Open Access Article
Open Access ArticleSynthesis of 8′,11′-dihydrospiro[cyclohexane-1,2′-oxepino[2,3-h] chromen]-4′(3′H)-ones with ring closing metathesis as a key step†
Prathima K a,
Ashok D
a,
Ashok D *a,
Sarasija Mb,
Prabhakar Sripadic,
Madhu Vemulac,
Venkata S. Komarrajud,
Biswajit Goraid and
Shyam Prakashd
*a,
Sarasija Mb,
Prabhakar Sripadic,
Madhu Vemulac,
Venkata S. Komarrajud,
Biswajit Goraid and
Shyam Prakashd
aDepartment of Chemistry, Osmania University, Hyderabad, India. E-mail: ashokdou@gmail.com
bDepartment of Chemistry, Satavahana University, Karimnagar, Telangana, India 505001
cAnalytical Chemistry & Mass Spectrometry Division, CSIR – Indian Institute of Chemical Technology, India
dDepartment of Laboratory Medicine, All India Institute of Medical Sciences, India
First published on 16th November 2018
Abstract
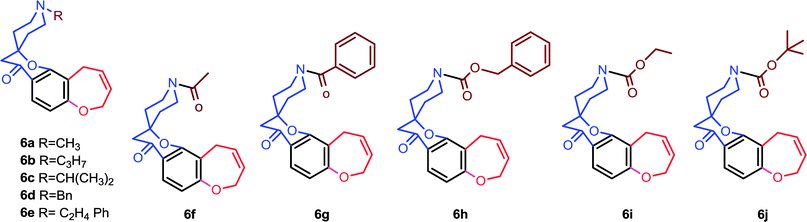
A series of novel hybrid molecular entities incorporating various spiro chromanone scaffolds onto the benzannulated oxepine core moiety were synthesised using allylation, Claisen rearrangement, Kabbe condensation and Ring Closing Metathesis (RCM) as a key step. During the synthesis we found that the nitrogen functionality in the substrate influences significantly the catalyst load due to electronic effects. Several iterations have been carried out to achieve complete conversion to products 6a–6e.
Introduction
The combination of two or more pharmacophores into a single molecule is an effective method used in medicinal chemistry to synthesize plausible lead molecules. Incorporation of drug pharmacophores into a single entity in order to enhance or amplify its mode of action is gaining popularity in medicinal chemistry. This type of combination of two or more pharmacophores either linked or fused together to create a new molecule is known as molecular hybridization and the resulting hybrid molecules may exhibit synergistic or additive biological effects. Hybrid structures, obtained from combining at least two biologically significant moieties, have emerged as a novel approach in finding new chemical entities.1–4 They are characterized by increased biological activity. Despite considerable efforts expended in this area, it is still a challenge to develop efficient methodologies and strategies for producing such compounds with the desired substituents and functional groups. One such pharmacophoric unit is chromone which is recognized as a useful motif for the design of biologically relevant entities and is also prevalent in many natural products.5–10Spiro chromanones too are known to be bio-active molecules with activities ranging from anti-inflammatory, anti-cancer, anti-bacterial and anti-fungal properties.
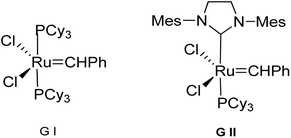
Spiro-heterocycles in general have a wide range of biological properties11–15 like spiro oxindoles have antitumor and antibiotic activity. Aza spiro cyclic compounds too are important medicinally and have been used to treat depression, anxiety and pain.12 Likewise, oxepines are structurally important motifs in many natural products and constitute the core structure of biologically active molecules. We envisioned that combination of both these important frameworks i.e. the spiro chromanone and oxepine will provide a novel and unprecedented scaffold. Oxepines are medium sized heterocycles and can be constructed by three distinct approaches viz intramolecular cyclization, annulation and ring expansion. Apart from this they can also be constructed by Ring Closing Metathesis (RCM).16–18 It is thought to be a powerful C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond forming reaction that occurs under very mild conditions. RCM of alkenes is indispensable to the synthesis of cyclic structures. It is a highly efficient reaction for the synthesis of carbocyclic, heterocyclic and fused ring frameworks and is used extensively in the synthesis of biologically active molecules. While most RCM reactions are performed using either Grubbs first-generation (G I) or Grubbs second-generation catalyst (G II) (Fig. 1), the latter has enjoyed wide application in organic synthesis due to its high functional group tolerance.19 However, in the case of metathetic cyclization, not every ring size is accessible with the same ease. The easiest reactions are those affording 5-to 7-membered rings with the entropic and enthalpic factors becoming less favourable with increasing size.
C bond forming reaction that occurs under very mild conditions. RCM of alkenes is indispensable to the synthesis of cyclic structures. It is a highly efficient reaction for the synthesis of carbocyclic, heterocyclic and fused ring frameworks and is used extensively in the synthesis of biologically active molecules. While most RCM reactions are performed using either Grubbs first-generation (G I) or Grubbs second-generation catalyst (G II) (Fig. 1), the latter has enjoyed wide application in organic synthesis due to its high functional group tolerance.19 However, in the case of metathetic cyclization, not every ring size is accessible with the same ease. The easiest reactions are those affording 5-to 7-membered rings with the entropic and enthalpic factors becoming less favourable with increasing size.
Results and discussion
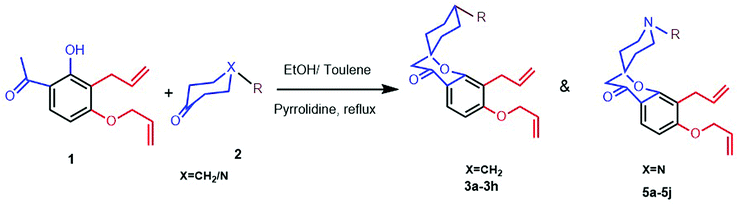
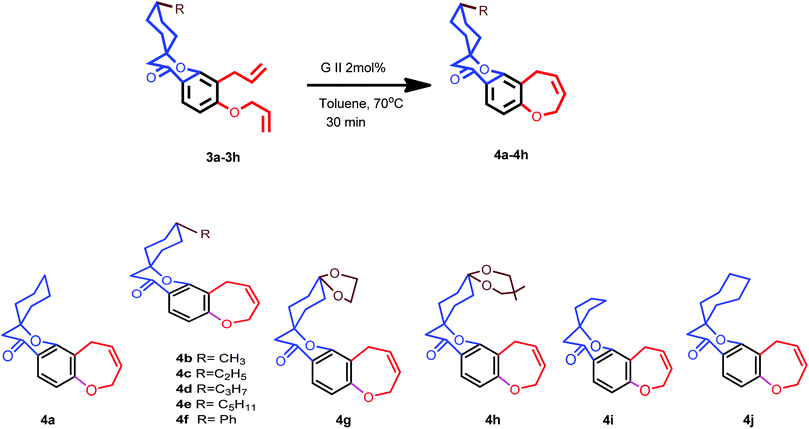
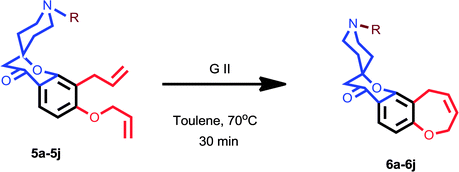
2,4-Dihydroxy acetophenone is allylated using allyl bromide and K2CO3 in acetone and the resulting 4-O-allylated product is made to undergo Claisen rearrangement in N,N-diethyl aniline to obtain a free OH group which is again allylated to yield product 1. This on Kabbe condensation with various cyclic ketones gave spiro cyclic substrates (3a–3h) and with different substituted piperidones gave aza spiro cyclic substrates (5a–5j) (Scheme1). 3i and 3j were also produced from their respective cyclic ketones. After screening several reaction conditions, it was concluded that this condensation was best affected by using pyrrolidine as secondary amine for in situ enamine generation and ethanol/toluene as solvent at 80 °C, which resulted in good yields.Exposure of dienes 3a–3h to G II under our optimized conditions (2 mol%, toluene, 70 °C) resulted in clean formation of the 4a–4h in good to excellent yields (65–91%) within 20–30 minutes (Scheme 2). 4i and 4j were also synthesised easily from their substrates 3i and 3j. Successful metathesis of above-mentioned spiro cyclic substrates to give good yields of benz-annulated oxepine spiro chromanones 4a–4j motivated us to explore the synthetic potential of G-II for our aza spiro cyclic substrates 5a–5j also. Thus, a series of products that could serve as precursors for the RCM was constructed in order to evaluate the scope of this reaction. In the first run, these substrates 5a–5j (0.18 mmol; Table 1) were stirred with G-II (2 mol%) in dry and degassed toluene at 70 °C maintaining the rest of the standard experimental protocol for metathesis reaction. Complete conversion to the products did not occur for 5a–5e (entries 1,5,8,11 and 13; Table 1) which is verified by GC-MS (Table 1). Metathesis at higher temperature of 110 °C keeping the catalyst load same, also did not lead to full conversion, (entry 9; Table 1). Substrates having either a protecting group or an electron withdrawing group have proceeded smoothly (entries 15–19; Table 1). In short, electron deficient aza spiro cycles are proven to be efficient substrates for RCM compared to those with excess electrons.20–22 The reason is attributed to the fact that the increased basicity on nitrogen is leading to the decomposition of catalyst, thus reducing availability at the site of action.23 When the reaction was monitored using a standard amount of 2 mol% of G II, carbene transfer must have occurred to a large extent at the basic nitrogen, where the ruthenium metal centre might have been stabilized by chelation. Such stabilization, in turn, could have reduced the concentration of the active catalyst in the reaction resulting in a negative impact on the rate of the metathesis.
