Open Access Article
Open Access ArticleCatalytic oxidation of ethyl acetate over LaBO3 (B = Co, Mn, Ni, Fe) perovskites supported silver catalysts†
Yu Qina,
Fangxia Shena,
Tianle Zhua,
Wei Honga and
Xiaolong Liu *b
*b
aSchool of Space and Environment, Beihang University, Beijing 100191, China
bBeijing Engineering Research Center of Process Pollution Control, National Engineering Laboratory for Hydrometallurgical Cleaner Production Technology, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: liuxl@ipe.ac.cn
First published on 27th September 2018
Abstract
A series of silver catalysts supported on lanthanum based perovskites LaBO3 (B = Co, Mn, Ni, Fe) were synthesized and evaluated in the catalytic oxidation of ethyl acetate. XRD, BET, TEM/HRTEM, HAADF-STEM, XPS and H2-TPR were conducted, and the results indicate that redox activity of the catalysts is of great importance to the oxidation reaction. Activity tests demonstrated that Ag/LaCoO3 was more active than the other samples in ethyl acetate oxidation. Moreover, the CO2 selectivity, COx yields and byproduct distributions for all catalysts were studied, and Ag/LaCoO3 showed the best catalytic performance. Besides, Ag/LaCoO3 also showed excellent catalytic activity for other OVOCs.
1. Introduction
As a common volatile organic compound (VOC) pollutant, ethyl acetate is usually used as a solvent for extracting natural flavors, cleaning textile materials and painting. Meanwhile, ethyl acetate emission may cause adverse health effects, such as headaches, nausea and even cancer.1 Currently, various techniques have been developed to remove ethyl acetate.2–4 Among them, catalytic oxidation is generally regarded as a highly efficient technique for reducing ethyl acetate emissions.4–7Numerous catalysts have been used in the catalytic oxidation of ethyl acetate, and usually include noble metals and metal oxides. Noble metals commonly exhibit high catalytic activity in VOCs abatements.8–10 Au catalysts have been well demonstrated in ethyl acetate oxidation, and Carabineiro et al. reported that Au/oxide catalysts possessed high catalytic activity for the oxidation of ethyl acetate, and pointed out that the role of gold is to enhance the reducibility of the surface of the oxide supports.11 However, the drawbacks of high cost, instability, and sensitivity to poison have greatly limited the practical applications of noble metals in the industrial process. Recently, metal oxides have been intensively studied due to their relatively low cost and poison resistance.12–14 Among the metal oxides, perovskite-type oxides with the general formula of ABO3, have been widely used in air purification,15–18 solar hydrogen production,19 sensor20 and diesel fuel reforming21 due to their advantages of low cost, high thermal stability, and great catalytic performance.22,23
Of all the perovskite-type oxides, lanthanum (La)-based perovskites, namely LaBO3, are recognized as one of the most potential catalysts with higher efficient for their B sites that can be filled with various transition metal cations.24–26 Recently, Taran et al. evaluated the effects of the variation of the B sites in LaBO3 (B = Cu, Fe, Mn, Co, Ni) catalysts for wet peroxide oxidation of phenol, and found that LaCuO3 was the most active one, whereas LaFeO3 was the most stable one.26 Zhu et al. studied a series of A-site substituted La0.8M0.2MnO3 catalysts (M = Ba, Ca, Ce, Mg and Sr) for plasma-catalytic oxidation of ethyl acetate, and found that La0.8Ce0.2MnO3 catalyst has the highest activity, corresponding to its highest reducibility among the tested catalysts.3 Subsequently, Zhu group investigated the plasma-catalytic oxidation of ethyl acetate with La1−xCexCoO3+δ (x = 0, 0.05, 0.1, 0.3 and 0.5) catalysts.1 The removal efficiency of ethyl acetate for the Ce-doped LaCoO3 catalyst was further enhanced, because of the increased specific surface area and a reduced crystallite size compare of pure LaCoO3. Giraudon et al. reported the oxidation of chlorobenzene over Pd/LaBO3 (B = Co, Mn, Fe, Ni), and found that palladium was totally reduced in hydrogen, while the perovskite network was either unaffected or transformed with partial destruction.27 Tang et al. found that LaCoO3 with 3DOM structures exhibited excellent catalytic activity in soot oxidation.28
Meanwhile, silver-based catalysts performed well for the oxidation of various VOCs pollutants, such as ethyl acetate,6 formaldehyde,29 toluene,30 methanol,31 acetone32 and naphthalene.33 Jodaei et al. studied bimetallic Ag–M (M = Fe, Co, Mn)-ZSM-5 for its catalytic oxidation ability of ethyl acetate, and found that Ag–Fe-ZSM-5 has the highest catalytic activity.6 Wang et al. reported that the Ag-doped LaMnO3 perovskite oxide exhibits high activity for simultaneous removal of NOx and diesel because of the abundant oxygen vacancy and over-stoichiometry oxygen in the perovskite lattice along with Ag0.34 However, few work has been done on the use of silver supported LaBO3 perovskite catalysts for ethyl acetate removal. Hence, it is of great interest to investigate the silver-based LaBO3 perovskite catalysts in ethyl acetate oxidation.
