Open Access Article
Open Access ArticleOrganoamine-induced isomerism of calixarene-based complexes: from 1D to 2D†
Xiaofei Zhuab,
Shentang Wangb,
Haitao Hanb and
Wuping Liao *b
*b
aSchool of Chemistry and Life Science, Changchun University of Technology, Changchun 130012, China. E-mail: wpliao@ciac.ac.cn
bState Key Laboratory of Rare Earth Resource Utilization, ERC for the Separation and Purification of REs and Thorium, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, China
First published on 23rd November 2018
Abstract
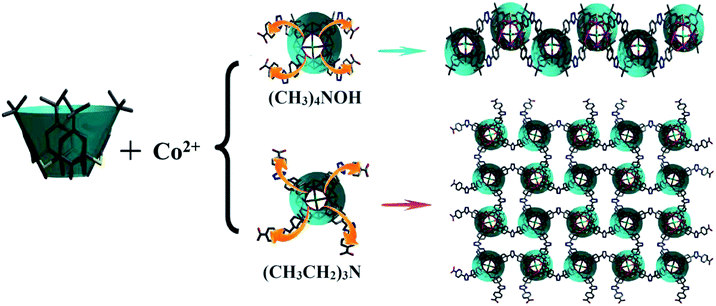
Two isomers of the calixarene-based cobalt complex [Co4Cl(TC4A)(BCPT)2]− (H4TC4A = p-tert-butylthiacalix[4]arene; H2BCPT = 3,5-bis (4′-carboxy-phenyl)-1,2,4-triazole) were obtained under the solvothermal conditions with tetramethylammonium/tetraethylammonium hydroxide (CIAC-236) and triethylamine (CIAC-237). Single crystal X-ray diffraction reveals that CIAC-236 has a 1D zigzag aggregate constructed by bridging the shuttlecock-like Co4–TC4A secondary building units (SBUs) with two pairs of opposite V-shaped BCPT ligands while CIAC-237 possesses a 2D layer assembly with each Co4–TC4A SBU bonded by four BCPT ligands in a same direction (clockwise or counterclockwise), which indicates that different shapes of the organoamines lead to different assembly of the BCPT ligands and the formation of different extended aggregates. For comparison, only the 1D structure ([Fe4Cl(TC4A)](BCPT)2]−, CIAC-238) was obtained for the iron complexes with all these three organoamines, which would be attributed to different property and coordination of iron element. Magnetic properties of all these three compounds were studied.
Introduction
The study of coordination polymers has stepped into a higher gear due to their fascinating topologies and intriguing properties in diverse fields such as host–guest chemistry, gas separation and storage, magnetics, catalysis, etc.1 However, it is still a challenge to design and prepare the desired structures with specific properties. One approach is to use the polynuclear clusters as the nodes and the multidentate ligands as the bridges.2 Calixarenes are a typical class of macrocyclic complexes used as the host materials and the platform for the designed receptors.3,4 They are also a kind of good ligand for the construction of coordination polymers. The shuttlecock-like polynuclear {MIIx–calixarene}n+ SBUs can be linked into a variety of structures such as the clusters,5 coordination cages,6 and extended entities/networks by some deliberately chosen O/N-donor ligands and bifunctional reagents.7–10 N-heterocyclic carboxylic acid H2BCPT would be an excellent candidate to connect {MIIx–calixarene}n+ SBUs into the coordination polymers due to its flexible backbone and rich coordination sites (carboxylate groups and the N-atoms).11 Here we present two isomeric coordination polymers constructed by bridging the Co4–TC4A SBUs with the V-shaped H2BCPT.As reported, many factors such as the metal cations, the linkers, the templates, reaction temperature, pH and even the solvent could affect the structures and the properties of the products.12 Notably, the template plays a decisive role in the structure of the product so that the product structure can be designed by selecting a specific template.13,14 Different from the study on the template effect of calixarenes themselves,15 we introduced different organoamines such as tetramethylammonium hydroxide, tetraehtylammonium hydroxide and triethylamine into the mixture of cobalt/iron chloride, p-tert-butylthiacalix[4]arene and 3,5-bis (4′-carboxy-phenyl)-1,2,4-triazole to study the effect of the amines. We successfully obtained two kinds of assemblies of cobalt, one 1D wavelike chain (CIAC-236) and one 2D network (CIAC-237). However, for the iron system, the template effect of the organoamines can be neglected and only the 1D motif (CIAC-238) was obtained with all these three organoamines. Magnetic properties of these compounds were studied.
