DOI:
10.1039/C8RA07830K
(Paper)
RSC Adv., 2018,
8, 38140-38145
Expansion of the photoresponse window of a BiVO4 photocatalyst by doping with chromium(VI)†
Received
20th September 2018
, Accepted 2nd November 2018
First published on 14th November 2018
Abstract
Doping of Cr6+ into BiVO4 was examined in this study. A new absorption band with a 1.84 eV energy threshold appeared with Cr-doping. The theoretical band calculation has revealed that the new absorption is ascribed to the electron transition from the valence band to acceptor levels formed by empty Cr 3d orbitals. It was confirmed that photocatalytic water oxidation in the presence of Ag+ or Fe3+ of an oxidizing reagent was induced by excitation of the new absorption although activity under band gap excitation decreased with Cr-doping. Characteristics of carrier dynamics were also investigated by transient absorption spectroscopy.
1. Introduction
Much attention has been paid to photocatalytic water splitting into H2 and O2 as one candidate method for solar hydrogen production.1,2 Although highly efficient water splitting has been achieved under ultraviolet irradiation,1–4 a visible-light response is highly demanded from the viewpoint of solar H2 production. Overall water splitting under visible light by suspended single photocatalyst systems has been achieved, but their efficiencies are still low.5,6 The Z-scheme system composed of two kinds of photocatalysts bringing about either H2 or O2 evolution is another strategy for photocatalytic water splitting under visible light.7 One of the merits of the Z-scheme system is that water splitting can be achieved by utilization of photocatalysts incapable of water splitting themselves. Monoclinic BiVO4 with a scheelite structure (ms-BiVO4) is one of the representative O2-evolving photocatalysts for the Z-scheme systems.7,8 ms-BiVO4 can be combined with many kinds of H2-evolving photocatalysts, such as SrTiO3:Rh, CuGaS2, Cu2ZnGeS4, CdS, LaMg1/3Ta2/3O2N and La5Ti2CuS5O7, to achieve Z-scheme water splitting.9–15 It is noteworthy that particulate ms-BiVO4 is used in photocatalyst sheets giving 1.1–1.2% solar-to-hydrogen energy conversion efficiencies.10,11 Electrodes based on ms-BiVO4 are also utilized as photoanodes for photoelectrochemical water splitting.16–18 Thus, ms-BiVO4 is an attractive visible-light-responsive photocatalyst for water oxidation. However, the development of photocatalysts working under longer wavelengths is still demanded because the photoresponse of ms-BiVO4 up to 520 nm wavelength is not sufficient in terms of solar energy conversion.19 A variety of attempts have so far been made to improve the performance of BiVO4, especially its photoelectrochemical efficiency,16–18,20–23 while there are no reports regarding extension of the photoresponse window of BiVO4.
As some partially oxidized metal cations such as Pb2+, Bi3+, Ag+, Sn2+ and Cu+ are known as constituents to form valence bands (VBs) or electron donor levels at shallow potential in comparison with O2−, these cations are used for visible light driven photocatalysts.1,24 Nevertheless, formation of electron acceptor levels at a deeper (more positive) position than the conduction band (CB) seems to be effective in expanding the photoresponse window of ms-BiVO4 rather than formation of electron donor levels because of the presence of a shallow VB by the contribution of Bi3+. A hexavalent chromium ion is known as one candidate constituent to form electron acceptor levels or CB at deep potentials.25,26 The Cr6+-substitution for PbMoO4 is a good example of narrowing the energy gaps of scheelite-type compounds.25 In this study, substitution of Cr6+ for V5+ in ms-BiVO4 was investigated with the aim of expanding the photoresponse window.
2. Experiments
2.1 Sample preparation
Powders of BiV1−xCrxO4 were synthesized by a liquid solid state reaction (LSR) method.8 Raw materials of 20 mmol of Bi(NO3)3·5H2O (Kanto, 99.9%), 9.5–10 mmol of V2O5 (Kanto, 99.0%) and 0–0.1 mmol of CaCrO4 (Strem, 95%) were added to 30 ml of 0.1 mol l−1 HNO3. The suspended solution was stirred for 26–168 h in the dark under ambient conditions. The resulting precipitation was washed with 1 mol l−1 HNO3 followed by distilled water, and was then dried at 80 °C for 5 h in air. Synthesis of the Cr-doped sample using Cr2O3 instead of CaCrO4 was also conducted by the same manner for comparison.