In this work, a series of B-site substituted Ag/LaBO3 (B = Co, Mn, Ni, Fe) catalysts were synthesized and evaluated regarding their catalytic oxidation potential of ethyl acetate. The structures of Ag/LaBO3 catalysts were characterized by BET, XRD, TEM/HR-TEM, HAADF-STEM, XPS and H2-TPR, and the role of the B-site substitutes on the reaction performance was further explored. The results indicate that the reducibility of the catalysts plays a key role in the removal of ethyl acetate.
2. Experimental
2.1. Catalyst preparation
LaBO3 (B = Co, Mn, Fe, Ni) perovskites were synthesized from citrate precursors as described in the literature.35 In brief, 20 mmol of La(NO3)3·6H2O and 20 mmol B(NO3)x nitrate salts (Co(NO3)2·6H2O; Mn(NO3)2 (50% solution); Ni(NO3)2·6H2O; Fe(NO3)3·9H2O) as well as 40 mmol citric acid were added to 100 mL of deionized water. The mixture was stirred for 2 h and then maintained at 100 °C for 12 h. A viscous gel was obtained with the rotary evaporator, followed by drying in an oven at 100 °C for 10 h. The obtained solid was finally calcined at 700 °C for 5 h giving LaBO3 perovskites. Ag/LaBO3 catalysts were prepared using wet impregnation method with silver nitrate aqueous solution. After the impregnation, the samples were dried at 100 °C for 12 h and calcined at 350 °C for 3 h in air. The loading of Ag in Ag/LaBO3 catalysts was 1.0 wt%.2.2. Catalyst characterization
X-ray diffraction (XRD) of the products was carried out on a powder diffractometer (Rigaku D/Max-RA) with Cu Kα radiation (120 mA, 40 kV). The nitrogen adsorption–desorption isotherm was performed using Autosorb iQ Quantachrome system at 77 K. Transmission electron microscopy (TEM) and high-angle annular dark-field scanning TEM (HAADF-STEM) images were taken on a FEI Tecnai G2 F20 field emission electron microscope operating at 200 kV. X-ray photoelectron spectroscopy (XPS) measurements were performed with a Thermo escalab 250Xi X-ray using Al Kα source. Hydrogen temperature programmed reduction (H2-TPR) was detected by an AutoChemII 2920 apparatus with a flow-type reactor. Hydrogen (10 vol% in Ar) was passed through a reaction tube containing the catalyst under atmospheric pressure at 30 mL min−1. The tube was heated with an electric furnace at a heating rate of 10 °C min−1 from 25 °C to 600 °C.2.3. Catalytic evaluation
The LaBO3 and Ag/LaBO3 samples were tested in a continuous flow fixed-bed quartz microreactor (i.d. = 4 mm) from 150 °C to 350 °C with 100 mg of catalyst (60–80 mesh). The solid was placed on the quartz sieve, which was fixed in the middle of the quartz microreactor. The reaction gas consisted of 500 ppm ethyl acetate, 20% O2, 1% H2O (when used), and was balanced with Ar. The concentration of reaction gas was calibrated with a gas chromatography (GC 2010 Plus, Shimadzu) through a by-pass. The total flow of the reactant mixture was 100 mL min−1 with the weight hourly space velocity (WHSV) of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL (g h)−1.
000 mL (g h)−1.
