Open Access Article
Open Access ArticleRecognition and optical sensing of amines by a quartz-bound 7-chloro-4-quinolylazopillar[5]arene monolayer†
Ilenia Pisagatti a,
Giuseppe Gattuso
a,
Giuseppe Gattuso a,
Anna Notti
a,
Anna Notti a,
Melchiorre F. Parisi
a,
Melchiorre F. Parisi *a,
Giovanna Brancatelli‡
*a,
Giovanna Brancatelli‡
 b,
Silvano Geremia
b,
Silvano Geremia b,
Francesco Greco
b,
Francesco Greco cd,
Salvatrice Millesicd,
Andrea Pappalardo
cd,
Salvatrice Millesicd,
Andrea Pappalardo cd,
Luca Spitalericd and
Antonino Gulino
cd,
Luca Spitalericd and
Antonino Gulino *cd
*cd
aDipartimento di Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, Università di Messina, Viale F. Stagno d'Alcontres 31, 98166 Messina, Italy. E-mail: mparisi@unime.it
bCentro di Eccellenza in Biocristallografia, Dipartimento di Scienze Chimiche e Farmaceutiche, Università di Trieste, via L. Giorgieri 1, 34127 Trieste, Italy
cDipartimento di Scienze Chimiche, Università di Catania, Viale Andrea Doria 6, 95125 Catania, Italy. E-mail: agulino@unict.it
dI.N.S.T.M. UdR of Catania, Viale Andrea Doria 6, 95125 Catania, Italy
First published on 26th September 2018
Abstract
Covalent bonding of 7-chloro-4-quinolylazo-octamethoxypillar[5]arene molecules to silylated quartz substrates readily produced a new chromogenic reusable pillararene-coated quartz slide, for the direct UV detection of “transparent” analytes in solution. This device provides an analyte-selective optical response towards linear (di)amines with a highly reproducible optical read-out.
Introduction
The construction of hybrid materials combining the robustness and inertness of inorganic substrates with the ample diversity of supramolecularly-active organic compounds is a topic of current academic and technological interest in connection with the development of stimuli-responsive materials (e.g., switches, memory devices, logic gates, sensors, etc.).1 Among these, substrates coated with molecular-based thin films – capable of detecting analytes at low concentrations – have received considerable attention in the context of sensing device construction,2 following the pioneering work by Reinhoudt.3 Cavitand-,4 and calixarene-based5 monolayers, for instance, owing to their electron-rich cavities, have been successfully used for the detection of ammonium-containing analytes,6,7 but have shown rather poor or no affinity for amines and polyamines unless a spontaneous host-to-guest proton transfer takes place during the recognition/binding process.8Despite the huge impact that pillararenes have had in the field of supramolecular chemistry9 since their discovery in 2008,10 with their scaffold being key to a number of different functional materials (e.g., drug delivery systems,11 supramolecular polymers,12 light harvesting complexes,13 photomodulated surfaces14), their potential as sensing agents for the detection of amine analytes has not yet been thoroughly explored. Very recently, the known proclivity of pillar[5]arenes to act as host molecules for alkanediamine neutral guests15,16 has led to the preparation of pillar[5]arene-modified silver nanoparticles for the visual detection of spermine analogues17 and the construction of a thiolated co-pillar[5]arene for the electrochemical sensing of linear biogenic amines.18
Given the UV- and fluorescence-transparency of both low molecular weight and biogenic amines and the usefulness of the latter in the direct monitoring/detection of foodstuff quality19 and human health,20 we have undertaken the design of an easy-to-make pillar[5]arene derivative containing a heteroarylazo chromophore suitable both as a sensing agent for “transparent” analytes and as a monolayer coating material.21 The key benefits of monolayers rest on their ability to display and possibly enhance the intrinsic molecular properties of single molecules bound to a solid surface. Specific advantages of monolayer-based sensors include: (i) the need for only a small amount of sensing agent to generate a large active surface, (ii) the absence of sensing material consumption and (iii) the lack of diffusion limitations because the surface-confined molecules are in direct contact with the solution of the target analyte.22 The aim of the present study was to obtain a sensing-device, namely a reusable pillararene-coated quartz slide, for the direct UV-vis and fluorescence detection of linear amines in solution. With this in mind, we have synthesised pillar[5]arene QAP5 and we now wish to report its covalent immobilization on quartz substrates together with the ability of the resulting monolayer to detect linear (di)amino analytes (i.e., n-butylamine, 1,8-diaminoctane and dansylcadaverine [5-dimethylaminonaphthalene-1-(N-(5-aminopentyl))sulfonamide]).
Results and discussion
7-Chloro-4-quinolylazo-octamethoxypillar[5]arene (QAP5) was selected as a potential sensing agent to be anchored to quartz substrates in view of the chromogenic properties of the quinolylazo moiety.23 The choice of QAP5 as a sensing agent was also motivated by the additional advantage of using a molecule suitably equipped with a chromogenic moiety bearing a nucleophilic nitrogen atom capable of undergoing, at a later stage, covalent bond formation with silylated quartz surfaces, to ultimately yield a robust linkage with the solid substrate.According to Scheme 1a, QAP5 was obtained in a single step (55% yield) from pillar[4]arene[1]quinone24 1 and 7-chloro-4-hydrazinoquinoline. NMR spectroscopy (Fig. S3 and S4, ESI†) as well as single-crystal X-ray diffraction analysis (Fig. 1), unambiguously confirmed the structure of the novel pillararene. In the solid state QAP5 adopts a semiregular pentagonal-prism shape (with dihedral angles between the hydroquinone-containing planes and the bridging-methylene mean-plane in the 80.88(7)–102.32(9)° range; ESI†). Several solvent molecules (tetrachloroethane (TCE), CH3OH and H2O; ESI†) either occlude or fill the hollow/cavity of the macrocycle.