| Entry | Compound | Ra | Cat loading (mol%) | Temp | Yieldb | Conversion | Isomerd ratio (6a–6j) |
|---|---|---|---|---|---|---|---|
| a Substrate conc of 0.18 mmol.b Isolated yield.c Reaction monitored by GC-MS.d Isomer ratio as deduced from 1H NMR. | |||||||
| 1 | 5a | CH3 | 2 | 70 | >53c | ||
| 2 | 5a | CH3 | 7 | 110 | >95c | ||
| 3 | 5a | CH3 | 11 | 70 | >95c | ||
| 4 | 5a | CH3 | 15 | 70 | 78 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
|
| 5 | 5b | C3H7 | 2 | 70 | |||
| 6 | 5b | C3H7 | 3 | 70 | >95c | ||
| 7 | 5b | C3H7 | 4 | 70 | 61 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
|
| 8 | 5c | CH(CH3)2 | 2 | 70 | >55c | ||
| 9 | 5c | CH(CH3)2 | 2 | 110 | >40c | ||
| 10 | 5c | CH(CH3)2 | 3.5 | 70 | 75 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
|
| 11 | 5d | Bn | 2 | 70 | >97c | ||
| 12 | 5d | Bn | 3 | 70 | 56 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 98 |
|
| 13 | 5e | C2H4Ph | 2 | 70 | >95c | ||
| 14 | 5e | C2H4Ph | 4 | 70 | 85 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
|
| 15 | 5f | COMe | 2 | 70 | 82 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
|
| 16 | 5g | Bz | 2 | 70 | 87 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98 98 |
|
| 17 | 5h | Cbz | 2 | 70 | 89 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
|
| 18 | 5i | CO2Et | 2 | 70 | 88 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
|
| 19 | 5j | Boc | 2 | 70 | 98 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
|
Conclusion
Sterically accessible Lewis bases are known to play an important role in the decomposition of metathesis catalysts. Many RCM reactions have been reported in case of nitrogen containing compounds where it is either protected or attached to an electron withdrawing group. But metathesis of substrates having a nitrogen functionality away from metathesis site does not seem to have been reported. We have tried to attempt RCM on substrates without protecting groups on nitrogen as in 5a–5e and studied the amount of catalyst load required for its completion. In short, we were able to achieve the synthesis of our target molecules though 5a–5e were troublesome and required multiple iterations.Experimental section
General
Column chromatography was performed using Finar 60–120 & 100–200 mesh silica gel and monitored using analytical thin-layer chromatography (TLC) carried out on 0.25 Merck silica gel plates (60 F-254). Visualization was by quenching of UV fluorescence (λmax = 254 nm) and by charring with freshly prepared anisaldehyde charring solution. Flash column chromatography was carried out using 60–120 mesh silica gel; samples were applied as saturated solutions in an appropriate solvent or pre-adsorbed onto the minimum quantity of silica. Except as otherwise indicated, reactions were carried out using oven-dried glassware under nitrogen with dry, freshly distilled solvents. Toluene was dried by refluxing with calcium chloride for 1 h, distilled under nitrogen atmosphere and dried using 4 Å molecular sieves. Ethanol, acetone, DMF and N,N-diethyl aniline were purchased as laboratory grade reagents. All the other solvents were used as obtained from commercial sources. Mass spectral analysis was done on gas chromatograph – time of flight (TOF) mass spectrometer. 1H NMR and 13C NMR spectra were recorded on a Bruker Biospin, Avance-III Fourier Transform Digital NMR spectrometer. Nuclear magnetic resonance (NMR) spectra (1H and 13C) are recorded using either 400 or 500 MHz instruments. Chemical shifts (δ) are referenced to the solvent signal and are quoted in ppm to the nearest 0.01 ppm. coupling constants (J) are reported in Hertz to the nearest 0.1 Hz. Multiplicities are recorded as broad peaks (brs/brd), singlets (s), doublets (d), triplets (t), doublet of doublets of triplets (ddt), doublet of quartets (dq), doublet of heptets (dhpt), pentet of triplets (pt), triplet of doublets (td), quartet of doublets (qd), doublet of pentet (dp) and multiplets (m). Isomeric δ values are indicated by *. All NMR samples were prepared in deuterated chloroform and all values are quoted in ppm relative to tetra methyl silane as internal reference.Preparation of 1
The monoallylated dihydroxy acetophenone of about 6 moles in 20 ml of N,N-diethyl aniline, was refluxed for about 3–4 h. Subsequently, the reaction mixture was cooled and diluted with ethyl acetate (100 ml), washed thrice with 1 N aqueous hydrochloric acid solution (3 × 50 ml). The organic phase was dried with Na2SO4 and evaporated in vacuo. The crude mixture products were purified via column chromatography using n-hexane/ethyl acetate as eluent (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) to obtain white powdery solid in about 60–65% yield. This rearranged product is allylated once again using K2CO3 & allyl bromide in acetone under reflux conditions. 1H NMR (CDCl3, 500 MHz) δ 12.77 (s, 1H), 7.61 (d, 3J = 9.0 Hz, 1H), 6.44 (d, 3J = 9.0 Hz, 1H), 6.02 (ddt, J = 15.8, 10.5, 5.0 Hz, 1H), 5.96 (ddt, J = 17.8, 10.5, 4.8 Hz, 1H), 5.29 (dq, 3J = 10.6, 2.0, 4J = 1.5 Hz, 1H), 5.03 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 4.96 (dq, 3J = 9.9, 2.0, 4J = 1.5 Hz, 1H), 4.62 (*4.59, dt, J = 5.0, 1.5 Hz, 2H), 3.45 (dt, J = 6.2, 1.5 Hz, 2H), 2.56 (*2.59, s, 3H). 13C (125 MHz, CDCl3) δ 221.1, 180.6, 180.3, 154.1, 150.9, 148.7, 135.8, 132.9, 121.5, 87.2, 45.0, 44.5.
90) to obtain white powdery solid in about 60–65% yield. This rearranged product is allylated once again using K2CO3 & allyl bromide in acetone under reflux conditions. 1H NMR (CDCl3, 500 MHz) δ 12.77 (s, 1H), 7.61 (d, 3J = 9.0 Hz, 1H), 6.44 (d, 3J = 9.0 Hz, 1H), 6.02 (ddt, J = 15.8, 10.5, 5.0 Hz, 1H), 5.96 (ddt, J = 17.8, 10.5, 4.8 Hz, 1H), 5.29 (dq, 3J = 10.6, 2.0, 4J = 1.5 Hz, 1H), 5.03 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 4.96 (dq, 3J = 9.9, 2.0, 4J = 1.5 Hz, 1H), 4.62 (*4.59, dt, J = 5.0, 1.5 Hz, 2H), 3.45 (dt, J = 6.2, 1.5 Hz, 2H), 2.56 (*2.59, s, 3H). 13C (125 MHz, CDCl3) δ 221.1, 180.6, 180.3, 154.1, 150.9, 148.7, 135.8, 132.9, 121.5, 87.2, 45.0, 44.5.
Preparation of 3a–3j & 5a–5j
To a stirring solution of 1 in ethanol/ACN/toluene (25 ml) was added catalytic amount of pyrrolidine (0.05 mmol), cyclic ketones 2 in about 10–15 mmol and the resultant brown solution was stirred at 80 °C for 18 h. After completion of the reaction, the reaction mixture was neutralised with 10% w/w HCl solution (5 ml) and extracted with ethyl acetate (3 × 20 ml). The combined organic layers were washed with brine solution and dried over anhydrous Na2SO4.3a 1H NMR (500 MHz, CDCl3) δ 7.74 (d, 3J = 8.7 Hz, 1H), 6.53 (d, 3J = 8.7 Hz, 1H), 6.03 (ddt, J = 17.7, 10.3, 4.8 Hz, 1H), 5.95 (ddt, J = 14.2, 12.8, 6.4 Hz, 1H), 5.42 (dq, 3J = 17.2, 4J = 1.6, 2.0 Hz, 1H), 5.28 (dq, 3J = 10.6, 4J = 1.2 Hz, 1H), 5.07 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 4.97 (dq, 3J = 9.9, 2.0, 4J = 1.3 Hz, 1H), 4.60 (dt, J = 4.8, 1.6 Hz, 2H), 3.45 (dt, J = 6.4, 1.3 Hz, 2H), 2.60 (s, 2H), 2.09–2.04 (m, 2H), 1.72–1.64 (m, 4H), 1.53–1.41 (m, 4H). 13C NMR (125 MHz, CDCl3) δ 210.2, 180.7, 176.5, 154.3, 151.0, 144.3, 135.7, 134.7, 133.4, 133.1, 123.3, 98.2, 87.3, 66.5, 53.2, 48.0, 45.8, 43.5, 39.9. MS (EI) LRMS: m/z 312, 269, 217 (100%), 175, 149, 97, 69, 55.