3. Results and discussion
3.1. Catalysts characterization
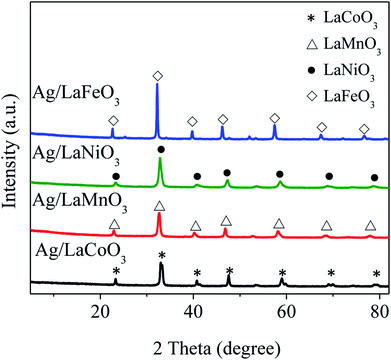
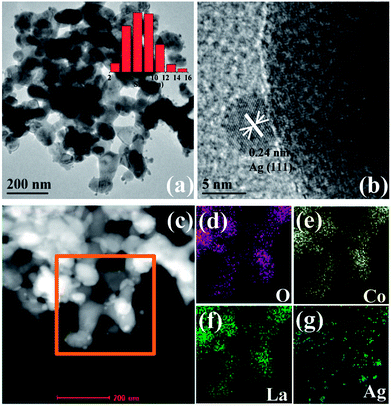
Fig. 1 shows XRD patterns of the Ag/LaBO3 catalysts, there appears no any new diffraction peak corresponding to Ag or Ag2O phase mainly due to the low loading (1 wt%) of Ag and the small size of Ag nanoparticles, which has been demonstrated in Au-based catalysts.11 All the diffraction peaks were clearly indexed which agreed with rhombohedral phase for LaCoO3 (JCPDS 48-0123) and LaNiO3 (JCPDS 33-711), cubic structure of LaMnO3 (JCPDS 75-0440), orthorhombic phase for LaFeO3 (JCPDS 15-0148), respectively. Besides, four catalysts were also characterized by BET method, and their specific surface areas (SBET) were 14.5, 19.3, 12.5, and 14.0 m2 g−1 for Ag/LaCoO3, Ag/LaMnO3, Ag/LaNiO3 and Ag/LaFeO3 (Table S1†), respectively, which were consistent with those of previously reported perovskite materials.27,36TEM images of Ag/LaCoO3 catalyst are shown in Fig. 2a with the inset of Ag particle size distribution calculated by Image-J software. The Ag particle sizes varied from 2 to 16 nm with the average value at 7.5 ± 2 nm. Moreover, the particle that corresponded to metallic Ag (111) facet with the lattice fringes of 0.24 nm was observed from the high-resolution TEM (HRTEM) (Fig. 2b). The Ag, La and Co distributions were studied by taking HAADF-STEM images and STEM-EDS mapping analysis (Fig. 2c–g). The LaCoO3 nanoparticles were presented as bright spots in Fig. 2c. In the STEM-EDS mappings (Fig. 2d–g), the observed O (purple), Co (yellow), La (green) and Ag (blue) shows similar distributions, indicating that Ag species was well-dispersed on the LaCoO3 support as metallic Ag nanoparticles.
The redox properties of Ag/LaBO3 and LaBO3 catalysts were investigated using H2-TPR from 50 to 600 °C (Fig. 3). The first reduction peaks can be attributed to the reduction of surface oxygen species, which were at 319, 316, 357 and 385 °C for LaCoO3, LaMnO3, LaNiO3 and LaFeO3, respectively (Fig. 3b, d, f and h). In addition, LaCoO3 (1.98 mol g−1) consumes more hydrogen than LaMnO3 (0.45 mol g−1) (Table S2†) because of a large number of adsorption and lattice oxygen on the LaCoO3 surface, suggesting that the sample with LaCoO3 support was easier to be reduced than samples with other supports. For the unsupported LaBO3 perovskites materials, two peaks were observed. The peak at low temperature was ascribed to the reduction of adsorbed oxygen, and the peak at high temperature was due to the reduction of lattice oxygen.3 For Ag/LaCoO3 and LaCoO3, Co3+/Co2+ and Co2+/Co0 transformation occurred in the reduction, and Co3+/Co2+ redox play an important role in the catalytic oxidation, generating abundant mobile oxygen for the realization of the Mars van Krevelen mechanism in ethyl acetate oxidation.11 The low hydrogen consumption by LaFeO3 induced by the low adsorption and lattice oxygen implies that the stable structure of LaFeO3 leads to its lowest oxidizing ability. LaNiO3 has higher hydrogen consumption but lower oxidizing ability than LaMnO3 which is mainly ascribed to the higher first reduction temperature of LaNiO3. Particularly, distinct shifts to low temperatures of the first reduction peak were observed when LaBO3 were doped with silver, indicating better oxygen mobility in silver-based catalysts compared to pure LaBO3. Furthermore, Ag/LaCoO3 catalyst had the highest hydrogen consumption rate of 2.04 mol g−1 (Table S2†), suggesting that the sample possessed the best redox among the tested catalysts.
