Open Access Article
Open Access ArticleDesigned formation of Co3O4@NiCo2O4 sheets-in-cage nanostructure as high-performance anode material for lithium-ion batteries†
Xuefeng Chu *,
Chao Wang,
Lu Zhou,
Xingzhen Yan,
Yaodan Chi and
Xiaotian Yang
*,
Chao Wang,
Lu Zhou,
Xingzhen Yan,
Yaodan Chi and
Xiaotian Yang *
*
Jilin Provincial Key Laboratory of Architectural Electricity & Comprehensive Energy Saving, School of Electrical and Electronic Information Engineering, Jilin Jianzhu University, Changchun 130118, China. E-mail: stone2009@126.com; hanyxt@163.com
First published on 29th November 2018
Abstract
Structural and compositional control of functional nanoparticles is considered to be an efficient way to obtain enhanced chemical and physical properties. A unique Co3O4@NiCo2O4 sheets-in-cage nanostructure is fabricated via a facile conversion reaction, involving subsequent hydrolysis and annealing treatment. Such hollow nanoparticles provide an excellent property for Li storage.
Recently, the development of low-cost and environment-friendly renewable energy conversion and storage systems has become a research focus to address the increasingly serious energy crisis and environment issues.1–3 Lithium-ion batteries (LIBs), as one of the most important energy storage technologies, have received tremendous attention in the past decades.4–8 Among the various anode materials, Co-based transition metal oxides have been widely investigated owing to their higher theoretical capacity compared with the traditional anode electrode, graphite (372 mA h g−1).9–13 However, such kind of anodes usually suffer from the large volume changes during the long-term charge/discharge process, as well as the low conductivity, which are the main reasons for the fast fading in capacity and poor rate performance.
One efficient way to solve the problem is the construction of complex hollow nanostructures instead of traditional solid ones.14–17 The complex hollow nanostructures have some unique advantages, such as the larger specific surface area, well-defined inner hollow space and increased surface permeability. These factors are favourable for the fast mass/charge transfer, resulting in highly increased property for lithium storage. To date, many kinds of synthetic strategies have been successfully developed and reported. As examples, Wang and co-workers reported a facile synthesis of multi-shelled Co3O4 nanospheres with controllable shells.18 Carbon nanospheres are pre-synthesized and used as the hard templates to adsorb the Co2+ ions, followed by an annealing treatment in air for the final conversion of Co3O4 crystals. Lou's group synthesized a series of Co-based complex hollow nanostructures.19–22 Co3O4@NiCo2O4,19 Co3O4@Co–Fe mixed oxide20 and Co3O4@Co3V2O8 triple-shelled nanocages21 are successfully fabricated. Otherwise, Co3O4 bubbles/N doped carbon,23 Co3O4 hollow nanospheres with ultra-thin nanosheets as building blocks,24 and Co3O4/N doped carbon nanocubes25 are also prepared. Benefiting from the unique structural features, these hollow materials always show highly enhanced electro-chemical properties. In addition, using heteroatoms to couple with Co metal to form the mixed metal compound is considered as another effective way to increase the LIB performance. Such mixed metal compounds are favourable for controlling the local chemical environment and modulating the synergistic effect between etch component. More importantly, the coupling of different kinds of metal ions together can bring richer redox reactions and higher electronic conductivity, resulting in significant enhancement of the LIB performance.26–31 NiCo2O4 hollow spheres,27,28 hollow polyhedrons,29 nanoarrays,30 and ZnCo2O4/carbon cloth hybrids31 are fabricated and used as high-performance anodes for LIBs. Therefore, a rational design and synthesis of Co-based functional nanomaterials with complex inner hollow nanostructure and multi-component-coupled outer surface is expected to yield the increased LIB performance.