2.2 Characterization
Crystal phases in the obtained samples were identified by X-ray diffraction (XRD; Bruker AXS, D2 PHASER). Photoabsorption spectra were taken by a diffuse reflection method using an ultraviolet-visible-near infrared (IR) spectrometer (Shimadzu, UV-3100) with an integrating sphere and obtained spectra were converted by the Kubelka–Munk method. Energy gaps of indirect transition in non-doped and Cr-doped samples were determined from the (αhν)1/2–hν plot (Tauc plot for indirect transition), where α, h and ν represent the Kubelka–Munk function, Planck constant and frequency, respectively. The particles were observed by scanning electron microscopy (SEM; Hitachi, SU-1510). Specific surface areas were determined by N2 adsorption and BET analysis (MicrotracBEL, BELSORP-miniII). Elemental compositions were analysed by inductively coupled plasma atomic emission spectroscopy (ICP-AES; SPECTRO, ARCOS EOP).
2.3 Photocatalytic reactions
Photocatalytic activity for O2 evolution from an aqueous AgNO3 (Kanto, 99.5%) solution was examined in a gas-closed circulation system with an on-line gas chromatograph (Shimadzu, GC-14B, TCD, MS-5A column, Ar carrier). Photocatalyst powder (0.1 g) was dispersed in 160 ml of a 0.02 mol l−1 aqueous AgNO3 solution containing 0.1 g of La2O3 as a pH buffer to adjust the pH to around 8.5. Photocatalytic O2 evolution from 2 mmol l−1 of an aqueous Fe(ClO4)3 (Kanto, 90%) solution, the pH of which was adjusted to 2.3 by HClO4 (Kanto, 60%), was also examined. The photocatalyst was irradiated by a 300 W Xe arc lamp (Excelitas, Cermax PE300BF) with two kinds of conditions (λ > 420 and 540 nm). The transmittance spectra of cut-off filters used are shown in Fig. S1†.
2.4 Theoretical calculations of band structures
The band structures were calculated by the plane wave based density functional theory (DFT) using the CASTEP program.27,28 The Perdew–Burke–Ernzerhof (PBE) functional was used together with the ultrasoft-core potentials.29–31 The cutoff energies were set to 300 eV. The electron configurations of the atoms were O: 2s22p4, Ca: 3s23p64s2, V: 3s23p63d34s2, Cr: 3s23p63d44s2 and Bi: 6s26p3. Super cells of Bi4V4O16 and Bi15CaV15CrO64 were employed as models of non-doped and Cr-doped BiVO4. For the Cr-doped model, one Bi atom was also replaced with a Ca atom accompanied by the substitution of Cr for V to maintain the charge balance in the Cr-doped BiVO4 system. The total numbers of electrons were 168 and 678 for Bi4V4O16 and Bi15CaV15CrO64, respectively. It was confirmed that transitions from the VB to CB minimum and from VB to the Cr 3d orbital are indirect.
2.5 Time resolved transient absorption measurements
The microsecond time-resolved visible to mid-IR absorption measurements were performed by using laboratory-built spectrometers as described in the previous paper.32 Briefly, in the mid-IR region (6000–1000 cm−1), the measurement was carried out in transmission mode, wherein the probe light emitted from a MoSi2 coil was focused on the sample, and then the transmitted light was introduced to a grating spectrometer. The monochromated light was then detected by an MCT detector (Kolmar), and the output electric signal was amplified with an AC-coupled amplifier (Stanford Research Systems, SR560, 1 MHz). In the visible to near IR region (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–6000 cm−1), the experiments were performed in the reflection mode, wherein the probe light that comes from a halogen lamp (50 W) was focused on the sample and detected using Si or InGaAs photodiodes. In each experiment, the pump ultraviolet laser pulses (355 nm) that originated from the Nd:YAG laser (Continuum, Surelite I, duration: 6 ns, power: 0.5 mJ, repetition rate: 10–0.01 Hz) were utilized to excite the samples. The time resolution of the spectrometers was limited to 1–2 μs by the bandwidth of the amplifier. To determine the decay processes and reactivity of photogenerated charge carriers, the measurements were performed in vacuum at room temperature.
000–6000 cm−1), the experiments were performed in the reflection mode, wherein the probe light that comes from a halogen lamp (50 W) was focused on the sample and detected using Si or InGaAs photodiodes. In each experiment, the pump ultraviolet laser pulses (355 nm) that originated from the Nd:YAG laser (Continuum, Surelite I, duration: 6 ns, power: 0.5 mJ, repetition rate: 10–0.01 Hz) were utilized to excite the samples. The time resolution of the spectrometers was limited to 1–2 μs by the bandwidth of the amplifier. To determine the decay processes and reactivity of photogenerated charge carriers, the measurements were performed in vacuum at room temperature.
