Open Access Article
Open Access ArticleIsolation of inorganic molecular chains from rod-like bulk V2Se9 crystal by liquid exfoliation
Sudong Chae†
a,
Akhtar J. Siddiqa†a,
Bum Jun Kimb,
Seungbae Oha,
Kyung Hwan Choib,
Keun Ho Lee a,
Hyo Yeol Kima,
Hak Ki Yu
a,
Hyo Yeol Kima,
Hak Ki Yu c and
Jae-Young Choi
c and
Jae-Young Choi *ab
*ab
aSchool of Advanced Materials Science & Engineering, Sungkyunkwan University, Suwon, 16419, Korea. E-mail: jy.choi@skku.edu
bSchool of Advanced Institute of Nanotechnology (SAINT), Sungkyunkwan University, Suwon, 16419, Korea
cDept. of Materials Science and Engineering, Dept. of Energy Systems Research, Ajou University, Suwon, 16499, Korea
First published on 15th October 2018
Abstract
We studied the optimum dispersion solvent for bulk V2Se9 material, which can be used as a new one-dimensional (1D) material, to separate into 1D chain units. Selected twelve solvents, which have different dielectric constants and surface tensions, were tested to exfoliate bulk V2Se9 into nano-scale chains. The atomic level (∼1 nm, mono-chain) exfoliation of V2Se9 was performed using acetone as the solvent. The dispersion concentration was high in solvents having medium dielectric constants ranging from 20 to 40 with surface tensions ranging from 25 to 35 mJ m−2. This result is similar to the dispersion results of previous transition metal dichalcogenides (TMDCs) such as MoS2, WS2, MoSe2, MoTe2, TaSe2, NbSe2, and NiTe2, indicating that the V2Se9 material and its dispersion to 1D units can be expected to play an important role in opening opportunities for new low-dimensional material studies.
Low-dimensional materials enable new research directions and advanced applications. Two-dimensional (2D) materials including graphene, transition metal dichalcogenides (TMDCs), and black phosphor have attracted much attention due to their unique physical, chemical, optical, and mechanical properties. These materials have multiple interesting applications in transistors, superconductors, and optoelectronic devices.1–5 Unlike previous low-dimensional materials, which were obtained by simply reducing the sizes of bulk materials, 2D materials with atomic thickness are prepared by isolating single atomic layers from three-dimensional materials with strong covalent in-plane bonds and weak interlayer interactions. When one atomic unit layer or several unit layers are isolated from the bulk crystal, excellent properties that are not present in the bulk state are observed due to the quantum confinement effect and the dangling bond-free surface.
Recently, new one-dimensional (1D) materials, such as LiMo3Se3,6–10 Mo6S3I6,11–14 and Mo6S4.5I4.5,15,16 having similar originality to 2D materials, have been investigated. These 1D materials have been prepared by isolating inorganic molecular chains from bulk crystals comprising strong covalently bonded unit chains connected by weak inter-chain interactions. When isolated from the bulk crystals, these molecular chains are as small as 1 nm in diameter and have unique surface characteristics. For example, LiMo3Se3 has a negatively charged chain surface because of ionic interactions between the chains.17 Mo6S3I6 and Mo6S4.5I4.5 have no dangling bonds on the chain surface, similar to graphene sheets, because of the van der Waals (vdW) forces between chains. These structural features give rise to unique physical and chemical properties including 1D quantum confinement effects, thus permitting applicability in composites, molecular connectors, transistors, sensors, and photovoltaic devices.11–13,18–23 In addition, new 1D inorganic materials, namely, Sb2S3 and Sb2Se3, have been reported to exhibit excellent optoelectronic properties because the chain surfaces lack dangling bonds.24–26
We have been studying the synthesis of new 1D inorganic materials and recently prepared a new semiconducting 1D bulk crystal of V2Se9. Exfoliation has been previously shown to separate CNTs into individual tubes, and has been used for many useful applications.27–29 Therefore, exfoliation is recognized as an important first step toward applications and thus, it is an indispensable scientific goal. The isolation of inorganic chains from this bulk crystal is necessary in order to study the materials' properties or device applications. For this purpose, we have exfoliated the V2Se9 bulk crystal in various solvents because exfoliation in a solvent is a simple and high-yield method compared with other exfoliation methods. In this study, we determined the optimal solvent for V2Se9 exfoliation and verified the isolation of single chains from the obtained dispersion solutions.
The dispersion of the solid material in the solvent may be affected by the wetting behavior and binding energy between the solvent and the solid surface. Among the various physical properties of solvents, surface tension (surface energy) affects the wetting property, and the dielectric constant affects the bonding energy between solid surface and liquid by dipole–dipole interactions. In other words, in order to be well dispersed, the solid material has to dissolve well in the solvent, and the theory explaining this phenomenon is the Hansen solubility parameter. Solids are most soluble in solvents with similar properties, such as dispersion forces, dipole intermolecular forces, and the hydrogen bonding energy between solvents and solid materials. The surface characteristics of the two-dimensional transition metal di-selenides and the 1D V2Se9 material used in this experiment show similar characteristics in the large frame of vdW bonding, but the detailed surface characteristics are different. In this experiment, it is meaningful to provide basic experimental data of dispersion for a new 1D material by analyzing the dispersion characteristics of V2Se9 in various solvents.
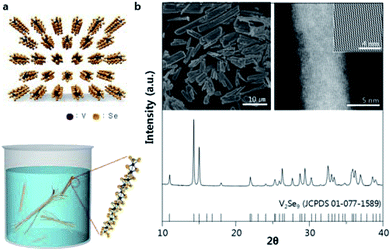
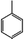
The V2Se9 chain is a 1D molecular chain of linearly connected V atoms decorated externally with Se atoms (top of Fig. 1a). Although the chain bonding is covalent, adjacent chains are stacked via vdW interactions to form the 3D crystal. In dispersion, single chains can be exfoliated from the 3D crystal because of the weak interactions between chains (bottom of Fig. 1a). Single-crystalline V2Se9 was prepared via the flux method using excess molten Se as a solvent. When the V–Se liquid at 330–340 °C is cooled to room temperature, dark grey bulks are precipitated, and X-ray diffraction (XRD) analysis confirms that the obtained material has a well crystallised V2Se9 phase (Fig. 1b). The inset of Fig. 1b shows the scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images of the as-prepared V2Se9 crystals. The resulting dark grey V2Se9 crystals have diameters of several tens of micrometres. Because of the weak attraction between V2Se9 chains, the V2Se9 crystals show axially cleaved sections. The TEM image shows that the V2Se9 crystal comprises 1D aligned chains with diameters < 1 nm. With the successful synthesis and structural identification of V2Se9, dispersion was performed in order to obtain mono- and few-chain nanowires in several solvents.
Liquid exfoliation is known to be insensitive to air and water, and is potentially scalable to yield large quantities of exfoliated materials.30 In order to determine the best exfoliation solvent for the V2Se9 chain material, twelve solvents were considered with varied dielectric constants (Table 1).
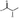
V2Se9 particles were dispersed in the solvents by sonication and further centrifuged to remove sediments of large and non-exfoliated particles. Photos of the dispersed solution before and after centrifugation are shown in Fig. 2a and b. The solvents isopropyl alcohol (IPA), isobutyl alcohol (IBA), acetone, and acetonitrile show a clear Tyndall effect, indicating that nanodispersions of V2Se9 chains are obtained (see the inductively coupled plasma (ICP) mass spectrometry results for different solvents in Fig. 2c).
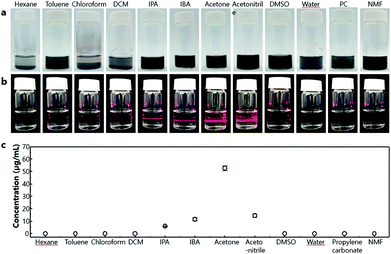
The exact concentration of V2Se9 in each solution was analysed by ICP mass spectrometry and plotted with the dielectric constants and the surface tensions of the solvents, as shown in Fig. 3a and b, respectively. The dispersion concentration is high in solvents having intermediate dielectric constants in the range of 20–40 and surface tensions in the range of 25–35 mJ m−2. To understand the dispersion behaviour of a material in a solvent, the surface structure of the exfoliated material must be understood. 1D V2Se9 is a transition metal chalcogenide (TMC) comprising chains of Se-decorated V atoms. These structural features are similar to those of well-known 2D TMDCs, which comprise hexagonal layers of metal atoms (M) sandwiched between two layers of chalcogenide atoms (X) with the stoichiometry MX2. Furthermore, 1D chains and 2D sheets, which are the unit structures of V2Se9 and TMCs, respectively, are linked by vdW interactions. Dispersions of TMCs in solvents have been studied extensively. TMDCs including MoS2, MoSe2, MoTe2, and NbSe2 are effectively dispersed in solvents with surface tensions between 30 and 40 mJ m−2.30 This result is similar to that obtained in this study because both V2Se9 and TMDCs have surface structures in which chalcogenide atoms encapsulate transition metals and vdW interactions between these unit material surfaces. The highest concentration of 52.8 μg mL−1 was obtained in acetone.
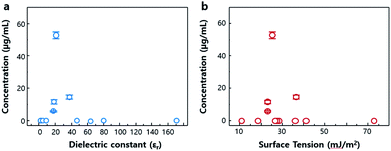
V2Se9 dispersed in acetone was spin-coated onto SiO2/Si substrates to analyse the size of the nanochain using an atomic force microscope (AFM); the results are shown in Fig. 4a and b. It is evident that the V2Se9 chains with 1D structure of and size < 10 nm are well dispersed. This confirms that monochains of size ≤ 1 nm can be obtained in a specific dispersion region (Fig. 4b). More details on the separation of 1D V2Se9 chains into atomic units will require the optimisation of the dispersion solvent and the dispersion process.
In summary, in this study, a novel 1D inorganic molecular chain material (V2Se9) was synthesised through a chemical reaction between V and Se. 1D nanoscale (≤10 nm) and mono-chain scale (∼1 nm) of V2Se9 chains were successfully obtained by the dispersion process, and the best solvent for V2Se9 dispersion was acetone. The covalently bonded V2Se9 chain, when isolated from its 3D bulk material, may have unique physical properties based on the confinement of electrons in the 1D chain structure and the absence of dangling bonds on the chain surface. Therefore, this 1D material may inspire the development of new nanoelectronic devices, similar to the various 2D materials currently under investigation.
Experimental section
Synthesis
V2Se9 was synthesised by a flux method using V (−325 mesh, 99.5%, Aldrich) and Se (>99%, Alfa Aesar) elemental powders. First, 1 g of mixed V and Se with the molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 97 was pelletized. Then, the pellets were sealed in a 10 cm-long evacuated quartz tube. The evacuated quartz tube was heated to 330 °C for 120 h, and then cooled at 10 °C h−1. The product was a dark grey powder. The unreacted Se was sublimated in a low-pressure tube furnace at 250 °C under Ar flow (100 sccm) for 24 h.
97 was pelletized. Then, the pellets were sealed in a 10 cm-long evacuated quartz tube. The evacuated quartz tube was heated to 330 °C for 120 h, and then cooled at 10 °C h−1. The product was a dark grey powder. The unreacted Se was sublimated in a low-pressure tube furnace at 250 °C under Ar flow (100 sccm) for 24 h.
Dispersion
V2Se9 powder (10 mg) was immersed in 10 mL of each solvent. The initial large powder particles were crushed using ultrasonication by a probe sonicator (VC 505, Sonics & Materials, Inc.), operated for 5 min at a 2 s on/2 s off interval. Subsequently, bath sonication (B2005S-68K, 68 kHz, 200 W, KODO Technical) was performed for 3 h. After ultrasonication, the solution was centrifuged at 6000 rpm for 10 min to remove the insufficiently exfoliated chains. For further analysis, 5 mL of the supernatant solution was used.Characterization
To examine the morphology of the as-synthesised V2Se9 powder, field-emission SEM (FE-SEM) was employed. An aberration-corrected scanning transmission electron microscope (STEM, JEM ARM 200F, JEOL) was operated at an acceleration voltage of 80 kV. The sample for STEM observation was prepared by drop-casting on a graphene-coated Quantifoil TEM grid. The concentrations of the dispersion solutions were confirmed by ICP-mass spectrometry (Agilent 7500, Agilent Technologies Inc.). To evaluate the morphology of the exfoliated V2Se9, AFM (Park Systems, NX10) was employed in non-contact mode, for which the samples were prepared by spin-coating on SiO2/Si wafers.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Nano Material Technology Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2017M3A7B8065561). This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (No. NRF-2017R1A4A1015770).References
- K. S. Novoselov, A. K. Geim, S. Morozov, D. Jiang, M. Katsnelson, I. Grigorieva, S. Dubonos and A. A. Firsov, Nature, 2005, 438, 197 CrossRef CAS PubMed.
- Y. Zhang, Y.-W. Tan, H. L. Stormer and P. Kim, Nature, 2005, 438, 201 CrossRef CAS PubMed.
- K. I. Bolotin, K. Sikes, Z. Jiang, M. Klima, G. Fudenberg, J. Hone, P. Kim and H. Stormer, Solid State Commun., 2008, 146, 351–355 CrossRef CAS.
- K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706 CrossRef CAS PubMed.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- L. Venkataraman and C. M. Lieber, Phys. Rev. Lett., 1999, 83, 5334 CrossRef CAS.
- L. Venkataraman, Y. S. Hong and P. Kim, Phys. Rev. Lett., 2006, 96, 076601 CrossRef PubMed.
- F. E. Osterloh, J. S. Martino, H. Hiramatsu and D. P. Hewitt, Nano Lett., 2003, 3, 125–129 CrossRef CAS.
- J. G. Sheridan, A. Heidelberg, D. F. Brougham, P. D. Nellist, R. M. Langford and J. J. Boland, Langmuir, 2012, 28, 15344–15349 CrossRef CAS PubMed.
- J. Lin, O. Cretu, W. Zhou, K. Suenaga, D. Prasai, K. I. Bolotin, N. T. Cuong, M. Otani, S. Okada and A. R. Lupini, Nat. Nanotechnol., 2014, 9, 436 CrossRef CAS PubMed.
- M. I. Ploscaru, S. J. Kokalj, M. Uplaznik, D. Vengust, D. Turk, A. Mrzel and D. Mihailovic, Nano Lett., 2007, 7, 1445–1448 CrossRef CAS PubMed.
- P. Topolovsek, C. Gadermaier, D. Vengust, M. Strojnik, J. Strle and D. Mihailovic, Nano Lett., 2015, 15, 813–818 CrossRef CAS PubMed.
- J. Strle, D. Vengust and D. Mihailovic, Nano Lett., 2009, 9, 1091–1095 CrossRef CAS PubMed.
- N. Ćelić, E. Pavlica, M. Borovšak, J. Strle, J. Buh, J. Zavašnik, G. Bratina, P. Denk, M. Scharber and N. S. Sariciftci, Synth. Met., 2016, 212, 105–112 CrossRef.
- M. McMullan, N. Sun, P. Papakonstantinou, M. Li, W. Zhou and D. Mihailovic, Biosens. Bioelectron., 2011, 26, 1853–1859 CrossRef CAS PubMed.
- H. Lin, H. Cheng, L. Liu, Z. Zhu, Y. Shao, P. Papakonstantinou, D. Mihailovič and M. Li, Biosens. Bioelectron., 2011, 26, 1866–1870 CrossRef CAS PubMed.
- J. Tarascon, F. DiSalvo, C. Chen, P. Carroll, M. Walsh and L. Rupp, J. Solid State Chem., 1985, 58, 290–300 CrossRef CAS.
- M. Uplaznik, B. Bercic, M. Remskar and D. Mihailovic, Phys. Rev. B, 2009, 80, 085402 CrossRef.
- H. Lin, H. Cheng, X. Miao, P. Papakonstantinou, D. Mihailovič and M. Li, Electroanalysis, 2009, 21, 2602–2606 CrossRef CAS.
- J. Andzane, J. Prikulis, D. Dvorsek, D. Mihailovic and D. Erts, Nanotechnology, 2010, 21, 125706 CrossRef CAS PubMed.
- A. Majkić, C. Gadermaier, N. Celic, P. Topolovsek, G. Bratina and D. Mihailovic, Sol. Energy Mater. Sol. Cells, 2014, 127, 63–66 CrossRef.
- D. Vrbanic, S. Pejovnik, D. Mihailovic and Z. Kutnjak, J. Eur. Ceram. Soc., 2007, 27, 975–977 CrossRef CAS.
- Y. Itzhaik, O. Niitsoo, M. Page and G. Hodes, J. Phys. Chem. C, 2009, 113, 4254–4256 CrossRef CAS.
- Y. C. Choi, D. U. Lee, J. H. Noh, E. K. Kim and S. I. Seok, Adv. Funct. Mater., 2014, 24, 3587–3592 CrossRef CAS.
- Y. Zhou, L. Wang, S. Chen, S. Qin, X. Liu, J. Chen, D.-J. Xue, M. Luo, Y. Cao and Y. Cheng, Nat. Photonics, 2015, 9, 409 CrossRef CAS.
- L. Wang, D.-B. Li, K. Li, C. Chen, H.-X. Deng, L. Gao, Y. Zhao, F. Jiang, L. Li and F. Huang, Nat. Energy, 2017, 2, 17046 CrossRef CAS.
- S. J. Tans, A. R. Verschueren and C. Dekker, Nature, 1998, 393, 49 CrossRef CAS.
- T. Rueckes, K. Kim, E. Joselevich, G. Y. Tseng, C.-L. Cheung and C. M. Lieber, Science, 2000, 289, 94–97 CrossRef CAS PubMed.
- P. G. Collins, M. S. Arnold and P. Avouris, Science, 2001, 292, 706–709 CrossRef CAS PubMed.
- J. N. Coleman, M. Lotya, A. O'Neill, S. D. Bergin, P. J. King, U. Khan, K. Young, A. Gaucher, S. De and R. J. Smith, Science, 2011, 331, 568–571 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2018 |