Open Access Article
Open Access ArticleChemical state changes of Nafion in model polymer electrolyte fuel cell under oxygen/hydrogen gas atmosphere observed by S-K XANES spectroscopy†
Kazuhisa Isegawa,
Daehyun Kim and
Hiroshi Kondoh *
*
Department of Chemistry, Keio University, 3-14-1 Hiyoshi, Kohoku-Ku, Yokohama, 223-8522 Japan. E-mail: kondoh@chem.keio.ac.jp
First published on 14th November 2018
Abstract
Changes in the chemical states of the sulfonic groups of Nafion in a model polymer electrolyte fuel cell under an oxygen/hydrogen gas atmosphere were studied using sulfur K-edge XANES spectroscopy. First, the chemical state changes in the sulfonic acid groups of both cathode and anode electrodes due to humidity under oxygen/hydrogen gas flow were observed. Reversible spectral changes ascribed to the hydration and dehydration of the sulfonic acid group were observed at both electrodes. This result is similar to the experimental results obtained without introducing oxygen (helium/hydrogen). On the anode, some of the sulfonic acid groups were decomposed to atomic sulfur adsorbed on platinum (Sad) and the amount increased with time. On the cathode, the formation of Sad was suppressed under the oxygen atmosphere. Next, the effects of oxygen gas introduction onto Sad were examined. Sad was at once formed on both electrodes under dry conditions without an oxygen supply. By supplying oxygen gas, Sad on the cathode disappears. Therefore, the catalyst of the cathode has the ability to recover against the poisoning Sad, while that on the anode accumulates.
Introduction
A prototype of fuel cells was developed by W. R. Grove in 1839.1 A century later, fuel cells were adopted as a power source for spacecraft and are nowadays used for green energy whose emission consists only of water. Among several types of fuel cells, polymer electrolyte fuel cells (PEFCs), which are used for fuel cell vehicles, home generators and so on, have been extensively studied to understand their working mechanisms as well as to improve their efficiency and durability. PEFCs are typically composed of carbon-supported platinum catalysts and polyelectrolytes. Nafion has been often used as a polyelectrolyte, consisting of a hydrophobic polytetrafluoroethylene (PTFE) backbone and hydrophilic side chains with sulfonic acid groups. The advantages of PEFCs are a solid electrolyte and low temperature operation. On the other hand, both the platinum catalyst and Nafion deteriorate easily and the power generation efficiency is lower than that of other types of fuel cells.Since proton transfer by Nafion is one of the key points of PEFCs, the hydration of the sulfonic acid groups2–5 and the interaction between Nafion and platinum6–12 have been investigated by various methods. In addition, it is known that a structure composed of a platinum catalyst, a carbon support and Nafion affects the performance and lifespan of PEFCs.13–19
The deterioration process of PEFCs is also important. Research combining electron microscopy and Pt-L EXAFS revealed the degradation processes of the platinum fine particle catalyst, such as dissolution, detachment, carbon corrosion and so on.20–24 Both the main chain and the side chain of Nafion are decomposed by hydroxyl radicals, which are generated by the decomposition of hydrogen peroxide arising from cross-leaked fuel gas on the platinum catalyst.25,26 The decomposition reaction is accelerated by the progress of decomposition,27 drying,28 over-humidification29 and high pressure.30 Contrary to the consensus, there is a report that the platinum catalyst detoxifies hydrogen peroxide and inhibits the decomposition of Nafion.31 It is also noted that Nafion is easily destroyed by exposure to radiation such as γ rays, β rays,32–36 XAFS measurement,37 XPS measurement38 and EELS measurement.39
