Open Access Article
Open Access ArticleInterface conjugation enhances the interface adhesion performance of carbon fiber-reinforced phthalonitrile composites by π–π stacking
Changping Yinb,
Liping Sheng *a,
Yudong Yanga,
Gengyuan Liangb,
Suli Xingb,
Jingcheng Zengb and
Jiayu Xiaob
*a,
Yudong Yanga,
Gengyuan Liangb,
Suli Xingb,
Jingcheng Zengb and
Jiayu Xiaob
aNational Local Joint Engineering Laboratory for New Petro-chemical Materials and Fine Utilization of Resources, College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha, 410081, P. R. China. E-mail: sleeping1217@126.com
bCollege of Aerospace Science and Engineering, National University of Defense Technology, Changsha, 410073, P. R. China
First published on 14th November 2018
Abstract
There has been little research focus on the interface problems of phthalonitrile (PN) resin and carbon fiber. However, interface performance is related to the overall mechanical properties of composites and is very important. This study focused on the interfacial performance and adhesion mechanism of a carbon fiber Cf/PN composite. Micro-composites of Cf/PN and Cf/epoxy resins were prepared, and their interfacial shear strengths (IFSS) were tested by micro-droplet testing. The result showed that the IFSS of Cf/PN was higher than that of Cf/epoxy resin, indicating that the interfacial adhesion of the PN matrix composite must be more effective. To explain the obtained results, a number of tests, including SEM, SEM-EDS, FTIR, and TGA, were carried out. From the SEM analyses, cured PN polymer films were found on the surface of de-bonded carbon fibers. With the aid of SEM-EDS, the elements on the de-bonded carbon fiber surface of the Cf/PN composite were detected in situ. An interesting synchronous relationship was observed in the IFSS and SEM-EDS results. Through the FTIR spectra, the chemical structures of the PN polymers were identified. From the detailed analyses and discussion in this work, the effective interfacial bond function in the Cf/PN composite appears to be a complex result for all relative functions. The functional advantage of the PN composite may be the interface conjugation between the PN polymers and the graphene layer on the surface of the carbon fiber.
Introduction
The interface between matrix and fibers manipulates the reintroduction of stress into either component at a given damage site. Therefore, the interface is highly responsible for the micromechanical failures and the durability of composite materials.1–5 Phthalonitrile (PN) resin is a high-performance matrix with excellent mechanical properties, high glass transition temperature, and flame-resistance properties. Recently, it has attracted more attention and has been applied in aviation, aerospace, boat construction and other fields.6–10 Carbon fiber (Cf) is widely used as a reinforcing phase in matrix composite materials because of its high strength, high modulus, and low density, among other advantages.11–14 Therefore, the study of the performance at the interface between the PN resin and carbon fibers is a particularly important, although underappreciated, area of research.Most research on interface performance has focused on the holistic and macroscopic performance of carbon fibers, for example, in studies of the surface energy,13,15 contact angle,16,17 element constitution,17–19 and acidity of carbon fiber.20–23 These holistic carbon-fiber-performance factors certainly influence the interface performance of composites; however, the de-bonded and failure regions of carbon fibers are weak but critical components of understanding the performance of fiber-reinforced composites. The properties and natures of these regions will be more important and meaningful than those of the carbon fibers alone. These interface regions exist at the micron scale, making in situ characterization significantly difficult, but with the help of Scanning Electron Microscopy combined with Energy Dispersive Spectrometry (SEM-EDS), in situ information from these micron-scale regions can be collected accurately. Using this advanced technology, the interface performance and adhesion mechanism of Cf/PN composites were investigated in this study.
Through micro-droplet testing, the interface-bonding performance between two kinds of resins (PN resin and epoxy resin) and carbon fiber were compared. The test results show that the interfacial shear strength of the Cf/PN composite is higher than that of the Cf/epoxy resin composite. This is a very significant result for high-performance PN resin. To understand this result, we examined the interface morphology of the de-bonded surface of carbon fiber with SEM, and the residual cured resin could be clearly seen. Then, using the combined SEM-EDS method, the in situ elements of the de-bonded interface of carbon fiber could be detected. As a result, it is found that an increase in nitrogen in de-bonded interfaces is synchronous with an increase in interfacial shear strength. This interesting discovery prompted us to perform an in-depth analysis of the molecular structure of a PN polymer derived from PN resin using infrared spectroscopy. This polymer is a polyheterocycle containing N, which is different from all other common polymers cured from resins. It is this distinctive polyheterocyclic structure that promotes the formation of interface conjugation between the PN polymer and graphene on the carbon-fiber surface. In addition, the ability of N to pull electrons is stronger than that of C, which can further strengthen the conjugate forces between them. This novel interface adhesion mechanism explains the good interfacial bonding performance observed for the Cf/PN composite and also suggests that the nitrogen-containing heterocyclic structure of PN polymers have promising applications in the field of interfacial modification.
Experimental
2.1 Materials
High strength carbon fibers (T700 grade) were purchased from Dongli Corporation, with a tensile strength of 4.9 GPa, tensile modulus of 230 GPa and an elongation at break of 2.1%. The fiber of this manufacturer is PAN (polyacrylonitrile) based carbon fiber. The fiber is surface treated before leaving the factory. The treatment includes two steps, first anodic electrolytic oxidation to increase surface activity, and then sizing treatment to protect the active surface and prevent burrs to facilitate further use. The PN resin was supplied by the Hunan Normal University laboratory. The major components were 4-(4-aminophenoxy)-phthalonitrile and poly-(arylene ether nitrile), among other aromatic nitrile compounds. Epoxy resin-51 was purchased from SINOPEC Baling Company. The curing agent for the epoxy resin was 4,4′-methylenebis(2-ethylbenzenamine) (DDM).2.2 Interfacial shear strength (IFSS) of the micro-composite
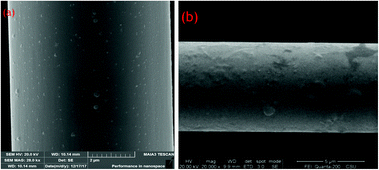
The carbon fibers are sized before leaving the factory and the sizing agent is dilute solution of epoxy resin. This sizing agent can be well removed by acetone and Soxhlet extraction. The specific steps are as follows.First, the carbon fiber was wound on a stainless steel cycle with a diameter of 3 cm, then placed in a Soxhlet extractor, and an appropriate amount of acetone was added, and heated to 60 °C to maintain the siphon 5–6 times per hour. After extraction for 10 hours, the acetone solution turned yellow, then stopped heating and taken out the cycle. The cycle with fiber was placed in a vacuum oven at −0.1 MPa and 80 °C for 2 hours. The desized and sized surfaces of the carbon fibers were observed by SEM. It was found that the resin particles on the surface of the fiber are removed, and the uneven oxidized surface is exposed (Fig. 1).
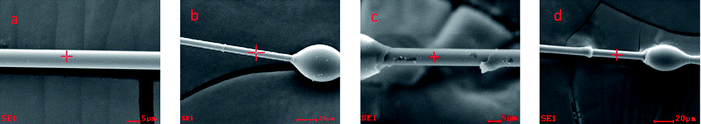
The micro-composite preparation and IFSS tests performed were similar to those previously reported in the literature.16 The IFSS of micro-composites were tested by micro-droplet testing. The matrix resin was wetted onto a carbon fiber filament to form a micro-droplet that surrounded the fiber diameter due to the function of surface tension. The micro-droplet resin was then cured in an oven. After resin curing, the single fiber was fixed in a concave mold. The knives came in contact with the solid resin droplet and the force required to debond the droplet from the fiber was recorded. The embedded length of each micro-droplet was measured through optical microscopic observation. The IFSS instrument (Model HM410, made in Japan) was used at the sensitivity of 1/100 gf with a moving speed of 0.03 mm min−1. According to the maximum tension of a single fiber, we selected for droplets with diameters that were suitable for the test. The micro-droplet resin was cured in an oven according to curing procedure in Table 1. The PN polymers 1, 2 and 3, are derived from the polymerization of PN resin, but the post-cure temperature is different. The different post-cure temperatures result in somewhat different degrees of cure cross-linking.