3b 1H NMR (400 MHz, CDCl3) δ 7.75 (d, 3J = 8.7 Hz, 1H), 6.53 (d, 3J = 8.7 Hz, 1H), 6.04 (ddt, J = 17.7, 10.3, 4.8 Hz, 1H), 4 (ddt, J = 14.2, 12.8, 6.4 Hz, 1H), 5.42 (dq, 3J = 17.2, 2.0, 4J = 1.6 Hz, 1H), 5.29 (dq, 3J = 10.6, 2.0, 4J = 1.2 Hz, 1H), 5.09 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 5.08–4.94 (m, 1H), 4.61 (dt, J = 4.8, 1.6 Hz, 2H), 3.46 (*3.42, dt, J = 6.4, 1.3 Hz, 2H), 2.60 (*2.76, s, 2H), 2.12–1.91 (m, 2H), 1.76–1.61 (m, 2H), 1.54–1.32 (m, 5H), 0.95–0.92 (m, 3H). 13C NMR (100 MHz, CDCl3) δ 191.9, 162.4, 158.1, 136.0, 132.7, 126.0, 117.4, 116.4, 115.0, 114.6, 115.1, 105.0, 104.9, 81.0, 79.2, 68.9, 48.7, 34.6, 31.6, 29.7, 27.5, 22.3. MS (EI) LRMS: m/z 326, 269, 217, 175, 97, 69, 57, 44 (100%).
3c 1H NMR (400 MHz, CDCl3) δ 7.68 (d, 3J = 8.7 Hz, 1H), 6.46 (d, 3J = 8.7 Hz, 1H), 5.95 (ddt, J = 17.7, 10.3, 4.8 Hz, 1H), 5.86 (ddt, J = 14.2, 12.8, 6.4 Hz, 1H), 5.35 (dq, 3J = 17.2, 2.0, 4J = 1.6 Hz, 1H), 5.21 (dq, 3J = 10.6, 2.0, 4J = 1.2 Hz, 1H), 5.00 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 4.94–4.87 (m, 1H), 4.52 (dt, J = 4.8, 1.6 Hz, 2H), 3.38 (*3.35, dt, J = 6.4, 1.3 Hz, 2H), 2.53 (*2.68, s, 2H), 2.08–2.02 (*1.91–1.85 (m), m, 2H), 1.75–1.59 (m, 1H), 1.56–1.47 (m, 2H), 1.36–1.18 (m, 6H), 0.82 (t, 3H). 13C NMR (100 MHz, CDCl3) δ 190.9, 161.4, 157.1, 135.0, 131.7, 125.0, 116.3, 115.3, 114.1, 113.9, 103.9, 78.5, 67.9, 47.7, 37.3, 33.5, 28.5, 26.5, 26.3, 10.4. MS (EI) LRMS: m/z 340, 299, 269, 217, 175, 149, 97, 69, 57, 41 (100%).
3d 1H NMR (400 MHz, CDCl3) δ 7.68 (d, 3J = 8.7 Hz, 1H), 6.46 (d, 3J = 8.7 Hz, 1H), 5.97 (ddt, J = 17.7, 10.3, 4.8 Hz, 1H), 5.86 (ddt, J = 14.2, 12.8, 6.4 Hz, 1H), 5.34 (dq, 3J = 17.2, 2, 4J = 1.6 Hz, 1H), 5.21 (dq, 3J = 10.6, 2.0, 4J = 1.2 Hz, 1H), 5.00 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 4.94–4.86 (m, 1H), 4.53 (dt, J = 4.8, 1.6 Hz, 2H), 3.38 (*3.35, dt, J = 6.4, 1.3 Hz, 2H), 2.52 (*2.68, s, 2H), 2.08–2.01 (*1.89–1.84 (m), m, 2H), 1.73–1.47 (m, 2H), 1.36–1.10 (m, 9H), 0.82 (td, 3H). 13C NMR (100 MHz, CDCl3) δ 192.0, 162.4, 158.1, 136.0, 132.7, 126.0, 117.4, 116.3, 114.9, 104.9, 79.5, 68.9, 48.7, 39.2, 36.3, 34.6, 28.2, 27.8, 27.5, 27.3, 19.9, 14.3. MS (EI) LRMS: m/z 354, 269, 217 (100%), 175, 149, 91, 41 (90%).
3e 1H NMR (400 MHz, CDCl3) δ 7.68 (d, 3J = 8.7 Hz, 1H), 6.46 (d, 3J = 8.7 Hz, 1H), 5.97 (ddt, J = 17.7, 10.3, 4.8 Hz, 1H), 5.87 (ddt, J = 14.2, 12.8, 6.4 Hz, 1H), 5.35 (dq, 3J = 17.2, 2.0, 4J = 1.6 Hz, 1H), 5.21 (dq, 3J = 10.6, 2.0, 4J = 1.2 Hz, 1H), 5.00 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 4.94–4.86 (m, 1H), 4.54 (dt, J = 4.8, 1.6 Hz, 2H), 3.39 (*3.35, dt, J = 6.4, 1.3 Hz, 2H), 2.53 (*2.68, s, 2H), 2.08–2.01 (*1.89–1.84, m, 2H), 1.73–1.56 (m, 2H), 1.37–1.10 (m, 13H), 0.81 (td, 3H). 13C NMR (100 MHz, CDCl3) δ 192.0, 162.4, 158.1, 136.0, 132.7, 126.0, 117.4, 114.9, 104.9, 79.6, 68.9, 48.7, 36.9, 36.6, 34.6, 32.1, 27.8, 27.5, 26.5, 22.6, 14.0. MS (EI) LRMS: m/z 382, 341, 269, 217 (100%), 175, 149, 43, 41 (100%).
3f 1H NMR (400 MHz, CDCl3) δ 7.78 (d, 3J = 8.7 Hz, 1H), 7.34–7.21 (m, 5H), 6.56 (d, 3J = 8.7 Hz, 1H), 6.09–5.96 (m, 2H), 5.43 (dq, 3J = 17.2, 2.0, 4J = 1.6 Hz, 1H), 5.30 (dq, 3J = 10.6, 2.0, 4J = 1.2 Hz, 1H), 5.09 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 5.01 (dq, 3J = 9.9, 2.0, 4J = 1.3 Hz, 1H), (m, 1H), 4.62 (dt, J = 5.0, 1.5 Hz, 2H), 3.55 (dt, J = 6.2, 1.5 Hz, 2H), 2.67 (*2.91, s, 2H), 2.57 (tt, 1H), 2.31–2.13 (m, 2H), 1.97 (qd, 2H), 1.78–1.72 (m, 2H), 1.52 (dd, 2H). 13C NMR (100 MHz, CDCl3) δ 191.7, 162.5, 158.0, 146.6, 136.0, 132.7, 128.4, 126.8, 126.1, 117.4, 116.3, 115.0, 105.1, 78.8, 69.0, 48.7, 43.3, 34.9, 28.8, 27.4. MS (EI) LRMS: m/z 388, 360 (98%), 241 (100%), 189, 91, 77.
3g 1H NMR (400 MHz, CDCl3) δ 7.73 (d, 3J = 8.5 Hz, 1H), 6.68 (d, 3J = 8.5, 1H), 5.91 (pt, 3J = 5.7 2.0 Hz, 1H), 5.58 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.64–4.61 (m, 2H), 4.01–3.93 (m, 4H), 3.59–3.57 (m, 2H), 2.67 (s, 2H), 2.14–2.09 (m, 2H), 1.91 (td, 2H), 1.78–1.71 (m, 2H), 1.64 (brd, 2H). 13C NMR (125 MHz, CDCl3) δ 209.7, 180.8, 176.2, 154.1, 150.9, 144.4, 135.8, 133.3, 126.3, 123.5, 97.1, 87.3, 82.7, 82.5, 65.9, 50.7, 48.5, 45.8. MS (EI) LRMS: m/z 330, 231, 177, 99 (100%), 86.
3h 1H NMR (400 MHz, CDCl3) δ 7.68 (d, 3J = 8.7 Hz, 1H), 6.47 (d, 3J = 8.7 Hz, 1H), 5.95 (ddt, J = 17.2, 10.3, 4.8 Hz, 1H), 5.85 (ddt, J = 14.2, 12.8, 6.4 Hz, 1H), 5.34 (dq, 3J = 17.2, 2.0, 4J = 1.6 Hz, 1H), 5.21 (dq, 3J = 10.3, 2.0, 4J = 1.2 Hz, 1H), 4.97 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 4.90 (dq, 3J = 9.9, 2.0, 4J = 1.3 Hz, 1H), 4.53 (dt, J = 4.8, 1.6 Hz, 2H), 3.46 (s, 2H), 3.38 (s, 4H), 2.56 (s, 2H), 1.96 (t, 4H), 1.78–1.71 (m, 2H), 1.56 (td, 2H), 0.89 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 190.4, 161.5, 156.9, 134.8, 131.6, 125.1, 116.4, 115.4, 113.9, 104.2, 95.8, 78.2, 69.1, 69.0, 67.9, 46.6, 30.0, 29.1, 26.4, 21.6. MS (EI) LRMS: m/z 412, 217, 141 (100%), 128, 69.