In the past two decades, metal–organic frameworks (MOFs) have been widely used as sacrificial templates for the synthesis of functional materials.19–21,32–39 The MOFs have some distinctive structural and compositional advantages, such as high porosity and easily controllable composition. Furthermore, the chemical and thermal instability makes the conversion reaction occur easily, that MOFs can transform to functional nanoparticles via a facile ion-exchange reaction or annealing treatment in a specific atmosphere. Among the variety of MOFs, Co-based zeolitic imidazolate framework-67 (ZIF-67) has received continues attention and been widely studied. As successful examples, 2-methylimidazole ligand in ZIF-67 can be easily replaced by S2−, Se2− and MoO42− to form the corresponding CoS,37 CoSe,38 and CoMoO4 hollow cages.39 Such MOF-assisted synthetic strategy provides more possibilities to obtain functional materials with improved physical and chemical properties.
Following the above considering, a new sheets-in-cage nanostructure composed by Co3O4 nanosheets inside and NiCo2O4 layer as outer shell has been designed and synthesized. The whole synthesis involves: (i) conversion reaction to produce ZIF-67@NiCo-LDH yolk@shell nanoparticles; (ii) hydrolysis reaction between ZIF-67@NiCo-LDH and hexamethylenetetramine (HMT) to form thin Co(OH)2 nanosheets in the NiCo-LDH nanocage; (iii) annealing treatment in air atmosphere at 300 °C for 2 hours for the fabrication of final Co3O4@NiCo2O4 sheets-in-cage hollow nanoparticles. In our design, three important structural advantages should be pointed out. First, strongly coupled NiCo2O4 as outer shell is expected to bring higher electro-conductivity. Second, the inner hollow space could accommodate the large volume changes during the lithiation/delithiation process. Last, the encapsulated Co3O4 nanosheets may provide more active sits for lithium storage. As expected, in the following LIB application, the as-obtained Co3O4@NiCo2O4 sheets-in-cage nanoparticles exhibit enhanced LIB performance.
The ZIF-67 crystals are synthesized according to the previous report.19 Scanning electron microscopy (SEM) transmission electron microscopy (TEM) and powder X-ray diffraction (XRD) are used as characterization methods. As shown in Fig. S1,† the product is uniform polyhedron with a particle size of ≈800 nm in average. The corresponding XRD pattern in Fig. S2† (black line) matches well with the previous reports,19,37,38 indicating the product is in the pure phase of ZIF-67. Next, the above obtained ZIF-67 crystals are further reacted with Ni(NO3)2 in absolute ethanol. 40 min later, the products are collected and washed with ethanol for three times (details are shown in the ESI†).
With the data displayed in Fig. 1a–c, the as-obtained particles are uniform and monodisperse. Compared with the original ZIF-67 templates, they keep the dodecahedral morphology with the similar average particle size (≈800 nm). However, the outer surface becomes much rougher. Ultra-thin nanosheet as the building block can be clearly observed. A solid core can be found in each particle. And the clear gap is formed between the shell and solid core, indicating the formation of yolk@shell nanostructure. The corresponding XRD pattern is shown in Fig. S2† (red line). The specific diffraction peaks can be assigned to ZIF-67 crystal. By referring to the report,40 it is considered Co2+ is slowly dissolved from ZIF-67 in such synthesis, then oxidized to Co3+ by NO3−. Further co-precipitation with Ni2+ causes the formation of NiCo-LDH. Along with the hydrolysis reaction, H+ is generated, and ZIF-67 is etched continuously. As a result, ZIF-67@NiCo-LDH yolk@shell nanostructure is obtained. Further prolonging the reaction time to three hours can cause the complete dissolution of the inner ZIF-67 core. As revealed by the TEM images in Fig. S3,† single-shelled NiCo-LDH nanocages are successfully produced.