3. Results and discussion
3.1 Synthesis of Cr-doped ms-BiVO4
A single phase of ms-BiVO4 was obtained in the non-doped sample after 26 h of reaction, while formation of the ms-BiVO4 phase by the LSR slowly proceeded in the Cr(2%)-doped sample (Fig. S2†). At 26 h of reaction time, the Cr(2%)-doped sample was still a mixture of V2O5, Bi6O5(OH)3(NO3)5(H2O)3 and a tiny amount of tetragonal BiVO4 with a zircon structure (tz-BiVO4). Even for the Cr(2%)-doped sample, the single phase of ms-BiVO4 was eventually obtained with 100 h of reaction time and longer. Both samples with and without Cr-doping showed no changes in the intensity and width of diffraction peaks with a prolonged reaction time of up to 1 week. Synthesis of BiVO4 with different amounts of Cr-doping (0.1–5%) was attempted by the LSR method with 1 week of reaction time (Fig. 1). The single phase of ms-BiVO4 was obtained with up to 2% Cr-doping while mixtures of ms- and tz-BiVO4 were obtained for samples with larger amounts of doping (3–5%). Synthesis of the pure phase of ms-BiVO4 with Cr(3–5%) has not been achieved by the LSR method even with a further prolonged reaction time of up to 4 weeks. Doping with Cr resulted in no significant changes in the morphology and size of particles (Fig. S3†). Elemental analysis by ICP-AES proved that, in the samples obtained as the pure phase of ms-BiVO4, the amounts of Cr incorporated in BiVO4 increased with the increases in the intended values although actual values were slightly lower than the nominal ones (Table 1). The fact that the supernatant solution obtained after the synthesis of the Cr-doped sample was yellow due to the presence of Cr6+ ions also supported the slightly lower amounts of Cr in the obtained samples. In the substitution of aliovalent ions, that is, substitution of Cr6+ for V5+ in the present case, co-substitution of Ca2+ for Bi3+ was thought to be one kind of ideal charge compensation. However, the elemental analysis proved that such co-substitution of Ca did not occur and suggested the formation of V defects to keep the charge balance. The samples synthesized using Cr2O3 instead of CaCrO4 were obtained as a pure phase of ms-BiVO4, but no Cr was incorporated in the sample as shown in Table 1. The excessively large ionic radius of Cr3+ against V5+ is the reason for no substitution of Cr3+ into ms-BiVO4 using the LSR method. No report of the ionic radius of Cr3+ in a 4-fold coordination in the paper by Shannon also supports the difficulty of substitution of Cr3+ for tetrahedral sites.33 Thus, it is obvious that Cr ions incorporated into ms-BiVO4 by the LSR method are not Cr3+ but Cr6+.
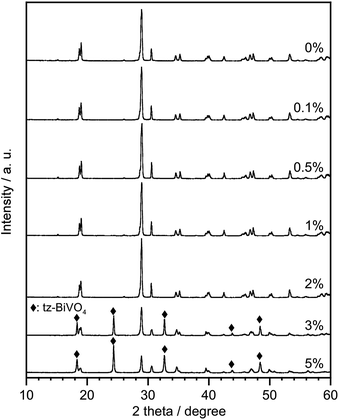 |
| | Fig. 1 XRD patterns of BiVO4 doped with various amounts of Cr synthesized by the LSR method with 1 week of reaction time. | |
Table 1 Elemental composition determined by ICP-AES analysis
| Cr Contenta/mol% |
Atomic composition |
| Bi |
Ca |
V |
Cr |
| In the starting mixture. Cr2O3 was used as the source of Cr. |
| 0 |
1 |
— |
1.018 |
— |
| 0.5 |
1 |
0 |
0.990 |
0.004 |
| 1 |
1 |
0 |
0.981 |
0.007 |
| 2 |
1 |
0 |
0.975 |
0.016 |
| 2b |
1 |
0 |
1.024 |
0 |
| 3 |
1 |
0 |
1.024 |
0.025 |
| 5 |
1 |
0 |
1.099 |
0.016 |
3.2 Photoabsorption properties and band structure