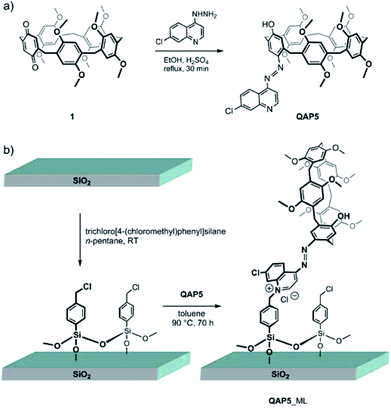 | ||
| Scheme 1 The syntheses of: (a) 7-chloro-4-quinolylazo-octamethoxypillar[5]arene (QAP5) and (b) the corresponding quartz-grafted monolayer (QAP5_ML). | ||
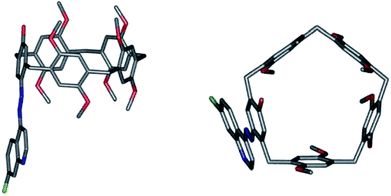 | ||
| Fig. 1 Side (left) and top (right) views of the solid-state structure of QAP5. Hydrogen atoms and solvent molecules have been omitted for the sake of clarity. | ||
The quinolylazo-pillar[5]arene monolayer (QAP5_ML) was synthesized by covalently grafting QAP5 macrocycles to silylated quartz slides§ (Scheme 1b). The silylation reaction was carried out under a rigorously inert atmosphere, using trichloro[4-(chloromethyl)phenyl]silane as a convenient bifunctional coupling agent (CA) capable of covalently linking, via the nucleophilic quinoline nitrogen atom, the QAP5 sensing agent to the quartz surface.25
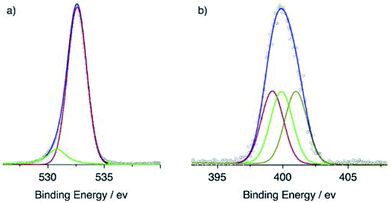
This QAP5_ML was found to be insoluble in toluene, DMSO, CH3CN, THF, Et2O and EtOH, thermally robust and stable for long-term storage (vide infra). The successful siloxane-mediated immobilization of QAP5_ML onto quartz (and Si(100)) substrates was assessed by X-ray photoelectron spectroscopy (XPS), via elemental composition analysis of the surface. Data fitting of the O 1s binding-energy region of the QAP5_ML spectrum revealed the presence of two components one of high- and one of low-intensity assigned to the oxygen atoms of the SiO2 substrate (532.6 eV) and the pillar[5]arene methoxy groups (530.8 eV), respectively (Fig. 2a). Similar fitting of the N 1s binding-energy region of the QAP5_ML experimental spectrum (Fig. 2b) showed the presence of three, equally intense, Gaussian components centred at 401.0, 399.9 and 399.2 eV which were respectively assigned to the quaternized nitrogen atom26 of the quinoline moiety and the two nitrogen atoms of the azo group27 adjacent to the quinoline and the phenol rings.
The reaction between QAP5 and the chlorobenzyl-coated monolayer was not quantitative owing to the high molecular footprint of the pillararene (vide infra). The observed Cl/N XPS ratio of 3.2 ± 0.5 indicates ∼11.9% yield, after taking into account the presence of one chlorine and three nitrogen atoms in QAP5. Physisorption was excluded on the basis of a control experiment carried out on a model substrate with a CA-uncoated hydrophilic SiO2-terminated surface, which showed that exposure of the substrate to a 10−3 M toluene solution of QAP5 (at 90 °C for 36 h) did not reveal any nitrogen signal, upon XPS analysis, thus excluding the presence of QAP5 molecules on the substrate surface. AFM analysis of a QAP5_ML showed a uniform surface (Fig. S5, ESI†) with structure heights of 2.5 ± 0.15 nm, compatible with the dimension of a QAP5 molecule covalently linked to a [4-(chloromethyl)phenyl]siloxane moiety (∼2.15 nm, from molecular models).
The UV-vis spectra (Fig. S6a and S6b; ESI†) of the pillarene as such ([QAP5] = 1.8 × 10−7 M, in toluene) and as a quartz-immobilized monolayer (QAP5_ML) display very similar absorption bands (λmax 384.2 and 406.0 nm, respectively). A QAP5_ML surface coverage of 5.4 × 1013 molecules per cm2 was then calculated from the absorption intensity of the monolayer (5.7 × 10−3 O.D.), by means of the Lambert–Beer law (ε = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 M−1 cm−1 for a toluene solution of QAP5), ultimately corresponding to a pillararene footprint of 185 Å2, compatible with the value estimated from X-ray measurements (120 Å2) for QAP5.28
700 M−1 cm−1 for a toluene solution of QAP5), ultimately corresponding to a pillararene footprint of 185 Å2, compatible with the value estimated from X-ray measurements (120 Å2) for QAP5.28
Owing to the ability of pillar[5]arenes to host solvent molecules inside their cavity29 and as a result impede analyte entry, preliminary UV-vis tests were carried out to assess the response of QAP5 and QAP5_ML to the addition of model (di)amines (i.e., n-butylamine, 1,8-diaminoctane and dansylcadaverine) in different solvents. Initial attempts, using QAP5, to sense/detect n-butylamine or 1,8-diaminoctane in toluene solution were to no avail, as the pillararene/amine mixtures under screening produced no significant UV-vis spectral changes (Fig. S6b, ESI†). Similarly unsatisfactory results were obtained in CH3CN, where the same QAP5/amine mixtures were found to be sparingly soluble. DMSO was also judged to be unsuitable as it promoted the ionization of the phenol moiety to the corresponding phenolate anion (Fig. S6b, ESI†). Further UV-vis studies were carried out in TCE, which is known to be too bulky to fit inside the pillar[5]arene cavity.30 In this solvent QAP5 showed a single absorption band (λmax = 397.2 nm; ε = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1). Aspecific interactions between n-butylamine and the simple 7-chloro-4-quinolylazophenol chromophore (QA) – lacking the pillararene macrocyclic scaffold – were preliminarily ruled out on the basis of the insignificant spectral variation observed in the chromophore spectrum upon addition of an excess of amine (Fig. S7, ESI†). Addition of increasing amounts (up to 50–100 equiv.) of n-butylamine, 1,8-diaminooctane and dansylcadaverine (Fig. S8–S10, ESI†) to a TCE solution of QAP5 produced, in all instances, a sizeable optical response consistent with a (small but significant) red shift (from 397.2 nm to 408.4, 407.6 and 403.4 nm for n-butylamine, 1,8-diaminoctane, and dansylcadaverine, respectively) as well as an hyperchromic effect (ca., 14, 3 and 20% for n-butylamine, 1,8-diaminooctane and dansylcadaverine, respectively).
