Open Access Article
Open Access ArticleDeep eutectic solvents as a new kind of dispersive solvent for dispersive liquid–liquid microextraction†
Andrey Shishov *,
Natalia Volodina,
Daria Nechaeva
*,
Natalia Volodina,
Daria Nechaeva ,
Svetlana Gagarinova and
Andrey Bulatov
,
Svetlana Gagarinova and
Andrey Bulatov
Department of Analytical Chemistry, Institute of Chemistry, Saint-Petersburg University, Saint Petersburg State University, SPbSU, SPbU, 7/9 Universitetskaya nab., St. Petersburg 199034, Russia. E-mail: a.y.shishov@spbu.ru
First published on 14th November 2018
Abstract
In this paper, deep eutectic solvents (DESs) were investigated as a new kind of dispersive solvent for effective dispersive liquid–liquid microextraction (DLLME) for the first time. It was established that the DESs can increase the extraction efficiency compared to conventional polar, water-miscible organic dispersive solvents.
One of the most common sample pretreatment methods used in chemical analysis is liquid–liquid extraction (LLE), which is characterized by simplicity, reliability and compatibility with modern analytical instrumentation. However, conventional LLE is time consuming and requires large amounts of organic solvents. Currently miniaturization, which means the significant reduction of the volume of hazardous organic solvents, is the main demand, which corresponds to the concept of green analytical chemistry (GAC).1 Thus, development of miniaturized, eco-friendly, less expensive, faster and simpler sample pretreatment methods is a very important task in GAC.
Dispersive liquid–liquid microextraction (DLLME) is an attractive tool for the miniaturized sample pretreatment of a wide range of sample matrices (water samples,2 biological fluids,3 foods,4 etc) introduced in 2006 by Rezaee et al.5 The DLLME has grown increasingly popular (ESM Fig. 1) due to its simplicity, low cost and high availability. In the DLLME, a cloudy solution of fine organic droplets is formed when a mixture of extraction solvent (immiscible with water) and dispersive solvent (miscible with both water and the extractant solvent) is rapidly injected into an aqueous phase. In the DLLME, large surface contact between fine organic droplets and sample speeds up the processes regarding mass-transfer of analytes from aqueous phase to organic phase which greatly enhances extraction efficiency. The DLLME can be coupled with various analytical methods (HPLC-UV,6 HPLC-MS,7 GC-MS,8 spectrophotometry,9 electrochemical methods,10 AAS,11 etc) and can by automated based on flow systems.12–14 However, one of the main disadvantages of the conventional DLLME is in need of using an additional dispersive organic solvent that can increase solubility of the hydrophobic analytes in an aqueous phase reducing the extraction efficiency.15 Moreover, relatively large volumes of hazardous dispersive organic solvents such as methanol, acetonitrile, acetone,16 etc are required to disperse extraction solvents in aqueous phase.
In this work, a new phenomenon – fast dispersion of extraction solvent in aqueous phase in the presence of deep eutectic solvent (DES) resulting excellent analyte extraction was established for the first time and used for the DLLME of bisphenol-A (BPA) as a proof-of-concept analyte in beverages followed by its determination by high-performance liquid chromatography with fluorescence detection (HPLC-FLD).
BPA is a polyphenol compound used in the manufacturing of polycarbonates-based plastic packaging. It is known, that BPA as a carcinogen can be found in food products stored in the plastic packaging.17 Therefore, monitoring of BPA's content in foods and drinks is an important task for analytical chemistry.