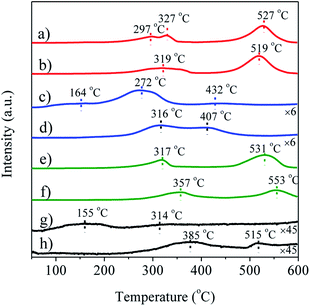 | ||
| Fig. 3 H2-TPR profiles of (a) Ag/LaCoO3, (b) LaCoO3; (c) Ag/LaMnO3, (d) LaMnO3; (e) Ag/LaNiO3, (f) LaNiO3; (g) Ag/LaFeO3, (h) LaFeO3. | ||
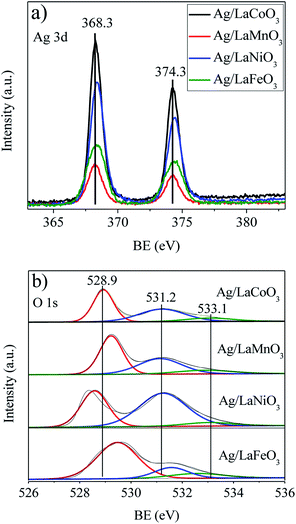
XPS spectra were collected to obtain the element chemical states and oxygen vacancies on the catalyst surface. Fig. 4a shows the Ag 3d spectra of the Ag/LaBO3 catalysts. The two peaks with binding energy at 368.3 and 374.3 eV correspond to the Ag 3d5/2 and Ag 3d3/2 states, respectively, which could be attributed to the Ag0 of the metallic Ag. As listed in Table 1, Ag/LaCoO3 showed the highest Ag content (2.64%), the highest ratio of Ag/La (0.14%) and B/La (0.72%) among the catalysts. The surface content of Ag on Ag/LaNiO3 catalyst was 2.50%, which is lower than surface content of Ag/LaCoO3 but higher than those of others, while it possessed the lowest content of B (5.07%). The Ag/La ratio on the surface of Ag/LaMnO3 catalyst was the lowest, while the activity of catalytic oxidation of ethyl acetate was higher than those of Ag/LaNiO3 and Ag/LaFeO3. Unsupported LaMnO3 contributed apparently higher catalytic activity than LaNiO3 and LaFeO3, due to the easy Mn3+/Mn2+ redox, which has been demonstrated in the H2-TPR results. Addition of Ag increased the catalytic activity of the perovskites, revealing that the Ag dispersion and B sites both play important roles in the oxidation process.
| Sample | Atomic composition (%) | Ag/La (%) | B/La (%) | Oβ/Ototal (%) | |||
|---|---|---|---|---|---|---|---|
| Ag | B | La | O | ||||
| Ag/LaCoO3 | 2.64 | 13.65 | 18.83 | 64.88 | 0.14 | 0.72 | 41.39 |
| Ag/LaMnO3 | 0.74 | 13.26 | 20.57 | 65.43 | 0.04 | 0.64 | 38.12 |
| Ag/LaNiO3 | 2.50 | 5.07 | 18.59 | 73.44 | 0.13 | 0.27 | 57.12 |
| Ag/LaFeO3 | 1.60 | 13.9 | 20.05 | 64.45 | 0.08 | 0.69 | 17.70 |
The O 1s spectra were measured to obtain the chemical states of oxygen on Ag/LaBO3 catalysts (Fig. 4b). Previous reports showed that the oxygen states in perovskite-like systems were rather complex and ambiguous to interpret.37,38 Nevertheless, the O 1s peak at the lowest binding energy may be assigned to the lattice oxygen O2− and the peak at Eb = 530.8–531.5 eV assigned to oxide defects or surface oxygen ions with low coordination which is denoted as Oβ, while the peak at the highest binding energy is assigned to surface carbonate or water molecules adsorbed on the surface. The relative concentration of Oβ, defined as Oβ/Ototal, was calculated based on the area of the corresponding peaks (Table 1). Ag/LaNiO3 with the highest Oβ/Ototal ratio showed relatively higher surface chemisorbed oxygen, although the catalytic activity was inconsistent with the amount of chemisorbed oxygen. The XPS results here suggest that the chemical states of Ag/LaBO3 catalysts were affected by the B-site of the perovskite, and the catalytic activity was related to the mutual effect of content of Ag0, ratio of Ag/La, B/La and Oβ/Ototal.