000 M−1 cm−1). Aspecific interactions between n-butylamine and the simple 7-chloro-4-quinolylazophenol chromophore (QA) – lacking the pillararene macrocyclic scaffold – were preliminarily ruled out on the basis of the insignificant spectral variation observed in the chromophore spectrum upon addition of an excess of amine (Fig. S7, ESI†). Addition of increasing amounts (up to 50–100 equiv.) of n-butylamine, 1,8-diaminooctane and dansylcadaverine (Fig. S8–S10, ESI†) to a TCE solution of QAP5 produced, in all instances, a sizeable optical response consistent with a (small but significant) red shift (from 397.2 nm to 408.4, 407.6 and 403.4 nm for n-butylamine, 1,8-diaminoctane, and dansylcadaverine, respectively) as well as an hyperchromic effect (ca., 14, 3 and 20% for n-butylamine, 1,8-diaminooctane and dansylcadaverine, respectively).
Based on this initial screening, (di)amine detection by QAP5 was closely looked into by 1H NMR spectroscopy using deutero TCE (TCE-d2) as the solvent of choice. Addition of increasing aliquots of analyte (up to ∼5 equiv.) to 1.0 mM solutions of QAP5 produced steady shifts of selected resonances (e.g., ArH at δ = 7.80 ppm), thus allowing the determination of the pertinent binding constants (Kass = 286 ± 20 and 509 ± 40 M−1 for n-butylamine and 1,8-diaminoctane, respectively) under a fast host–guest association/dissociation regime (see Fig. S11 and S12 in the case of 1,8-diaminoctane, ESI†). Fluorescence spectroscopy was alternatively used for the determination, in TCE, of the binding constant between QAP5 and dansylcadaverine (Kass = (4.3 ± 0.4) × 104 M−1), by looking at the quenching of the amine emission (at λ = 510 nm) upon addition of increasing amounts of pillararene (Fig. S13–S14, ESI†).
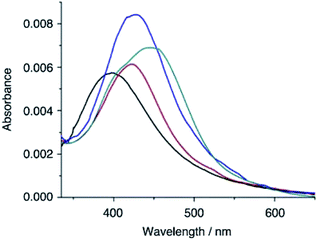
The ability of QAP5_ML to sense the model (di)amines in TCE solutions was tested spectrophotometrically (Fig. 3). However, owing to the presence of toluene molecules (used as the solvent in the QAP5-coating step) tightly hosted inside the pillararene cavity,29b it was deemed necessary to prime the monolayer (see the Experimental section) to expel these residual solvent molecules from the monolayer binding/recognition sites. Successful monolayer priming was ultimately confirmed by a shift in the QAP5_ML absorption from 384.2 to 397.0 nm. Dipping of the monolayer (5 min) into either n-butylamine, 1,8-diaminoctane or dansylcadaverine TCE solutions consistently caused a red shift of the original QAP5_ML band (λmax = 397.0 nm), as well as, a substantial hyperchromic effect. In particular, the endo-cavity inclusion of n-butylamine, 1,8-diaminoctane and dansylcadaverine inside the QAP5_ML cavities, was diagnostically revealed by the presence of new absorption bands centred at 422.6 (8% intensity increase), 426.4 (47%) and 445.6 nm (21%), respectively. These data indicate that, depending on the amine included inside the QAP5_ML cavities, the grafted pillarazo derivative returns an analyte-selective optical response (λmax).
In analogy with very closely structurally-related pillar[5]arene derivatives, the host–guest complexation between QAP5 and uncharged aliphatic amines and diamines is believed to occur via the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 pseudorotaxane-type complexes.15 The threading of the guests inside QAP5 most likely involves multiple C–H⋯π interactions between the aliphatic chain of the (di)amine and the pillar[5]arene cavity, as well as hydrophobic interactions.15c,31 The additional formation of hydrogen bonds between the primary amino group(s) and the oxygen atoms on the pillararene rims may also provide further stabilization to the pseudorotaxane. As a result, analyte detection relies on the transient presence of the guest inside the QAP5 cavity and consequently on the strength of the concomitant host–guest interactions which in turn affect the absorption spectrum of the chromogenic pillararene.
1 pseudorotaxane-type complexes.15 The threading of the guests inside QAP5 most likely involves multiple C–H⋯π interactions between the aliphatic chain of the (di)amine and the pillar[5]arene cavity, as well as hydrophobic interactions.15c,31 The additional formation of hydrogen bonds between the primary amino group(s) and the oxygen atoms on the pillararene rims may also provide further stabilization to the pseudorotaxane. As a result, analyte detection relies on the transient presence of the guest inside the QAP5 cavity and consequently on the strength of the concomitant host–guest interactions which in turn affect the absorption spectrum of the chromogenic pillararene.