X-ray absorption fine structure spectroscopy (XAFS: further divided into XANES and EXAFS depending on the energy region) enables us to carry out element-selective observations of chemical states and local structures. Changes in the chemical state of a noble metal catalyst due to potential have been reported in detail by time resolved Pt-Lm EXAFS.40,41 It is reported that C-K ELNES (energy loss near edge spectroscopy) reflects sensitively the helical structure of the main chain of Nafion.42,43 ELNES is a core-level spectroscopy similar to XANES and has better light element detectability and spatial resolution capability. Research on fuel cells using S-K XANES has clarified the effect of sulfur dioxide in air on the catalyst.44,45 However, this study did not necessarily focus on the sulfonic acid groups of Nafion. Therefore, previously we tried S-K XANES measurements of PEFC under He/H2 gas-flow and revealed that this technique gives information on the hydration of sulfonic acid groups and the formation of atomic sulfur adsorbed on platinum under dry condition.37
In this paper, we conducted an S-K XANES study on changes in the chemical state of the sulfonic acid groups of Nafion in a model PEFC under oxygen atmosphere. First, the influence of oxygen gas due to humidity on the changes in the chemical state of a sulfonic acid group was investigated. Next, XANES observations were conducted under the introduction to atomic sulfur adsorbed on platinum of oxygen which is produced under dry conditions. The chemical state of Nafion on the anode electrode was also analysed under each condition. Since water emitted by power generation absorbs and scatters X rays significantly and inhibits the drying of the catalyst, we investigated the effects of oxygen gas and of dry conditions on Nafion without a flowing electric current.
Experimental
Soft X-ray near in situ electrochemical XAFS system
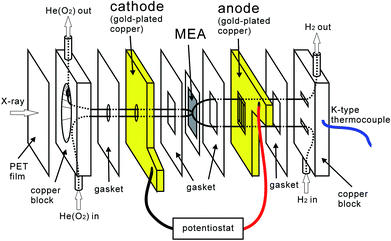
The experimental setup is shown in our previous paper.37 The apparatus was modified to supply a mixed gas of helium and oxygen.The fuel cell used here has a multilayer structure, as shown in Fig. 1. Membrane electrode assemblies (MEAs) consisting of Nafion-212 and 50 wt% Pt/C (Vulcan XC-72) (1 mgPt cm−2) (ElectroChem Inc., USA) were peeled off a gas diffusion layer (GDL) on the cathode side and loaded into the fuel cell. Each layer was electrically connected by fixing with insulated screws. For the anode electrode, hydrogen gas was supplied onto the MEA through a GDL, which can be used to define the potential of the catalyst. The cathode electrode has a conical hole for passing X rays, and the fuel gases are supplied from the hole without GDL. The conical hole is capped by a PET film (thickness of 6 μm) to seal the flow gas from the He-path. Since the catalyst ink layer is thick enough to prevent transmission of 2500 eV X rays through the layer, the XANES spectra from one electrode are not influenced by the contribution from the polymer electrolyte or from another electrode. The electrodes are floating electrically from the chamber by the insulation between the metal plates connected to the gas line and the electrodes so that the anode and the cathode can be exchanged by changing the electric wiring and the gas line system.
H2 was supplied at 30 mL min−1 to the anode of the cell, and He to the cathode at 30 mL min−1. When introducing oxygen, O2–He mixed gas (O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) He = 1
He = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was supplied to the cathode at 30 mL min−1. The humidity of the cell was defined by the dew point of the supplied fuel gases and the temperature of the cell. The temperature of the cell was measured by a thermocouple attached to the gas introduction block (Fig. 1). Under wet conditions, the flow gases passed through bubblers at room temperature and the fuel cell was also at room temperature, which results in a dew point of 26 °C. The humidity under these gas conditions was 30–70% depending on the cell temperature. Under dry conditions, the gases were supplied without bubblers and the fuel cell was heated to 55 °C (−40° CDP, RH 0%). The MEA tends to swell with water and cross-leakage of the fuel gases occurs, which could be induced by the conical hole of the cathode electrode for X-ray introduction. The humidity of the wet conditions was set slightly lower in order to prevent the catalysts from burning due to the cross-leakage. A potentiostat/galvanostat (Hokuto Denko corp. HA-151B) was used for electrochemical measurements.