Experimental section
Materials, syntheses and characterization
H4TC4A was synthesized by the literature method,16 and the other reagents were obtained commercially and used as received. FT-IR spectra (KBr pellets) were recorded in the range of 400–4000 cm−1 on a Bruker IFS 66 V/S FT/IR spectrometer. Elemental analyses for C, H and N were performed on a Perkin-Elmer 2400. Powder X-ray diffraction (PXRD) patterns were collected on a Bruker X-ray Diffractometer with graphite monochromatized Cu-Kα radiation (λ = 1.5418 Å) with an increment of 0.02° in 2θ between 5 to 50° and a scanning rate of 5° min−1. Thermogravimetric analyses (TGA) were performed on a Perkin-Elmer Thermal Analyzer. Field-cooled DC magnetic susceptibility measurements were performed on polycrystalline samples using a Quantum Design MPMS XL-7 SQUID system in the temperature range 2–300 K and under the applied magnetic field of 1000 Oe. Diamagnetic corrections for the sample and sample holder were applied.Synthesis of the 1D isomer of [Co4Cl(TC4A)(BCPT)2]− (CIAC-236)
A 23 ml Teflon-lined stainless steel container charged with a mixture of CoCl2·6H2O (100 mg, 0.4 mmol), H4TC4A (72 mg, 0.1 mmol), H2BCPT (46 mg, 0.15 mmol), N,N-dimethylformamide (DMF) (5 ml), CH3OH (5 ml) and tetramethylammonium hydroxide solution (25%, 0.2 ml) was sealed and heated at 130 °C for 3 days, and then cooled to room temperature at the rate of 4 °C h−1. The product was isolated as purple block crystals in 62% yield based on Co. Elemental analysis (%): calcd for C76H74ClCo4N7O12S4 (formula weight: 1676.83): C, 54.39, H, 4.41, N, 5.84; found: C, 53.92, H, 4.44, N, 5.76. IR bands (KBr pellet, cm−1): 3738(s), 3001(s), 1852(m), 1657(m), 1587(s), 1527(w), 1384(m), 1253(m), 1030(m), 847(w), 775(w), 751(w), 644(w), 649(w), 471(w).Synthesis of the 2D isomer of [Co4Cl(TC4A)(BCPT)2]− (CIAC-237)
CIAC-237 was synthesized by a similar method as that of CIAC-236 except replacing tetramethylammonium hydroxide solution with triethylamine (0.3 ml). The product was isolated as purple block crystals in 68% yield based on Co. Elemental anal. (%): calcd for C76H74ClCo4N7O12S4 (formula weight: 1676.83): C, 54.39, H, 4.41, N, 5.84; found: C, 53.96, H, 4.54, N, 5.71. IR bands (KBr pellet, cm−1): 3729(s), 3001(s), 1850(m), 1587(m), 1562(s), 1503(w), 1420(m), 1324(m), 1055(m), 930(w), 834(w), 755(w), 617(w), 477(w).Synthesis of 1D [Fe4Cl(TC4A)(BCPT)2]− (CIAC-238)
Compound CIAC-238 was synthesized with FeCl2·6H2O in place of CoCl2·6H2O following an otherwise identical procedure with either CIAC-236 or CIAC-237. The product was isolated as yellow block crystals in 68% yield based on Fe. Elemental anal. (%): calcd for C76H74ClFe4N7O12S4 (formula weight: 1664.51): C, 54.79, H, 4.44, N, 5.88; found: C, 54.29, H, 3.96, N, 5.25. IR bands (KBr pellet, cm−1): 3714(s), 3006(s), 1736(m), 1646(m), 1562(s), 1492(w), 1395(m), 1228(m), 1001(m), 915(w), 847(w), 811(w), 751(w), 644(w), 465(w).Single crystal X-ray diffraction