3i 1H NMR (400 MHz, CDCl3) δ 7.76 (d, 3J = 8.7 Hz, 1H), 6.54 (d, 3J = 8.7 Hz, 1H), 6.03 (ddt, J = 17.7, 10.3, 4.8 Hz, 1H), 5.90 (ddt, J = 14.2, 12.8, 6.4 Hz, 1H), 5.42 (dq, 3J = 17.2, 2.0, 4J = 1.6 Hz, 1H), 5.29 (dq, 3J = 10.6, 2.0, 4J = 1.2 Hz, 1H), 5.01 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 4.94 (dq, 3J = 9.9, 2.0, 4J = 1.3 Hz, 1H), 4.60 (dt, J = 4.8, 1.6 Hz, 2H), 3.39 (dt, J = 6.4, 1.3 Hz, 2H), 2.77 (s, 2H), 2.09–2.03 (m, 2H), 1.91–1.83 (m, 2H), 1.76–1.67 (m, 2H), 1.65–1.58 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 191.7, 162.1, 159.0, 136.0, 132.7, 126.1, 117.3, 116.5, 115.3, 114.5, 105.1, 90.0, 68.8, 46.6, 37.4, 27.2, 23.8. MS (EI) LRMS: m/z 298, 269, 217 (100%), 175, 149, 91, 69.
3j 1H NMR (400 MHz, CDCl3) δ 7.73 (d, 3J = 8.7 Hz, 1H), 6.52 (d, 3J = 8.7 Hz, 1H), 6.03 (ddt, J = 17.7, 10.3, 4.8 Hz, 1H), 5.93 (ddt, J = 14.2, 12.8, 6.4 Hz, 1H), 5.41 (dq, 3J = 17.2, 2.0, 4J = 1.6 Hz, 1H), 5.28 (dq, 3J = 10.6, 2.0, 4J = 1.2 Hz, 1H), 5.05 (dq, 3J = 17.0, 2.0, 4J = 1.6 Hz, 1H), 4.96 (dq, 3J = 9.9, 2.0, 4J = 1.3 Hz, 1H), 4.59 (dt, J = 4.8, 1.6 Hz, 2H), 3.44 (dt, J = 6.4, 1.3 Hz, 2H), 2.66 (s, 2H), 2.13–2.03 (m, 2H), 1.77–1.63 (m, 4H), 1.59–1.51 (m, 4H), 1.47–1.38 (m, 2H)·13C NMR (100 MHz, CDCl3) δ 191.9, 162.3, 158.5, 136.0, 132.7, 125.9, 117.3, 116.4, 115.1, 114.7, 104.9, 84.2, 68.9, 48.7, 38.4, 29.3, 27.4, 22.0. MS (EI) LRMS: m/z 326, 269, 217 (100%), 175, 149, 91, 55.
5a1H NMR (400 MHz, CDCl3) δ 7.69 (d, 3J = 8.7 Hz, 1H), 6.48 (d, 3J = 8.7 Hz, 1H), 5.99 (ddt, J = 14.3, 10.3, 5.0 Hz, 1H), 5.90 (ddt, J = 21.0, 9.3, 3.0 Hz, 1H), 5.37 (dq, 3J = 17.0, 2.0, 4J = 1.7 Hz, 1H), 5.20 (dq, 3J = 10.7, 2.0, 4J = 1.2 Hz, 1H), 5.02 (dq, 3J = 17.0, 2.0, 4J = 1.5 Hz, 1H), 4.93 (dq, 3J = 10.0, 2.0, 4J = 1.2 Hz, 1H), 4.52 (dt, J = 5.0, 1.5 Hz, 2H), 3.39 (dt, J = 6.2, 1.5 Hz, 2H), 2.57 (s, 2H), 2.50 (td, 2H), 2.36 (td, 2H), 2.24 (s, 3H), 1.99 (brd, 2H), 1.78–1.71 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 190.2, 161.5, 156.8, 134.8, 131.6, 125.1, 116.4, 115.3, 114.0, 104.2, 67.9, 49.8, 45.0, 33.4, 26.4. MS (EI) LRMS: m/z 327, 326, 175, 110, 109, 96, 70 (100%).
5b 1H NMR (400 MHz, CDCl3) δ 7.69 (d, 3J = 8.7 Hz, 1H), 6.48 (d, 3J = 8.7 Hz, 1H), 6.03 (ddt, J = 14.3, 10.3, 5.0 Hz, 1H), 5.94 (ddt, J = 21.0, 9.3, 3.0 Hz, 1H), 5.42 (dq, 3J = 17.0, 2.0, 4J = 1.7 Hz, 1H), 5.29 (dq, 3J = 10.7, 2.0, 4J = 1.2 Hz, 1H), 5.06 (dq, 3J = 17.0, 2.0, 4J = 1.5 Hz, 1H), 4.99 (dq, 3J = 10.0, 2.0, 4J = 1.2 Hz, 1H), 4.60 (dt, J = 5.0, 1.5 Hz, 2H), 3.47 (dt, J = 6.2, 1.5 Hz, 2H), 2.58 (s, 2H) 2.39 (td, 3H), 2.25 (*t, d, 2H), 1.96 (brd, 2H), 1.78–1.71 (m, 4H), 1.42 (sxt, 2H), 0.85 (t, 3H). 13C NMR (100 MHz, CDCl3) δ 190.3, 161.5, 156.8, 134.9, 131.6, 125.1, 116.4, 114.0, 104.2, 76.8, 67.9, 59.6, 47.8, 33.4, 26.4, 19.1, 10.9. MS (EI) LRMS: m/z 355, 326 (100%), 110, 98, 70.
5c 1H NMR (400 MHz, CDCl3) δ 7.76 (d, 3J = 8.7 Hz, 1H), 6.55 (d, 3J = 8.7 Hz, 1H), 6.03 (ddt, J = 12.8, 10.3, 5.0 Hz, 1H), 5.94 (ddt, J = 16.3, 10.0, 3.7 Hz, 1H), 5.42 (dq, 3J = 17.0, 2.0, 4J = 1.7 Hz, 1H), 5.29 (dq, 3J = 10.7, 2.0, 4J = 1.5 Hz, 1H), 5.06 (dq, 3J = 17.0, 2, 4J = 1.5 Hz, 1H), 4.99 (dq, 3J = 10.0, 2.0, 4J = 1.2 Hz, 1H), 4.60 (dt, J = 5.0, 1.5 Hz, 2H), 3.47 (dt, J = 6.2, 1.5 Hz, 2H), 2.77 (hpt, 1H), 2.65 (s, 2H) 2.64–2.56 (m, 4H), 2.06 (brd, 2H), 1.77–1.70 (m, 2H), 1.07 (d, 6H).·13C NMR (100 MHz, CDCl3) δ 191.3, 162.5, 157.8, 135.9, 132.6, 126.1, 120.4, 118.3, 117.5, 116.3, 114.9, 105.2, 3.9, 78.0, 69.0, 54.6, 47.7, 44.0, 34.6, 33.3, 27.9, 27.4, 24.8, 21.5, 18.3. MS (EI) LRMS: m/z 355, 340 (100%), 284, 124, 56, 41.
5d 1H NMR (400 MHz, CDCl3) δ 7.75 (d, 3J = 8.7 Hz, 1H), 7.31 (s, 5H), 6.54 (d, 3J = 8.7 Hz, 1H), 6.04 (ddt, J = 17.1, 10.5, 5.0 Hz, 1H), 5.94 (ddt, J = 14.7, 12.7, 6.2 Hz, 1H), 5.40 (dq, 3J = 17.3, 2.0, 4J = 1.5 Hz, 1H), 5.28 (dq, 3J = 10.0, 2.0, 4J = 1.2 Hz, 1H), 5.08 (dq, 3J = 17.3, 2.0, 4J = 1.5 Hz, 1H), 5.01 (dq, 3J = 10.0, 2.0, 4J = 1.2 Hz, 1H), 4.60 (dt, J = 4.7, 1.7 Hz, 2H), 3.55 (s, 2H), 3.44 (d, 2H), 2.64 (s, 2H) 2.63–2.60 (m, 2H), 2.46 (t, 2H), 2.04 (d, 2H), 1.76–1.70 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 191.3, 162.5, 157.9, 132.6, 129.2, 127.1, 128.2, 127.1, 126.1, 138.0, 117.5, 116.4, 115.0, 105.2, 77.9, 69.0, 63.1, 48.7, 47.7, 34.5, 27.4. MS (EI) LRMS: m/z 403, 402, 185, 146, 91 (100%), 69.