3.2. Catalytic evaluation
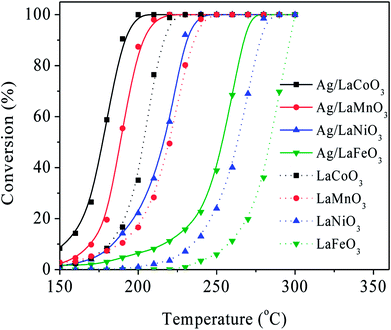
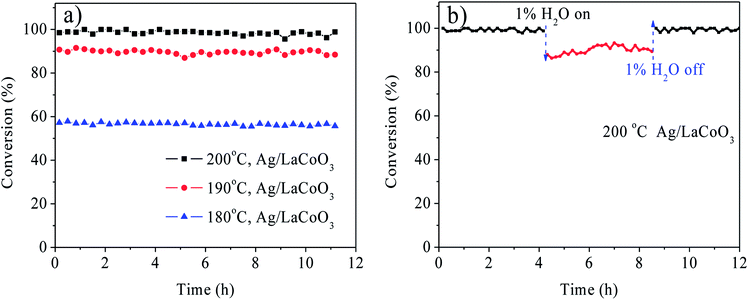
The Ag/LaBO3 and LaBO3 (B = Co, Mn, Ni, Fe) samples were evaluated in the catalytic oxidation of ethyl acetate, and the results are shown in Fig. 5. The catalysts were ranked by decreasing activity based on T90 as follow: Ag/LaCoO3 (190 °C) > Ag/LaMnO3 (203 °C) > LaCoO3 (216 °C) > Ag/LaNiO3 (230 °C) > LaMnO3 (236 °C)> Ag/LaFeO3 (267 °C) > LaNiO3 (278 °C) > LaFeO3 (296 °C). The result indicates that LaCoO3 contributed the highest catalytic activity for the unsupported LaBO3 perovskite materials, and addition of Ag facilitated apparently facilitated the catalytic oxidation process due to the formation Ag0 nanoparticles and its stronger reducibility, which has been proved in the HAADF-STEM mapping images and H2-TPR results. Ag/LaCoO3 showed the highest reactivity for catalytic oxidation of ethyl acetate. According to the H2-TPR result, Ag/LaCoO3 has higher reducibility than other samples, which was crucial to the catalytic oxidation of ethyl acetate.In order to evaluate the catalytic stability, on-stream ethyl acetate oxidation for Ag/LaCoO3 was performed at 180, 190 and 200 °C, respectively. The results are summarized in Fig. 6a. When the reaction temperature was 180 °C, the ethyl acetate conversion was stabilized at 58% within 12 h, and no apparent conversion decrease was observed. With the increase of the reaction temperature, the conversion could be maintained at 90 and 99% at 190 and 200 °C, respectively, revealing that Ag/LaCoO3 sample was catalytically durable.
Water vapor commonly exists in the VOCs-containing waste gases, and the anti-moisture ability is crucial to the industrial application of the catalysts. Hence, the anti-moisture experiment was evaluated for Ag/LaBO3 in presence of 1% H2O at 200 °C. As illustrated in Fig. 6b, the conversion was maintained at 99% in the first 4 h. Introduction of 1% H2O led to the decrease of the ethyl acetate conversion from 99% to 88%, while the catalytic activity was recovered when 1% H2O was cut off. Therefore, the water exhibited an inhibitory and reversible effect in the catalytic oxidation of ethyl acetate.