The detection limit for the current prototypical device is 100 ppm. Longer contact periods of the QAP5_ML with the amine solutions did not change the optical absorbance. This detection limit is most likely related to the relatively weak absorption intensity of the 7-chloro-4-quinolylazo chromophore present in QAP5 (ε = 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in TCE). In all instances, reactivation of the monolayer with TCE (see the Experimental section), produces full recovery of the optical absorbance, as verified by UV-vis spectroscopy. Fig. 4 shows that the ability of the monolayer to sense n-butylamine remains substantially unchanged after several exposure/recovery cycles. Remarkably, heating of the QAP5_ML at 100 °C, in the presence of air for 10 days, does not affect its performance. Similarly, sensitivity was not compromised when the sensor was left in air at room temperature for a period as long as 4 months.
000 in TCE). In all instances, reactivation of the monolayer with TCE (see the Experimental section), produces full recovery of the optical absorbance, as verified by UV-vis spectroscopy. Fig. 4 shows that the ability of the monolayer to sense n-butylamine remains substantially unchanged after several exposure/recovery cycles. Remarkably, heating of the QAP5_ML at 100 °C, in the presence of air for 10 days, does not affect its performance. Similarly, sensitivity was not compromised when the sensor was left in air at room temperature for a period as long as 4 months.
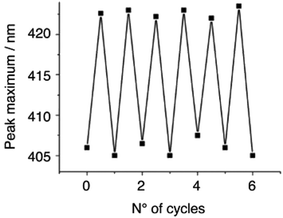 | ||
| Fig. 4 Peak position variations for the QAP5_ML upon sequential 5 min. Dipping in a 100 ppm TCE solution of n-butylamine followed by rinsing (10 min) with TCE. | ||
Experimental
Materials and methods
1H NMR titration studies were carried out at a fixed QAP5 concentration (1 mM) and samples were routinely prepared by dissolving solid QAP5 in TCE-d2. Stock solutions of n-butylamine and 1,8-diaminooctane (20 mM) were, in turn, prepared by using the above-mentioned 1 mM QAP5 TCE-d2 solution as a convenient solvent so that, during the titration, the host concentration did not vary upon addition of increasing aliquots of the guest. The association constants were calculated by a nonlinear regression method (assuming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation model) using the WinEQNMR.32
1 complexation model) using the WinEQNMR.32
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 slit-widths. The association constant for the dansylcadaverine⊂QAP5 complex was calculated by a nonlinear regression method (assuming a 1
10 slit-widths. The association constant for the dansylcadaverine⊂QAP5 complex was calculated by a nonlinear regression method (assuming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation model) using the MicroMath Scientist program.
1 complexation model) using the MicroMath Scientist program.Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4 mL) containing 900 μL of conc. H2SO4 was added dropwise to a boiling solution of the known pillar[4]arene[1]quinone24 1 (294 mg, 0.408 mM) in EtOH (30 mL) under stirring. The starting red-coloured solution of 1 quickly darkened. After 30 min reflux the solution was stirred overnight at room temperature. The reaction mixture was concentrated to dryness and the resulting residue was partitioned between water and CH2Cl2. The organic layer was washed with a saturated NaHCO3 solution, dried over anhydrous Na2SO4 and subjected to column chromatography (eluent CH2Cl2/AcOEt 100
1, 4 mL) containing 900 μL of conc. H2SO4 was added dropwise to a boiling solution of the known pillar[4]arene[1]quinone24 1 (294 mg, 0.408 mM) in EtOH (30 mL) under stirring. The starting red-coloured solution of 1 quickly darkened. After 30 min reflux the solution was stirred overnight at room temperature. The reaction mixture was concentrated to dryness and the resulting residue was partitioned between water and CH2Cl2. The organic layer was washed with a saturated NaHCO3 solution, dried over anhydrous Na2SO4 and subjected to column chromatography (eluent CH2Cl2/AcOEt 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 95
0 to 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to afford two main fractions:
5, v/v) to afford two main fractions:Fraction A gave 7-chloro-4-quinolylazo-octamethoxypillar[5]arene O-ethyl ether (8–10% yield) as red crystals (mp 109–110 °C, from EtOH): 1H NMR δ 9.00 (d, 2-QuinH, J = 4.4 Hz, 1H), 8.80 (d, 5-QuinH, J = 8.8 Hz, 1H), 8.19 (d, 8-QuinH, J = 2.2 Hz, 1H), 7.90 (s, ArH, 1H), 7.64 (dd, 6-QuinH, J = 8.8, 2.2 Hz, 1H), 7.51 (d, 3-QuinH, J = 4.4 Hz, 1H), 6.65, 6.645, 6.639, 6.62 (4 × s, ArH, 1H each), 6.60 (s, ArH, 2H), 6.58, 6.54, 6.52 (3 × s, ArH, 1H each), 4.46, 3.86, 3.76, 3.75, 3.74 (5 × s, ArCH2Ar, 2H each), 3.79 (q, OCH2CH3, J = 7.0 Hz, 2H), 3.54, 3.52, 3.51, 3.504, 3.496, 3.47, 3.43, 3.30 (8 × s, OCH3, 3H each) and 0.86 (t, J = 7.0 Hz, OCH2CH3, 3H) ppm; 1H NMR (CD2Cl2) δ 9.02 (d, 2-QuinH, J = 5.0 Hz, 1H), 8.84 (d, 5-QuinH, J = 9.0 Hz, 1H), 8.17 (d, 8-QuinH, J = 2.0 Hz, 1H), 8.04 (s, ArH, 1H), 7.66 (d, 3-QuinH, J = 5.0 Hz, 1H), 7.59 (dd, 6-QuinH, J = 9.0, 2.0 Hz, 1H), 7.09 (s, ArH, 1H), 6.89 (s, ArH, 2H), 6.88, 6.86, 6.824, 6.817, 6.71, 6.56 (6 × s, ArH, 1H each), 4.40, 3.83, 3.73, 3.67, 3.64 (5 × s, ArCH2Ar, 2H each), 4.11 (q, OCH2CH3, J = 6.5 Hz, 2H), 3.80, 3.78, 3.77, 3.73, 3.72, 3.69, 3.56, 3.11 (8 × s, OCH3, 3H each) and 1.51 (t, J = 6.5 Hz, OCH2CH3, 3H) ppm; 13C NMR δ 160.7, 153.4, 152.1, 150.8, 150.7, 150.56, 150.52 (×2), 150.48, 150.47, 150.42, 150.1, 144.9, 143.9, 135.4, 129.1, 128.7, 128.5, 128.4, 128.3 (×2), 128.2, 128.0, 127.57, 127.56, 127.54, 125.2, 123.8, 118.1, 114.0, 113.9, 113.8 (×2), 113.7, 113.6, 112.4, 105.2, 63.2, 55.82 (×2), 55.76, 55.68, 55.64 (×2) 55.5 (×2), 30.4, 30.2, 29.8, 29.7, 29.6 and 13.8 ppm; ESI MS m/z 923.7 (M+, 100%). Anal. calcd for C54H54ClN3O9: C, 70.16; H, 5.89; Cl, 3.83; N, 4.55. Found: C, 69.83; H, 6.02; Cl, 3.75; N, 4.45.