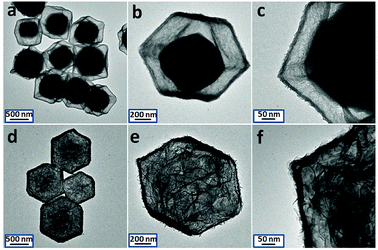 | ||
| Fig. 1 TEM images of ZIF-67@NiCo-LDH yolk@shell nanoparticles (a to c) and Co(OH)2@NiCo-LDH sheets-in-cage nanoparticles (d to f). | ||
In next step, HMT is chosen as the weak alkali source. After heated at 90 °C for two hours, the color of the solution becomes from purple to yellow, indicating the complete conversion of inner ZIF-67 core. TEM images are first used to investigate the structure information. As shown in Fig. 1d–f, there is no obverse change in appearance. The overall structure is well maintained. The particles have the similar particle size, the outer surface is still rough and the thin nanosheets are clear to be observed. As expected, the inner solid cores are disappeared. Unexpected, large amount of thicker Co(OH)2 sheets are formed and criss-cross in the nanocage. The formation mechanism could be understood as follow: OH− ions are first generated from HMT at high temperature, and then reacted with ZIF-67 to form the Co(OH)2 nanosheets. The hollow space inside is large enough and the outer NiCo-LDH layer is stable to limit the expanded growth of Co(OH)2 nanosheets, thus, the unique sheets-in-cage hybrid nanostructure is successfully synthesized. The XRD pattern of the as-obtained product is investigated and shown in Fig. S4.† The specific diffraction peaks of the solid ZIF-67 crystals disappeared completely, no clear diffraction peaks could be found, indicating the poor crystallization degree of the product.
Some control experiments are carried out to get a deeper understand about the formation mechanism. First, we tried to study the solvent-effect towards the structure evolution. Pure ethanol is used to replace the ethanol/water mixture. Two hours later, the colour of the solution is still purple, indicating the remain of ZIF-67. Fig. S5† displays the typical TEM images, that the change of the inner solid core is not obvious. Otherwise, there is no Co(OH)2 nanosheets formed during the process. It reveals that water is necessary, which can participate in the decomposition of HMT to release OH− ions. Then, we increase the volume ratio of H2O/ethanol to 2/1. With the TEM images shown in Fig. S6,† the sheets-in-cage hybrid nanostructure is also obtained. The similar phenomenon is also appeared when doubling the feeding amount of HMT (Fig. S7†). In such two conditions, large amount of OH− ions are generated, and the inner ZIF-67 cores can be dissolved completely. A control experiment is also done by using pure water as the solvent. In such condition, no precipitation is obtained. It is considered that OH− is generated fast, which causes the seriously damage of the three-dimensional cages. Thus, ultra-thin Co/Ni hydroxide nanosheets are well dispersed in water and hard to be collected by centrifugation. Next, we tried to use ZIF-67 crystals to instead of ZIF-67@NiCo-LDH yolk@shell nanoparticles to react with HMT directly. Four hours later, the original purple solution becomes to pink. The TEM images in Fig. S8† reveal that the as-obtained hollow particles show significant shrinkage with decreased size compared with the original ZIF-67 templates. No criss-crossed nanosheets formed inside the cages.
Such phenomenon indicates the outer NiCo-LDH layer is important for the formation of such sheets-in-cage nanostructure. The difference of crystal structure between NiCo-LDH and Co(OH)2 prevents the expanded growth of Co(OH)2 nanosheets. Otherwise, we also study the alkali effect. NH3H2O is used to instead of HMT. The TEM images in Fig. S9† reveal that the inner hollow space cannot be maintained in such condition. Small black particles are formed and loaded on the surface of the nanosheets. However, when urea is used, similar sheets-in-cage hybrids are successful synthesized. Urea is much similar with HMT, they can provide OH− ions slowly, which is considered as the necessary condition for the formation of inner nanosheets.