5e 1H NMR (400 MHz, CDCl3) δ 7.78 (d, 3J = 8.7 Hz, 1H), 7.40 (s, 5H), 6.56 (d, 3J = 8.7 Hz, 1H), 6.03 (ddt, J = 14.1, 10.5, 5.0 Hz, 1H), 5.97 (ddt, J = 16.3, 9.2, 3.0 Hz, 1H), 5.42 (dq, 3J = 17.0, 2.0, 4J = 1.7 Hz, 1H), 5.31 (dq, 3J = 10.7, 2.0, 4J = 1.2 Hz, 1H), 5.06 (dq, 3J = 17.0, 2.0, 4J = 1.5 Hz, 1H), 5.00 (dq, 3J = 10.0, 2.0, 4J = 1.2 Hz, 1H), 4.62 (dt, J = 5.0, 1.5 Hz, 2H), 3.49 (dt, J = 6.2, 1.5 Hz, 2H), 2.84–2.75 (m, 4H), 2.67 (s, 2H) 2.54 (t, 2H), 2.07 (d, 2H), 1.81–1.63 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 190.2, 161.5, 156.8, 139.2, 134.8, 131.6, 127.4, 127.6, 125.0, 116.4, 114.0, 115.3, 104.2, 76.7, 67.9, 59.4, 47.8, 46.7, 33.4, 32.7, 26.4. MS (EI) LRMS: m/z 417, 327, 326 (100%), 110.
5f 1H NMR (400 MHz, CDCl3) δ 7.71 (d, 3J = 8.7 Hz, 1H), 6.52 (d, 3J = 8.7 Hz, 1H), 5.99 (ddt, J = 17.1, 10.5, 5.0 Hz, 1H), 5.85 (ddt, J = 14.7, 12.7, 6.2 Hz, 1H), 5.37 (dd, J = 17.0, Hz, 1H), 5.23 (dd, J = 10.5, Hz, 1H), 4.95–4.90 (m, 2H), 4.54 (d, 2H), 4.33 (brd, 1H) 3.60–3.52 (m, 1H), 3.44 (d, 1H), 3.39 (d, 2H), 2.99 (t, 1H), 2.59 (s, 2H), 2.03 (s, 3H), 1.57–1.47 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 190.2, 169.1, 161.6, 158.0, 135.4, 126.5, 114.4, 114.4, 113.4, 110.0, 77.8, 47.5, 42.0, 37.1, 34.7, 34.0, 29.7, 27.2, 21.3. MS (EI) LRMS: m/z 355, 217, 175, 81, 69 (100%).
5g 1H NMR (400 MHz, CDCl3) δ 7.78 (d, 3J = 8.7 Hz, 1H), 7.40 (s, 5H), 6.58 (d, 3J = 8.7 Hz, 1H), 6.06 (ddt, J = 17.1, 10.5, 5.0 Hz, 1H), 5.95 (ddt, J = 15.7, 10.0, 3.7 Hz, 1H), 5.44 (dq, 3J = 17.3, 2.0, 4J = 1.5 Hz, 1H), 5.31 (dq, 3J = 10.5, 2.0, 4J = 1.5 Hz, 1H), 5.05–4.99 (m, 2H), 4.61 (dt, J = 5.0, 1.5 Hz, 2H), 4.52 (brs, 1H), 3.60 (brs, 1H), 3.48 (dt, J = 6.2, 1.5 Hz, 3H), 3.28 (brs, 1H), 2.68 (s, 2H), 2.18 (brd, 2H), 1.80–1.67 (m, 1H), 1.61–1.39 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 190.3, 170.4, 162.7, 157.4, 135.9, 132.5, 129.7, 126.8, 126.8, 126.3, 115.0, 16.2, 114.8, 105.6, 77.8, 69.1, 47.7, 27.4. MS (EI) LRMS: m/z 417, 376, 217, 175, 105 (100%), 77.
5h 1H NMR (400 MHz, CDCl3) δ 7.77 (d, 3J = 8.7 Hz, 1H), 7.35 (s, 5H), 6.58 (d, 3J = 8.7 Hz, 1H), 6.02 (ddt, J = 17.1, 10.5, 5.0 Hz, 1H), 5.91 (ddt, J = 14.7, 12.7, 6.2 Hz, 1H), 5.44 (dq, 3J = 17.3, 2, 4J = 1.5 Hz, 1H), 5.39 (dq, 3J = 10.5, 2.0, 4J = 1.2 Hz, 1H), 5.13 (s, 2H), 4.99–4.96 (m, 2H), 4.61 (dt, J = 5.0, 1.5 Hz, 2H), 4.00 (brs, 2H), 3.46 (d, 2H), 3.26 (brs, 2H), 2.65 (s, 2H), 2.03 (brs, 2H), 1.61 (brs, 2H). 13C NMR (100 MHz, CDCl3) δ 190.6, 162.6, 157.5, 155.2, 136.6, 135.8, 132.5, 128.5, 127.9, 126.3, 117.6, 116.3, 115.0, 105.5, 69.0, 67.2, 47.8, 39.5, 29.7, 27.4. MS (EI) LRMS: m/z 447, 357, 356, 312, 217, 215, 175, 92, 91 (100%), 44, 41.
5i 1H NMR (400 MHz, CDCl3): δ 7.78 (d, 3J = 8.7 Hz, 1H), 6.58 (d, 3J = 8.7 Hz, 1H), 6.07 (ddt, J = 17.1, 10.5, 5.0 Hz, 1H), 5.93 (ddt, J = 14.7, 12.7, 6.2 Hz, 1H), 5.44 (dq, 3J = 17.3, 2.0, 4J = 1.5 Hz, 1H), 5.31 (dq, 3J = 10.5, 2.0, 4J = 1.5 Hz, 1H), 5.04–4.97 (m, 2H), 4.61 (dt, J = 5.0, 1.5 Hz, 2H), 4.13 (q, J = 7.0, 2H) 3.93 (brs, 2H), 3.47 (dt, J = 6.0, 1.2 Hz, 2H), 3.25 (brt, 2H) 2.65 (s, 2H), 2.02 (d, 2H), 1.64–1.56 (m, 2H), 1.24 (t, J = 7.0, 3H). 13C NMR (100 MHz, CDCl3) δ 190.6, 162.6, 157.5, 155.4, 135.8, 132.5, 126.3, 117.6, 116.3, 115.0, 105.5, 77.8, 69.0, 61.4, 47.8, 39.3, 34.1, 29.7, 27.4, 14.6. MS (EI) LRMS: m/z 385, 344, 217, 175 (100%), 69.
5j 1H NMR (400 MHz, CDCl3) δ 7.77 (d, 3J = 8.7 Hz, 1H), 6.57 (d, 3J = 8.7 Hz, 1H), 6.04 (ddt, J = 17.1, 10.5, 5.0 Hz, 1H), 5.92 (ddt, J = 14.7, 12.7, 6.2 Hz, 1H), 5.42 (dq, 3J = 17.3, 2.0, 4J = 1.7 Hz, 1H), 5.30 (dq, 3J = 10.5, 2.0, 4J = 1.5 Hz, 1H), 5.03–4.96 (m, 2H), 4.61 (dt, J = 5.0, 1.5 Hz, 2H), 3.89 (brs, 2H), 3.47 (dt, J = 6.2, 1.5 Hz, 2H), 3.20 (brs, 2H) 2.65 (s, 2H), 2.04 (d, 2H), 1.60 (s, 2H), 1.46 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 190.8, 162.6, 157.6, 154.7, 135.8, 132.5, 126.2, 117.6, 116.3, 115.0, 105.5, 79.7, 77.9, 69.0, 47.8, 28.4, 27.4. MS (EI) LRMS: m/z 413![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 357, 312, 217, 175, 96, 57 (100%).
357, 312, 217, 175, 96, 57 (100%).
Ring closing metathesis (4a–4j) & (6a–6j)
To an oven dried Schlenk tube containing the substrate (0.18 mmol) was added dry toluene (10 ml) under inert atmosphere and purged with nitrogen gas for about 30 min. This reaction mixture was then heated to 70 °C, on reaching the temperature, the catalyst (G II) in solvent (2 ml) was then added using an oven dried syringe. Reaction was monitored by charring the TLC plate. After completion of the reaction, the reaction mass was then concentrated in vacuo to give brown oil. Purification was done using column chromatography.4a 1H NMR (400 MHz, CDCl3) δ 7.72 (*7.67, d, 3J = 8.5 Hz, 1H), 6.66 (*6.58, d, 3J = 8.5, 1H), 5.92{*6.08 (dt, J = 12.3, 4.4 Hz), pt, 3J = 5.5, 2.0 Hz, 1H}, 5.58 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.65–4.61{*4.30 (t, J = 4.8 Hz), m, 2H}, 3.61–3.59 (m, 2H), 2.64 (s, 2H), 2.02 (brd, 2H), 1.71–1.59 (m, 4H), 1.57–1.51 (m, 2H), 1.47–1.41 (m, 2H). 13C NMR (125 MHz, CDCl3) δ 210.4, 183.7, 175.3, 145.8, 144.9, 144.2, 142.1, 135.6, 133.0, 98.5, 88.7, 66.5, 53.0, 48.0, 43.5, 40.7, 39.9. MS (EI) LRMS: m/z 284, 241, 189 (100%); HRMS (EI): m/z calcd for C19H24O2 is 284.1776; found: 284.1775.