Fraction B yielded the desired QAP5 as red-brown crystals (mp 105–108 °C, from MeOH) (201 mg, 55% yield); 1H NMR δ 9.02 (d, J = 4.9 Hz, 2-QuinH, 1H), 8.79 (d, J = 9.0 Hz, 5-QuinH, 1H), 8.20 (s, ArH, 1H), 7.92 (s, OH, 1H), 7.80 (s, 8-QuinH, 1H), 7.63 (dd, J = 9.0, 1.8 Hz, 6-QuinH, 1H), 7.56 (d, J = 4.9 Hz, 3-QuinH, 1H), 6.91, 6.78, 6.69, 6.64, 6.63, 6.61, 6.59, 6.54, 6.49 (9 × s, ArH, 1H each), 4.41, 3.87, 3.78, 3.75 (4 × s, ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, ArCH2Ar, 10H), 3.85, 3.66, 3.533, 3.525, 3.46, 3.44, 3.36 and 3.29 (8 × s, OCH3, 3H each) ppm. 13C NMR δ 159.1, 153.5, 152.1, 151.9, 150.9, 150.8, 150.7, 150.6, 150.5, 150.3, 150.0, 147.9, 145.4, 144.3, 135.5, 130.0, 128.9, 128.4, 128.2, 128.1, 128.0, 127.9, 127.6, 126.0, 125.6, 125.1, 123.7, 117.7, 116.9, 114.5, 114.4, 114.1, 114.0, 113.7, 112.8, 105.6, 56.5, 55.93, 55.86, 55.82, 55.77, 52.5, 30.4, 30.2, 29.9, 29.8 and 29.4 ppm. ESI MS m/z 895.4 (M+, 100%). Anal. calcd for C52H50ClN3O9: C, 69.67; H, 5.62; Cl, 3.95; N, 4.69. Found: C, 69.73; H, 5.75; Cl, 4.07; N, 4.81.
2, ArCH2Ar, 10H), 3.85, 3.66, 3.533, 3.525, 3.46, 3.44, 3.36 and 3.29 (8 × s, OCH3, 3H each) ppm. 13C NMR δ 159.1, 153.5, 152.1, 151.9, 150.9, 150.8, 150.7, 150.6, 150.5, 150.3, 150.0, 147.9, 145.4, 144.3, 135.5, 130.0, 128.9, 128.4, 128.2, 128.1, 128.0, 127.9, 127.6, 126.0, 125.6, 125.1, 123.7, 117.7, 116.9, 114.5, 114.4, 114.1, 114.0, 113.7, 112.8, 105.6, 56.5, 55.93, 55.86, 55.82, 55.77, 52.5, 30.4, 30.2, 29.9, 29.8 and 29.4 ppm. ESI MS m/z 895.4 (M+, 100%). Anal. calcd for C52H50ClN3O9: C, 69.67; H, 5.62; Cl, 3.95; N, 4.69. Found: C, 69.73; H, 5.75; Cl, 4.07; N, 4.81.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30% H2O2, 7
30% H2O2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) at 90 °C for 1 h and then left to cool to r.t. Substrates were repeatedly rinsed with double-distilled water and then kept in a H2O
3 v/v) at 90 °C for 1 h and then left to cool to r.t. Substrates were repeatedly rinsed with double-distilled water and then kept in a H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30% H2O2
30% H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH3 (5
NH3 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v/v) mixture at r.t. for 1 h.34 A final wash with double-distilled water, followed by drying under vacuum was then carried out just prior to deposition of the coupling agent. Si(100) substrates, were first cleaned with the above-mentioned piranha solution for 10 min at 90 °C, rinsed with double-distilled water for 5 min, etched in a 2.5% hydrofluoric acid aqueous solution for 150 s (both piranha and hydrofluoric acid solutions need to be handled with caution!), washed, dried under N2 and then treated for 5 min with ozone (using a Fisher 500 ozone-generator system) to yield a SiO2 thin (∼10 Å) layer.35 Both types of freshly cleaned substrates were transferred in a glove-box under a N2 atmosphere and dipped, at r.t. for 1 h, in a n-pentane/trichloro[4-(chloromethyl)phenyl]silane (CA) (100
1 v/v/v) mixture at r.t. for 1 h.34 A final wash with double-distilled water, followed by drying under vacuum was then carried out just prior to deposition of the coupling agent. Si(100) substrates, were first cleaned with the above-mentioned piranha solution for 10 min at 90 °C, rinsed with double-distilled water for 5 min, etched in a 2.5% hydrofluoric acid aqueous solution for 150 s (both piranha and hydrofluoric acid solutions need to be handled with caution!), washed, dried under N2 and then treated for 5 min with ozone (using a Fisher 500 ozone-generator system) to yield a SiO2 thin (∼10 Å) layer.35 Both types of freshly cleaned substrates were transferred in a glove-box under a N2 atmosphere and dipped, at r.t. for 1 h, in a n-pentane/trichloro[4-(chloromethyl)phenyl]silane (CA) (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, v/v) solution, to afford siloxane-coated substrates.36 These substrates were washed with copious amounts of n-pentane, removed from the glove-box and heated to 135 °C for 15 min in an oven to complete the grafting of the coupling agent (CA). CA-grafted substrates were sonicated in n-pentane for 10 min, to remove any physisorbed CA, and subsequently dipped for 72 h in a stirred 8.56 × 10−4 M toluene solution of QAP5 and kept at 90 °C. The monolayer thus obtained (QAP5_ML) was allowed to reach r.t. and then sonicated in turn with toluene, CH3CN and THF to remove any residual unreacted QAP5.
