures increases with increasing |q|. In the meantime, Ws is increased and decreased with increasing ZX (ZS < ZSe < ZTe) for the 1H and 1T structures, respectively. On the other hand, Ws of the MoX2 monolayers is smaller than that of the WX2 monolayers for both 1H and 1T structures. Therefore, the highest Ws is found in the WTe2 (41.66 MJ m−3) and WS2 (43.30 MJ m−3) monolayers with the 1H and 1T structures at q = −0.1 e/atom, respectively. These work density per cycle are more than 1000 times that of skeleton muscle (∼0.04 MJ m−3)39 and 7 times that of Au–Pt alloys (∼6 MJ m−3)40,41 due to their high Young's modulus and high actuator strain. For the hole doping (q > 0), the maximum Ws is found in the WTe2 (16.4 MJ m−3 at 0.1 e/atom) and WS2 monolayers (7.03 MJ m−3 at 0.06 e/atom) for the 1H and 1T structures, respectively. Ws at the electron doping case is thus higher than that of the hole doping case, which suggests that the electron doping should be good to achieve high-performance actuator in the artificial muscle application. All the periodic trends strictly obeyed by our data are summarized for the electron doping at q = −0.1 e/atom are shown in Fig. 8.
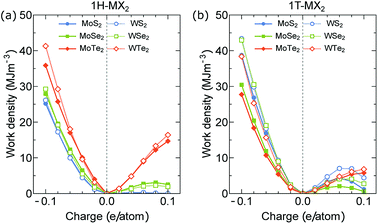 | ||
| Fig. 7 Work density per cycle of the (a) 1H-MX2 and (b) 1T-MX2 monolayers plotted as function of charge (electron and hole) doping per atom. | ||
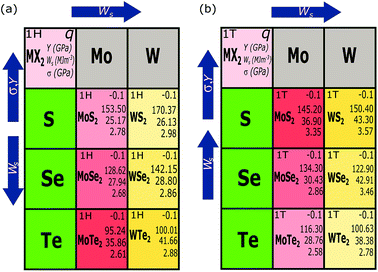 | ||
| Fig. 8 Trends in work density per cycle, stress and Young's modulus of (a) the 1H-MX2 and (b) 1T-MX2 monolayers for the electron doping at q = −0.1 e/atom. | ||
4 Conclusion
In summary, we have investigated the actuator performance of the two-dimensional MX2 monolayers with the 1H and 1T structures by first principles calculations. We find that the actuator performance of the MX2 monolayers not only depend on the charge doping level but also the atomic numbers ZM and ZX of M and X atoms, respectively. The 1H-WTe2 and 1T-WS2 monolayers have the best electromechanical performances in the MX2 compounds with the work density per cycle can be achieved up to 41.66 MJ m−3 and 43.30 MJ m−3 under charge doping, respectively. Moreover, the MX2 monolayers show the reversible strain up to 3%. The results of this study are useful for the design and fabricating of artificial muscles with the MX2 compounds.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is funded by the Hanoi University of Science and Technology (HUST) under project number T2017-LN-10.References
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- K. S. Novoselov, V. Fal, L. Colombo, P. Gellert, M. Schwab and K. Kim, et al., Nature, 2012, 490, 192 CrossRef CAS PubMed.
- K. Hu, D. D. Kulkarni, I. Choi and V. V. Tsukruk, Prog. Polym. Sci., 2014, 39, 1934–1972 CrossRef CAS.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- S. Park, J. An, J. W. Suk and R. S. Ruoff, Small, 2010, 6, 210–212 CrossRef CAS PubMed.
- X. Xie, H. Bai, G. Shi and L. Qu, J. Mater. Chem., 2011, 21, 2057–2059 RSC.
- Y. Ge, R. Cao, S. Ye, Z. Chen, Z. Zhu, Y. Tu, D. Ge and X. Yang, Chem. Commun., 2018, 54, 3126–3129 RSC.
- C. Lu, Y. Yang, J. Wang, R. Fu, X. Zhao, L. Zhao, Y. Ming, Y. Hu, H. Lin and X. Tao, et al., Nat. Commun., 2018, 9, 752 CrossRef PubMed.
- Y. Ding, Y. Wang, J. Ni, L. Shi, S. Shi and W. Tang, Physica B Condens. Matter., 2011, 406, 2254–2260 CrossRef CAS.
- A. Ostadhossein, A. Rahnamoun, Y. Wang, P. Zhao, S. Zhang, V. H. Crespi and A. C. Van Duin, J. Phys. Chem. Lett., 2017, 8, 631–640 CrossRef CAS PubMed.