4) was supplied to the cathode at 30 mL min−1. The humidity of the cell was defined by the dew point of the supplied fuel gases and the temperature of the cell. The temperature of the cell was measured by a thermocouple attached to the gas introduction block (Fig. 1). Under wet conditions, the flow gases passed through bubblers at room temperature and the fuel cell was also at room temperature, which results in a dew point of 26 °C. The humidity under these gas conditions was 30–70% depending on the cell temperature. Under dry conditions, the gases were supplied without bubblers and the fuel cell was heated to 55 °C (−40° CDP, RH 0%). The MEA tends to swell with water and cross-leakage of the fuel gases occurs, which could be induced by the conical hole of the cathode electrode for X-ray introduction. The humidity of the wet conditions was set slightly lower in order to prevent the catalysts from burning due to the cross-leakage. A potentiostat/galvanostat (Hokuto Denko corp. HA-151B) was used for electrochemical measurements.
XANES measurements
Sulfur K-edge XANES spectra were measured in the fluorescence mode using a silicon drift detector (XR-100SDD, Amptek) at beam line 11B of the Photon-Factory of High Energy Accelerator Research Organization (KEK-PF) in Tsukuba, Japan. Sulfur powder (S, 99.9999%, Wako Pure Chemical Industries, Ltd) and an aqueous solution of sulfuric acid (H2SO4, 96.0%, Kanto Chemical Co., Inc.) were used as the standard substances to calibrate the sulfur K-edge energy (sulfur powder: 2472.4 eV, sulfuric acid: 2482.1 eV).44 The aqueous solution of H2SO4 was adjusted to 0.1 M and sealed in polypropylene films.Since we found in the previous study that intense X rays decompose Nafion into sulfuric acid,37 polyethylene membranes were properly installed on the X-ray path in order to reduce the X-ray intensity.
Result and discussion
Effects of humidity change under oxygen atmosphere
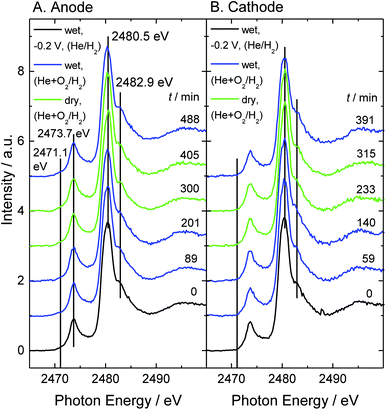
S-K XANES spectra of model PEFCs measured under oxygen gas-flow are shown in Fig. 2. For observation of the anode shown in Fig. 2A, hydrogen was supplied to the electrode with the conical hole for X-ray irradiation and helium (and oxygen) was supplied to another electrode. While for observation of the cathode shown in Fig. 2B, the supplied gases were exchanged with each other. All the spectra were normalized by the intensity of the post-edge region.First, at −0.2 V under wet helium/hydrogen gas-flow (RH 70%), the break-in operation was performed and the reference S-K XANES spectra (black lines) and cyclic voltammetry (Fig. S1†) were measured for a performance test. Next, under oxygen gas-flow, the effects of humidity change on the S-K XANES spectra were investigated. Changes in cell temperature and open circuit voltage are shown in Fig. S3.† Nafion exhibits a main peak at 2480.5 eV and a shoulder structure at 2482.9 eV. A large peak that appears at 2473.7 eV in the pre-edge region is attributed to the thioether group (2473.1–2474.3 eV).46,47 This peak is not detected in the S-K XANES spectrum of a pristine Nafion-212 membrane44,45 but is exclusively observed for Pt/C catalysts. Therefore, this sulfur species is assumed to be included in the carbon support, Vulcan XC-72. Since modification of the carbon support with sulfonic acid or amine affects the performance of fuel cells,17,19 this sulfur species may have some effect on the activity. The thioether peak seems to be large relative to the sulfonic acid peaks. However, assuming the ion exchange capacity to be almost 1 mEq g−1 and the ionomer/carbon ratio to be 1.0 or less, the molar fraction of the sulfur impurity in the carbon support is estimated to be less than 1%.