0.1, v/v) solution, to afford siloxane-coated substrates.36 These substrates were washed with copious amounts of n-pentane, removed from the glove-box and heated to 135 °C for 15 min in an oven to complete the grafting of the coupling agent (CA). CA-grafted substrates were sonicated in n-pentane for 10 min, to remove any physisorbed CA, and subsequently dipped for 72 h in a stirred 8.56 × 10−4 M toluene solution of QAP5 and kept at 90 °C. The monolayer thus obtained (QAP5_ML) was allowed to reach r.t. and then sonicated in turn with toluene, CH3CN and THF to remove any residual unreacted QAP5.Conclusions
In conclusion, we have described a simple strategy for the construction of reliable quartz-supported pillararene-based optical devices for the sensing of “transparent” analytes. In particular, a new pillararene-based sensing agent (QAP5) for the detection of UV-inactive or dansyl-derivatized linear (di)amines has been synthesized and covalently grafted to a silylated quartz substrate, with the resulting device displaying an analyte-selective optical response. Linear amine detection – in the 100 ppm range – involves simple immersion of the pillararene-coated quartz substrate (QAP5_ML) into a tetrachloroethane solution of the amine to be revealed. The synthesis of new quartz-bound monolayers based on pillararenes incorporating a variety of different chromogenic moieties is currently in progress.Conflicts of interest
There are no conflicts to declare.Acknowledgements
For financial support, A. G. thanks the “Piano della Ricerca di ateneo 2016–2018” M. F. P. thanks the Ministero dell'Istruzione, dell’Università e della Ricerca (MIUR).Notes and references
- (a) J. L. Zhang, J. Q. Zhong, J. D. Lin, W. P. Hu, K. Wu, G. Q. Xu, A. T. S. Wee and W. Chen, Chem. Soc. Rev., 2015, 44, 2998–3022 RSC; (b) M. D. Yilmaz and J. Huskens, Soft Matter, 2012, 8, 11768–11780 RSC; (c) G. de Ruiter and M. E. van der Boom, Acc. Chem. Res., 2011, 44, 563–573 CrossRef CAS PubMed.
- (a) P. Murugan, M. Krishnamurthy, S. N. Jaisankar, D. Samanta and A. B. Mandal, Chem. Soc. Rev., 2015, 44, 3212–3243 RSC; (b) L. Basabe-Desmonts, D. N. Reinhoudt and M. Crego-Calama, Chem. Soc. Rev., 2007, 36, 993–1017 RSC.
- K. D. Schierbaum, T. Weiss, E. U. T. van Veizen, J. F. J. Engbersen, D. N. Reinhoudt and W. Gopel, Science, 1994, 265, 1413–1415 CrossRef CAS PubMed.
- (a) E. Biavardi, M. Favazza, A. Motta, I. L. Fragalà, C. Massera, L. Prodi, M. Montalti, M. Melegari, G. G. Condorelli and E. Dalcanale, J. Am. Chem. Soc., 2009, 131, 7447–7455 CrossRef CAS PubMed; (b) F. Tancini, D. Genovese, M. Montalti, L. Cristofolini, L. Nasi, L. Prodi and E. Dalcanale, J. Am. Chem. Soc., 2010, 132, 4781–4789 CrossRef CAS PubMed.
- (a) F. Lupo, C. Capici, G. Gattuso, A. Notti, M. F. Parisi, A. Pappalardo, S. Pappalardo and A. Gulino, Chem. Mater., 2010, 22, 2829–2834 CrossRef CAS; (b) D. A. Cristaldi, I. Fragalà, A. Pappalardo, R. M. Toscano, F. P. Ballistreri, G. A. Tomaselli and A. Gulino, J. Mater. Chem., 2012, 22, 675–683 RSC; (c) N. Feng, H. Zhao, J. Zhan, D. Tian and H. Li, Org. Lett., 2012, 14, 1958–1961 CrossRef CAS PubMed.
- (a) R. Pinalli, G. Brancatelli, A. Pedrini, D. Menozzi, D. Hernàndez, P. Ballester, S. Geremia and E. Dalcanale, J. Am. Chem. Soc., 2016, 138, 8569–8580 CrossRef CAS PubMed; (b) R. Pinalli and E. Dalcanale, Acc. Chem. Res., 2013, 46, 399–411 CrossRef CAS PubMed.