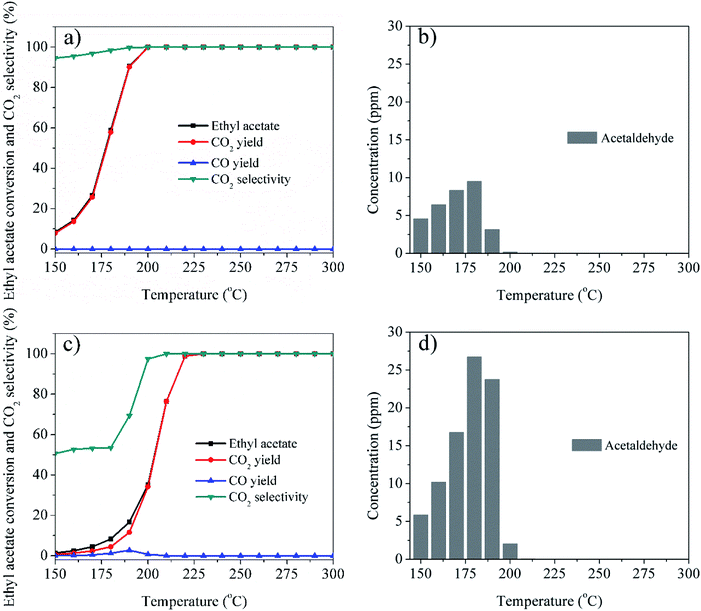
To further investigate the catalytic performance, CO2 selectivity, COx yields, distributions of the organic byproducts for Ag/LaCoO3 and LaCoO3 were studied and compared for Ag/LaCoO3 and LaCoO3, and the results are shown in Fig. 7. Clearly, the addition of Ag significantly promoted the removal of ethyl acetate and the selectivity of CO2, and the CO2 yield are almost equal to the ethyl acetate conversions in the whole experimental temperature for Ag/LaCoO3. Notably, no CO was observed in the catalytic oxidation over Ag/LaCoO3, whereas the CO yield reached 2.8% at 190 °C for LaCoO3. As shown in Fig. 7b and d, acetaldehyde was observed and detected as the main byproduct due to the partial oxidation of ethyl acetate. It is known that ethyl acetate oxidation is a stepwise process with a preliminary hydrolysis to ethanol and acetic acid.1 Further oxidation of ethanol contributed acetaldehyde, which was easily released from the catalyst surface as byproduct. It has been reported that surface acidity facilitates the adsorption of VOCs,39 whereas perovskites materials commonly exhibits weak surface acidity, which might be a reason for the byproduct releasing.40 The maximum concentration of acetaldehyde reached 9.5 and 26.7 ppm at 180 °C for Ag/LaCoO3 and LaCoO3, respectively, suggesting that Ag/LaCoO3 possesses the advantages of higher CO2 selectivity and less byproduct due to Ag addition. For comparison, the CO2 selectivity, COx yields, distributions of the organic byproduct for Ag/LaMnO3 and LaMnO3 (Fig. S1†), Ag/LaNiO3 and LaNiO3 (Fig. S2†), Ag/LaFeO3 and LaFeO3 (Fig. S3†) have been collected and summarized in ESI.† It can be seen that Ag/LaCoO3 and LaCoO3 generates far less byproducts than other perovskite materials at similar conversions, demonstrating their high catalytic activities, which were well-correlated with their physicochemical properties. Addition of Ag commonly decreased the byproduct generations. The maximum concentrations of acetaldehyde were 22.1, 28.1, and 30.8 ppm for Ag/LaMnO3, Ag/LaNiO3, and Ag/LaFeO3, respectively, apparently higher than that of Ag/LaCoO3.
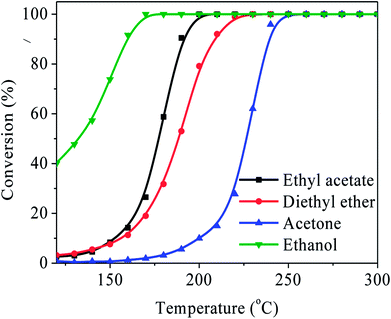
Other kinds of OVOCs such as the ethers, alcohols, and ketones, commonly exist in the industrial waste gases. Hence, the most efficient catalyst Ag/LaCoO3 was also employed in the catalytic oxidation of diethyl ether, ethanol and acetone to test its oxidation ability to various OVOCs abatements. As summarized in Fig. 8, the T90 of ethanol, ethyl acetate, acetone, and diethyl ether were 159 °C, 190 °C, 209 °C, and 239 °C, respectively, demonstrating that Ag/LaCoO3 exhibits outstanding applicability to various OVOCs purifications.