The spectral shape and peak position in Fig. 2 seem to be maintained against changes in humidity and supplied gas. So, the intensity ratio of the main peak (2480.5 eV) and the shoulder structure (2482.9 eV) are plotted as a function of elapsed time (Fig. 3). The ratio became smaller exclusively under dry conditions, which is the same as the results without an oxygen gas supply.37 According to the density functional theory transition-potential (DFT-TP) calculation48 of the S-K XANES of fluoroalkylsulfonic acid, the main peak is attributed to excitation from the S 1s level into the antibonding σ*(S–C) molecular orbital and the shoulder structure is attributed to excitation to the antibonding π*(S–C) molecular orbital.49 Three bonds between sulfur and oxygen could not be easily named because of the mixing of σ* and π* molecular orbitals. By combining experiments and DFT-TP calculations, we revealed that the intensity ratio of the main peak and the shoulder structure reflects the adsorption and desorption of a proton to/from the sulfonic acid group.37 Similar intensity changes were observed for the interaction between sulfonic acid groups and vanadium ions.50
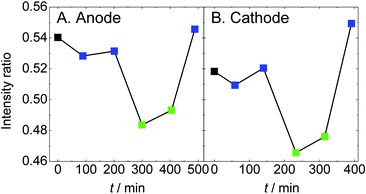 | ||
| Fig. 3 The intensity ratio averaged over 5 points of the main peak (2480.5 eV) and the shoulder structure (2482.9 eV) in the spectra shown in Fig. 2 are plotted as a function of elapsed time. The color code is the same as that in Fig. 2. | ||
Our previous study revealed that the sulfonic acid group is decomposed into atomic sulfur adsorbed on the platinum electrode (Sad) on the cathode under the condition of dry helium gas-flow irrespective of the X-ray irradiation time.37 Although it is presumed that the sulfonic acid groups losing the coordination water are pulled to the platinum catalyst under dry conditions,51 the detailed decomposition mechanism is still unclear. The intensity of Sad (2471.1 eV)44,45 in the spectra of Fig. 2 is plotted as shown in Fig. 4. Sad gradually increases on the anode, while it is much lower on the cathode. In this study, it is possible that not only sulfonic acid in Nafion but also the sulfur-containing species observed at 2473.7 eV (thioether in the carbon support) is a source of sulfur species for the generation of Sad. However, thioether in the carbon support is not involved in the production of Sad because the intensity change in Sad is independent of that of thioether. Details are discussed in Section 3 of the ESI.†
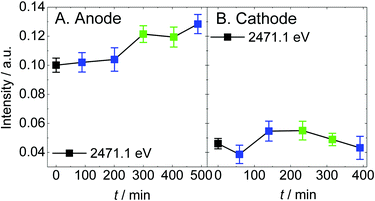 | ||
| Fig. 4 The intensity averaged over 5 points around 2471.1 eV in the spectra shown in Fig. 2 are plotted as a function of elapsed time. The color code is the same as that in Fig. 2. | ||
Enlarged views of the pre-edge region of the spectra in Fig. 2 are shown in Fig. S5.† For the anode, the spectral curves appear to be convex upward at 2471.1 eV even in the first spectrum, while for the cathode, the spectra remain convex downward under any conditions. This means that, on the anode, the sulfonic acid group decomposes not only under dry conditions but also with the supply of hydrogen gas. Comparing A and B in Fig. 4, the intensity of Sad on the anode during the break-in operation under hydrogen gas becomes larger by about 0.05 arb. units than that on the cathode and an additional 0.03 arb. units was generated during 8 hours of experiment. 1 arb. unit corresponds to the total amount of all kinds of sulfur species of the sample. On the anode, it is suggested that the sulfonic acid groups are reduced by hydrogen on the platinum catalyst regardless of humidity. Although the catalyst and Nafion deteriorate by only a trace amount during 8 hours of experiment, this will have a non-negligible negative effect in a long-term experiment. On the other hand, Sad on the cathode does not increase. The generation of sulfur species is suppressed under oxygen atmosphere at 1 V (open circuit voltage).