The Cr-doped ms-BiVO4 showed a new absorption band in addition to the intrinsic band gap excitation of ms-BiVO4 as shown in Fig. 2. The intensity of the new absorption band became stronger as the amount of Cr doping increased. There are no doubts that chromium is responsible for the appearance of the low energy absorption band. It was expected that the new absorption could be attributed to electron transition from VB to acceptor levels formed by the empty 3d orbitals of Cr6+. Theoretical calculations of band structures based on DFT were performed for super cells of Bi4V4O16 and Bi15CaV15CrO64 as models of non-doped and Cr-doped samples. Although the charge balance was kept not by Ca2+ ions but V5+ defects in the real samples, the co-substituted model was used to simplify the calculation. Fig. 3 depicts the projected density of states (PDOS) near the band gap of Bi4V4O16 and Bi15CaV15CrO64. The empty Cr 3d orbitals appeared below CB in Bi15CaV15CrO64 and no other significant differences were seen between Bi4V4O16 and Bi15CaV15CrO64 in PDOS near the band gap. Thus, it has been proven that Cr6+ ions doped into ms-BiVO4 form acceptor levels in the band gap and that the new absorption band is attributed to electron transition from the valence band to the acceptor levels formed by Cr6+ similar to Cr-doped PbMoO4.25 The threshold energy of the new absorption band was determined from the Tauc plot to be 1.86 and 1.84 eV for Cr(0.1%)- and Cr(0.5–2%)-doped samples (Fig. S4†) and this is narrower by 0.52–0.54 eV than the band gap energy of non-doped ms-BiVO4 (2.38 eV). These experimental values are in good agreement with the theoretically calculated values, 1.59 and 2.23 eV for Bi4V4O16 and Bi15CaV15CrO64, although the theoretical values are slightly smaller than the experimental values as is usually observed. Moreover, a 1.84 eV energy gap is narrower than those of other Cr6+-containing photocatalysts, PbMoO4:Cr (2.26 eV) and PbCrO4 (2.3 eV).25,26
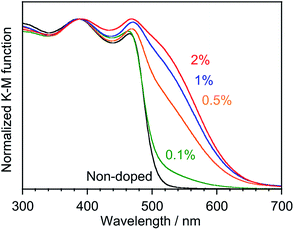 |
| | Fig. 2 Absorption spectra of Cr-doped ms-BiVO4. | |
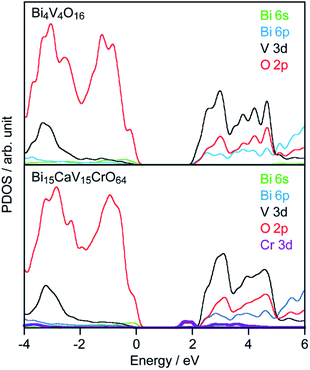 |
| | Fig. 3 PDOS of Bi4V4O16 and Bi15CaV15CrO64. | |
3.3 Photocatalytic activity
Table 2 summarizes the photocatalytic activities of Cr-doped ms-BiVO4 for the O2 evolution reaction using Ag+ as an oxidizing reagent. Two kinds of irradiation conditions were examined to see photocatalytic activity induced by the excitation of the new absorption band. Whole visible light (λ > 420 nm) enables band gap excitation while a portion of the longer wavelength side of visible light (λ > 540 nm) is insufficient for band gap excitation but enables excitation of the new absorption. Indeed, the rate of O2 evolution over non-doped ms-BiVO4 was very low under the long wavelength light irradiation. Activity under whole visible light decreased with doping with Cr up to 0.5% and then increased with further doping while activity under the long wavelength light irradiation was monotonically increased with the amount of Cr. It has been reported that large particles are favorable for O2 evolution with it being a 4-electron reaction.34 However, such effects of the particle size were negligible in the present samples possessing similar particle sizes of around 1.5 μm and the almost constant specific surface area (SBET) of 1.3 ± 0.2 m2 g−1. The improvements of the activity induced by excitation of the new absorption are due to enhancement of absorption with the amount of Cr doping. It was also confirmed that Cr(2%)-doped ms-BiVO4 produced O2 from an aqueous Fe(ClO4)3 solution (2 mmol l−1, pH 2.3) at a rate of 0.14 μmol h−1 under long wavelength light irradiation despite there being no O2 evolution over non-doped ms-BiVO4. The results described above prove that the carriers photogenerated by excitation of the new absorption band, electrons in acceptor levels formed by empty Cr 3d and holes in VB, are available for photocatalytic water oxidation reactions using oxidizing reagents despite low activity; 0.06% and 0.03% apparent quantum yields for O2 evolution over a Cr(2%)-doped sample from AgNO3 at 540 and 580 nm, respectively.