4b 1H NMR (400 MHz, CDCl3) δ 7.72 (d, 3J = 8.5 Hz, 1H), 6.67 (d, 3J = 8.5, 1H), 5.90 (pt, 3J = 5.7 2.0 Hz, 1H), 5.58 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.64–4.61 (m, 2H), 3.60–3.55 (m, 2H), 2.62 (*2.77, s, 2H), 2.05 (m, 2H), 1.74–1.66 (m, 2H), 1.59–1.35 (m, 4H), 1.28–1.12 (m, 1H), 0.97 (m, 3H). 13C NMR (100 MHz, CDCl3) δ 192.1, 165.3, 156.8, 136.0, 127.5, 126.5, 125.9, 123.8, 120.6, 118.3, 117.4, 114.6, 79.5, 70.4, 48.6, 34.7, 31.5, 29.9, 25.1, 21.4, 22.4, 22.3. MS (EI) LRMS: m/z 298, 255, 205, 203 (100%), 189, 131, 69, 41; HRMS (EI): m/z calcd for C19H22O3 is 298.1568; found: 298.1550.
4c 1H NMR (400 MHz, CDCl3) δ 7.72 (d, 3J = 8.5 Hz, 1H), 6.66 (d, 3J = 8.5, 1H), 5.90{*6.05 (dd, J = 12.2, 4.5 Hz), pt, 3J = 5.7 2.0 Hz, 1H}, 5.58 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.63–4.62 (*4.85–4.82, m, 2H), 3.59–3.57 (m, 2H), 2.62 (*2.76, s, 2H), 2.16–2.10 (m, 2H), 1.62–1.56 (m, 2H), 1.40–1.19 (m, 7H), 0.90 (t, 3H). 13C NMR (100 MHz, CDCl3) δ 192.2, 165.3, 156.8, 127.5, 126.5, 125.9, 123.8, 117.4, 114.7, 79.9, 70.4, 48.6, 38.1, 34.4, 29.6, 27.4, 22.3, 11.3. MS (EI) LRMS: m/z 312, 242, 241, 190, 189 (100%), 160, 77, 41; HRMS (EI): m/z calcd for C20H24 O3 is 312.1725; found: 312.1695.
4d 1H NMR (400 MHz, CDCl3) δ 7.72 (d, 3J = 8.5 Hz, 1H), 6.66 (d, 3J = 8.5, 1H), 5.91 (pt, 3J = 5.7 2.0 Hz, 1H), 5.58 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.64–4.61 (m, 2H), 3.60–3.55 (m, 2H), 2.62 (*2.76, s, 2H), 2.10 (brd, 2H), 1.62–1.57 (m, 3H), 1.37–1.22 (m, 8H), 0.90 (m, 3H). 13C NMR (100 MHz, CDCl3) δ 192.1, 165.3, 156.8, 127.5, 126.6, 125.9, 123.8, 117.4, 114.7, 79.9, 70.4, 48.6, 39.4, 36.1, 34.4, 27.9, 22.3, 20.3, 19.9, 14.3. MS (EI) LRMS: m/z 326, 255, 241 (99%), 190, 189 (100%), 149, 69, 57, 55, 43, 41; HRMS (EI): m/z calcd for C21H26O3 is 326.1881; found: 326.1863.
4e 1H NMR (400 MHz, CDCl3) δ 7.72 (d, 3J = 8.5 Hz, 1H), 6.58 (d, 3J = 8.5, 1H), 5.94 (pt, 3J = 5.7 2.0 Hz, 1H), 5.58 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.64–4.61 (m, 2H), 3.60–3.55 (m, 2H), 2.61 (*2.76, s, 2H), 2.10 (brd, 2H), 1.62–1.58 (m, 2H), 1.36–1.22 (m, 13H), 0.89 (m, 3H). 13C NMR (100 MHz, CDCl3) δ 192.1, 165.3, 156.8, 127.5, 126.6, 125.9, 123.8, 117.4, 114.7, 79.9, 70.4, 48.6, 37.0, 36.4, 34.4, 32.1, 27.9, 26.5, 22.3, 14.0. MS (EI) LRMS: m/z 354, 283, 241 (100%), 231, 190, 189 (100%), 69, 55, 41; HRMS (EI): m/z calcd for C23H30O3 is 354.2194; found: 354.2200.
4f 1H NMR (400 MHz, CDCl3) δ 7.75 (d, 3J = 8.5 Hz, 1H), 7.35–7.29 (m, 2H), 7.24–7.20 (m, 3H), 6.69 (d, 3J = 8.5, 1H), 5.96 (*6.13 (dd, J = 12.2, 4.5 Hz), pt, 3J = 5.7, 2.0 Hz, 1H), 5.58 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.65–4.62 (m, 2H), 3.70–3.68 (*3.59–3.56 (m), 2H), 2.69 (*2.91, s, 2H), 2.59 (tt, J = 12.5, 11.2, 4.0 Hz, 1H), 2.24 (brd, 2H), 1.98–1.84 (m, 2H), 1.82–1.71 (m, 2H), 1.54 (td, 2H). 13C NMR (100 MHz, CDCl3) δ 192.0, 165.4, 156.7, 146.5, 128.5, 127.7, 126.7, 126.0, 114.9, 79.2, 70.4, 48.5, 43.1, 34.7, 29.8, 28.8, 22.4. MS (EI) LRMS: m/z 360, 286, 241, 229, 189, 177, 149, 91, 86, 84, 69, 57, 49, 41; HRMS (EI): m/z calcd for C24H24O3 is 360.1725; found: 360.1700.
4g 1H NMR (400 MHz, CDCl3) δ 7.73 (d, 3J = 8.5 Hz, 1H), 6.68 (d, 3J = 8.5, 1H), 5.91 (pt, 3J = 5.7 2.0 Hz, 1H), 5.58 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.64–4.61 (m, 2H), 4.01–3.93 (m, 4H), 3.59–3.57 (m, 2H), 2.67 (s, 2H), 2.14–2.09 (m, 2H), 1.91 (td, 2H), 1.78–1.71 (m, 2H), 1.64 (brd, 2H). 13C NMR (100 MHz, CDCl3) δ 191.5, 156.6, 127.4, 126.5, 126.0, 123.8, 117.2, 115.0, 107.9, 79.1, 70.4, 64.4, 64.3, 47.5, 32.2, 30.2, 22.3. MS (EI) LRMS: m/z 342, 269, 217, 189 (100%), 175, 99, 86, 84; HRMS (EI): m/z calcd for C20H22O5 is 342.1467; found: 342.1462.
4h 1H NMR (400 MHz, CDCl3) δ 7.73 (d, 3J = 8.5 Hz, 1H), 6.68 (d, 3J = 8.5, 1H), 5.91 (pt, 3J = 5.7 2.0 Hz, 1H), 5.56 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.64–4.61 (m, 2H), 3.58–3.57 (m, 2H), 3.54 (s, 2H), 3.46 (s, 2H), 2.65 (s, 2H), 2.11–1.99 (m, 4H), 1.77–1.70 (m, 2H), 1.66–1.61 (m, 2H), 0.97 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 191.5, 165.4, 156.7, 127.4, 126.6, 125.9, 123.8, 117.2, 114.9, 96.7, 79.5, 70.1, 47.6, 30.9, 30.2, 27.6, 22.4. MS (EI) LRMS: m/z 384, 298, 189, 141 (100%), 128, 84, 69; HRMS (EI): m/z calcd for C23H28O5 is 384.1936; found: 384.1933.
4i 1H NMR (400 MHz, CDCl3) δ 7.65 (*7.68, d, 3J = 8.5 Hz, 1H), 6.58 (*d, 3J = 8.5, 1H), 5.81{*5.95 (dt, J = 12.2, 3.7 Hz), pt, 3J = 5.5, 2.0 Hz, 1H}, 5.50{*4.77–4.74 (m), dhpt, J = 11.2, 1.7 Hz, 1H}, 4.56–4.52{*4.21 (t, J = 4.7 Hz), m, 2H}, 3.46–3.43{*4.12 (dd, J = 12.5, 1.7 Hz), m, 2H}, 2.71 (s, 2H), 2.03–1.97 (m, 2H), 1.83–1.73 (m, 2H), 1.71–1.63 (m, 2H), 1.60–1.51 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 192.3 (*191.5), 165.7 (*165.2), 159.8, 157.9, 130.7, 127.7 (*127.4), 126.7 (*126.1), 125.6, 124.0, 114.8, 113.6, 91.2 (*90.3), 82.5, 70.4 (*70.5), 46.4 (*46.2), 37.8, 37.4, 37.1, 23.8, 22.3. MS (EI) LRMS: m/z 270, 241, 189 (100%); HRMS (EI): m/z calcd for C17H18O3 is 270.1255; found: 270.1250.
4j 1H NMR (400 MHz, CDCl3) δ 7.71 (d, 3J = 8.5 Hz, 1H), 6.65 (d, 3J = 8.5, 1H), 5.91 (pt, 3J = 5.5, 2.0 Hz, 1H), 5.58 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.64–4.61 (m, 2H), 3.60–3.58 (m, 2H), 2.68 (s, 2H), 2.43–2.40 (m, 1H), 2.12–2.07 (m, 2H), 1.91–1.85 (m, 1H), 1.76–1.63 (m, 3H), 1.61–1.52 (m, 3H), 1.49–1.40 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 192.1, 165.3, 157.2, 127.4, 126.6, 125.8, 123.6, 117.3, 114.5, 84.3, 70.4, 48.7, 38.2, 28.9, 22.5, 22.0. MS (EI) LRMS: m/z 298, 255, 241, 189 (100%), 134, 98, 84, 55. HRMS (EI): m/z calcd for C19H22O3 is 298.1568; found: 298.1558.
6a 1H NMR (400 MHz, CDCl3) δ 7.73 (*7.69, d, 3J = 8.5 Hz, 1H), 6.70 (*6.62, d, 3J = 8.5 Hz, 1H), 5.92{pt, 3J = 5.5, 2.2 Hz,*6.10 (dt, 3J = 12.0, 4.5 HZ), *6.39 (dt, J = 7.2, 1.7 Hz, 1H}, 5.59{dhpt, 3J = 11.2, 1.7 Hz, *4.90 (dt, 3J = 7.5, 4.5 Hz), *5.05–5.02 (m), 1H}, 4.65–4.62{m, *4.30 (t, 3J = 4.7 Hz), 2H}, 3.61–3.59 {m,*3.29–3.27 (m),*3.17–3.14 (m), 2H}, 2.68 (s, 4H), 2.38 (s, 4H), 2.10–1.98 (m, 3H), 1.75–1.79 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 191.3, 165.4, 127.6, 126.4, 126.0, 123.8, 117.37, 115.0, 70.4, 51.0, 46.1, 34.2, 29.6, 26.3, 22.4. MS (EI) LRMS: m/z 299, 110, 96, 81, 70, 69 (100%), 57, 55. HRMS (EI): m/z calcd for C18H21NO3 is 299.1521; found: 299.1519.
6b 1H NMR (400 MHz, CDCl3) δ 7.73 (*7.68, d, 3J = 8.5 Hz, 1H), 6.68 (*6.60, d, 3J = 8.5 Hz, 1H), 5.92{pt, 3J = 5.5, 2.0 Hz,*6.09 (dt, 3J = 12.0, 4.5 Hz), 1H}, 5.58{dhpt, 3J = 11.2, 1.7 Hz,*5.45–5.39 (m), 1H}, 4.66–4.61{m, *4.30 (t, 3J = 4.7 Hz), 2H}, 3.61–3.59 (m, 2H), 2.72–2.69 (m, 2H), 2.67 (s, 2H), 2.39–2.28 (m, 4H), 2.04 (d, 2H), 1.79–1.71 (m, 2H), 1.54 (sxt, J = 7.5, 2H), 0.91 (t, 3H).·13C NMR (100 MHz, CDCl3) 191.4, 165.4, 156.5, 127.5, 126.5, 126.0, 123.8, 117.4, 115.0, 78.2, 70.4, 60.7, 48.9, 34.2, 22.5, 20.1, 11.9. MS (EI) LRMS: m/z 327, 299, 198 (100%), 187, 110, 70. HRMS (EI): m/z calcd for C20H25NO3 is 327.1834; found: 327.1814.
6c 1H NMR (400 MHz, CDCl3) δ 7.73 (*7.68, d, 3J = 8.5 Hz, 1H), 6.68 (*6.62, d, 3J = 8.5 Hz, 1H), 5.91{pt, J = 5.7, 2.0 Hz, *6.09 (dt, 3J = 12.2, 4.5 HZ), 1H}, 5.58 (dhpt, 3J = 11.2, 1.7 Hz, 1H), 4.64–4.62{m, *4.30 (t, 3J = 5.0 Hz), 2H}, 3.63–3.59 (m, 2H), 2.76 (hpt, 1H), 2.70–2.68 (m, 1H), 2.66 (s, 2H), 2.66–2.63 (m, 1H), 2.55 (td, 2H), 2.05 (brd, 2H), 1.80–1.74 (m, 2H), 1.07 (d, 6H).·13C NMR (100 MHz, CDCl3) δ 191.5, 165.4, 156.5, 127.6, 126.4, 126.0, 123.8, 117.4, 115.0, 78.4, 70.4, 54.5, 47.7, 44.0, 34.5, 22.4, 18.2. MS (EI) LRMS: m/z 327, 313, 236, 98, 83, 71, 69, 57, 44 (100%). HRMS (EI): m/z calcd for C20H25NO3 is 327.1834; found: 327.1810.
6d 1H NMR (400 MHz, CDCl3) δ 7.72 (d, 3J = 8.7 Hz, 1H), 7.32 (s, 5H), 6.67 (d, 3J = 8.7 Hz, 1H), 5.87 (pt, J = 5.5, 2.0 Hz, 1H), 5.60 (dhpt, J = 11.2, 1.7 Hz, 1H), 4.64–4.61 (m, 2H), 3.56 (s, 4H), 2.65 (s, 4H), 2.38 (td, J = 11.5 Hz, 2H), 2.03 (brd, 2H), 1.80–1.68 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 191.5, 165.4, 156.6, 137.8, 129.2, 128.2, 127.5, 127.1, 126.5, 125.9, 123.8, 117.3, 115.0, 78.2, 70.4, 63.1, 48.7, 34.3, 22.5. MS (EI) LRMS: m/z 375, 189, 97, 91 (100%), 71, 69, 57. HRMS (EI): m/z calcd for C24H25NO3 is 375.1834; found: 375.1813.
6e 1H NMR (400 MHz, CDCl3) δ 7.73 (*7.68, d, 3J = 8.5 Hz, 1H), 7.31–7.26 (m, 3H), 7.24–7.19 (m, 2H), 6.67 (*6.61, d, 3J = 8.7 Hz, 1H), 5.93{pt, 3J = 5.5, 2.0 Hz,*6.38 (dt, 3J = 7.2, 1.5 HZ), 1H}, 5.57{dhpt, J = 11.2, 1.5 Hz, *5.43 (brs), 1H}, 4.64–4.62{m,*4.30 (t, 3J = 4.7 Hz), 2H}, 3.61–3.60{m, 2H, *3.66 (t, J = 7.0 Hz, 2H)}, 2.85–2.77 (m, 4H), 2.68 (s, 2H) 2.66–2.63 (m, 2H), 2.52–2.40 (m, 2H), 2.10 (brd, 2H), 1.81–1.73 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 191.4, 165.4, 156.5, 140.2, 131.0, 128.6, 128.4, 127.6, 126.4, 126.1, 126.0, 123.8, 119.7, 117.3, 115.0, 78.1, 70.4, 60.5, 49.0, 34.3, 33.7, 22.5. MS (EI) LRMS: m/z 389, 360, 359 (100%), 298, 110, 105, 91, 83, 71. HRMS (EI): m/z calcd for C25H27NO3 is 389.1990; found: 389.1989.
6f 1H NMR (400 MHz, CDCl3) δ 7.68 (*7.60, d, 3J = 8.5 Hz, 1H), 6.65 (*6.57, d, 3J = 8.7 Hz, 1H), 5.82{pt, 3J = 5.7, 2.0 Hz,*6.04, (dt, J = 12.0, 4.2 Hz), 1H}, 5.51{dhpt, J = 11.2, 1.5 Hz, *5.38–5.33 (m), 1H}, 4.58–4.55 (m, 2H), 4.37 (dp, J = 13.5, 2.0 Hz, 1H), 3.61 (dp, J = 13.5, 2.0 Hz, 1H), 3.52–3.51 (m, 2H), 3.36 (td, 1H), 2.92 (td, 1H), 2.61 (s, 2H), 2.04 (s, 5H), 1.54 (qd, 2H). 13C NMR (100 MHz, CDCl3) δ 190.6, 169.0, 165.6, 156.0, 131.6, 127.7, 126.1, 123.8, 117.1, 115.5, 114.2, 78.0, 70.4, 47.6, 42.0, 37.0, 34.4, 33.8, 22.4, 21.4. MS (EI) LRMS: m/z, 312, 214, 189 (100%), 160, 96, 91, 82, 55. HRMS (EI): m/z calcd for C20H25NO3 is 327.1834: found: 327.1831.
6g 1H NMR (400 MHz, CDCl3) δ 7.69 (*7.73, d, 3J = 8.7 Hz, 1H), 7.34 (s, 5H), 6.63{dt, J = 12.2, 1.7 Hz}, 6.64 (*6.71, d, 3J = 8.7 Hz, 1H), 6.19{dt, J = 12.2, 4.5 Hz, *5.92 (pt, J = 5.5, 2.0 Hz), 1H}, 5.59 (t, J = 4.7 Hz, 2H), 4.57 (s, 2H), 4.48 (brs, 1H), 3.53 (s, 2H), 3.22 (brd, 2H), 2.65 (s, 2H) 1.86–1.73 (m, 2H), 2.01–1.85 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 190.2, 170.5, 166.0, 157.7, 142.7, 135.6, 131.7, 129.8, 128.5, 126.9, 126.1, 119.2, 116.0, 115.7, 114.2, 78.6, 70.4, 47.4, 34.1. MS (EI) LRMS: m/z 389, 229, 177, 149, 105 (100%). HRMS (EI): m/z calcd for C24H23NO4; found: 389.1600.
6h 1H NMR (400 MHz, CDCl3) δ = 7.74 (d, 3J = 8.5 Hz, 1H), 7.37–7.35 (m, 5H), 6.71 (d, 3J = 8.7 Hz, 1H), 5.91 (pt, 3J = 5.5, 2.0 Hz, 1H), 5.58 (dhpt, J = 11.2, 1.7 Hz, 1H), 5.13 (s, 2H), 4.64–4.62 (m, 2H), 4.02 (brs, 2H), 3.59 (m, 2H), 3.19 (brs, 2H), 2.67 (s, 2H), 2.06 (brs, 2H), 1.64–1.56 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 190.7, 165.6, 156.1, 155.2, 136.6, 128.5, 128.1, 127.9, 127.6, 126.2, 126.1, 123.7, 117.2, 115.4, 78.0, 70.4, 67.2, 47.7, 39.5, 33.9, 22.4. MS (EI) LRMS: m/z 419, 328 (100%), 284, 187, 91. HRMS (EI): m/z calcd for C25H25NO5 is 419.1732; found: 419.1750.
6i 1H NMR (400 MHz, CDCl3) δ 7.74 (*7.69, d, 3J = 8.5 Hz, 1H), 6.71 (*6.53, d, 3J = 8.7 Hz, 1H), 5.92{pt, 3J = 5.5, 2.0 Hz,*6.08 (dt, J = 12.2, 4.5 Hz), *6.37 (dt, J = 7.2, 2.0 Hz), 1H }, 5.60{dhpt, J = 11.2, 1.7 Hz, 1H}, 4.63{(dt, J = 7.5, 4.2 Hz), *4.96–4.83 (m), 2H}, 4.14 (q, J = 7.2 Hz, 2H), 3.97 (brs, 2H), 3.58–3.50{m, *3.68–3.63 (m), 2H}, 3.27–3.10 (m, 2H), 2.68 (s, 2H), 2.07 (brd, 2H), 1.62–1.57 (m, 2H), 1.25 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 189.8, 164.5, 155.2, 154.4, 126.6, 125.2, 125.1, 116.2, 114.3, 122.7, 77.1, 69.4, 60.5, 46.7, 38.3, 32.9, 21.4, 13.6. MS (EI) LRMS: m/z 357, 342, 189 (100%), 160. HRMS (EI): m/z calcd for C20 H23NO5 is 357.1576; found 357.1570.
6j 1H NMR (400 MHz, CDCl3) δ 7.69 (*7.61, d, 3J = 8.5 Hz, 1H), 6.64 (*6.54, d, 3J = 8.7 Hz, 1H), 5.83{pt, 3J = 5.7, 2.0 Hz, *6.04, (dt, J = 12.0, 4.2 Hz), 1H}, 5.50 (dhpt, J = 11.2, 1.5 Hz, 1H), 4.57–4.55 (m, 2H), 3.89 (brs, 2H), 3.52–3.50 (m, 2H), 3.06 (brs, 2H), 2.60 (s, 2H), 1.95 (brd, 2H), 1.64–1.52 (m, 2H), 1.39 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 189.9, 164.5, 155.2, 153.6, 126.6, 125.3, 125.0, 122.7, 116.2, 114.3, 78.8, 77.1, 69.4, 46.7, 33.0, 27.4, 21.4. MS (EI) LRMS: m/z 385, 330, 329 (100%) 314, 189, 96. HRMS (EI): m/z calcd for C22H27NO5: 385.1889; found: 385.1900.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Department of Science and Technology (DST), India for supporting this research, under the scheme “Disha Programme for Women in Science”. This project was also funded initially by KIRAN—Societal Research Fellowship (SoRF), DST.References
- L. F. Tietze, H. P. Bell and S. Chandrasekhar, Angew. Chem., 2003, 42, 3996–4028 CrossRef CAS.
- R. Romagnoli, P. Baraldi, M. K. Salvador, M. E. Camacho, J. Balzarini, J. Bermejo and F. Estévez, Eur. J. Med. Chem., 2013, 63, 544–557 CrossRef CAS.
- E. M. Guantai, K. Ncokazi, T. J. Egan, J. Gut, P. J. Rosenthal, P. J. Smith and K. Chibale, Bioorg. Med. Chem. Lett., 2010, 18, 8243–8256 CrossRef CAS.
- B. Meunier, Acc. Chem. Res., 2008, 41, 69–77 CrossRef CAS PubMed.
- Y. Wu, M. Hu, L. Yang, X. Li, J. Bian, F. Jiang, H. Sun, Q. You and X. Zhang, Bioorg. Med. Chem. Lett., 2015, 25, 2584–2588 CrossRef CAS PubMed.
- L. He, Y. Ling, L. Fu, D. Yin, X. Wang and Y. Zhang, Bioorg. Med. Chem. Lett., 2012, 22, 289–292 CrossRef CAS PubMed.
- X. Zhang, X. Li, H. Sun, Z. Jiang, L. Tao, Y. Gao, Q. Guo and Q. You, Org. Biomol. Chem., 2012, 10, 3288–3299 RSC.
- K. M. Elbel, G. Guizzunti, M. A. Theodoraki, J. Xu, A. Batova, M. Dakanali and E. A. Theodorakis, Org. Biomol. Chem., 2013, 11, 3341–3348 RSC.
- X. Zhang, X. Li, H. Sun, X. Wang, L. Zhao, Y. Gao, X. Liu, S. Zhang, Y. Wang, Y. Yang, S. Zeng, Q. Guo and Q. You, J. Med. Chem., 2013, 56, 276–292 CrossRef CAS PubMed.
- L. Feng, M. M. Maddox, M. Z. Alam, L. S. Tsutsumi, G. Narula, D. F. Bruhn, X. Wu, S. Sandhaus, R. B. Lee, C. J. Simmons, Y.-C. Tse-Dinh, J. G. Hurdle, R. E. Lee and D. Sun, J. Med. Chem., 2014, 57, 8398–8420 CrossRef CAS PubMed.
- H. G. Bonacorso, W. C. Rosa, S. M. Oliveira, I. Brusco, E. S. Brum, M. B. Rodrigues, C. P. Frizzo and N. Zanatta, Bioorg. Med. Chem. Lett., 2017, 27, 1551–1556 CrossRef CAS PubMed.
- U. M. Battisti, S. Corrado, C. Sorbi, A. Cornia, A. Tait, D. Malfacini, M. C. Cerlesi, G. Calo and L. Brasili, Med. Chem. Commun., 2014, 5, 973–983 RSC.
- M. Mujahid, R. G. Gonnade, P. Yogeeswari, D. Sriram and M. Muthukrishnan, Bioorg. Med. Chem. Lett., 2013, 23, 1416–1419 CrossRef CAS PubMed.
- L. Zhang, V. Dewan and H. Yin, J. Med. Chem., 2017, 60, 5029–5044 CrossRef CAS PubMed.
- D. Ashok, E. V. L. Madhuri, M. Sarasija, S. Sree Kanth, M. Vijjulatha, M. D. Alaparthi and S. R. Sagurthi, RSC Adv., 2017, 7, 25710–25724 RSC.
- K. Sambasivarao, M. Kalyaneswar, T. Arti and M. M. Shaikh, Chem.–Eur. J., 2006, 12, 8024–8038 CrossRef PubMed.
- L. Yet, Chem. Rev., 2000, 100, 2963–3008 CrossRef CAS PubMed.
- G. A. Molander, Acc. Chem. Res., 1998, 31, 603–609 CrossRef CAS.
- A. K. Chatterjee, J. P. Morgan, M. Scholl and R. H. Grubbs, J. Am. Chem. Soc., 2000, 122, 3783–3784 CrossRef CAS.
- V. Declerck, H. Allouchi, J. Martinez and F. Lamaty, J. Org. Chem., 2007, 72, 1518–1521 CrossRef CAS PubMed.
- M. Arisawa, A. Nishida and M. Nakagawa, J. Organomet. Chem., 2006, 691, 5109–5121 CrossRef CAS.
- J. Burnley, Z. J. Wang, W. R. Jackson and A. J. Robinson, J. Org. Chem., 2017, 82, 8497–8505 CrossRef CAS PubMed.
- B. J. Ireland, B. T. Dobigny and D. E. Fogg, ACS Catal., 2015, 5, 4690–4698 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07920j |
| This journal is © The Royal Society of Chemistry 2018 |