The intensity data were recorded on a Bruker D8 QUEST system with Cu-Kα radiation (λ = 1.54178 Å). The crystal structures were solved by means of Direct Methods and refined employing full-matrix least squares on F2 (SHELXTL-97). The diffraction data were treated by the “SQUEEZE” method as implemented in PLATON.17 All non-hydrogen atoms were refined anisotropically, and hydrogen atoms of the organic ligands were generated theoretically onto the specific atoms and refined isotropically with fixed thermal factors. The unidentified solvent molecules and counter ions were not included for all the structures. CCDC 1864223–1864225 contain the supplementary crystallographic data for this paper.Results and discussion
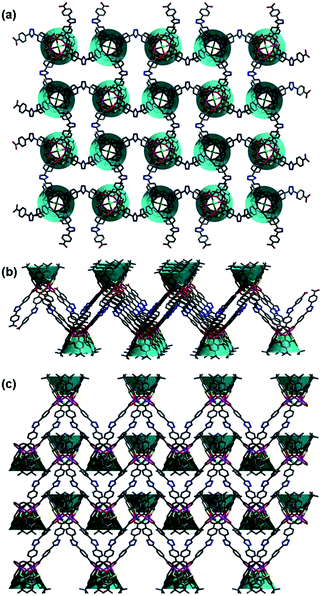
Single crystal X-ray diffraction analyses revealed that all these three compounds are feathered with the shuttlecock-like M4–TC4A (M = Co, Fe) SBUs which are further interconnected into 1D chains or 2D network. The arrangement of the BCPT ligand plays a key role in the formation of these isomers (Scheme 1). But the BCPT ligand acts as a bidentate linker with its nitrogen atoms un-bonded.CIAC-236 was obtained from the system with tetramethylammonium hydroxide. It crystallizes in the monoclinic system with space group P21/m and has some 1D zigzag chains. As shown in Fig. 1, four Co(II) atoms are capped by one TC4A molecule with a cone conformation to give a shuttlecock-like Co4–TC4A SBU. This SBU is bonded by two pairs of BCPT ligands through their carboxyl groups and further bridged into an infinite wave-like chain (Fig. 2). All the calixarene molecules are located on the wave crests and troughs as those in the system with 1,3-di(4-carboxyphenyl)benzene (DCPB) being the linker.7a When viewed along the b axis, the chain exhibits an oval pore so that the chain can also be thought as a coordination tube (Fig. 2b). The extended structure is stacked by these chains/coordination tubes in the ac plane (Fig. 2c). It should be noted that the BCPT ligands in a chain are divided into some oppositely aligned pairs as the DCPB ligands.7a However, compared with DCPB in which the angle between two carboxyl groups is 116.2° and the dihedral angle between two carboxyl group planes is of 55.3°, BCPT has different angles of 141.3° and 70.1° (Fig. 3), which can be attributable to the fact that CIAC-236 has some oval-like tubular channels but no quadrangular ones.