Table 2 Photocatalytic activity of Cr-doped ms-BiVO4a
| Cr/mol% |
SBET/m2 g−1 |
O2 evolution/μmol h−1 |
| λ > 420 nm |
λ > 540 nm |
| Catalyst: 0.1 g, reactant solution: 0.02 mol l−1 AgNO3 with 0.1 g of La2O3, light source: 300 W Xe lamp. |
| 0 |
1.1 |
5.9 |
0.2 |
| 0.1 |
1.1 |
2.2 |
0.3 |
| 0.5 |
1.3 |
1.7 |
0.4 |
| 1.0 |
1.1 |
2.5 |
0.5 |
| 2.0 |
1.5 |
3.5 |
0.9 |
3.4 Transient absorption analysis
Doping with foreign elements frequently decreases photocatalytic activity due to enhancement of carrier recombination by dopants.35 Transient absorption measurements were performed to analyse carrier dynamics. Fig. 4 shows transient absorption spectra of non-doped and Cr(2%)-doped ms-BiVO4 taken under vacuum. It is known that transient absorption owing to trapped holes appears near the band gaps whereas deeply trapped electrons give absorption at 5000–19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1.32 Non-doped ms-BiVO4 gave two obvious kinds of absorption bands ascribed to trapped holes at 18
000 cm−1.32 Non-doped ms-BiVO4 gave two obvious kinds of absorption bands ascribed to trapped holes at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–21
000–21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and deeply trapped electrons at 6000–18
000 cm−1 and deeply trapped electrons at 6000–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1. In addition, absorption ascribed to free and/or shallowly trapped electrons, which is usually observed at lower energy than 5000 cm−1, was very weak in non-doped ms-BiVO4. Cr(2%)-doped ms-BiVO4 showed three different features in the transient absorption spectra in comparison with the non-doped sample. (1) The absorption attributed to trapped holes remarkably shifted to 15
000 cm−1. In addition, absorption ascribed to free and/or shallowly trapped electrons, which is usually observed at lower energy than 5000 cm−1, was very weak in non-doped ms-BiVO4. Cr(2%)-doped ms-BiVO4 showed three different features in the transient absorption spectra in comparison with the non-doped sample. (1) The absorption attributed to trapped holes remarkably shifted to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1 in the Cr(2%)-doped sample accompanied with a lower energy gap (threshold energy: 14
600 cm−1 in the Cr(2%)-doped sample accompanied with a lower energy gap (threshold energy: 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 cm−1). (2) The shape of absorption for deeply trapped electrons was changed by Cr-doping and three obvious bands were seen at 9400, 11
840 cm−1). (2) The shape of absorption for deeply trapped electrons was changed by Cr-doping and three obvious bands were seen at 9400, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 13
000 and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1. (3) The absorption at the lowest energy side attributed to free or shallowly trapped electrons slightly increased by doping with Cr6+. The significant changes in the structure of transient absorption relating to the deeply and shallowly trapped electrons could be caused by the electron acceptor levels formed by Cr6+ ions and/or the V5+ defects. Decay curves of shallowly trapped electrons at 4500 cm−1, deeply trapped electrons at 11
600 cm−1. (3) The absorption at the lowest energy side attributed to free or shallowly trapped electrons slightly increased by doping with Cr6+. The significant changes in the structure of transient absorption relating to the deeply and shallowly trapped electrons could be caused by the electron acceptor levels formed by Cr6+ ions and/or the V5+ defects. Decay curves of shallowly trapped electrons at 4500 cm−1, deeply trapped electrons at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and trapped holes at 20
000 cm−1 and trapped holes at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 15
000 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1 for non-doped and Cr(2%)-doped ms-BiVO4 are presented in Fig. 5. The shallowly trapped electrons monitored at 4500 cm−1 slowly decreased in the Cr(2%)-doped sample compared to the non-doped one. The decay curves at 11
600 cm−1 for non-doped and Cr(2%)-doped ms-BiVO4 are presented in Fig. 5. The shallowly trapped electrons monitored at 4500 cm−1 slowly decreased in the Cr(2%)-doped sample compared to the non-doped one. The decay curves at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 indicated that the deeply trapped electrons also decreased faster in the non-doped sample than in the Cr(2%)-doped one. Both samples showed almost the same slope in the decay curves for trapped holes, where it should be noted that the direct comparison of absorbance between the samples was difficult because of the remarkable difference in the peak positions. These carrier dynamics analyses prove that Cr6+ ions doped into ms-BiVO4 do not dominantly facilitate the recombination of electrons and holes despite formation of electron traps.
000 cm−1 indicated that the deeply trapped electrons also decreased faster in the non-doped sample than in the Cr(2%)-doped one. Both samples showed almost the same slope in the decay curves for trapped holes, where it should be noted that the direct comparison of absorbance between the samples was difficult because of the remarkable difference in the peak positions. These carrier dynamics analyses prove that Cr6+ ions doped into ms-BiVO4 do not dominantly facilitate the recombination of electrons and holes despite formation of electron traps.