Effects of oxygen gas on atomic sulfur adsorbed on platinum
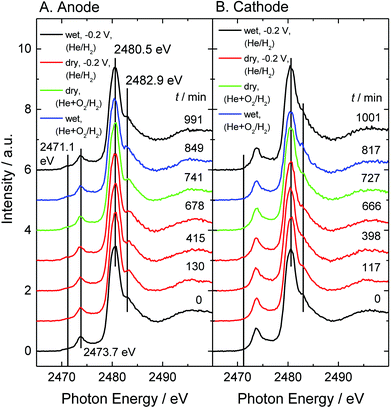
In the previous section, we found the generation of sulfur on the catalyst under He + O2/H2 gas-flow conditions. In this section, we intentionally generated Sad on both electrodes by dry treatment and subsequently attempted to clarify the behaviour of the once-generated sulfur under He + O2/H2 gas-flow conditions. A series of spectra are shown in Fig. 5. First, the break-in operation was performed at −0.2 V under wet helium/hydrogen gas-flow conditions (black lines (RH 70%)). Next, the dried gases were supplied for 9 hours to generate Sad (red lines (RH 0%)). After that, a mixed gas of oxygen and helium (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), which was initially dry and then moistened afterwards, was supplied to the cathode (green (RH 0%) and blue lines (RH 30%)). Cyclic voltammograms were measured at each condition (Fig. S2†). Changes in cell temperature and open circuit voltage are shown in Fig. S4.†
4), which was initially dry and then moistened afterwards, was supplied to the cathode (green (RH 0%) and blue lines (RH 30%)). Cyclic voltammograms were measured at each condition (Fig. S2†). Changes in cell temperature and open circuit voltage are shown in Fig. S4.†
The intensity ratio of the main peak and the shoulder structure and the intensity of Sad are plotted as a function of elapsed time, as shown in Fig. 6 and 7, respectively. The Sad species increase at both electrodes under the dry helium/hydrogen gas-flow conditions. On the anode, Sad increases steadily under a reducing atmosphere irrespective of humidity. On the other hand, exposing oxygen gas onto the cathode gives rise to a drastic decrease in Sad. This result suggests that Sad is removed from the platinum surface via oxidation. Thus the platinum catalyst on the cathode can be regenerated under wet oxygen gas-flow even if it is contaminated by sulfur under overly dry conditions or SO2 gas-flow.44,45 However, the conduction of MEAs was not completely recovered, as shown in Fig. S2,† because of the irreversible decomposition of Nafion and the increase in Sad on the anode electrode. Although the deterioration of the cathode catalysts has been paid much attention so far, the present experiment under gas-flow reveals renewed deterioration pathways relating to the anode catalysts as well as to the electrolyte.