4. Conclusions
In summary, LaBO3 and Ag/LaBO3 (B= Co, Mn, Ni, Fe) were investigated for catalytic oxidation of ethyl acetate. The synergistic effect of silver and LaBO3 is a significant factor which influences the activity of catalytic oxidation. Silver supported LaBO3 samples were more active than perovskite alone for ethyl acetate oxidation. Among the catalysts, Ag/LaCoO3 exhibited the highest conversion rate of ethyl acetate and possessed the highest redox activity, which indicates that the reducibility of the catalysts plays a significant role in the catalytic oxidation process. Ag/LaCoO3 shows excellent catalytic stability and anti-moisture performance. Ag/LaCoO3 shows higher CO2 selectivity and produces less byproduct than other samples. Moreover, Ag/LaCoO3 also shows outstanding applicability for other OVOCs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Key R&D Program of China (Grant no. 2016YFC0207103) and Natural Science Foundation of China (Grant no. 21607154).Notes and references
- X. Zhu, S. Zhang, Y. Yang, C. Zheng, J. Zhou, X. Gao and X. Tu, Appl. Catal., B, 2017, 213, 97–105 CrossRef CAS.
- C. H. Wu and C. W. Lin, J. Taiwan Inst. Chem. Eng., 2016, 68, 332–337 CrossRef CAS.
- X. Zhu, X. Tu, M. Chen, Y. Yang, C. Zheng, J. Zhou and X. Gao, Catal. Commun., 2017, 92, 35–39 CrossRef CAS.
- Y. Zhou, H. Zhang and Y. Yan, J. Taiwan Inst. Chem. Eng., 2018, 84, 162–172 CrossRef CAS.
- G. N. Pirogova, R. I. Korosteleva, N. M. Panich, T. A. Lagutina and Y. V. Voronin, Russ. Chem. Bull., 1994, 43, 551–554 CrossRef.
- A. Jodaei, D. Salari, A. Niaei, M. Khatamian and N. Çaylak, Environ. Technol., 2011, 32, 395–406 CrossRef CAS PubMed.
- A. Niaei, D. Salari, S. A. Hosseini, R. Nabavi and A. Jodaei, Int. J. Chem. React. Eng., 2008, 6, 77–90 Search PubMed.
- J. E. Sawyer and M. A. Abraham, Ind. Eng. Chem. Res., 1994, 33, 2084–2089 CrossRef CAS.
- S. Akram, L. Chen, Q. Wang, X. Zhang, N. Han, G. Shen, Z. Wang and G. Ge, Catal. Lett., 2017, 147, 1–13 CrossRef.
- V. P. Santos, S. A. C. Carabineiro, P. B. Tavares, M. F. R. Pereira, J. J. M. Órfão and J. L. Figueiredo, Appl. Catal., B, 2010, 99, 198–205 CrossRef CAS.
- S. A. C. Carabineiro, X. Chen, O. Martynyuk, N. Bogdanchikova, M. Avalos-Borja, A. Pestryakov, P. B. Tavares, J. J. M. Órfão, M. F. R. Pereira and J. L. Figueiredo, Catal. Today, 2015, 244, 103–114 CrossRef CAS.
- B. Aellach, A. Ezzamarty, J. Leglise, C. Lamonier and J. F. Lamonier, Catal. Lett., 2010, 135, 197–206 CrossRef CAS.
- X. Chen, S. A. C. Carabineiro, S. S. T. Bastos, P. B. Tavares, J. J. M. Órfão, M. F. R. Pereira and J. L. Figueiredo, J. Environ. Chem. Eng., 2013, 1, 795–804 CrossRef CAS.
- Z. Zhang, Z. Jiang and W. Shangguan, Catal. Today, 2016, 264, 270–278 CrossRef CAS.
- B. Białobok, J. Trawczyński, W. Miśta and M. Zawadzki, Appl. Catal., B, 2007, 72, 395–403 CrossRef.
- M. M. E. Geurts, J. Talsma, J. R. B. J. Brouwers and J. J. De Gier, Catal. Today, 2011, 176, 449–452 CrossRef.