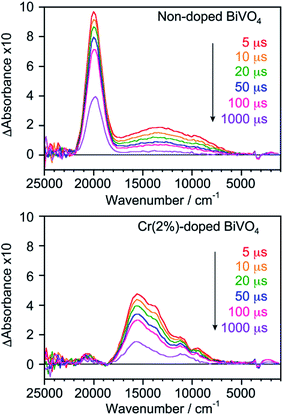 |
| | Fig. 4 Transient absorption spectra of non-doped and Cr(2%)-doped ms-BiVO4 taken under vacuum. | |
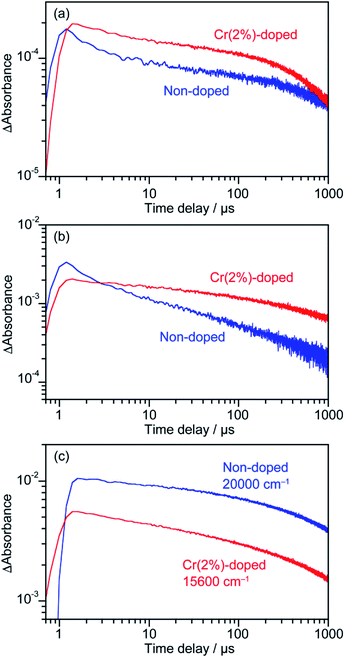 |
| | Fig. 5 Decay curves of transient absorption of non-doped and Cr(2%)-doped ms-BiVO4 monitored at (a) 4500 cm−1, (b) 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and (c) 20 000 cm−1 and (c) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 15 000 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1, respectively. 600 cm−1, respectively. | |
4. Conclusions
It has been confirmed that substitution of Cr6+ for V5+ in ms-BiVO4 is possible by the LSR method. The Cr-doping results in the appearance of a new absorption band with a 1.84 eV energy threshold. Photocatalytic O2 evolution can be induced by excitation of the new absorption band. Thus, it has been demonstrated that doping with Cr6+ ions is a way of expanding the photoresponse window of ms-BiVO4. Transient carrier dynamics analyses indicate that decreases in the photocatalytic activity by doping with Cr6+ are not due to enhanced carrier recombination and suggest that the low mobility of electrons in the discrete deep traps (empty Cr 3d) limits the activity at this time. It implies the possibility of further improvements of activity by modification. O2 evolution using Fe3+ as an electron acceptor suggests the possibility of applying Cr-doped ms-BiVO4 to Z-scheme systems after some improvements.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This study was partly supported by KAKENHI Grant Numbers JP16H04186, JP16H04188 and JP17H05491 and Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials.
References
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253 RSC.
- X. Chen, S. Shen, L. Guo and S. Mao, Chem. Rev., 2010, 110, 6503 CrossRef CAS PubMed.
- Y. Sakata, Y. Matsuda, T. Nakagawa, R. Yasunaga, H. Imamura and K. Teramura, ChemSusChem, 2010, 4, 181 Search PubMed.
- Y. Ham, T. Hisatomi, Y. Goto, Y. Moriya, Y. Sakata, A. Yamakata, J. Kubota and K. Domen, J. Mater. Chem. A, 2016, 4, 3027 RSC.
- C. Pan, T. Takata, M. Nakabayashi, T. Matsumoto, N. Shibata, Y. Ikuhara and K. Domen, Angew. Chem., Int. Ed., 2015, 54, 295 Search PubMed.
- W. Liu, L. Cao, W. Cheng, u. Cao, X. Liu, W. Zhang, Z. Mou, L. Jin, X. Zheng, W. Che, Q. Liu, T. Yao and S. Wei, Angew. Chem., Int. Ed., 2017, 56, 9312 CrossRef CAS PubMed.
- K. Maeda, ACS Catal., 2013, 3, 1486 CrossRef CAS.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459 CrossRef CAS.
- H. Kato, Y. Sasaki, N. Shirakura and A. Kudo, J. Mater. Chem. A, 2013, 1, 12327 RSC.
- Q. Wang, T. Hisatomi, Q. Jia, H. Tokudome, M. Zhong, C. Wang, Z. Pan, T. Takata, M. Nakabayashi, N. Shibata, Y. Li, I. D. Sharp, A. Kudo, T. Yamada and K. Domen, Nat. Mater., 2016, 15, 611 CrossRef CAS PubMed.
- Q. Wang, T. Hisatomi, Y. Suzuki, Z. Pan, J. Seo, M. Katayama, T. Minegishi, H. Nishiyama, T. Takata, K. Seki, A. Kudo, T. Yamada and K. Domen, J. Am. Chem. Soc., 2017, 139, 1675 CrossRef CAS PubMed.
- A. Iwase, S. Yoshino, T. Takayama, Y. H. Ng, R. Amal and A. Kudo, J. Am. Chem. Soc., 2016, 138, 10260 CrossRef CAS PubMed.
- Z. Pan, T. Hisatomi, Q. Wang, S. Chen, M. Nakabayashi, N. Shibata, C. Pan, T. Takata, M. Katayama, T. Minegishi, A. Kudo and K. Domen, ACS Catal., 2016, 6, 7188 CrossRef CAS.
- Y.-J. Yuan, D. Chen, S. Yang, L.-X. Yang, J.-J. Wang, D. Cao, W. Tu, Z.-T. Yu and Z.-G. Zou, J. Mater. Chem. A, 2017, 5, 21205 RSC.
- S. Sun, T. Hisatomi, Q. Wang, S. Chen, G. Ma, J. Liu, S. Nandy, T. Minegishi, M. Katayama and K. Domen, ACS Catal., 2018, 8, 1690 CrossRef CAS.
- K. Sayama, A. Nomura, T. Arai, T. Sugita, R. Abe, M. Yanagida, T. Oi, Y. Iwasak, Y. Abe and H. Sugihara, J. Phys. Chem. B, 2006, 110, 11352 CrossRef CAS PubMed.
- F. F. Abdi, L. Han, A. H. M. Smets, M. Zeman, B. Dam and R. van de Krol, Nat. Commun., 2013, 4, 2195 CrossRef PubMed.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990 CrossRef CAS PubMed.
- K. Maeda and K. Domen, J. Phys. Chem. Lett., 2010, 1, 2655 CrossRef CAS.
- S. J. Hong, S. Lee, J. S. Jang and J. S. Lee, Energy Environ. Sci., 2011, 4, 1781 RSC.
- R. Li, F. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Commun., 2013, 4, 1432 CrossRef PubMed.
- R. Li, H. Han, F. Zhang, D. Wang and C. Li, Energy Environ. Sci., 2014, 7, 1369 RSC.
- Z. Jiang, Y. Liu, T. Jing, B. Huang, X. Zhang, X. Qin, Y. Dai and M.-H. Whangbo, J. Phys. Chem. C, 2016, 120, 2058 CrossRef CAS.
- I. Sullivan, B. Zoellner and P. A. Maggard, Chem. Mater., 2016, 28, 5999 CrossRef CAS.
- Y. Shimodaira, H. Kato, H. Kobayashi and A. Kudo, Bull. Chem. Soc. Jpn., 2007, 80, 885 CrossRef CAS.
- Y. Miseki, O. Kitao and K. Sayama, RSC Adv., 2015, 5, 1452 RSC.
- M. C. Payne, M. P. Teter, D. C. Allan, T. A. Arias and J. D. Johnnopoulos, Rev. Mod. Phys., 1992, 64, 1045 CrossRef CAS.
- V. Milman, B. Winkler, J. A. White, C. J. Pickard, M. C. Payne, E. V. Akhmatskaya and R. H. Nobes, Int. J. Quantum Chem., 2000, 77, 895 CrossRef CAS.
- D. Vanderbilt, Phys. Rev. B, 1991, 43, 6796 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396 CrossRef CAS.
- A. Yamakata, J. J. M. Vequizo and H. Matsunaga, J. Phys. Chem. C, 2015, 119, 24538 CrossRef CAS.
- R. D. Shannon, Acta Crystallogr., 1976, A32, 751 CrossRef CAS.
- F. Amano, E. Ishinaga and A. Yamakata, J. Phys. Chem. C, 2013, 117, 22584 CrossRef CAS.
- T. Ikeda, T. Nomoto, K. Eda, Y. Mizutani, H. Kato, A. Kudo and H. Onishi, J. Phys. Chem. C, 2008, 112, 1167 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Transmittance spectra of optical filters used, XRD patterns of samples obtained, scanning electron microscopy images and a Tauc plot. See DOI: 10.1039/c8ra07830k |
|
| This journal is © The Royal Society of Chemistry 2018 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Junie Jhon M. Vequizo
*a,
Junie Jhon M. Vequizo b,
Akira Yamakata
b,
Akira Yamakata *b,
Hisayoshi Kobayashic,
Makoto Kobayashi
*b,
Hisayoshi Kobayashic,
Makoto Kobayashi a and
Masato Kakihanaa
a and
Masato Kakihanaa
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–6000 cm−1), the experiments were performed in the reflection mode, wherein the probe light that comes from a halogen lamp (50 W) was focused on the sample and detected using Si or InGaAs photodiodes. In each experiment, the pump ultraviolet laser pulses (355 nm) that originated from the Nd:YAG laser (Continuum, Surelite I, duration: 6 ns, power: 0.5 mJ, repetition rate: 10–0.01 Hz) were utilized to excite the samples. The time resolution of the spectrometers was limited to 1–2 μs by the bandwidth of the amplifier. To determine the decay processes and reactivity of photogenerated charge carriers, the measurements were performed in vacuum at room temperature.