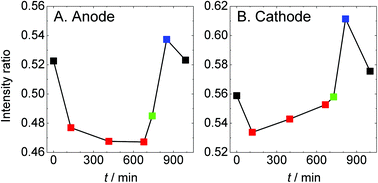 | ||
| Fig. 6 The intensity ratio averaged over 5 points of the main peak (2480.6 eV) and the shoulder structure (2483.0 eV) in the spectra shown in Fig. 5 are plotted as a function of elapsed time. The color code is the same as that in Fig. 5. | ||
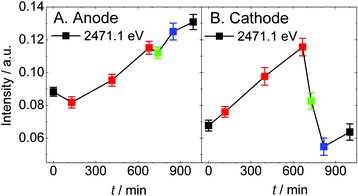 | ||
| Fig. 7 The intensity averaged over 5 points around 2471.1 eV in the spectra shown in Fig. 5 are plotted as a function of elapsed time. The color code is the same as that in Fig. 5. | ||
Conclusions
We developed a model fuel cell electrically insulated from the gas line and used it for a soft X-ray electrochemical XAFS system. First, we investigated the chemical state of Nafion under an He + O2(cathode)/H2(anode) atmosphere by S-K XANES. We observed the hydration/dehydration of the sulfonic acid group due to humidity change and sulfur poisoning of the platinum catalyst on the anode. Next, we examined the effects of introduction of oxygen gas on the poisoning sulfur. After supplying the He + O2/H2 gas, the generated Sad species on the cathode significantly decrease. This indicates that the cathode has regenerative capability against sulfur contamination on the platinum catalyst. Whereas on the anode the sulfur accumulates.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was supported by the research fund from Keio University and the Grants-in-Aid for scientific research (18K19064). The experiments have been performed under the approval of the Photon Factory Program Advisory Committee (PF PAC No. 2015G601).References
- W. R. Grove, Philos. Mag., 1839, 14, 127–130 Search PubMed.
- K. Kunimatsu, B. Bae, K. Miyatake, H. Uchida and M. Watanabe, J. Phys. Chem. B, 2011, 115, 4315–4321 CrossRef CAS PubMed.
- C. Wakai, T. Shimoaka and T. Hasegawa, Anal. Chem., 2013, 85, 7581–7587 CrossRef CAS PubMed.
- T. Shimoaka, C. Wakai, T. Sakabe, S. Yamazaki and T. Hasegawa, Phys. Chem. Chem. Phys., 2015, 17, 8843–8849 RSC.
- Y. Cui, Y. Harada, T. Hatanaka, N. Nakamura, M. Ando, T. Yoshida, E. Ikenaga, K. Ishii, D. Matsumura, R. Li and M. Oshima, ECS Trans., 2016, 72(8), 131–136 CrossRef CAS.
- R. Subbaraman, D. Strmcnik, V. Stamenkovic and N. M. Markovic, J. Phys. Chem. C, 2010, 114, 8414–8422 CrossRef CAS.
- I. Kendrick, D. Kumari, A. Yakaboski, N. Dimakis and E. S. Smotkin, J. Am. Chem. Soc., 2010, 132, 17611–17616 CrossRef CAS PubMed.
- M. Ahmed, D. Morgan, G. A. Attard, E. Wright, D. Thompsett and J. Sharman, J. Phys. Chem. C, 2011, 115, 17020–17027 CrossRef CAS.
- H. Hanawa, K. Kunimatsu, M. Watanabe and H. Uchida, J. Phys. Chem. C, 2012, 116, 21401–21406 CrossRef CAS.
- T. Masuda, K. Ikeda and K. Uosaki, Langmuir, 2013, 29, 2420–2426 CrossRef CAS PubMed.
- T. Masuda, F. Sonsudin, P. R. Singh, H. Naohara and K. Uosaki, J. Phys. Chem. C, 2013, 117, 15704–15709 CrossRef CAS.
- T. Masuda, H. Fukumitsu, T. Kondo, H. Naohara, K. Tamura, O. Sakata and K. Uosaki, J. Phys. Chem. C, 2013, 117, 12168–12171 CrossRef CAS.
- R. Subbaraman, D. Strmcnik, A. P. Paulikas, V. R. Stamenkovic and N. M. Markovic, ChemPhysChem, 2010, 11, 2825–2833 CrossRef CAS PubMed.
- R. Lin, T. Zhao, M. Shang, J. Wang, W. Tang, V. E. Guterman and J. Ma, J. Power Sources, 2015, 293, 274–282 CrossRef CAS.
- S. M. Andersen, R. Dhiman, M. J. Larsen and E. Skou, Appl. Catal., B, 2015, 172–173, 82–90 CrossRef CAS.