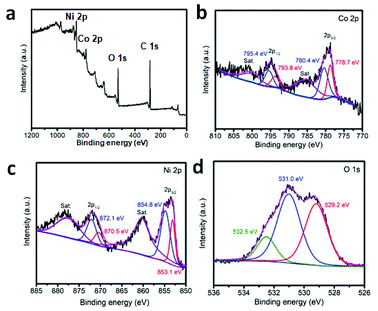
Finally, the pre-synthesized sample is annealed in air at 300 °C for the formation of crystallized product. SEM, TEM, XRD and XPS have been used to characterize the structural and composition information. The typical SEM image is displayed in Fig. 2a and b. The particles can withstand the annealing treatment. Compared with the un-heated precursor, the polyhedron morphology has been maintained well with rough surface. The inner nanostructure is further investigated by TEM images. With the data shown in Fig. 2d–f, the hollow cage is filled with thin Co3O4 nanosheets. XRD pattern of the annealed products are taken and shown in Fig. 2c. Three diffraction peaks appear at 2θ = 31.2°, 36.8°, 44.8°, 59.4° and 65.3° which can be indexed to the (220), (311), (400), (511) and (400) planes of Co3O4. In addition, the composition of the product is further determined by EDX analysis. As shown in Fig. S10,† Co and Ni signals are clear observed. X-ray photoelectron spectroscopy (XPS) is further used to determine the chemical state of Co, Ni and O in Co3O4/NiCo2O4 sheets-in-cage sample. With the data shown in Fig. 3, there are two peaks appear at 779 eV and 795 eV, corresponding to the Co 2p3/2 and 2p1/2 spin orbit peaks, respectively. As shown in Fig. 3c, two strong peaks are observed at 854 and 872 eV, which could be assigned to the Ni 2p3/2 and 2p1/2 spin orbit, respectively. These results reveal that the presence of Co2+, Co3+ and Ni2+ in the final product. Otherwise, the contents of Co and Ni are also calculated based on the XPS analysis, that the molar ratio of Co/Ni is ≈23/6. For better comparison, NiCo-LDH cages and ZIF-67 are also annealed in air under the same condition. With the typical TEM images shown in Fig. S11 and S12,† the single-shelled NiCo2O4 and Co3O4 nanocages are obtained.
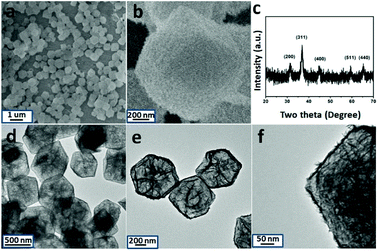 | ||
| Fig. 2 SEM (a and b), TEM images (d to f) and XRD pattern (c) of Co3O4@NiCo2O4 sheets-in-cage hybrid nanoparticle. | ||
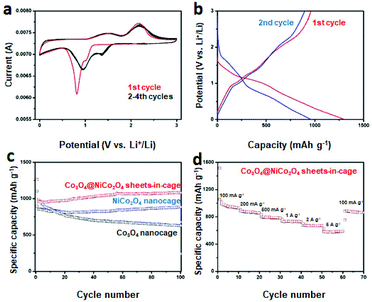
Next, we further test the Li storage performance of the as-obtained Co3O4@NiCo2O4 sheets-in-cage nanoparticles. Fig. 4a displays the first four cyclic voltammogram (CV) curves of the Co3O4@NiCo2O4 electrode at a scan rate of 0.1 mV s−1 in the potential window of 3 V to 0.01 V. There is a big difference between the first cycle and the others. It is clear two reduction peaks appeared at about 1.3 V and 0.8 V in the first discharge process, corresponding to the destruction and/or amorphization of their crystal structure and the reduction reaction between Co3O4/NiCo2O4 and Li, respectively.41,42 During this process, irreversible solid electrolyte interface is also generated. For the first charge process, two wide peaks at 1.5 and 2.2 V are clearly observed, which could be attributed to the oxidation reaction between Co/Ni metal and LixO. Starting from the second cycle, there is an obverse shift of the main reduction peak from 0.8 to 1.0 V, which could be assign to the irreversible processes in first cycle. Importantly, the following CV curves overlap well, indicating the excellent reversibility and stability.
Fig. 4b is the charge–discharge voltage profiles of the Co3O4@NiCo2O4 sheets-in-cage nanocages at a current density of 100 mA g−1 for 1st and 2nd cycle. In the first cycle, the discharge voltage plateaus related the reduction reaction of Co3O4/NiCo2O4 and the charge voltage about the corresponding oxidation reaction appears at 1.2 and 2.1 V, respectively. Starting from the second cycle, the discharge voltage plateau is shifted, indicating the changes of the structure during the charging and discharging process. These results match well with the CV measurements.
Fig. 4c shows the cycling performance of Co3O4@NiCo2O4 sheets-in-cage nanocages in the current density of 100 mA g−1. In the first discharge process, an ultra-high capacity of 1262 mA h g−1 with a coulombic efficiency of 62% is achieved. After then, the second cycle delivers a capacity of 959 mA h g−1. The capacity is slightly decreased in the following ten cycles, and then increased to 1083 mA h g−1 after 100 cycles. The increase of the capacities could be attributed to the activation effect. The electrode material can become more and more accessible to host lithium ions during the charge/discharge process.43–45 Furthermore, each cycle exhibits a high coulombic efficiency of around 99% excepted the first cycle. For comparison, single-shelled NiCo2O4 and Co3O4 nanocages are also used as anode materials for LIBs. As shown in Fig. 4c blue line, NiCo2O4 nanocage delivers a specific capacity of 882 mA h g−1 after 100 cycles at a current density of 100 mA g−1. And the capacity of Co3O4 nanocage fads fast, a capacity of only 626 mA h g−1 is obtained after 100 cycles (Fig. 4c black line). In addition, the long-term cycling performance of Co3O4@NiCo2O4 sheets-in-cage nanocage at higher current density of 1 A g−1 is also tested. With the data displayed in Fig. S13,† a high capacity of 658 mA h g−1 is remained after 200 successful cycles. We also collect the Co3O4@NiCo2O4 sheets-in-cage samples after 30 cycles at the current density of 100 mA g−1. The corresponding TEM images are shown in Fig. S14.† Although the shrink of the nanostructure is observed, the overall sheets-in-cage hollow nanostructure is maintained well, indicating the high structural stability.
The rate performance is another important factor for anode materials. Fig. 4d shows the rate performance of the as-obtained Co3O4@NiCo2O4 sheets-in-cage nanocage by gradually increasing the current density from 0.1 to 5 A g−1 and then returning it to 0.1 A g−1. It delivers specific capacities of 942, 872, 786, 734, 668, and 578 mA h g−1 at the current densities of 0.1, 0.2, 0.5, 1, 2 and 5 A g−1, respectively. Impressively, when the current density returned to 100 mA g−1, the capacity is fast increased to 890 mA h g−1, indicating the excellent rate performance and structural stability. For comparison, the average discharge capacities of the as-obtained NiCo2O4 and Co3O4 nanocages are measured to be 926, 845, 760, 705, 642, 550, and 735, 587, 542, 503, 475, 311 mA h g−1 at a current density of 0.1, 0.2, 0.5, 1, 2 and 5 A g−1 respectively, which is much lower than the Co3O4@NiCo2O4 sheets-in-cage material (with the data shown in Fig. S15†).
Electrochemical impedance spectroscopy (EIS) is adopted to further investigate the electrochemical kinetics difference among the as-obtained three samples. With the data shown in Fig. S16,† the Nyquist plots are consisted of a semicircle at the high to medium frequencies followed by a sloped line at the low frequencies. It is obvious that Co3O4@NiCo2O4 sheets-in-cage electrode has the lowest charge transfer resistance, which could be a big benefit to the LIB performance.
Such enhanced LIB property of the Co3O4@NiCo2O4 sheets-in-cage nanocages could be attributed to the following features. First, the inner thin Co3O4 nanosheets have larger surface area, which can provide massive active sites for lithium storage. Second, the formation of hollow space in NiCo2O4 nanocages is considered as the efficient way to alleviate the volume change and buffer the induced strain during quick charge/discharge process. Thirdly, the outer surface is strongly coupled NiCo2O4 mixed metal oxide, which is expected to bring higher electro-conductivity compared with the Co3O4 component.
Conclusions
In summary, a facile synthesis has been developed for the fabrication of Co3O4@NiCo2O4 sheets-in-cage nanostructures. Uniform ZIF-67 crystals are chosen as the starting hard templates, following by an ion-exchange reaction to produce ZIF-67@NiCo-LDH yolk@shell nanoparticles, and subsequent hydrolyzation reaction with HMT realize the conversion of inner ZIF-67 cores to Co(OH)2 thin nanosheets. An annealing process is finally applied under air atmosphere at 300 °C for the final formation of Co3O4@NiCo2O4 sheets-in-cage nanostructures. In the following LIB test, the as-prepared Co3O4@NiCo2O4 sheets-in-cage nanoparticles exhibit excellent performance for Lithium storage, not only the high specific capacity after long-term cycling, but also the excellent rate performance. Such structural design and wet-chemistry synthetic strategy may hold great promise for the fabrication of functional nanomaterials with enhanced properties for energy storage and conversion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 51672103) and Natural Science Foundation of Jilin Province (No. 20160204069GX, No. JJKH20170236KJ and JJKH20170243KJ).Notes and references
- B. Dunn, H. Kamath and J.-M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS PubMed.
- J. W. Choi and D. Aurbach, Nat. Rev. Mater., 2016, 1, 16013 CrossRef CAS.
- P. Simon, Y. Gogotsi, P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- Y. Yang, G. Zheng, S. Misra, J. Nelson, M. Toney and Y. Cui, J. Am. Chem. Soc., 2012, 134, 15387–15394 CrossRef CAS PubMed.
- Y. Wang, J. Wu, Y. Tang, X. Lü, C. Yang, M. Qin, F. Huang, X. Li and X. Zhang, ACS Appl. Mater. Interfaces, 2012, 4, 4246–4250 CrossRef CAS PubMed.
- F. Wang, X. Wu, C. Li, Y. Zhu, L. Fu, Y. Wu and X. Liu, Energy Environ. Sci., 2016, 9, 3570–3611 RSC.
- F. Hu and T. Song, RSC Adv., 2017, 7, 54203–54212 RSC.
- Z. Gong and Y. Yang, Energy Environ. Sci., 2011, 4, 3223–3242 RSC.
- M. Aslam, S. Shah, S. Li and C. Chen, J. Mater. Chem. A, 2018, 6, 14083–14090 RSC.
- C. Zhang, B. Lu, F. Cao, Z. Yu, H. Cong and S. Yu, J. Mater. Chem. A, 2018, 6, 1428–1436 Search PubMed.
- C. Yan, G. Chen, X. Zhou, J. Sun and C. Lv, Angew. Chem., Int. Ed., 2016, 26, 7060–7064 Search PubMed.
- D. Gu, W. Li, F. Wang, H. Bongard, B. Spliethoff, W. Schmidt, C. Weidenthaler, Y. Xia, D. Zhao and F. Schüth, Angew. Chem., Int. Ed., 2015, 54, 7060–7064 CrossRef CAS PubMed.
- B. Wang, X. Lu and Y. Tang, J. Mater. Chem. A, 2015, 3, 9689–9699 RSC.
- B. Guan, X. Yu, H. Wu and X. Lou, Adv. Mater., 2017, 29, 1703614 CrossRef PubMed.
- R. Wu, X. Qian, K. Zhou, J. Wei, J. Lou and P. Ajayan, ACS Nano, 2014, 8, 6297–6303 CrossRef CAS PubMed.
- J. Qi, X. Lai, J. Wang, H. Tang, H. Ren, Y. Yang, Q. Jin, L. Zhang, R. Yu, G. Ma, Z. Su, H. Zhao and D. Wang, Chem. Soc. Rev., 2015, 44, 6749–6773 RSC.
- J. Zhang, H. Ren, J. Wang, J. Qi, R. Yu, D. Wang and Y. Liu, J. Mater. Chem. A, 2016, 4, 17673–17677 RSC.
- J. Wang, N. Yang, H. Tang, Z. Dong, Q. Jin, M. Yang, D. Kisailus, H. Zhao, Z. Tang and D. Wang, Angew. Chem., Int. Ed., 2013, 52, 6417–6420 CrossRef CAS PubMed.
- H. Hu, B. Guan, B. Xia and X. Lou, J. Am. Chem. Soc., 2015, 137, 5590–5595 CrossRef CAS PubMed.
- X. Wang, L. Yu, B. Guan, S. Song and X. Lou, Adv. Mater., 2017, 29, 1801211 Search PubMed.
- Y. Lu, J. Nai and X. Lou, Angew. Chem., Int. Ed., 2018, 57, 2899–2903 CrossRef CAS PubMed.
- B. Guan, A. Kushima, L. Yu, S. Li, J. Li and X. Lou, Adv. Mater., 2018, 30, 1605902 Search PubMed.
- S. Parka, J. Kima, J. Kimb and Y. Kang, Mater. Charact., 2017, 132, 320–329 CrossRef.
- J. Zhao, Y. Zou, X. Zou, T. Bai, Y. Liu, R. Gao, D. Wang and G. Li, Nanoscale, 2014, 6, 7255–7262 RSC.
- X. Yang, J. Chen, Y. Chen, Pi. Feng, H. Lai, J. Li and X. Luo, Nano-Micro Lett., 2017, 10, 15 CrossRef PubMed.
- X. Yu and X. Lou, Adv. Energy Mater., 2018, 8, 1701592 CrossRef.
- J. Guo, Z. Yin, X. Zang, Z. Dai, Y. Zhang, W. Huang and X. Dong, Nano Res., 2017, 10, 405–414 CrossRef CAS.
- X. Qi, W. Zheng, G. He, T. Tian, N. Du and L. Wang, Chem. Eng. J., 2017, 309, 426–434 CrossRef CAS.
- S. Park, S. Yang and Y. Kan, Chem. Eng. J., 2018, 349, 214–222 CrossRef CAS.
- J. Cheng, Y. Lu, K. Qiu, H. Yan, J. Xu, L. Han, X. Liu, J. Luo, J. Kim and Y. Luo, Sci. Rep., 2015, 5, 12099 CrossRef PubMed.
- B. Liu, J. Zhang, X. Wang, G. Chen, D. Chen, C. Zhou and G. Shen, Nano Lett., 2012, 12, 3005–3011 CrossRef CAS PubMed.
- W. Huang, S. Li, X. Cao, C. Hou, Z. Zhang, J. Feng, L. Ci, P. Si and Q. Chi, ACS Sustainable Chem. Eng., 2017, 5, 5039–5048 CrossRef CAS.
- X. Xu, R. Cao, S. Jeong and J. Cho, Nano Lett., 2012, 12, 4988–4991 CrossRef CAS PubMed.
- D. Sun, L. Ye, F. Sun, H. García and Z. Li, Inorg. Chem., 2017, 56, 5203–5209 CrossRef CAS PubMed.
- S. Hou, X. Xu, M. Wang, Y. Xu, T. Lu, Y. Yao and L. Pan, J. Mater. Chem. A, 2017, 5, 19054–19061 RSC.
- T. Wang, Q. Zhou, X. Wang, J. Zheng and X. Li, J. Mater. Chem. A, 2015, 3, 16435–16439 RSC.
- P. Zeng, J. Li, M. Ye, K. Zhuo and Z. Fang, Chem.–Eur. J., 2017, 23, 9517–9524 CrossRef CAS PubMed.
- S. Park, J. Park and Y. Kang, ACS Appl. Mater. Interfaces, 2018, 10, 16531–16540 CrossRef CAS PubMed.
- Y. Li, H. Xu, H. Huang, C. Wang, L. Gao and T. Ma, Chem. Commun., 2018, 54, 2739–2742 RSC.
- Z. Jiang, Z. Li, Z. Qin, H. Sun, X. Jiao and D. R. Chen, Nanoscale, 2013, 5, 11770–11775 RSC.
- Y. H. Li, X. W. Wu, S. L. Wang, W. Q. Wang, Y. H. Xiang, C. H. Dai, Z. X. Liu, Z. Q. He and X. m. Wu, RSC Adv., 2017, 7, 36909–36916 RSC.
- L. L. Li, Y. L. Cheah, Y. Ko, P. Teh, G. Wee, C. L. Wong, S. J. Peng and M. Srinivasan, J. Mater. Chem. A, 2013, 1, 10935–10941 RSC.
- J. Jamnik and J. Maier, Phys. Chem. Chem. Phys., 2003, 5, 5215–5220 RSC.
- J. F. Chen, Q. Ru, Y. D. Mo, S. J. Hu and X. H. Hou, Phys. Chem. Chem. Phys., 2016, 18, 18949–18957 RSC.
- F. X. Wang, C. Wang, Y. J. Zhao, Z. C. Liu, Z. Chang, L. J. Fu, Y. S. Zhu, Y. P. Wu and D. Y. Zhao, Small, 2016, 12, 853–859 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra07396a |
| This journal is © The Royal Society of Chemistry 2018 |