Recently, DESs are presently getting increased attention from researchers in analytical chemistry as high-efficiency extraction solvents.18 DESs are formed by mixing two or three cheap components that are capable of interacting through the formation of hydrogen bonds, to form a eutectic mixture with a melting point lower than that of each individual component.19 DESs are regarded as a cheap alternative to ionic liquids. DES are easy to be produced and cheaper due to lower cost of the raw materials.20
In this research, long-chain alcohols (heptanol, octanol, decanol) as extraction solvents mixed with various DESs as dispersive solvents were studied. DESs were prepared by mixing tetrabutylammonium bromide (TBABr) as a hydrogen bond acceptor and ethylene glycol, glycerol, acetic and formic acids as donors of hydrogen bond in the molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
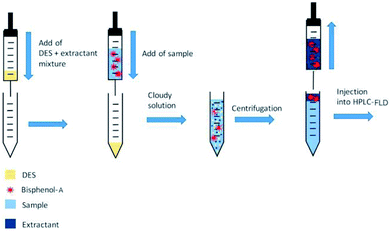
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 at 80 °C.21 The obtained DESs were viscous liquids at ambient condition. The kinematic viscosity values of the DESs were estimated using micro capillary viscometers (17.1, 15.7, 154.5 and 471 mm2 s−1 at 30 °C for the DES based on TBABr and formic acid, acetic acid, ethylene glycol and glycerol, respectively). Unlike conventional DLLME, the aqueous sample phase was injected into an extraction mixture of dispersive solvent (DES) and extraction solvent by a syringe because the extraction mixtures were viscous and their aspiration into the syringe was difficult. As result fast dissolution and decomposition of the DES and dispersion of the extraction solvent in aqueous sample phase was observed resulting fast extraction of BPA into extraction solvent droplets Fig. 1. After centrifugation (2 min, 4000 rpm) the upper phase was collected and injected into a HPLC-FLD system (LC-20 Prominence liquid chromatograph system, Shimadzu, Japan). Chromatographic separation was performed using a Zorbax Bonus-RP column (Agilent, 2500 × 2.1 mm, 3.5 μm). Mobile phase was deionized water-methanol (1
2 at 80 °C.21 The obtained DESs were viscous liquids at ambient condition. The kinematic viscosity values of the DESs were estimated using micro capillary viscometers (17.1, 15.7, 154.5 and 471 mm2 s−1 at 30 °C for the DES based on TBABr and formic acid, acetic acid, ethylene glycol and glycerol, respectively). Unlike conventional DLLME, the aqueous sample phase was injected into an extraction mixture of dispersive solvent (DES) and extraction solvent by a syringe because the extraction mixtures were viscous and their aspiration into the syringe was difficult. As result fast dissolution and decomposition of the DES and dispersion of the extraction solvent in aqueous sample phase was observed resulting fast extraction of BPA into extraction solvent droplets Fig. 1. After centrifugation (2 min, 4000 rpm) the upper phase was collected and injected into a HPLC-FLD system (LC-20 Prominence liquid chromatograph system, Shimadzu, Japan). Chromatographic separation was performed using a Zorbax Bonus-RP column (Agilent, 2500 × 2.1 mm, 3.5 μm). Mobile phase was deionized water-methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). The analyte was eluted with a flow rate of 1 mL min−1. Fluorescence detection was carried out with excitation and emission at 230 and 305 nm, respectively.
3, v/v). The analyte was eluted with a flow rate of 1 mL min−1. Fluorescence detection was carried out with excitation and emission at 230 and 305 nm, respectively.
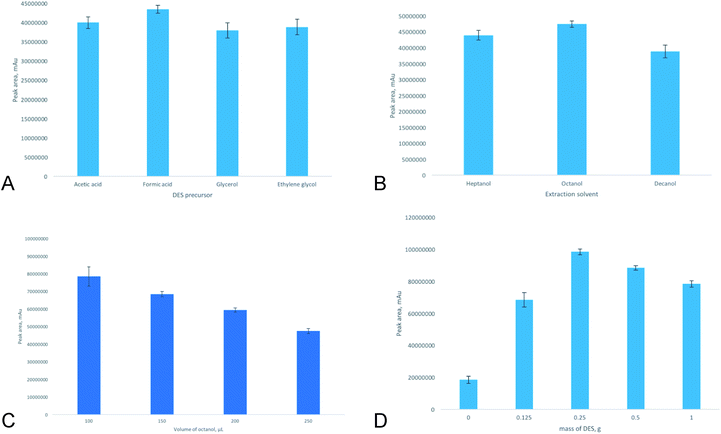
Criterion for the choice of DES as a dispersive solvent was the ability to be mixed well with the aqueous phase and extraction solvent as well to provide the maximum extraction efficiency and reproducibility. All DESs studied were mixed with long-chain alcohols used as extractants as well as with aqueous phase forming homogeneous solutions. The maximum peak areas were found for the DESs based on acetic and formic acids (Fig. 2A). This fact can be explained by suppression of analyte dissociation in acidic media resulting more effective analyte extraction. During aqueous sample injection into extraction mixture dissolution and decomposition of the DES is observed resulting acidification of the aqueous phase. Moreover, TBABr acts as salting-out agent and promotes BPA extraction. It should be pointed out that the DES based on the formic acid was characterized by less RSD value due to its less viscosity and it was chosen for future studies.
The selection of the extraction solvent is important in the DLLME to achieve good extraction efficiency and efficient analyte enrichment. Long-chain alcohols as green extraction solvents were studied for extraction of BPA. In the experiments, 5 mL of aqueous BPA solution (1 μg L−1) was injected into extraction mixture containing 250 μL of long-chain alcohol and 1 g of DES prepared (in all cases viscous liquid phase). It was found, that the use of octanol provided maximum extraction efficiency (Fig. 2B).