- M. Acerce, E. K. Akdoğan and M. Chhowalla, Nature, 2017, 549, 370 CrossRef CAS PubMed.
- N. T. Hung, A. R. Nugraha and R. Saito, J. Phys. D: Appl. Phys., 2018, 51, 075306 CrossRef.
- H. Ramakrishna Matte, A. Gomathi, A. K. Manna, D. J. Late, R. Datta, S. K. Pati and C. Rao, Angew. Chem., Int. Ed., 2010, 49, 4059–4062 CrossRef PubMed.
- J. N. Coleman, M. Lotya, A. ONeill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De and R. J. Smith, et al., Science, 2011, 331, 568–571 CrossRef CAS PubMed.
- Z. Zeng, Z. Yin, X. Huang, H. Li, Q. He, G. Lu, F. Boey and H. Zhang, Angew. Chem., 2011, 123, 11289–11293 CrossRef.
- A. Castellanos-Gomez, M. Barkelid, A. Goossens, V. E. Calado, H. S. van der Zant and G. A. Steele, Nano Lett., 2012, 12, 3187–3192 CrossRef CAS PubMed.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni and I. Dabo, et al., J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- K. Lejaeghere, G. Bihlmayer, T. Björkman, P. Blaha, S. Blügel, V. Blum, D. Caliste, I. E. Castelli, S. J. Clark and A. Dal Corso, et al., Science, 2016, 351, aad3000 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 5188 CrossRef.
- C. G. Broyden, IMA Journal of Applied Mathematics, 1970, 6, 222–231 CrossRef.
- R. Fletcher, Comput. J., 1970, 13, 317–322 CrossRef.
- D. Goldfarb, Math. Comput., 1970, 24, 23–26 CrossRef.
- D. F. Shanno, Math. Comput., 1970, 24, 647–656 CrossRef.
- N. T. Hung, A. R. Nugraha and R. Saito, Carbon, 2017, 118, 278–284 CrossRef CAS.
- N. T. Hung, A. R. Nugraha and R. Saito, Carbon, 2017, 125, 472–479 CrossRef CAS.
- A. Dal Corso, J. Phys.: Condens. Matter, 2016, 28, 075401 CrossRef PubMed.
- X. Sun, Z. Wang, Z. Li and Y. Q. Fu, Sci. Rep., 2016, 6, 26666 CrossRef CAS PubMed.
- N. Savjani, E. A. Lewis, M. A. Bissett, J. R. Brent, R. A. Dryfe, S. J. Haigh and P. OBrien, Chem. Mater., 2016, 28, 657–664 CrossRef CAS.
- Q. D. Truong, N. T. Hung, Y. Nakayasu, K. Nayuki, Y. Sasaki, D. Murukanahally Kempaiah, L.-C. Yin, T. Tomai, R. Saito and I. Honma, RSC Adv., 2018, 8, 33391–33397 RSC.
- E. Torun, H. Sahin, S. Cahangirov, A. Rubio and F. M. Peeters, J. Appl. Phys., 2016, 074307 CrossRef.
- Y. Du, J. Maassen, W. Wu, Z. Luo, X. Xu and P. D. Ye, Nano Lett., 2016, 16, 6701–6708 CrossRef CAS PubMed.
- J.-W. Jiang and H. S. Park, Nano Lett., 2016, 16, 2657–2662 CrossRef CAS PubMed.
- H. Wang, X. Li, P. Li and J. Yang, Nanoscale, 2017, 9, 850–855 RSC.
- R. H. Baughman, J. M. Shacklette, A. A. Zakhidov and S. Stafström, Nature, 1998, 392, 362 CrossRef CAS.
- F. Mouhat and F. Coudert, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 224104 CrossRef.
- J. Y. Rho, R. B. Ashman and C. H. Turner, J. Biomech., 1993, 26, 111–119 CrossRef CAS PubMed.
- G. W. Rogers and J. Z. Liu, J. Am. Chem. Soc., 2011, 133, 10858–10863 CrossRef CAS PubMed.
- J. D. Madden, N. A. Vandesteeg, P. A. Anquetil, P. G. Madden, A. Takshi, R. Z. Pytel, S. R. Lafontaine, P. A. Wieringa and I. W. Hunter, IEEE J. Oceanic Eng., 2004, 29, 706–728 CrossRef.
- J. Weissmüller, R. Viswanath, D. Kramer, P. Zimmer, R. Würschum and H. Gleiter, Science, 2003, 300, 312–315 CrossRef PubMed.
- H.-J. Jin, X.-L. Wang, S. Parida, K. Wang, M. Seo and J. Weissmuller, Nano Lett., 2009, 10, 187–194 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |