Open Access Article
Open Access ArticleNew type of alginate/chitosan microparticle membranes for highly efficient pervaporative dehydration of ethanol†
Gabriela Dudek * and
Roman Turczyn
* and
Roman Turczyn
Department of Physical Chemistry and Technology of Polymers, Faculty of Chemistry, Silesian University of Technology, Strzody 9, 44-100 Gliwice, Poland. E-mail: gmdudek@polsl.pl; Fax: +48 32 2371509; Tel: +48 32 2371427
First published on 27th November 2018
Abstract
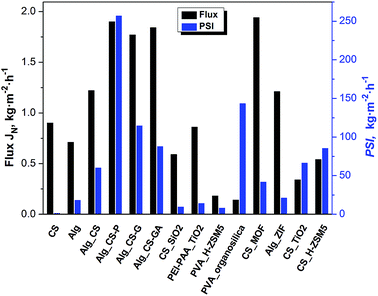
A new type of composite alginate membranes filled with chitosan (CS) and three different modified chitosan submicron particles, i.e. phosphorylated (CS-P), glycidol (CS-G) or glutaraldehyde (CS-GA) crosslinked ones, were prepared, and the pervaporation of water/ethanol mixture was investigated. The influence of various chitosan particles and their content on the transport properties of membranes was discussed. It was found that the addition of chitosan particles into the alginate matrix has a prominent effect on the ethanol/water separation efficiency. All tested membranes are characterized simultaneously by a high flux and selectivity, exhibiting advantageous properties, and outperforming numerous conventional materials. The best results were achieved for alginate membranes filled with phosphorylated chitosan particles at 10 wt%, for which separation factor, flux and PSI were equal to 136.2, 1.90 kg m−2 h−1 and 256.9 kg m−2 h−1, respectively.
1. Introduction
Hybrid membranes are an emerging class of innovative nano-structured materials, suitable for a wide range of practical applications.1–5 Over the last two decades, a rapid growth in fabrication methods, mass transport mechanisms and practical applications of hybrid membranes in pervaporation was observed.6–8 For the preparation of hybrid membranes several methods have been frequently used, e.g. physical blending method, sol–gel method, in situ polymerization method, etc. Among them, physical blending is the most common and facile fabrication technique because of its independency on the filler preparation or modification. Besides, the respective synthesis of filler phase and membrane favours incorporation of various fillers with different sizes, shapes, porosities and surface functionalities into hybrid membranes, thus enriching their hierarchical structures.Different kinds of materials can be used as fillers, e.g. metal oxide nanoparticles,9–13 silica nanoparticles,14–19 zeolites,20–23 graphene oxides,24–26 carbon nanotubes (CNTs)27–29 and metal–organic frameworks (MOFs).30–33 Nevertheless, the most popular inorganic additives are metal oxide nanoparticles. The broad spectrum of metal oxide nanoparticles that can be introduced into the polymer membrane contains Fe3O4, ZrO2 TiO2, MgO, CaO, Al2O3,CuO, SiO2 and ZnO.9–13 Each of them can be easily incorporated into the majority of polymeric materials used for the fabrication of membrane, showing the fine synergistic properties between the polymeric materials and nanoparticles.
One of the vital advantages of chitosan is the possibility of multifarious modifications, from chemical to physical viewpoints, tailoring for specific purposes. Additionally, biopolymers possess the benefit of widespread availability from marine (chitin and chitosan) or agriculture (cellulose, starch, pectin) resources, as well as the environmental safety because of their biodegradability, biocompatibility and non-toxicity. Furthermore, chitosan nanoparticles (ChNPs) benefit both from the chitosan as a material and its nano-size, e.g. as surface and interface effects, and quantum effects.34 Chitosan beads often possess an open-porous structure with interconnected micropores and channels extending from surface to the bead's centre. In this case, bead surface presents a uniform porosity. The rigidity of chitosan beads is supposed to increase the mechanical strength of a membrane material, whereas their pores should enhance the flux affecting the mechanism of diffusion and transport.35–37
Keeping above in mind, we have prepared novel organic–organic alginate hybrid membranes filled with pristine chitosan (CS) and three type of fillers of amended chitosan particles with submicron size, i.e. phosphorylated (CS-P), glycidol modified (CS-G) and glutaraldehyde (CS-GA) crosslinked chitosan. Proposed new type hybrid alginate/chitosan membranes differ from described in literature. Generally this both biopolymers are blended together to produce hybrid membranes or are casted layer by layer to form multilayer, asymmetric membranes. Chitosan is soluble in acidic solutions, e.g. 0.1 M acetic acid, but sodium alginate as anionic polymer is better soluble in basic solutions, which could rise some problems with the mutual solubility and miscibility. Typically alginate membranes demonstrate better separation performance than chitosan membranes, whereas chitosan one usually exhibits higher fluxes. The approach proposed in this work is very simple, but allows to overcome the aforementioned difficulties and benefits from the advantages of both polymers. In our case membranes consist of alginate matrix with submicron chitosan particles acting as a filler. As it can be seen in the following part, the new type of hybrid alginate/chitosan membranes demonstrate high efficiency in ethanol dehydration process, one of the best mentioned in the literature. What more, they keep the very high selectivity at simultaneous high fluxes.
We evaluated and compared transport as well as separation properties38–42 of investigated membranes in the pervaporation process of a water/ethanol mixture and discussed the influence of chitosan particles and their content on the overall effectiveness of separation. The results were compared with a previously described/investigated pristine sodium alginate membrane. Additionally, the physico-chemical properties of the resulting membranes were studied by FTIR spectroscopy, SEM microscopy, EDS analysis, DSC thermal analysis, contact angle and swelling experiments.
2. Experimental
2.1 Materials
Sodium alginate, chitosan, urea (purity ≥ 98%), glutaricdialdehyde (2.5 wt% solution in water), sodium hydroxide (purity ≥ 98%), orthophosphoric acid (purity ≥ 100%), dimethylformamide (for analysis), glycidol (purity ≥ 96%), sodium borohydride (purity ≥ 99%), sodium periodate (purity ≥ 99%), were obtained from Acros Organic. Calcium chloride (purity ≥ 96%), aceticacid (purity ≥ 99%)were purchased from Avantor Performance Materials.2.2 Modification of chitosan particles
Phosphorylated chitosan (CS-P) particles were prepared based on Sakaguchi et al. method.43 In this case, 20 g of chitosan, 100 g of urea, and 20 g of 100% orthophosphoric acid were added into 200 ml of dimethylformamide. The mixture was stirred and heated at 150 °C for 1 h. The obtained suspension was cooled to room temperature and separated by centrifugation. The precipitate was thoroughly washed with deionised water and freeze-dried. Glycidol modified chitosan (CS-G) particles were prepared using the modified method of Shainoff.44 Briefly, 200 ml of 3% chitosan solution in 0.15 M acetic acid was mixed with 12 ml of glycidol and 100 ml of 1 M NaOH containing sodium borohydride NaBH4 (2 mg ml−1) as antioxidant. The solution was then stirred vigorously at 25 °C for 18 h. Fabricated CS-G particles were washed with distilled water until neutral pH (7 ± 0.5). These one step modifications yield glycerol groups, CS–O–CH2–CHOH–CH2OH. The resultant CS-G was further suspended in 250 ml of water and 70 ml of 0.16 M sodium periodate (NaIO4) to transform the vicinal –OH groups into aldehyde groups and formaldehyde molecules. The suspension was kept under slow stirring for 2 h at room temperature.Chitosan-glutaraldehyde (CS-GA) crosslinked particles were synthesized based on modified Poon et al. procedure.45 Thus, an aqueous 3 wt% chitosan solution was prepared by dissolving 2 g of chitosan in 100 ml of 2 vol% acetic acid solution. Next, the pH of solution was adjusted to 5.6 by drop-wise adding of 0.01 M NaOH. Then, 1.4 ml of 50 vol% glutaraldehyde was added to the stirred polymer solution. The precipitated gel was left overnight. Thereafter, 2 M NaOH was added drop-wise into the aged gel phase for 3 h until a dark brown suspension was produced and the final pH reached ca. 7. The insoluble crosslinked products were vacuum filtered, subsequently washed with several portions of distilled water and cold acetone. After partial air-drying, prepared CS-GA was crushed and further dried in air for additional 24 h.
2.3 Membrane preparation
1.5 wt% sodium alginate solution was synthesized by dissolving an appropriate amount of sodium alginate powder in deionised water. The solution was mixed with an appropriate portion of chitosan (CS), and modified chitosan particles, such as phosphorylated chitosan (CS-P), glycidol modified chitosan (CS-G) and chitosan-glutaraldehyde (CS-GA) crosslinked particles (0; 5; 10; 15; 25 wt%). The sodium alginate solution was then casted onto a levelled glass plate and evaporated to dryness at 40 °C. After 24 h, the membrane was crosslinked using calcium chloride. In this case, sodium alginate membrane was immersed in 2.5 wt% calcium chloride solution for 120 min at room temperature. The pristine Alg membrane was prepared in the same manner as above except for the addition of metal oxide nanoparticles. Optimization of the casting method allows for the preparation of homogeneous membranes with reproducible thickness. The membrane thickness was measured using waterproof precise coating thickness gauge MG-401 ELMETRON, estimated as a mean value of at least 10 measurements in different points and equals to 15.0 ± 1.0 μm.2.4 Membrane characterization
Membranes were characterized using scanning electron microscope (SEM), differential scanning calorimeter (DSC) and FTIR spectroscopy. Basing on the results of the sorption tests, the degree of swelling was calculated. Contact angles of dry membranes were measured using MDA1300 Handheld USB Metal Microscope. The contact angle was measured immediately after dropping and at intervals of 10 s. To measure the effect of fouling, 1 μl droplets of deionised water were placed on the membranes. Scanning electron microscope characterization was done using Phenom Pro-X microscope. FTIR measurements were performed using Perkin Elmer Spectrum Two FTIR spectrometer for scanning the films at ambient temperature, in the spectral range of 650–4000 cm−1, and with the spectral resolution of 2 cm−1. The thermal measurements were performed on Mettler-Toledo DSC 822e calorimeter under nitrogen atmosphere (flow rate 250 ml min−1) and heating rate of 10 K min−1. The degree of swelling (DS) of the pristine and hybrid Alg membranes with different chitosan and modified chitosan particles loading was determined using sorption test. In this case, membranes were immersed in water or ethanol and the mass changes of analysed samples were determined during one week using analytical balance. The degree of swelling (DS) was calculated using following equation:
 | (1) |
2.5 Pervaporation experiments
PV experiments were carried out using the apparatus described in previously published paper under the same conditions. As the feed, an aqueous solution of 96 wt% ethanol was used. The permeate was collected in a cold trap cooled with liquid nitrogen. Flux was calculated from the measured weight of liquid collected in the cold traps during a certain time intervals at steady-state conditions. The composition of feed, permeate and retentate was analysed using gas chromatography.46 For each membrane, the experiment was repeated three times. The results showed the repeatability of measurements and the errors were of the order of few percent. The permeation flux of component i was calculated using the following equation:47,48
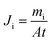 | (2) |
Two parameters were used for the description of the separation properties of the membrane, namely separation factor (αAB) and selectivity coefficient (ScAB). Separation factor was calculated by:47,48
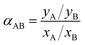 | (3) |
Based on the Ist Fick's law, the permeation coefficient was determined according to the formula:
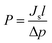 | (4) |
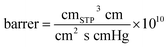 , l is the membrane thickness, cm, Δp is the difference of vapour pressure at both sides of the membrane, cmHg, Js is the diffusive mass flux,
, l is the membrane thickness, cm, Δp is the difference of vapour pressure at both sides of the membrane, cmHg, Js is the diffusive mass flux, 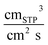 .
.
Selectivity coefficient was equal to the ratio of permeability of separated components:47,48
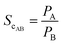 | (5) |
In order to compare the separation efficiency of investigated membranes, pervaporation separation index expressed by following equation was used:47,48
| PSI = J(αAB − 1) | (6) |
3. Results and discussion
3.1 Membrane characterization
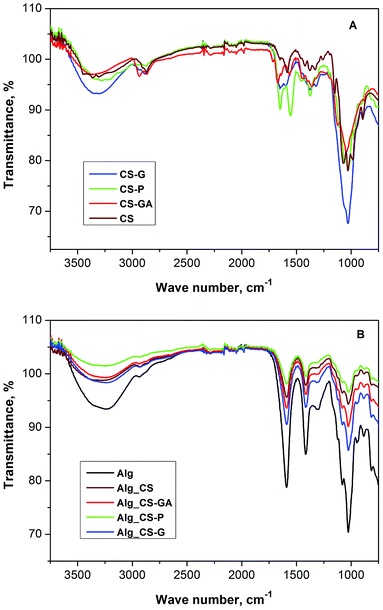
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching) at 1651 cm−1, amide II (N–H bending overlapped by the amide I band) at 1576 cm−1, CH2 wagging coupled with OH in plane deformation at 1380 cm−1, ether group (C–O–C stretching) at 1159 cm−1, secondary hydroxyl group (C–OH stretching) and primary hydroxyl and amine groups (C–OH and C–NH2 stretching) at 1065 cm−1 and 1030 cm−1, respectively. Phosphorylation led to an emerging shoulder at 1374 cm−1 which can be attributed to P
O stretching) at 1651 cm−1, amide II (N–H bending overlapped by the amide I band) at 1576 cm−1, CH2 wagging coupled with OH in plane deformation at 1380 cm−1, ether group (C–O–C stretching) at 1159 cm−1, secondary hydroxyl group (C–OH stretching) and primary hydroxyl and amine groups (C–OH and C–NH2 stretching) at 1065 cm−1 and 1030 cm−1, respectively. Phosphorylation led to an emerging shoulder at 1374 cm−1 which can be attributed to P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O asymmetric stretching from phosphates. The peaks found at 1059, 1029 and 972 cm−1 were due to P–OH group vibration. Protonation of chitosan amine functionalities is suggested by the presence of two peaks, both attributed to –NH3+ groups, namely the asymmetrical deformation at 1650 cm−1 and the symmetric deformation at 1553 cm−1. The initial amide I and II bands were possibly overlapped by these vibrations.
O asymmetric stretching from phosphates. The peaks found at 1059, 1029 and 972 cm−1 were due to P–OH group vibration. Protonation of chitosan amine functionalities is suggested by the presence of two peaks, both attributed to –NH3+ groups, namely the asymmetrical deformation at 1650 cm−1 and the symmetric deformation at 1553 cm−1. The initial amide I and II bands were possibly overlapped by these vibrations.
 | ||
| Fig. 1 ATR FTIR spectra of (A) – pristine and modified chitosan particles, and (B) – pristine Alg membrane and series of hybrid Alg membranes filled with modified chitosan particles. | ||
Poon et al. considered two possible reactions that may occur during the crosslinking of chitosan with glutaraldehyde. The first one may occur through an amine-catalyzed aldol addition or become an acetal derivative, where a substitution reaction occurs between the amine of chitosan and the acetal group.45 The crosslinking of chitosan membranes with glutaraldehyde leads to the increase in the absorption at 1675 cm−1. As already stated, it is proposed that mechanism of crosslinking between glutaraldehyde and a free amine on chitosan follows a Schiff's base reaction that results in formation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. According to Knaul et al.,49 the signal of C
N bond. According to Knaul et al.,49 the signal of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration can show up anywhere between 1620 and 1680 cm−1, depending on the reacted compounds. The presence of shoulders at 1564 and 1718 cm−1, that are due to the ethylenic and free-aldehydic bonds, respectively, can be observed relatively to the intensity of other peaks. Signal of the C–H stretch increase at 2938 cm−1 and the presence of aliphatic amino group decreases as the intensity of a peak at 1113 cm−1. It indicates that the crosslinking with glutaraldehyde turns the membrane more hydrophobic as the primary amine groups are substituted with an aliphatic chain.
N vibration can show up anywhere between 1620 and 1680 cm−1, depending on the reacted compounds. The presence of shoulders at 1564 and 1718 cm−1, that are due to the ethylenic and free-aldehydic bonds, respectively, can be observed relatively to the intensity of other peaks. Signal of the C–H stretch increase at 2938 cm−1 and the presence of aliphatic amino group decreases as the intensity of a peak at 1113 cm−1. It indicates that the crosslinking with glutaraldehyde turns the membrane more hydrophobic as the primary amine groups are substituted with an aliphatic chain.
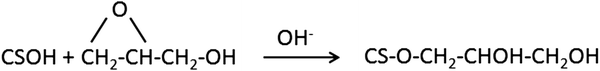
In case of the chitosan film reacting with glycidol and sodium periodate, the significantly larger band at 1035 cm−1 is observed. It should be associated with the etherification, acetal and C–N bond formation both from vicinal hydroxyl groups of glycidol moiety and oxidised to aldehyde. According to Shainoff et al. the addition of glycidol to the chitosan solution leads to etherification in presented reaction:44
Treatment of the product of glycidol addition with sodium periodate resulted in the generation of reactive aldehyde functionalities through the oxidation of the glycol moieties:50
FT-IR spectra of pristine and hybrid alginate membranes filled with chitosan and modified chitosan particles are shown in Fig. 1B. Pristine alginate membrane shows characteristic bands at 3310, 1591, 1415, and 1027 cm−1 corresponding to O–H stretching, –COO– asymmetric and symmetric stretching, and C–O–C (ring) vibrational modes, respectively. For hybrid alginate membranes the characteristic peaks of the pristine alginate membranes remain at the same positions but their intensities are significantly smaller. It is due to the fact that the addition of chitosan particles into polymer matrix equals to 10 wt% in relation to the amount of alginate, so the peaks of chitosan particles are weaker than the peaks of alginate matrix.
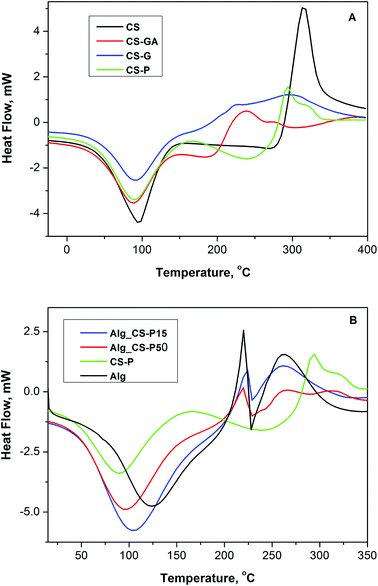 | ||
| Fig. 2 DSC curves of (A) – series of modified chitosan particles (CS, CS-GA, CS-G, CS-P) and (B) – pristine Alg membrane and two hybrid Alg membranes loaded with 15 wt% and 50 wt% of CS-P particles. | ||
The glutaraldehyde-crosslinked chitosan tends to retain less water, which in turn volatilizes more easily than it does in the pristine membrane. These finding indicates how intense the membrane–water interactionis, and enables to asset whether the water is loosely or strongly bounded to the chitosan chains. The lower affinity of water in glutaraldehyde-treated membranes indicates more hydrophobic character of chitosan after crosslinking.51
The CS-P also shows two stages of weight loss. The first stage of weight loss starts at 25 °C and continues up to 150 °C. The second stage from 180 to 360 °C may be contributed to the decomposition of different structures of the phosphorylated chitosan, demonstrating that CS-P is less thermally stable than pristine chitosan. Sodium alginate exhibits an endothermic peak with onset the temperature of 75 °C and an exothermic peak with the onset at 190 °C and more complicated shape, caused by the overlapping with another endothermic process. First, endothermic peaks are correlated with the loss of water associated with hydrophilic groups of alginate while exothermic peaks results from the degradation of polymer backbone due to dehydration, depolymerisation, saccharide ring destruction, most probably to the partial decarboxylation of the protonated carboxylic groups, than CO2 elimination and chain scission. Satishbabu et al. showed DSC thermograms of the physical mixture of sodium alginate and chitosan showing broad endothermic peak at 81.72 °C, and suggested that it probably represents the coalescence of isolated endothermic dehydration peaks resulting from individual contribution of both polymers.52 Formation of the new chemical bonds is expected due to the interaction and complexation between alginate and chitosan. Many researchers postulate the formation of strong insoluble ionic complex layer at the boundary.53 The shift of peaks position is expected for such complexes compared to those of the physical mixture, and could be interpreted as an evidence of interaction between both polymers. The intermediate and broader endothermic peak at 80.6 °C is reported that differ from the one of isolated polyelectrolytes.52
In Fig. 2B the thermograms of pristine alginate membrane, pristine CS-P filler and membranes filled with 15 and 50 wt% of CS-P are compared. Observed changes in the peak position of water loss in case of Alg membranes loaded with CS-P, likewise, points the changes of the hydrophilic character depending on the composition. As expected, it is the unfilled pristine Alg membrane that strongest bounds water, and it is exhibited as the presence of the peak of water loss at higher temperature (125 °C).
This emphatic hydrophilicity of Alg should be associated with the ionic structure of this polymer, i.e. to the presence of carboxylic group in form of anion or salt. With the presence of CS-P particles, the composite becomes gradually less hydrophilic. The temperature of water loss decreases and is equal to 105, 96 and 89 °C for Alg_CS-P15, Alg_CS-P50 and CS-P itself, respectively. The shift of peaks position as well their symmetry pronounce the strong interaction between Alg matrix and modified CS filler in the investigated composite membranes, independent on the ratio between both components.
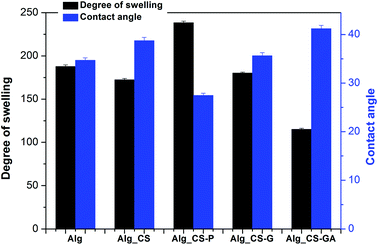 | ||
| Fig. 3 Contact angles θ and degree of swelling DS measured for pristine and hybrid alginate membranes filled with modified chitosan particles (average from ten measurements). | ||
The lowest value of DS (115.22%) is observed for alginate membranes filled with chitosan-glutaraldehyde particles. This was attributed to the decrease of free –OH or –NH2 hydrophilic groups in the chitosan particles resulting from the reaction between –OH or –NH2 groups with aldehyde groups of the GA.55 The highest value of DS (238%) is obtained in case of sodium alginate membranes filled with phosphorylated chitosan. The presence of phosphate group in chitosan matrix impacts on the increase in hydroxyl groups content into obtained particles. The hydroxyl groups have hydrophilic character, influencing the value of the degree of swelling.
The consistent results were obtained in case of contact angle measurement, describing the use of surface hydrophilicity of the membrane for the prediction of the membrane performance. Each experiment was performed eight times to guarantee a statistical integrity. All investigated membranes are characterized as hydrophilic (q < 90°). The contact angle of pristine sodium alginate membrane is equal to 34.7 ± 0.20 and the hydrophilicity of the composites is dependent on the kind of chitosan filler and increases in the following order: Alg_CS-GA < Alg_CS < Alg_CS-G < Alg < Alg_CS-P. Like in the case of swelling, the three types of membrane show similar contact angles and are more hydrophilic that Alg_CS-GA. The higher contact angle of Alg_CS-GA membrane results from the hydrophobic nature of the glutaric aldehyde used as a crosslinking agent. The lowest value of contact angle (27.5°) is obtained in case of Alg_CS-P membrane, corresponding with the highest value of a degree of swelling.
3.2 Pervaporation performance of hybrid alginate membranes
| CS particles content, wt% | 0 | 5 | 10 | 15 | 25 |
| Flux JN, kg m−2 h−1 | 0.71 | 1.41 | 1.22 | 1.09 | 0.86 |
| Permeation coefficient of water PH2O × 105, barrer | 4.83 | 8.73 | 8.48 | 8.12 | 6.25 |
| Permeation coefficient of ethanol PEtOH × 105, barrer | 0.23 | 0.34 | 0.32 | 0.30 | 0.27 |
| Diffusion coefficient of water DH2O × 107, cm2 s−1 | 1.44 | 5.12 | 4.99 | 4.81 | 4.23 |
| Diffusion coefficient of ethanol DEtOH × 107, cm2 s−1 | 0.22 | 0.33 | 0.32 | 0.32 | 0.31 |
| Solubility coefficient of water SH2O, cmSTP3 cm−3 cmHg−1 | 335.4 | 170.5 | 169.9 | 168.8 | 147.7 |
| Solubility coefficient of ethanol SEtOH, cmSTP3 cm−3 cmHg−1 | 104.5 | 103.0 | 100.0 | 93.7 | 87.1 |
| Selectivity coefficient Sc | 21.0 | 25.7 | 26.5 | 27.1 | 23.1 |
| Separation factor, αH2O/EtOH | 26.5 | 47.3 | 50.2 | 53.6 | 22.1 |
| Pervaporation separation index PSI, kg m−2 h−1 | 18.2 | 65.3 | 60.0 | 57.3 | 18.1 |
| CS-P particles content, wt% | 0 | 5 | 10 | 15 | 25 |
| Flux JN, kg m−2 h−1 | 0.71 | 1.85 | 1.90 | 1.92 | 1.14 |
| Permeation coefficient of water PH2O × 105, barrer | 4.83 | 12.13 | 12.11 | 12.02 | 8.52 |
| Permeation coefficient of ethanol PEtOH × 105, barrer | 0.23 | 0.28 | 0.32 | 0.41 | 0.31 |
| Diffusion coefficient of water DH2O × 107, cm2 s−1 | 1.44 | 5.22 | 5.51 | 5.90 | 4.41 |
| Diffusion coefficient of ethanol DEtOH × 107, cm2 s−1 | 0.22 | 0.33 | 0.38 | 0.48 | 0.32 |
| Solubility coefficient of water SH2O, cmSTP3 cm−3 cmHg−1 | 335.4 | 232.5 | 219.8 | 203.7 | 193.3 |
| Solubility coefficient of ethanol SEtOH, cmSTP3cm−3 cmHg−1 | 104.5 | 84.8 | 84.2 | 85.4 | 84.4 |
| Selectivity coefficient Sc | 21.0 | 43.3 | 37.8 | 29.3 | 27.5 |
| Separation factor, αH2O/EtOH | 26.5 | 96.1 | 136.2 | 102.3 | 64.5 |
| Pervaporation separation index PSI, kg m−2 h−1 | 18.2 | 175.93 | 256.9 | 194.5 | 72.4 |
| CS-GA particles content, wt% | 0 | 5 | 10 | 15 | 25 |
| Flux JN, kg m−2 h−1 | 0.71 | 1.73 | 1.84 | 1.89 | 1.98 |
| Permeation coefficient of water PH2O × 105, barrer | 4.83 | 8.19 | 8.56 | 8.63 | 7.12 |
| Permeation coefficient of ethanol PEtOH × 105, barrer | 0.23 | 0.41 | 0.42 | 0.42 | 0.40 |
| Diffusion coefficient of water DH2O × 107, cm2 s−1 | 1.44 | 8.39 | 7.96 | 7.54 | 6.32 |
| Diffusion coefficient of ethanol DEtOH × 107, cm2 s−1 | 0.22 | 0.25 | 0.25 | 0.24 | 0.23 |
| Solubility coefficient of water SH2O, cmSTP3 cm−3 cmHg−1 | 335.4 | 97.6 | 107.5 | 114.4 | 112.6 |
| Solubility coefficient of ethanol SEtOH, cmSTP3 cm−3 cmHg−1 | 104.5 | 164.0 | 168.1 | 175.3 | 173.9 |
| Selectivity coefficient Sc | 21.0 | 20.0 | 20.4 | 20.5 | 17.8 |
| Separation factor, αH2O/EtOH | 26.5 | 50.5 | 48.7 | 42.8 | 34.2 |
| Pervaporation separation index PSI, kg m−2 h−1 | 18.2 | 85.6 | 87.8 | 79.0 | 65.7 |
The addition of CS-GA particles has also the influence on the solubility coefficient of membranes, especially when compared with alginate membranes filled with pristine and phosphorylated chitosan particles. The values of water solubility coefficients are lower and the values of ethanol solubility coefficients are higher. The higher values of water diffusion coefficients and the lower values of solubility coefficients of water compared with alginate membranes filled with pristine chitosan particles result in the similarity of water permeation throughout both membranes (water permeation coefficients of 8.50 barrer). Although the values of permeation of coefficient are higher, the evaluated selectivity coefficients are lower than for Alg_CS membranes, and reach the similar values as pristine alginate membrane. As a consequence of increased affinity to ethanol, the values of separation factors of Alg_CS-GA membranes are lower than for Alg_CS-P membranes and reach the similar values as for Alg_CS membranes (50.5 for alginate membrane with 5 wt% of CS-GA filler). However, for both type of membranes – Alg_CS and Alg_CS-GA – the values of separation factors are similar, notwithstanding, the bigger values of fluxes in case of Alg_CS-GA membranes, have positive impact on the pervaporation separation index. The highest value of PSI (87.8, kg m−2 h−1), is obtained for Alg membrane with 10 wt% of CS-GA particles. This value is about 5 times higher than for pristine alginate membrane and about 1.3 times higher than for alginate membrane with 10 wt% of pristine chitosan particles which reaches the highest value of PSI.
| CS-G content, wt% | 0 | 5 | 10 | 15 | 25 |
| Flux JN, kg m−2 h−1 | 0.71 | 1.77 | 1.88 | 1.82 | 1.95 |
| Permeation coefficient of water PH2O × 105, barrer | 4.83 | 14.90 | 11.93 | 9.25 | 6.92 |
| Permeation coefficient of ethanol PEtOH × 105, barrer | 0.23 | 0.43 | 0.45 | 0.49 | 0.50 |
| Diffusion coefficient of water DH2O × 107, cm2 s−1 | 1.44 | 7.41 | 7.75 | 7.91 | 8.12 |
| Diffusion coefficient of ethanol DEtOH × 107, cm2 s−1 | 0.22 | 0.31 | 0.33 | 0.37 | 0.42 |
| Solubility coefficient of water SH2O, cmSTP3 cm−3 cmHg−1 | 335.4 | 201.1 | 153.9 | 117.0 | 85.2 |
| Solubility coefficient of ethanol SEtOH, cmSTP3 cm−3 cmHg−1 | 104.5 | 103.2 | 93.9 | 81.1 | 71.4 |
| Selectivity coefficient Sc | 21.0 | 34.6 | 26.5 | 18.9 | 13.8 |
| Separation factor, αH2O/EtOH | 26.5 | 65.8 | 57.9 | 51.7 | 40.5 |
| Pervaporation separation index PSI, kg m−2 h−1 | 18.2 | 114.7 | 107.0 | 92.3 | 77.0 |
| Polymer | Filler type | T, °C | Flux, kg m−2 h−1 | Separation factor | PSI, kg m−2 h−1 | References |
|---|---|---|---|---|---|---|
| CS | — | 20 | 0.9 | 2.6 | 1.4 | 60 |
| Alg | — | 20 | 0.71 | 26.5 | 18.2 | This work |
| Alg | CS | 20 | 1.22 | 50.2 | 60.02 | This work |
| Alg | CS-P | 20 | 1.90 | 136.2 | 256.88 | This work |
| Alg | CS-G | 20 | 1.77 | 65.8 | 114.70 | This work |
| Alg | CS-GA | 20 | 1.84 | 48.7 | 87.77 | This work |
| PEI-PAA | TiO2 | 60 | 0.86 | 17.25 | 13.97 | 12 |
| CS | TiO2 | 80 | 0.34 | 196.00 | 66.30 | 13 |
| Alg | ZIF | 30 | 1.21 | 18.42 | 21.08 | 14 |
| PVA | Organosilica | 40 | 0.14 | 1026 | 143.50 | 15 |
| CS | SiO2 | 30 | 0.59 | 17.25 | 9.59 | 16 |
| PVA | H-ZSM5 | 30 | 0.18 | 46.00 | 8.10 | 20 |
| CS | H-ZSM-5 | 80 | 0.54 | 158.02 | 85.33 | 22 |
| CS | MOF | 40 | 1.94 | 22.52 | 41.76 | 61 |
4. Conclusions
Novel composite organic–organic membranes consisting of organic filler in the form of modified chitosan particles (CS, CS-P, CS-GA, CS-G), and crosslinked with calcium cations alginate matrix were prepared for the pervaporation dehydration of ethanol/water mixtures.The results of conducted separation tests shows that the addition of one polymer in a form of particles as a filler to the another polymer matrix allows to prepare interesting class of membranes and is a very simple and effective manner of significant improvement of the final transport properties. In presented system chitosan filler/alginate matrix, the values of flux and separation factor were about 2.0 times higher, and the PSI was about 3.5 times higher for Alg_CS membrane with 5 wt% of filler, when compared with the pristine alginate membrane. The further improvement of transport parameters and separation effectiveness was realized by the chemical modifications of chitosan particles. Two of modified chitosan particles (Alg_CS-P, Alg_CS-G) showed enhanced hydrophilicity, thus resulting in higher water affinity of membranes contained them. The best effectiveness of pervaporative ethanol dehydration were obtained for alginate membranes filled with 10 wt% of phosphorylated chitosan particles. In this case, separation factor, flux and PSI were equalled to 136.2, 1.90 kg m−2 h−1 and 256.9 kg m−2 h−1, respectively. The presented organic/organic hybrid membranes possess superior separation factor which comes together with a high flux, what is highly advantageous and not of ten among membranes described in the literature.
Conflicts of interest
The authors ensure that there are no conflicts to declare.Acknowledgements
The authors would like to thank The National Centre for Research and Development and The Silesian University of Technology for providing partial financial support under the following projects: 2018/02/X/ST8/00309 and 04/040/RGH18/0088.References
- R. Singh, Hybrid Membrane Systems for Water Purification, 2005 Search PubMed.
- F. Gao, Advances in Polymer Nanocomposites: Types and Applications, 2012 Search PubMed.
- D. E. Suk and T. Matsuura, Sep. Sci. Technol., 2006, 41, 595–626 CrossRef.
- Inorganic Polymeric and Composite Membranes - Structure, Function and Other Correlations, ed. S. T. Oyama and S. Stagg-Williams, Elsevier, Oxford, 1st edn, 2011 Search PubMed.
- H.-C. Yang, J. Hou, V. Chen and Z.-K. Xu, J. Mater. Chem. A, 2016, 4, 9716–9729 RSC.
- A. Basile, A. Figoli and M. Khayet, Pervaporation, vapour permeation and membrane distillation: Principles and applications, 2015 Search PubMed.
- B. Smitha, D. Suhanya, S. Sridhar and M. Ramakrishna, J. Membr. Sci., 2004, 241, 1–21 CrossRef CAS.
- G. Jyoti, A. Keshav and J. Anandkumar, J. Eng., 2015, 2015, 1–24 CrossRef.
- X. Cheng, F. Pan, M. Wang, W. Li, Y. Song, G. Liu, H. Yang, B. Gao, H. Wu and Z. Jiang, J. Membr. Sci., 2017, 541, 329–346 CrossRef CAS.
- A. Strzelewicz, M. Krasowska, G. Dudek, A. Rybak, R. Turczyn and M. Cieśla, Acta Phys. Pol. B, 2013, 44, 955–965 CrossRef CAS.
- Y. Wang, L. Yang, G. Luo and Y. Dai, Chem. Eng. J., 2009, 146, 6–10 CrossRef CAS.
- L. Gong, L. Zhang, N. Wang, J. Li, S. Ji, H. Guo, G. Zhang and Z. Zhang, Sep. Purif. Technol., 2014, 122, 32–40 CrossRef CAS.
- D. Yang, J. Li, Z. Jiang, L. Lu and X. Chen, Chem. Eng. Sci., 2009, 64, 3130–3137 CrossRef CAS.
- G. Liu, Z. Jiang, K. Cao, S. Nair, X. Cheng, J. Zhao, H. Gomaa, H. Wu and F. Pan, J. Membr. Sci., 2017, 523, 185–196 CrossRef CAS.
- L. L. Xia, C. L. Li and Y. Wang, J. Membr. Sci., 2016, 498, 263–275 CrossRef CAS.
- R. P. Pandey and V. K. Shahi, J. Membr. Sci., 2013, 444, 116–126 CrossRef CAS.
- P. V. Naik, L. H. Wee, M. Meledina, S. Turner, Y. Li and G. Van Tendeloo, J. Mater. Chem. A, 2016, 4, 12790–12798 RSC.
- P. H. T. Ngamou, J. P. Overbeek, R. Kreiter, H. M. Van Veen, J. F. Vente and I. M. Wienk, J. Mater. Chem. A, 2013, 1, 5567–5576 RSC.
- S. Claes, P. Vandezande, S. Mullens, K. De Sitter, R. Peeters and M. K. Van Bael, J. Membr. Sci., 2012, 389, 265–271 CrossRef CAS.
- D. P. Suhas, T. M. Aminabhavi and A. V. Raghu, Polym. Eng. Sci., 2014, 54, 1774–1782 CrossRef CAS.
- Y. Wang, X. Zou, L. Sun, H. Rong and G. Zhu, Chem. Sci., 2018, 9, 2533–2539 RSC.
- H. Sun, L. Lu, X. Chen and Z. Jiang, Sep. Purif. Technol., 2008, 58, 429–436 CrossRef CAS.
- A. Malekpour, B. Mostajeran and G. A. Koohmareh, Chem. Eng. Process. Process Intensif., 2017, 118, 47–53 CrossRef CAS.
- J. Zhao, Y. Zhu, F. Pan, G. He, C. Fang and K. Cao, J. Membr. Sci., 2015, 487, 162–172 CrossRef CAS.
- B. Liang, W. Zhan, G. Qi, S. Lin, Q. Nan and Y. Liu, J. Mater. Chem. A, 2015, 3, 5140–5147 RSC.
- H. K. Dave and K. Nath, J. Water Process Eng., 2016, 14, 124–134 CrossRef.
- J. Zhang, Z. Xu, W. Mai, C. Min, B. Zhou and M. Shan, J. Mater. Chem. A, 2013, 1, 3101–3111 RSC.
- A. M. Sajjan, B. K. Jeevan Kumar, A. A. Kittur and M. Y. Kariduraganavar, J. Membr. Sci., 2013, 425–426, 77–88 CrossRef CAS.
- B. Gao, Z. Jiang, C. Zhao, H. Goma and F. Pan, J. Membr. Sci., 2015, 492, 230–241 CrossRef CAS.
- B. Gao, Z. Jiang, M. Zhao, H. Wu, F. Pan, J. Q. Mayta, Z. Chang and X. Bu, J. Membr. Sci., 2018, 546, 31–40 CrossRef CAS.
- A. Kasik and Y. S. Lin, Sep. Purif. Technol., 2014, 121, 38–45 CrossRef CAS.
- W. Liang, L. Li, J. Hou, N. D. Shepherd, T. D. Bennett, D. M. D'Alessandro and V. Chen, Chem. Sci., 2018, 9, 3508–3516 RSC.
- M. S. Denny, J. C. Moreton, L. Benz and S. M. Cohen, Nat. Rev. Mater., 2016, 16078 CrossRef CAS.
- K. Divya and M. S. Jisha, Environ. Chem. Lett., 2018, 16(1), 101–112 CrossRef CAS.
- A. Ouyang and J. Liang, RSC Adv., 2014, 4(49), 25835–25842 RSC.
- A. L. Angelim, S. P. Costa, B. C. S. Farias, L. F. Aquino and V. M. M. Melo, J. Environ. Manage., 2013, 127, 10–17 CrossRef CAS PubMed.
- M. Nitsae, A. Madjid, L. Hakim and A. Sabarudin, Chem. Chem. Technol., 2016, 10(1), 105–114 CrossRef CAS.
- G. Dudek, M. Krasowska, R. Turczyn, M. Gnus and A. Strzelewicz, Sep. Purif. Technol., 2017, 182, 101–109 CrossRef CAS.
- G. Dudek, M. Gnus, A. Strzelewicz, R. Turczyn and M. Krasowska, Sep. Sci. Technol., 2018, 53(8), 1178–1190 CrossRef CAS.
- G. Dudek, A. Strzelewicz, R. Turczyn, M. Krasowska and A. Rybak, Sep. Sci. Technol., 2014, 49(11), 1761–1767 CrossRef CAS.
- G. Dudek, M. Gnus, R. Turczyn and K. Konieczny, Desalin. Water Treat., 2017, 64, 339–344 CrossRef CAS.
- G. Dudek, R. Turczyn, M. Gnus and K. Konieczny, Sep. Purif. Technol., 2018, 193, 398–407 CrossRef CAS.
- T. Sakaguchi, T. Horikoshi and A. Nakajima, Agric. Biol. Chem., 1981, 45(10), 2191–2195 CAS.
- J. R. Shainoff, Top. Catal., 1980, 95(2), 690–695 CAS.
- L. Poon, S. Younus and L. D. Wilson, J. Colloid Interface Sci., 2014, 420, 136–144 CrossRef CAS PubMed.
- G. Dudek, M. Gnus, R. Turczyn, A. Strzelewicz and M. Krasowska, Sep. Purif. Technol., 2014, 133, 8–15 CrossRef CAS.
- J. G. Wijmans and R. W. Baker, J. Membr. Sci., 1995, 107, 1–21 CrossRef CAS.
- C. J. Geankoplis,Transport Processes and Separation Process Principles. Transport processes and separation process principles, Upper Saddle River: Prentice-Hall International, 2014 Search PubMed.
- J. Z. Knaul, S. M. Hudson and K. A. M. Creber, J. Polym. Sci., Part B: Polym. Phys., 1999, 37, 1079–1094 CrossRef CAS.
- J. M. Guisán, Enzyme Microb. Technol., 1988, 10(6), 375–382 CrossRef.
- M. M. Beppu, R. S. Vieira, C. G. Aimoli and C. C. Santana, J. Membr. Sci., 2007, 301(1–2), 126–130 CrossRef CAS.
- B. K. Satishbabu, V. R. Sandeep, R. B. Ravi and R. Shrutinag, Indian J. Pharm. Sci., 2010, 72(6), 738–744 CrossRef CAS PubMed.
- S. V. Kononova, A. V. Volod'ko, V. A. Petrova, E. V. Kruchinina, Y. G. Baklagina, E. A. Chusovitin and Y. A. Skorik, Carbohydr. Polym., 2018, 181, 86–92 CrossRef CAS PubMed.
- M. G. Carneiro-da-Cunha, M. A. Cerqueira, B. W. S. Souza, S. Carvalho, M. A. C. Quintas, J. A. Teixeira and A. A. Vicente, Carbohydr. Polym., 2010, 82(1), 153–159 CrossRef CAS.
- J. H. Chen, X. F. Dong and Y. S. He, RSC Adv., 2016, 6(65), 60765–60772 RSC.
- R. Y. M. Huang, R. Pal and G. Y. Moon, J. Membr. Sci., 2000, 167(2), 275–289 CrossRef CAS.
- G. Lawrie, I. Keen, B. Drew, A. Chandler-Temple, L. Rintoul, P. Fredericks and L. Grøndahl, Biomacromolecules, 2007, 8(8), 2533–2541 CrossRef CAS PubMed.
- K. Sunitha, S. V. Satyanarayana and S. Sridhar, Carbohydr. Polym., 2012, 87(2), 1569–1574 CrossRef CAS.
- L. Wang, J. Li, Y. Lin and C. Chen, Chem. Eng. J., 2009, 146(1), 71–78 CrossRef CAS.
- M. Gnus, G. Dudek, R. Turczyn, A. Tórz, D. Łącka, K. Konieczny and M. Lapkowski, Prog. Chem. Appl. Chitin Its Deriv., 2015, 20, 54–63 CAS.
- Q. Li, Q. Liu, J. Zhao, Y. Hua, J. Sun, J. Duan and W. Jin, J. Membr. Sci., 2017, 544, 68–78 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07868h |
| This journal is © The Royal Society of Chemistry 2018 |

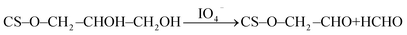
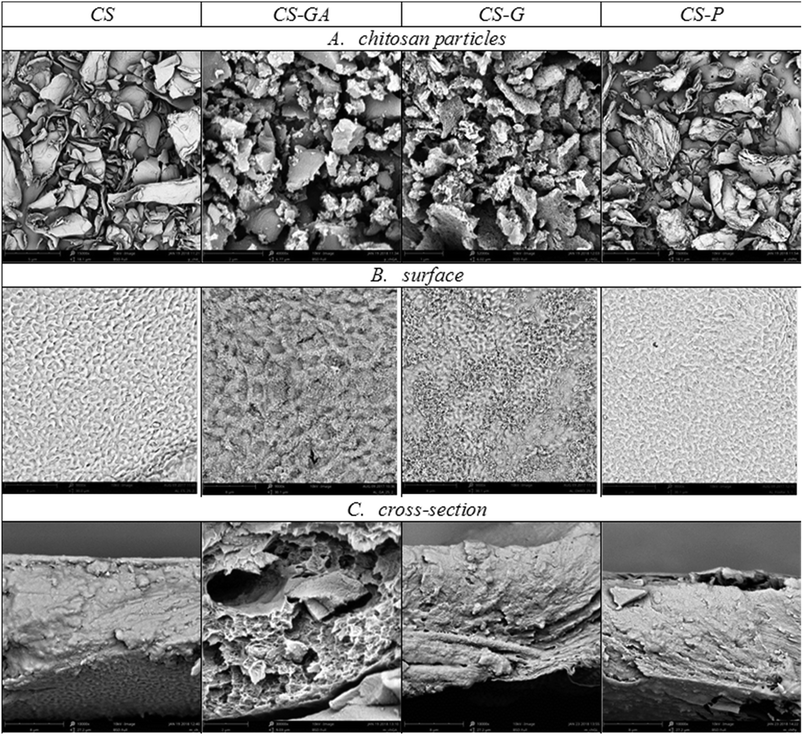
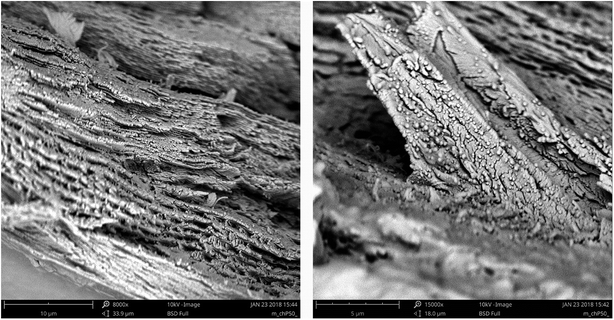
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000×); (B) surface and (C) cross-sectional view of hybrid Alg_CS membranes, respectively (magnification 10
000×); (B) surface and (C) cross-sectional view of hybrid Alg_CS membranes, respectively (magnification 10