The volume of extraction solvent may result in preconcentration or dilution of extracted analyte. The volume of extraction solvent should be as small as possible to achieve high enrichment factor. 5 mL of aqueous BPA solution (1 mg L−1) was injected into extraction mixture containing 1 g of DES (TBABr–formic acid) and extraction solvent (octanol). The octanol volume was varied from 100 to 250 μL. It was shown (Fig. 2C), that maximum peak area was observed for 100 μL of octanol. However, in this case it was difficult to withdraw the upper organic phase resulting poor reproducibly (RSD = 15%). The use of 150 μL of octanol was characterized by satisfactory extraction efficiency and better reproducibly (RSD = 4%). Thus, 150 μL of octanol was chosen as the optimum volume.
To reduce the consumption of DES, the effect of its amount on the extraction efficiency was also studied. As can be seen from the data (Fig. 2D), amount of DES in the extraction mixture significantly influenced on the extraction process. The analytical signal was increased with DES mass decreasing up to 250 mg. BPA extraction into octanol phase without DES and in the presence of TBABr (190 mg) in aqueous phase was characterized by poor extraction efficiency (extraction recovery (ER) values were 32% and 41%, respectively). ER was calculated as ER = (CsopVsop/C0V0) × 100%, were Csop and C0 – analyte concentration in the separated organic phase and in the initial sample phase, respectively; Vsop and V0 – separated organic phase and sample volumes, respectively. If the DES content was higher than 250 mg dilution effect of extractant phase was predominant and solubility of analyte in aqueous phase was increased and as result analytical signal was reduced. This fact was confirmed by the HPLC-FLD determination of BPA in aqueous phase after the DLMME. Thus, DES mass of 250 mg was chosen as the optimum value.
After optimization of extraction conditions, the proposed approach was compared with the DLMME procedure using conventional polar, water-miscible organic dispersive solvents. For this purpose, the extraction mixture containing 150 μL of octanol and 250 μL of dispersive solvent (acetone, acetonitrile, methanol, ethanol or isopropyl alcohol) was injected into 5 mL of aqueous BPA solution (1 mg L−1) by the syringe. Moreover, formic acid was also studied as dispersive solvent. After centrifugation organic phase was analyzed. As can be seen from the obtained results (Fig. 3), the proposed approach provided significantly higher extraction efficiency. It was established, that ER values obtained for DES, acetone, acetonitrile, methanol, ethanol, isopropyl alcohol and formic acid were 95%, 49%, 61%, 58%, 55%, 59% and 47%, respectively. The obtained results shown that polar, water-miscible organic dispersive solvents increase solubility of BPA in an aqueous phase.
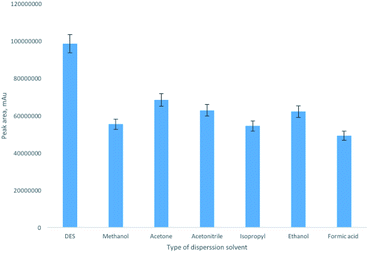 | ||
| Fig. 3 Comparison of the extraction efficiency using DES and polar organic solvents as dispersive solvents. | ||
To evaluate the proposed procedure, some relevant parameters such as enrichment factor (EF), linearity correlation coefficient (R2), precision, limit of detection (LOD) and limit of quantification (LOQ) were determined in the optimized condition. EF value was 33. EF was estimated as the ratio between the analyte concentration in the separated organic phase (Csop) and the initial concentration of analyte (C0) in the sample phase: EF = Csop/C0. The calibration graph was linear over the concentration ranges from 0.001 to 1 mg L−1 with linearity correlation coefficient of 0.996. The LOD and LOQ were calculated as three and ten times the standard deviation of the blank test (n = 10), respectively. The LOD was 0.0003 mg L−1 and the LOQ was 0.0009 mg L−1. The method's repeatability expressed as RSD (n = 5) was 4.0% and 2% at 0.001 mg L−1 and 1 mg L−1, respectively.
The developed procedure was used for the determination of BPA in three beverages samples (still water, sparkling water and Orange juice) purchased from local supermarkets (Saint Petersburg, Russia). As can be seen from the obtained data, BPA was found in sparkling water sample stored in a plastic bottle (Table 1). Accuracy and reliability of the resulting information were further studied by add-found method. The recoveries obtained for each of the samples were within of 93–110% range which is considered acceptable for this type of samples.