- (a) M. De Rosa, C. Talotta, C. Gaeta, A. Soriente, P. Neri, S. Pappalardo, G. Gattuso, A. Notti, M. F. Parisi and I. Pisagatti, J. Org. Chem., 2017, 82, 5162–5168 CrossRef CAS PubMed; (b) G. Gattuso, A. Notti, S. Pappalardo, M. F. Parisi, T. Pilati and G. Terraneo, CrystEngComm, 2012, 14, 2621–2625 RSC; (c) G. Gattuso, A. Notti, M. F. Parisi, I. Pisagatti, M. E. Amato, A. Pappalardo and S. Pappalardo, Chem.–Eur. J., 2010, 16, 2381–2385 CrossRef CAS PubMed; (d) D. Garozzo, G. Gattuso, A. Notti, A. Pappalardo, S. Pappalardo, M. F. Parisi, M. Perez and I. Pisagatti, Angew. Chem., Int. Ed., 2005, 44, 4892–4896 CrossRef CAS PubMed; (e) G. Cafeo, D. Garozzo, F. H. Kohnke, S. Pappalardo, M. F. Parisi, R. Pistone Nascone and D. J. Williams, Tetrahedron, 2004, 60, 1895–1902 CrossRef CAS; (f) F. Arnaud-Neu, S. Fuangswasdi, A. Notti, S. Pappalardo and M. F. Parisi, Angew. Chem., Int. Ed., 1998, 37, 112–114 CrossRef CAS.
- (a) G. Brancatelli, G. Gattuso, S. Geremia, N. Manganaro, A. Notti, S. Pappalardo, M. F. Parisi and I. Pisagatti, CrystEngComm, 2016, 18, 5012–5016 RSC; (b) G. Brancatelli, G. Gattuso, S. Geremia, A. Notti, S. Pappalardo, M. F. Parisi and I. Pisagatti, Org. Lett., 2014, 16, 2354–2357 CrossRef CAS PubMed; (c) G. Brancatelli, S. Pappalardo, G. Gattuso, A. Notti, I. Pisagatti, M. F. Parisi and S. Geremia, CrystEngComm, 2014, 16, 89–93 RSC; (d) C. Capici, G. Gattuso, A. Notti, M. F. Parisi, S. Pappalardo, G. Brancatelli and S. Geremia, J. Org. Chem., 2012, 77, 9668–9675 CrossRef CAS PubMed.
- (a) T. Kakuta, T. Yamagishi and T. Ogoshi, Acc. Chem. Res., 2018, 51, 1656–1666 CrossRef CAS PubMed; (b) T. Ogoshi, T. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed; (c) K. Yang, Y. Pei, J. Wen and Z. Pei, Chem. Commun., 2016, 52, 9316–9326 RSC; (d) N. L. Strutt, H. Zhang, S. T. Schneebeli and J. F. Stoddart, Acc. Chem. Res., 2014, 47, 2631–2642 CrossRef CAS PubMed; (e) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS PubMed; (f) P. J. Cragg and K. Sharma, Chem. Soc. Rev., 2012, 41, 597–607 RSC.
- T. Ogoshi, S. Kanai, S. Fujinami, T. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed.
- (a) L. Barbera, L. M. De Plano, D. Franco, G. Gattuso, S. P. P. Guglielmino, G. Lando, A. Notti, M. F. Parisi and I. Pisagatti, Chem. Commun., 2018, 54, 10203–10206 RSC; (b) X. Wu, L. Gao, X.-Y. Hu and L. Wang, Chem. Rec., 2016, 16, 1216–1227 CrossRef CAS PubMed; (c) L. Barbera, D. Franco, L. M. De Plano, G. Gattuso, S. P. P. Guglielmino, G. Lentini, N. Manganaro, N. Marino, S. Pappalardo, M. F. Parisi, F. Puntoriero, I. Pisagatti and A. Notti, Org. Biomol. Chem., 2017, 15, 3192–3195 RSC; (d) Y. Zhou, L.-L. Tan, Q.-L. Li, X.-L. Qiu, A.-D. Qi, Y. Tao and Y.-W. Yang, Chem.–Eur. J., 2014, 20, 2998–3004 CrossRef CAS PubMed; (e) H. Zhang, X. Ma, K. T. Nguyen and Y. Zhao, ACS Nano, 2013, 7, 7853–7863 CrossRef CAS PubMed.
- (a) X. Yang, W. Cai, S. Dong, K. Zhang, J. Zhang, F. Huang, F. Huang and Y. Cao, ACS Macro Lett., 2017, 6, 647–651 CrossRef CAS; (b) Y. Wang, M.-Z. Lv, N. Song, Z.-J. Liu, C. Wang and Y.-W. Yang, Macromolecules, 2017, 50, 5759–5766 CrossRef CAS; (c) M. Fathalla, N. L. Strutt, S. Sampath, K. Katsiev, K. J. Hartlieb, O. M. Bakr and J. F. Stoddart, Chem. Commun., 2015, 51, 10455–10458 RSC; (d) C. Li, Chem. Commun., 2014, 50, 12420–12433 RSC; (e) N. Song, D.-X. Chen, Y.-C. Qiu, X.-Y. Yang, B. Xu, W. Tian and Y.-W. Yang, Chem. Commun., 2014, 50, 8231–8234 RSC.
- Y. Sun, F. Guo, T. Zuo, J. Hua and G. Diao, Nat. Commun., 2016, 7, 12042 CrossRef CAS PubMed.
- S. Pan, M. Ni, B. Mu, Q. Li, X.-Y. Hu, C. Lin, D. Chen and L. Wang, Adv. Funct. Mater., 2015, 25, 3571–3580 CrossRef CAS.
- (a) N. L. Strutt, R. S. Forgan, J. M. Spruell, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 5668–5671 CrossRef CAS PubMed; (b) H. Deng, X. Shu, X. Hu, J. Li, X. Jia and C. Li, Tetrahedron Lett., 2012, 53, 4609–4612 CrossRef CAS; (c) G. Yu, B. Hua and C. Han, Org. Lett., 2014, 16, 2486–2489 CrossRef CAS PubMed.