- J. Chlistunoff and B. Pivovar, J. Electrochem. Soc., 2015, 162(8), F890–F900 CrossRef CAS.
- S. M. Andersen, Appl. Catal., B, 2016, 181, 146–155 CrossRef CAS.
- T. Kim, J. Yi, C. Jung, E. Jeong and S. Yi, Int. J. Hydrogen Energy, 2017, 42, 478–485 CrossRef CAS.
- F. Yang, L. Xin, A. Uzunoglu, Y. Qiu, L. Stanciu, J. Ilavsky, W. Li and J. Xie, ACS Appl. Mater. Interfaces, 2017, 9, 6530–6538 CrossRef CAS PubMed.
- J. C. Meier, C. Galeano, I. Katsounaros, A. A. Topalov, A. Kostka, F. Schüth and K. J. J. Mayrhofer, ACS Catal., 2012, 2, 832–843 CrossRef CAS.
- H. Yoshida, T. Kinumoto, Y. Iriyama, Y. Uchimoto and Z. Ogumi, ECS Trans., 2007, 11(1), 1321–1329 CAS.
- Y. Yu, H. L. Xin, R. Hovden, D. Wang, E. D. Rus, J. A. Mundy, D. A. Muller and H. D. Abruña, Nano Lett., 2012, 12, 4417–4423 CrossRef CAS PubMed.
- O. A. Baturina, A. Epshteyn, P. Northrup and K. E. Swider-Lyons, J. Electrochem. Soc., 2014, 161(4), F365–F372 CrossRef CAS.
- J. A. Gilbert, A. J. Kropf, N. N. Kariuki, S. DeCrane, X. Wang, S. Rasouli, K. Yu, P. J. Ferreira, D. Morgan and D. J. Myers, J. Electrochem. Soc., 2015, 162(14), F1487–F1497 CrossRef CAS.
- L. Ghassemzadeh, K.-D. Kreuer, J. Maier and K. J. Müller, J. Phys. Chem. C, 2010, 114, 14635–14645 CrossRef CAS.
- T. Kinumoto, M. Inaba, Y. Nakayama, K. Ogata, R. Umebayashi, A. Tasaka, Y. Iriyama, T. Abe and Z. Ogumi, J. Power Sources, 2006, 158, 1222–1228 CrossRef CAS.
- K. Ono, Y. Yasuda, K. Sekizawa, N. Takeuchi, T. Yoshida and M. Sudoh, Electrochim. Acta, 2013, 97, 58–65 CrossRef CAS.
- M. Inaba, T. Kinumoto, M. Kiriake, R. Umebayashi, A. Tasaka and Z. Ogumi, Electrochim. Acta, 2006, 51, 5746–5753 CrossRef CAS.
- D. G. Sanchez, T. Ruiu, I. Biswas, M. Schulze, S. Helmly and K. A. Friedrich, J. Power Sources, 2017, 352, 42–55 CrossRef CAS.
- A. Kusoglu, M. Calabrese and A. Z. Weber, ECS Electrochem. Lett., 2014, 3(5), F33–F36 CrossRef CAS.
- M. Bodner, B. Cermenek, M. Rami and V. Hacker, Membranes, 2015, 5, 888–902 CrossRef CAS PubMed.
- Y. Iwai, T. Yamanishi, M. Nishi, T. Yagi and M. Tamada, J. Nucl. Sci. Technol., 2005, 42, 636–642 CrossRef CAS.
- Y. Iwai, A. Hiroki, M. Tamada and T. Yamanishi, J. Membr. Sci., 2008, 322, 249–255 CrossRef CAS.
- Y. Iwai, A. Hiroki, M. Tamada, K. Isobe and T. Yamanishi, Radiat. Phys. Chem., 2010, 79, 46–51 CrossRef CAS.