| Sample | Concentration, mg L−1 | Recovery, % | |
|---|---|---|---|
| Added | Found | ||
| Still water | 0 | <LOD | — |
| 0.01 | 0.0094 ± 0.0001 | 94 | |
| Sparkling water | 0 | 0.020 ± 0.002 | — |
| 0.01 | 0.028 ± 0.002 | 93 | |
| Orange juice | 0 | <LOD | — |
| 0.01 | 0.011 ± 0.002 | 110 | |
In conclusion, really effective DLMME approach using DES as a dispersive solvent was developed and successfully applied for the HPLC-FLD determination of BPA in beverages. Above all, DES allowed to significantly improve the extraction efficiency in comparison with conventional polar, water-miscible organic dispersive solvents. The benefits observed during the dispersion process are related to the action of the DES and individual components of the DES system. During aqueous sample injection into homogeneous octanol – DES mixture decomposition of the DES is observed resulting dispersion of octanol phase and extraction of analyte. In this case, TBABr as quaternary ammonium salt containing bromide ion can act as salting-out agent and promote mass transfer between the two phases. Comparative studies have indicated that the developed approach is a reliable analytical tool with wide potential applications in analysis of various sample matrixes and it has the opportunity to be coupled with other instrumental methods.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Andrey Shishov gratefully acknowledges financial support for research from Russian Scientific Foundation (project no. 18-73-00111). HPLC-FLD assay was performed at the Chemistry Educational Centre of Research Park of St. Petersburg State University.Notes and references
- S. Armenta, S. Garrigues and M. de la Guardia, TrAC, Trends Anal. Chem., 2008, 27, 497–511 CrossRef CAS.
- M. Rezaee, Y. Assadi, M.-R. Milani Hosseini, E. Aghaee, F. Ahmadi and S. Berijani, J. Chromatogr. A, 2007, 66, 8–86 Search PubMed.
- H. A. Mashayekhi, P. Abroomand-Azar, M. Saber-Tehrani and S. W. Husain, Chromatographia, 2010, 71, 517–521 CrossRef CAS.
- H. Abdolmohammad-Zadeh and G. H. Sadeghi, Talanta, 2010, 81, 778–785 CrossRef CAS PubMed.
- M. Rezaee, Y. Assadi, M.-R. Milani Hosseini, E. Aghaee, F. Ahmadi and S. Berijani, J. Chromatogr. A, 2006, 1116, 1–9 CrossRef CAS PubMed.
- X. W. Lai, D. L. Sun, C. Q. Ruan, H. Zhang and C. L. Liu, J. Sep. Sci., 2014, 37, 92–98 CrossRef CAS PubMed.
- R. S. Zhao, X. Wang, J. Sun, C. Hu and X. K. Wang, Microchim. Acta, 2011, 174, 145–151 CrossRef CAS.
- A. Melo, S. C. Cunha, C. Mansilha, A. Aguiar, O. Pinho and I. M. P. L. V. O. Ferreira, Food Chem., 2015, 38, 3552–3559 Search PubMed.
- N. Pourreza, S. Rastegarzadeh and A. Larki, Talanta, 2015, 134, 24–29 CrossRef CAS PubMed.
- E. Fernández, L. Vidal, D. Martín-Yerga, M. D. C. Blanco, A. Canals and A. Costa-García, Talanta, 2015, 135, 34–40 CrossRef PubMed.
- N. N. Meeravali, K. Madhavi and S. J. Kumar, J. Anal. At. Spectrom., 2016, 31, 1582–1589 RSC.
- A. N. Anthemidis and K.-I. G. Ioannou, Talanta, 2009, 79, 86–91 CrossRef CAS PubMed.
- V. Andruch, C. Cecilia Acebal, J. Skrlikova, H. Sklenarova, P. Solich, I. S. Balogh, F. Billes and L. Kocurova, Microchem. J., 2012, 100, 77–82 CrossRef CAS.
- A. Bulatov, K. Medinskaia, D. Aseeva, S. Garmonov and L. Moskvin, Talanta, 2015, 133, 66–70 CrossRef CAS PubMed.
- J. Regueiro, M. Llompart, C. Garcia-Jares, J. C. Garcia-Monteagudo and R. Cela, J. Chromatogr. A, 2008, 1190, 27–38 CrossRef CAS PubMed.
- N. Campillo, P. Viñas, J. Šandrejová and V. Andruch, Appl. Spectrosc. Rev., 2017, 52, 267–415 CrossRef.
- N. Salgueiro-González, S. Castiglioni, E. Zuccato, I. Turnes-Carou, P. López-Mahía and S. Muniategui-Lorenzo, Anal. Chim. Acta, 2018, 1024, 39–51 CrossRef PubMed.
- A. Shishov, A. Bulatov, M. Locatelli, S. Carradori and V. Andruch, Microchem. J., 2017, 135, 33–38 CrossRef CAS.
- A. P Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, J. Am. Chem. Soc., 2004, 126, 9142–9147 CrossRef PubMed.
- Y. Cui, C. Li, J. Yin, S. Li, Y. Jia and M. Bao, Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride, J. Mol. Liq., 2017, 236, 338–343 CrossRef CAS.
- R. Yusof, E. Abdulmalek, K. Sirat and M. B. A. Rahman, Molecules, 2014, 19, 8011–8026 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Number of publications on dispersive liquid–liquid microextraction (DLLME) for the past 11 years. See DOI: 10.1039/c8ra07300g |
| This journal is © The Royal Society of Chemistry 2018 |