- Pillar[n]arenes are also able to recognize a range of ammonium-containing substrates, see: (a) S. Dasgupta and P. S. Mukherjee, Org. Biomol. Chem., 2017, 15, 762–772 RSC; (b) W. Cheng, H. Tang, R. Wang, L. Wang, H. Meier and D. Cao, Chem. Commun., 2016, 52, 8075–8078 RSC.
- Y. Yao, Y. Zhou, J. Dai, S. Yue and M. Xue, Chem. Commun., 2014, 50, 869–871 RSC.
- R. R. Kothur, B. A. Patel and P. J. Cragg, Chem. Commun., 2017, 53, 9078–9080 RSC.
- V. Ladero, M. Calles-Enríquez, M. Fernández and M. A. Alvarez, Curr. Nutr. Food Sci., 2010, 6, 145–156 CrossRef CAS.
- E. W. Gerner and F. L. Meyskens Jr, Nat. Rev. Cancer, 2017, 4, 706–707 Search PubMed.
- For an example of silica-bound pillar[5,6]arenes employed as adsorbent for paraquat see: T. Zhou, N. Song, H. Yu and Y.-W. Yang, Langmuir, 2015, 31, 1454–1461 CrossRef CAS PubMed.
- (a) A. Gulino, F. Lupo, M. E. Fragalà and S. Lo Schiavo, J. Phys. Chem. C, 2009, 113, 13558–13564 CrossRef CAS; (b) F. Lupo, M. E. Fragalà, T. Gupta, A. Mamo, A. Aureliano, M. Bettinelli, A. Speghini and A. Gulino, J. Phys. Chem. C, 2010, 114, 13459–13464 CrossRef CAS.
- M. El-Behery and M. El-Twigry, Spectrochim. Acta, Part A, 2007, 6, 28–36 CrossRef PubMed.
- D. N. Shurpik, P. L. Padnya, L. I. Makhmutova, L. S. Yakimova and I. I. Stoikov, New J. Chem., 2015, 39, 9215–9220 RSC , and references therein.
- D. Li, M. A. Ratner, T. J. Marks, C. Zhang, J. Yang and G. K. Wong, J. Am. Chem. Soc., 1990, 112, 7389–7390 CrossRef CAS.
- M. Morozov, L. Motiei, J. Choudhury, A. Gulino, M. Lahav and M. E. van der Boom, Chem. Commun., 2014, 50, 8154–8156 RSC.
- A. M. Ricci, L. P. Méndez De Leo, F. J. Williams and E. J. Calvo, ChemPhysChem, 2012, 13, 2119–2127 CrossRef CAS PubMed.
- A. Kumar, M. Chhatwal, P. C. Mondal, V. Singh, D. A. Cristaldi, R. D. Gupta and A. Gulino, Chem. Commun., 2014, 50, 3783–3785 RSC.
- (a) L.-L. Tan, Y. Zhang, B. Li, K. Wang, S. X.-A. Zhang, Y. Tao and Y.-W. Yang, New J. Chem., 2014, 38, 845–851 RSC; (b) C. Schönbeck, H. Li, B.-H. Han and B. W. Laursen, J. Phys. Chem. B, 2015, 119, 6711–6720 CrossRef PubMed.
- T. Ogoshi, T. Furuta, Y. Hamada, T. Kakuta and T. Yamagishi, Mater. Chem. Front., 2018, 2, 597–602 RSC.
- (a) X. Shu, S. Chen, J. Li, Z. Chen, L. Weng, X. Jia and C. Li, Chem. Commun., 2012, 48, 2967–2969 RSC; (b) Z. Zhang, B. Xia, C. Han, Y. Yu and F. Huang, Org. Lett., 2010, 12, 3285–3287 CrossRef CAS PubMed.
- M. J. Hynes, J. Chem. Soc., Dalton Trans., 1993, 311–312 RSC.
- (a) A. Gulino, Anal. Bioanal. Chem., 2013, 405, 1479–1495 CrossRef CAS PubMed; (b) D. Briggs, in Practical Surfaces Analysis, ed, D. Briggs, M. P. Seah, Wiley-VCH, Weinheim, Germany, 2nd edn, 1995, vol. 1, p. 244 Search PubMed.
- L. Motiei, M. Altman, T. Gupta, F. Lupo, A. Gulino, G. Evmenenko, P. Dutta and M. E. van der Boom, J. Am. Chem. Soc., 2008, 130, 8913–8915 CrossRef CAS PubMed.
- D. A. Cristaldi, A. Motta, S. Millesi, T. Gupta, M. Chhatwal and A. Gulino, J. Mater. Chem. C, 2013, 1, 4979–4984 RSC.
- (a) A. Gulino, T. Gupta, M. Altman, S. Lo Schiavo, P. G. Mineo, I. L. Fragalà, G. Evmenenko, P. Dutta and M. E. van der Boom, Chem. Commun., 2008, 2900–2902 RSC; (b) A. Gulino, T. Gupta, P. G. Mineo and M. E. van der Boom, Chem. Commun., 2007, 4878–4880 RSC; (c) E. Frydman, H. Cohen, R. Maoz and J. Sagiv, Langmuir, 1997, 13, 5089–5106 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures and characterizations; AFM and crystallographic images, NMR, UV-vis and fluorescence spectra. CCDC crystal data for QAP5. CCDC 1536000. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8ra06792a |
| ‡ Current address: Crystallics B.V., Meibergdreef 31 1105 AZ, Amsterdam, The Netherlands. |
| § For XPS and AFM characterizations, monolayers were also prepared by grafting quinolylazo-pillar[5]arene molecules onto silylated Si(100) slides (ESI†). |
| This journal is © The Royal Society of Chemistry 2018 |