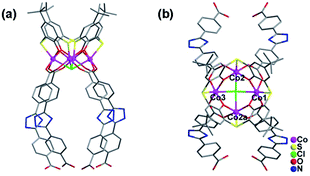 | ||
| Fig. 1 Coordination of the Co4–TC4A SBU in CIAC-236. (a) Side view and (b) top view. Symmetry codes: a: x, 1.5 − y, z. | ||
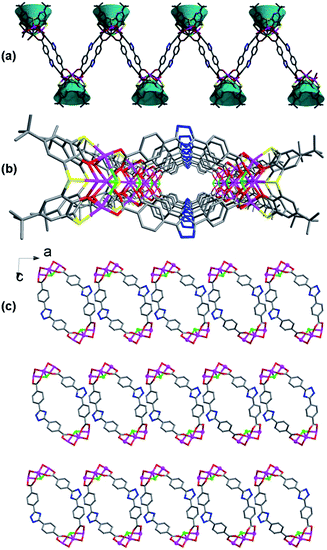 | ||
| Fig. 2 1D zigzag chain of CIAC-236 (a) and its view along the chain (b), and the extended structure of CIAC-236 (c). | ||
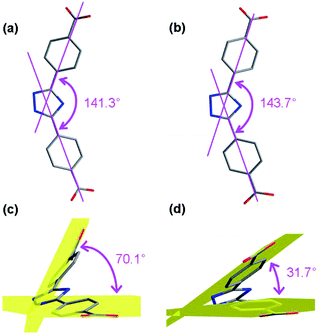 | ||
| Fig. 3 Comparison of the angles between two carboxyl groups and their dihedral angles of BCPT in CIAC-236 (a and c) and CIAC-237 (b and d). | ||
When tetramethylammonium hydroxide was replaced by triethylamine, a layer structure of CIAC-237 was obtained. CIAC-237 crystallizes in a tetragonal system with space group P4212. In CIAC-237, four adjacent cobalt atoms are also capped by a TC4A ligand adopting a cone conformation to form a Co4–TC4A shuttlecock-like SBU (Fig. 4). Then these SBUs are bonded by four BCPT ligands arranging in clockwise direction and further bridged into some wave-like 2D motifs (Fig. 5a). The Co4–TC4A SBUs on the troughs are arranged in an opposite direction to those on the crests while in the reported 2D network connected by 5-(4-pyridyl)tetrazolate,7b the ones on the troughs are rotated by 120.2° compared to those on the crests. The different assembly would be caused by different coordination methods of BCPT and 5-(4-pyridyl)tetrazolate and different coordination of metal atoms in these SBUs. The wave-like layers are stacked along the b axis into an extended structure by supramolecular interactions (Fig. 5c). A major difference between CIAC-237 and CIAC-236 is the different arrangement of the BCPT linkers, that is, CIAC-237, each four of them are located in a same direction so that the SBUs can be interconnected in four directions, while in CIAC-236, BCPT are arranged into some oppositely aligned pairs helpful to the formation of 1D structure. In addition, although the angle between two carboxyl groups is comparable to that in CIAC-236 (143.7° vs. 141.3°), the dihedral angle between the carboxyl group planes is of 31.7° which is much smaller than that in CIAC-236 (70.1°).
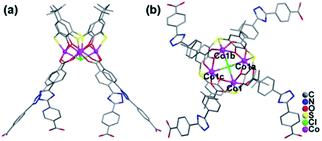 | ||
| Fig. 4 Coordination of the Co4–TC4A SBU in CIAC-237. (a) Side view and (b) top view. Symmetry codes: a: 0.5 + y, 0.5 − x, z; b: 1 − x, −y, z; c: 0.5 − y, −0.5 + x, z. | ||
 | ||
| Fig. 5 Top-view (a) and side-view (b) of the 2D meta-calixarene aggregate, and the arrangement of the coordination layers into an extended structure (c). | ||
It should be noted that the replace of tetramethylammonium hydroxide by tetraethylammonium hydroxide did not affect the product structure and 1D motif was also obtained. So it can be deduced that the formation of different isomers under analogous conditions would be attributed to the template effect of the organoamines. Actually, the tetrahedral ligands tetramethylammonium/tetraethylammonium lead to the formation of 1D structure while the cone-like ligand triethylammonium to 2D structure. However, the substitution of Co2+ by Fe2+ resulted in only one kind of product, 1D entity of ([Fe4Cl(TC4A)](BCPT)2]− (CIAC-238)), which might be attributed to different property and coordination of iron element.
To further investigate the template effect of organoammonium, some control experiments with inorganic bases such as NaOH and KOH being used to adjust the pH value of the mixtures or without any base were processed. No single crystals suitable for the X-ray diffraction measurement were obtained with these inorganic bases or without any base. Furthermore, the metal ions also affect the formation of such two structures, and no suitable single crystals were obtained for other transition metals such as Ni2+, Mn2+ and Cu2+ even with organoamines.
Magnetic studies
Calixarenes are some ideal macrocycle molecules to bond and separate the metal centers and the magnetic properties of many metal–calixarene compounds was investigated.18 The solid static dc magnetic susceptibility for CIAC-236–238 was also recorded in the temperature range 2–300 K under a magnetic field of 1000 Oe. For CIAC-236 and 237, the room temperature χMT values are 10.2 and 10.5 cm3 K mol−1, respectively, higher than the spin-only value 7.5 cm3 K mol−1 expected for four high-spin CoII (S = 3/2) ions. As shown in Fig. 6, the χMT values are gradually decreased to 0.2 and 0.3 cm3 K mol−1, respectively, upon cooling to 2 K. The steady decline of χMT cooling from 300 to 2 K indicates the antiferromagnetic coupling within the molecules. As described above, CIAC-236 features a 1D zigzag structure based on tetra-nuclear Co4–TC4A SBUs interconnected by BCPT ligands (Co⋯Co distance: 3.2–4.6 Å). The Co⋯Co distance between the adjacent Co4–TC4A SBUs ranges from 16.3 Å to 18.5 Å. Due to the long distance between the adjacent SBUs, the magnetic structure of CIAC-236 could be simplified as four isotropic CoII4 coupling unit. Similarly, the magnetic structure of CIAC-237/238 could also be regarded as isotropic CoII4/FeII4 coupling unit. As depicted in Fig. 6, the χM−1 data for CIAC-236–238 obey the Curie–Weiss law χM−1 = (T − θ)/C (CIAC-236/237: 300–50 K; CIAC-238: 300–75 K), where the Curie constant (C) and the Weiss constant (θ) could be deduced as C = 11.5 cm3 K mol−1, θ = −41.3 K (CIAC-236); C = 12.0 cm3 K mol−1, θ = −42.8 K (CIAC-237) and C = 23.7 cm3 K mol−1, θ = −169.8 K (CIAC-238). The negative θ values reveal the antiferromagnetic interactions between the metal centers of CIAC-236/237/238.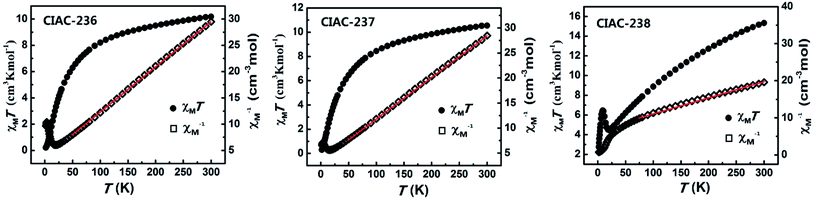 | ||
| Fig. 6 Plots of χMT versus T and χM−1 versus T for compounds CIAC-236, -237 and -238. The red lines represent the best fitting based on the Curie–Weiss equation. | ||
Conclusions
In summary, we successfully obtained two cobalt–calixarene coordination isomers with a V-shaped ligand BCPT using different organoamines as the template. BCPT is coordinated as a bidentate ligand by two carboxyl groups but not the nitrogen atoms. The different arrangement of BCPT ligands deduced by the template of organoamines leads to different structures of the products. However, this template effect does not work on the system with iron. This work gives another example for the template effect of organoamines and offers new information on the crystal engineering of calixarene-based crystalline materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 21571172, 21521092 and 21471022). SKLRERU Open Research Fund (RERU2016017). The 13th Five-Year Plan for Science & Technology Research sponsored by Department of Education of Jilin Province (No. JJKH20170542KJ) and Jilin Provincial Science Research Foundation of China (No. 20170101097JC).Notes and references
- (a) H. C. Zhou and S. Kitagawa, Chem. Soc. Rev., 2014, 43, 5415 RSC; (b) H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed; (c) B. Li, M. Chrzanowski, Y. Zhang and S. Ma, Coord. Chem. Rev., 2016, 307, 106 CrossRef CAS; (d) L. B. Li, R. B. Lin, R. Krishna, X. Q. Wang, B. Li, H. Wu, J. P. Li, W. Zhou and B. L. Chen, J. Am. Chem. Soc., 2017, 139, 7733 CrossRef CAS PubMed; (e) M. Meilikhov, S. Furukawa, K. Hirai, R. A. Fischer and S. Kitagawa, Angew. Chem., Int. Ed., 2013, 52, 341 CrossRef CAS PubMed; (f) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196 CrossRef CAS PubMed.
- (a) Q. X. Yang, X. Q. Chen, J. H. Cui, J. S. Hu, M. D. Zhang, L. Qin, G. F. Wang, Q. Y. Lu and H. G. Zheng, Cryst. Growth Des., 2012, 12, 4072 CrossRef CAS; (b) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS PubMed; (c) P. Kanoo, K. L. Gurunatha and T. K. Maji, Cryst. Growth Des., 2009, 9, 4147 CrossRef CAS.
- (a) S. J. Dalgarno, P. K. Thallapally, L. J. Barbour and J. L. Atwood, Chem. Soc. Rev., 2007, 36, 236 RSC; (b) S. J. Dalgarno, N. P. Power and J. L. Atwood, Coord. Chem. Rev., 2008, 252, 825 CrossRef CAS; (c) P. Jin, S. J. Dalgarno and J. L. Atwood, Coord. Chem. Rev., 2010, 254, 1760 CrossRef CAS; (d) B. S. Creaven, D. F. Donlon and J. McGinley, Coord. Chem. Rev., 2009, 253, 893 CrossRef CAS.
- (a) D. M. Homden and C. Redshaw, Chem. Rev., 2008, 108, 5086 CrossRef CAS PubMed; (b) J. L. Atwood, L. J. Barbour, M. J. Hardie and C. L. Raston, Coord. Chem. Rev., 2001, 222, 3 CrossRef CAS; (c) N. Morohashi, F. Narumi, N. Iki, T. Hattori and S. Miyano, Chem. Rev., 2006, 106, 5291 CrossRef CAS PubMed; (d) R. Kumar, Y. O. Lee, V. Bhalla, M. Kumar and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4824 RSC.
- (a) G. Karotsis, S. Kennedy, S. J. Teat, C. M. Beavers, D. A. Fowler, J. J. Morales, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, J. Am. Chem. Soc., 2010, 132, 12983 CrossRef CAS PubMed; (b) S. M. Taylor, G. Karotsis, R. D. McIntosh, S. Kennedy, S. J. Teat, C. M. Beavers, W. Wernsdorfer, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem.–Eur. J., 2011, 17, 7521 CrossRef CAS PubMed; (c) H. Kumari, A. V. Mossine, S. R. Kline, C. L. Dennis, D. A. Fowler, S. J. Teat, C. L. Barnes, C. A. Deakyne and J. L. Atwood, Angew. Chem., Int. Ed., 2012, 51, 1452 CrossRef CAS PubMed; (d) Y. F. Bi, X. T. Wang, W. P. Liao, X. F. Wang, X. W. Wang, H. J. Zhang and S. Gao, J. Am. Chem. Soc., 2009, 131, 11650 CrossRef CAS PubMed; (e) S. T. Wang, Y. F. Bi and W. P. Liao, CrystEngComm, 2015, 17, 2896 RSC; (f) T. Kajiwara, N. Iki and M. Yamashita, Coord. Chem. Rev., 2007, 251, 1734 CrossRef CAS.
- (a) Y. F. Bi, S. C. Du and W. P. Liao, Coord. Chem. Rev., 2014, 15, 61 CrossRef; (b) H. Q. Tan, S. C. Du, Y. F. Bi and W. P. Liao, Inorg. Chem., 2014, 53, 7083 CrossRef CAS PubMed; (c) K. Z. Su, F. L. Jiang, J. J. Qian, L. Chen, J. D. Pang, S. M. Bawaked, M. Mokhtar, S. A. Al-Thabaiti and M. C. Hong, Inorg. Chem., 2015, 54, 3183 CrossRef CAS PubMed; (d) F. R. Dai and Z. Q. Wang, J. Am. Chem. Soc., 2012, 134, 8002 CrossRef CAS PubMed; (e) S. T. Wang, X. H. Gao, X. X. Hang, X. F. Zhu, H. T. Han, W. P. Liao and W. Chen, J. Am. Chem. Soc., 2016, 138, 16236 CrossRef CAS PubMed; (f) X. X. Hang, B. Liu, X. F. Zhu, S. T. Wang, H. T. Han, W. P. Liao, Y. L. Liu and C. H. Hu, J. Am. Chem. Soc., 2016, 138, 2969 CrossRef CAS PubMed; (g) X. X. Hang, S. T. Wang, X. F. Zhu, H. T. Han and W. P. Liao, CrystEngComm, 2016, 18, 4938 RSC; (h) Y. Fang, Z. F. Xiao, J. L. Li, C. Lollar, L. J. Liu, X. Z. Lian, S. Yuan, S. Banerjee, P. Zhang and H. C. Zhou, Angew. Chem., Int. Ed., 2018, 57, 5283 CrossRef CAS PubMed.
- (a) M. Liu, S. C. Du and W. P. Liao, J. Mol. Struct., 2013, 1049, 310 CrossRef CAS; (b) X. X. Hang, S. C. Du, S. T. Wang and W. P. Liao, Inorg. Chem. Commun., 2014, 47, 152 CrossRef CAS; (c) S. T. Wang, X. H. Gao, X. X. Hang, X. F. Zhu, H. T. Han, W. P. Liao and W. Chen, J. Am. Chem. Soc., 2018, 140, 6271 CrossRef CAS PubMed.
- (a) Y. F. Bi, W. P. Liao, G. C. Xu, R. P. Deng, M. Y. Wang, Z. J. Wu, S. Gao and H. J. Zhang, Inorg. Chem., 2010, 49, 7735 CrossRef CAS PubMed; (b) H. Q. Tan, S. C. Du, Y. F. Bi and W. P. Liao, Chem. Commun., 2013, 49, 8211 RSC; (c) F. R. Dai, U. Sambasivam, A. J. Hammerstrom and Z. Q. Wang, J. Am. Chem. Soc., 2014, 136, 7480 CrossRef CAS PubMed; (d) N. L. Netzer, F. R. Dai, Z. Q. Wang and C. Y. Jiang, Angew. Chem., Int. Ed., 2014, 53, 1 CrossRef PubMed; (e) C. M. Liu, D. Q. Zhang, X. Hao and D. B. Zhu, Eur. J. Inorg. Chem., 2012, 26, 4210 CrossRef.
- (a) C. Chen, J. F. Ma, B. Liu, J. Yang and Y. Y. Liu, Cryst. Growth Des., 2011, 11, 4491 CrossRef CAS; (b) Y. F. Bi, W. P. Liao, X. F. Wang, Y. L. Li, Z. M. Su, Y. B. Liu, H. J. Zhang and D. Q. Lia, CrystEngComm, 2009, 11, 597 RSC; (c) W. P. Liao, Y. L. Li, X. F. Wang, Y. F. Bi, Z. M. Su and H. J. Zhang, Chem. Commun., 2009, 14, 1861 RSC.
- (a) Y. F. Bi, W. P. Liao and H. J. Zhang, Cryst. Growth Des., 2008, 8, 3630 CrossRef CAS; (b) R. G. Lin, L. S. Long, R. B. Huang and L. S. Zheng, Cryst. Growth Des., 2008, 8, 791 CrossRef CAS.
- (a) X. Y. Hou, X. Wang, F. Fu, J. J. Wang and L. Tang, J. Coord. Chem., 2013, 66, 3126 CrossRef CAS; (b) X. Y. Hou, X. Wang, H. Wang, L. J. Gao, F. Fu, J. J. Wang, L. Tang and J. Cao, J. Coord. Chem., 2015, 68, 1814 CrossRef CAS.
- (a) A. Bilyk, J. W. Dunlop, R. O. Fuller, A. K. Hall, J. M. Harrowfield, M. W. Hosseini, G. A. Koutsantonis, I. W. Murray, B. W. Skelton, R. L. Stamps and A. H. White, Eur. J. Inorg. Chem., 2010, 2106 CrossRef CAS; (b) Q. L. Guo, W. X. Zhu, S. L. Ma, S. J. Dong and M. Q. Xu, Polyhedron, 2004, 23, 1461 CrossRef CAS; (c) D. Yuan, W. X. Zhu, S. Ma and X. Yan, J. Mol. Struct., 2002, 616, 241 CrossRef CAS; (d) D. Q. Yuan, Y. Q. Xu, M. C. Hong, W. H. Bi, Y. F. Zhou and X. Li, Eur. J. Inorg. Chem., 2005, 1182 CrossRef CAS; (e) N. Morohashi, N. Iki, A. Sugawara and S. Miyano, Tetrahedron, 2001, 57, 5557 CrossRef CAS; (f) X. X. Hang, B. Liu, X. F. Zhu, S. T. Wang, H. T. Han, W. P. Liao, Y. L. Liu and C. H. Hu, J. Am. Chem. Soc., 2016, 138, 969 CrossRef PubMed.
- (a) F. N. Dai, H. Y. He, D. L. Gao, F. Ye, X. L. Qiu and D. F. Sun, CrystEngComm, 2009, 11, 2516 RSC; (b) Y. Takashima, S. Furukawa and S. Kitagawa, CrystEngComm, 2011, 13, 3360 RSC; (c) M. Chen, S. S. Chen, T.-A. Okamura, Z. Su, M. S. Chen, Y. Zhao, W. Y. Sun and N. Ueyama, Cryst. Growth Des., 2011, 11, 1901 CrossRef CAS.
- J. H. Li, S. Meng, J. F. Zhang, Y. L. Song, Z. P. Huang, H. J. Zhao, H. Y. Wei, W. J. Huang, M. P. Cifuentes, M. G. Humphrey and C. Zhang, CrystEngComm, 2012, 14, 2787 RSC.
- A. Ignaszak, N. Patterson, M. Radtke, M. R. J. Elsegood, J. W. A. Frese, J. L. Z. F. Lipman, T. Yamato, S. Sanz, E. K. Brechin, T. J. Prior and C. Redshaw, Dalton Trans., 2018, 47, 15983 RSC.
- N. Iki, C. Kabuto, T. Fukushima, H. Kumagai, H. Takeya, S. Miyanari, T. Miyashi and S. Miyano, Tetrahedron, 2000, 56, 1437 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- (a) G. Mislin, E. Graf, M. W. Hosseini, A. Bilyk, A. K. Hall, J. M. Harrowfield, B. W. Skelton and A. H. White, Chem. Commun., 1999, 373 RSC; (b) H. Akdas, E. Graf, M. W. Hosseini, A. De Cian, A. Bilyk, B. W. Skelton, G. A. Koutsantonis, I. Murray, J. M. Harrowfield and A. H. White, Chem. Commun., 2002, 1042 RSC; (c) A. Bilyk, A. K. Hall, J. M. Harrowfield, M. W. Hosseini, G. Mislin, B. W. Skelton, C. Taylor and A. H. White, Eur. J. Inorg. Chem., 2000, 823 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: X-ray crystallographic data in CIF format for CIAC-236–238 and TG analysis of the as-synthesized samples. CCDC 1864223–1864225. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra07833e |
| This journal is © The Royal Society of Chemistry 2018 |