- M. Alifanti, M. Florea and V. Pârvulescu, Appl. Catal., B, 2007, 70, 400–405 CrossRef CAS.
- P. R. Zonouz, A. Niaei and A. Tarjomannejad, J. Taiwan Inst. Chem. Eng., 2016, 65, 276–285 CrossRef CAS.
- S. Hu and M. Zhu, Chempluschem, 2016, 81, 1202–1208 CrossRef CAS.
- P. K. Sekhar, R. Mukundan, E. Brosha and F. Garzon, Sens. Actuators, B, 2013, 183, 20–24 CrossRef CAS.
- J. A. Villoria, M. C. Alvarez-Galvan, S. M. Al-Zahrani, P. Palmisano, S. Specchia, V. Specchia, J. L. G. Fierro and R. M. Navarro, Appl. Catal., B, 2011, 105, 276–288 CrossRef CAS.
- M. A. Peña and J. L. Fierro, Chem. Rev., 2001, 32, 1981–2017 CrossRef.
- Q. Yang, G. Liu and Y. Liu, Ind. Eng. Chem. Res., 2017, 57, 1–17 CrossRef.
- M. Ghasdi and H. Alamdari, Sens. Actuators, B, 2010, 148, 478–485 CrossRef CAS.
- J. Gallego, F. Mondragon and C. Batiot-Dupeyrat, Appl. Catal., A, 2013, 450, 73–79 CrossRef CAS.
- O. P. Taran, A. B. Ayusheev, O. L. Ogorodnikova, I. P. Prosvirin, L. A. Isupova and V. N. Parmon, Appl. Catal., B, 2016, 180, 86–93 CrossRef CAS.
- J.-M. Giraudon, A. Elhachimi and G. Leclercq, Appl. Catal., B, 2008, 84, 251–261 CrossRef CAS.
- L. Tang, Z. Zhao, Y. Wei, J. Liu, Y. Peng and K. Li, Catal. Today, 2017, 297, 131–142 CrossRef CAS.
- M. Lei, D. Wang, J. Li, B. Bai, L. Fu and Y. Li, Appl. Catal., B, 2014, 148–149, 36–43 Search PubMed.
- H. Yang, J. Deng, Y. Liu, S. Xie, Z. Wu and H. Dai, J. Mol. Catal. A: Chem., 2016, 414, 9–18 CrossRef CAS.
- N. Shimoda, S. Umehara, M. Kasahara, T. Hongo, A. Yamazaki and S. Satokawa, Appl. Catal., A, 2015, 507, 56–64 CrossRef CAS.
- S. Scirè, P. M. Riccobene and C. Crisafulli, Appl. Catal., B, 2010, 101, 109–117 CrossRef.
- M. Liu, X. Wu, S. Liu, Y. Gao, Z. Chen, Y. Ma, R. Ran and D. Weng, Appl. Catal., B, 2017, 219, 231–240 CrossRef CAS.
- K. Wang, L. Qian, L. Zhang, H. Liu and Z. Yan, Catal. Today, 2010, 158, 423–426 CrossRef CAS.
- J. M. Giraudon, A. Elhachimi, F. Wyrwalski, S. Siffert, A. Aboukaïs, J. F. Lamonier and G. Leclercq, Appl. Catal., B, 2007, 75, 157–166 CrossRef CAS.
- K. Y. A. Lin, Y. C. Chen and Y. F. Lin, Chem. Eng. Sci., 2017, 160, 96–105 CrossRef CAS.
- J. L. Hueso, A. Caballero, M. Ocaña and A. R. González-Elipe, J. Catal., 2008, 257, 334–344 CrossRef CAS.
- J. N. Kuhn and U. S. Ozkan, J. Catal., 2008, 253, 200–211 CrossRef CAS.
- F. Bertinchamps, C. Grégoire and E. M. Gaigneaux, Appl. Catal., B, 2006, 66, 1–9 CrossRef CAS.
- G. Pecchi, P. Reyes, R. Zamora, L. E. Cadús and J. L. G. Fierro, J. Solid State Chem., 2008, 181, 905–912 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Specific surface areas, H2 consumption of the samples, and byproduct distributions. See DOI: 10.1039/c8ra06933f |
| This journal is © The Royal Society of Chemistry 2018 |