- Y. Iwai, A. Hiroki and M. Tamada, J. Membr. Sci., 2011, 369, 397–403 CrossRef CAS.
- Y. Iwai, K. Sato and T. Yamanishi, Fusion Eng. Des., 2014, 89, 1534–1538 CrossRef CAS.
- K. Isegawa, T. Nagami, S. Jomori, M. Yoshida and H. Kondoh, Phys. Chem. Chem. Phys., 2016, 18, 25183–25190 RSC.
- M. Schulze, M. Lorenz, N. Wagner and E. Gülzow, Fresenius. J. Anal. Chem., 1999, 365, 106–113 CrossRef CAS.
- Q. He, J. Chen, D. J. Keffer and D. C. Joy, Scanning, 2014, 36, 338–346 CrossRef CAS PubMed.
- M. Tada, S. Murata, T. Asakoka, K. Hiroshima, K. Okumura, H. Tanida, T. Uruga, H. Nakanishi, S. Matsumoto, Y. Inada, M. Nomura and Y. Iwasawa, Angew. Chem., Int. Ed., 2007, 46, 4310–4315 CrossRef CAS PubMed.
- O. Sekizawa, T. Uruga, K. Higashi, T. Kaneko, Y. Yoshida, T. Sakata and Y. Iwasawa, ACS Sustainable Chem. Eng., 2017, 5, 3631–3636 CrossRef CAS.
- C. Wang, G. Duscher and S. J. Paddison, Microscopy, 2014, 63(1), 73–83 CrossRef CAS PubMed.
- C. Wang, G. Duscher and S. J. Paddison, RSC Adv., 2015, 5, 2368–2373 RSC.
- O. A. Baturina, B. D. Gould, A. Korovina, Y. Garsany, R. Stroman and P. A. Northrup, Langmuir, 2011, 27, 14930–14939 CrossRef CAS PubMed.
- O. A. Baturina, B. D. Gould, P. A. Northrup and K. E. Swider-Lyons, Catal. Today, 2013, 205, 106–110 CrossRef CAS.
- J. Prietzel, J. Thieme, U. Neuhäusler, J. Ssusini and I. Kögel-Knabner, Eur. J. Soil Sci., 2003, 54, 423–433 CrossRef CAS.
- F.-R. Orthous-Daunay, E. Quirico, L. Lemelle, P. Beck, V. deAndrade, A. Simionovici and S. Derenne, Earth Planet. Sci. Lett., 2010, 300, 321–328 CrossRef CAS.
- K. Hermann, L. G. M. Pettersson, M. E. Casida, C. Daul, A. Goursot, A. Koester, E. Proynov, A. St-Amant and D. R. Salahub. Contributing authors: V. Carravetta, H. Duarte, C. Friedrich, N. Godbout, M. Gruber, J. Guan, C. Jamorski, M. Leboeuf, M. Leetmaa, M. Nyberg, S. Patchkovskii, L. Pedocchi, F. Sim, L. Triguero and A. Vela, StoBe-deMon version 3.3, 2014 Search PubMed.
- E. D. Risberg, L. Eriksson, J. Mink, L. G. M. Pettersson, M. Yu. Skripkin and M. Sandströlm, Inorg. Chem., 2007, 46, 8332–8348 CrossRef CAS PubMed.
- M. Vijayakumar, N. Govind, B. Li, X. Wei, Z. Nie, S. Thevuthasan, V. Sprenkle and W. Wang, Front. Energy Res., 2015, 3, 10 Search PubMed.
- K. Kodama, R. Jinnouchi, T. Suzuki, H. Murata, T. Hatanaka and Y. Morimoto, Electrochem. Commun., 2013, 36, 26–28 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Cyclic voltammetry curves for the MEAs depending on humidity, open circuit voltage changes due to humidity and temperature, and peak analysis of low-energy region of the S-K XANES spectra. See DOI: 10.1039/c8ra06426a |
| This journal is © The Royal Society of Chemistry 2018 |