000–6000 cm−1), the experiments were performed in the reflection mode, wherein the probe light that comes from a halogen lamp (50 W) was focused on the sample and detected using Si or InGaAs photodiodes. In each experiment, the pump ultraviolet laser pulses (355 nm) that originated from the Nd:YAG laser (Continuum, Surelite I, duration: 6 ns, power: 0.5 mJ, repetition rate: 10–0.01 Hz) were utilized to excite the samples. The time resolution of the spectrometers was limited to 1–2 μs by the bandwidth of the amplifier. To determine the decay processes and reactivity of photogenerated charge carriers, the measurements were performed in vacuum at room temperature.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1.32 Non-doped ms-BiVO4 gave two obvious kinds of absorption bands ascribed to trapped holes at 18
000 cm−1.32 Non-doped ms-BiVO4 gave two obvious kinds of absorption bands ascribed to trapped holes at 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–21
000–21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and deeply trapped electrons at 6000–18
000 cm−1 and deeply trapped electrons at 6000–18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1. In addition, absorption ascribed to free and/or shallowly trapped electrons, which is usually observed at lower energy than 5000 cm−1, was very weak in non-doped ms-BiVO4. Cr(2%)-doped ms-BiVO4 showed three different features in the transient absorption spectra in comparison with the non-doped sample. (1) The absorption attributed to trapped holes remarkably shifted to 15
000 cm−1. In addition, absorption ascribed to free and/or shallowly trapped electrons, which is usually observed at lower energy than 5000 cm−1, was very weak in non-doped ms-BiVO4. Cr(2%)-doped ms-BiVO4 showed three different features in the transient absorption spectra in comparison with the non-doped sample. (1) The absorption attributed to trapped holes remarkably shifted to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1 in the Cr(2%)-doped sample accompanied with a lower energy gap (threshold energy: 14
600 cm−1 in the Cr(2%)-doped sample accompanied with a lower energy gap (threshold energy: 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 cm−1). (2) The shape of absorption for deeply trapped electrons was changed by Cr-doping and three obvious bands were seen at 9400, 11
840 cm−1). (2) The shape of absorption for deeply trapped electrons was changed by Cr-doping and three obvious bands were seen at 9400, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 13
000 and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1. (3) The absorption at the lowest energy side attributed to free or shallowly trapped electrons slightly increased by doping with Cr6+. The significant changes in the structure of transient absorption relating to the deeply and shallowly trapped electrons could be caused by the electron acceptor levels formed by Cr6+ ions and/or the V5+ defects. Decay curves of shallowly trapped electrons at 4500 cm−1, deeply trapped electrons at 11
600 cm−1. (3) The absorption at the lowest energy side attributed to free or shallowly trapped electrons slightly increased by doping with Cr6+. The significant changes in the structure of transient absorption relating to the deeply and shallowly trapped electrons could be caused by the electron acceptor levels formed by Cr6+ ions and/or the V5+ defects. Decay curves of shallowly trapped electrons at 4500 cm−1, deeply trapped electrons at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and trapped holes at 20
000 cm−1 and trapped holes at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 15
000 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1 for non-doped and Cr(2%)-doped ms-BiVO4 are presented in Fig. 5. The shallowly trapped electrons monitored at 4500 cm−1 slowly decreased in the Cr(2%)-doped sample compared to the non-doped one. The decay curves at 11
600 cm−1 for non-doped and Cr(2%)-doped ms-BiVO4 are presented in Fig. 5. The shallowly trapped electrons monitored at 4500 cm−1 slowly decreased in the Cr(2%)-doped sample compared to the non-doped one. The decay curves at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 indicated that the deeply trapped electrons also decreased faster in the non-doped sample than in the Cr(2%)-doped one. Both samples showed almost the same slope in the decay curves for trapped holes, where it should be noted that the direct comparison of absorbance between the samples was difficult because of the remarkable difference in the peak positions. These carrier dynamics analyses prove that Cr6+ ions doped into ms-BiVO4 do not dominantly facilitate the recombination of electrons and holes despite formation of electron traps.
000 cm−1 indicated that the deeply trapped electrons also decreased faster in the non-doped sample than in the Cr(2%)-doped one. Both samples showed almost the same slope in the decay curves for trapped holes, where it should be noted that the direct comparison of absorbance between the samples was difficult because of the remarkable difference in the peak positions. These carrier dynamics analyses prove that Cr6+ ions doped into ms-BiVO4 do not dominantly facilitate the recombination of electrons and holes despite formation of electron traps.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1 and (c) 20
000 cm−1 and (c) 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 15
000 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cm−1, respectively.
600 cm−1, respectively.