| Cured polymer label | Curing procedure |
|---|---|
| Epoxy polymer | 95 °C, 1 h + 135 °C, 2 h + 170 °C, 2 h |
| PN polymer-1 | 2 °C min−1 from 30 °C to 320 °C + 320 °C, 15 min |
| PN polymer-2 | 2 °C min−1 from 30 °C to 350 °C + 350 °C, 15 min |
| PN polymer-3 | 2 °C min−1 from 30 °C to 380 °C + 380 °C, 15 min |
The IFSS was calculated by the equation,
| τ = F/πdl, |
2.3 Microtopography and elemental analysis of the de-bonded surface of micro-composites
The morphological changes on desized and de-bonded surfaces of carbon fibers were examined by SEM. Simultaneously, the elemental composition of the corresponding micro-area were probed by SEM-EDS (FEI Quanta-20).2.4 Thermal stabilities of different cured polymers
According to the curing procedure in Table 1, the cured polymers were prepared by thermo-gravimetric-analysis (TGA, Mettler-Toledo TGA/DSC1 STAR System) in a chamber under nitrogen atmosphere at a flow rate of 100 mL min−1.Thermal stabilities of the cured polymers were also evaluated by TGA at a heating rate of 20 °C min−1 under nitrogen atmosphere at a flow rate of 100 mL min−1, from 25 °C to 800 °C.
2.5 Chemical structure of polymers
The structure of the polymers derived from PN resin that had been treated under different curing procedures were examined by Fourier transform infrared spectra (FTIR) using a PerkinElmer Spectrum TWO spectrometer. FTIR spectra were recorded in infrared reflectance mode.Results and discussion
3.1 Interfacial shear strength
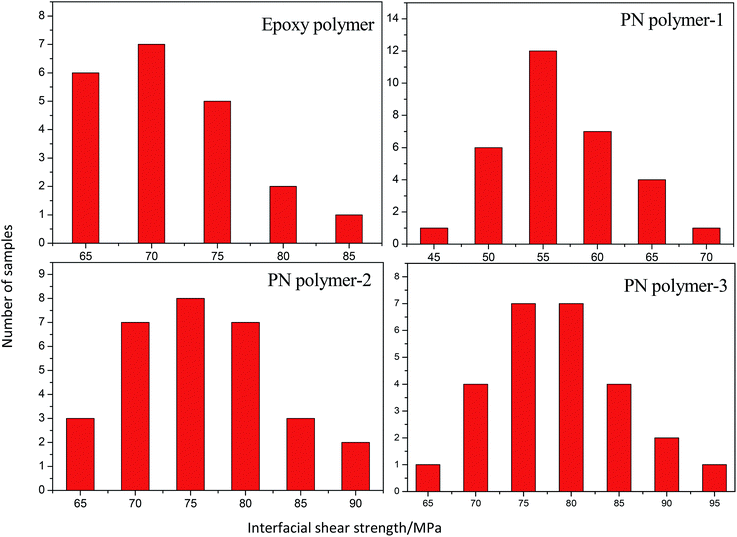
In order to reduce the errors from testing and sample variations, more than 20 droplets with diameters agreeing with the test requirements are selected and studied. The IFSS distributions of different resins and curing temperatures are shown in Fig. 2. These distributions show classic bell curves that appear frequently in statistics, indicating that all the IFSS values agreed with a normal distribution.The IFSS values of the polymers derived from different resins and curing procedures are listed in Table 2. Since epoxy resin is widely used in the preparation of composites, they are often used as reference for comparison in various literatures.24–26 The results show that the IFSS of the Cf/PN composite is higher than that of the epoxy micro-composite. This is a very significant result for the high performance PN resin. Furthermore, a gradually-increasing IFSS of the PN composite appeared along with a raise in curing temperature. This indicates that the interfacial adhesion strength of the Cf/PN composite is stronger than that of the Cf/epoxy composite. Moreover, the adhesion strength could be improved by increasing the temperature.
| Cured polymer label | Average of IFSS/MPa | Average deviation of IFSS/MPa | Relative average deviation of IFSS/% |
|---|---|---|---|
| PN polymer-1 | 56.726 | 5.863 | 10.168% |
| PN polymer-2 | 75.173 | 7.103 | 9.449% |
| PN polymer-3 | 77.721 | 7.487 | 9.634% |
| Epoxy polymer | 70.870 | 6.254 | 8.824% |
3.2 Micromorphology of interface
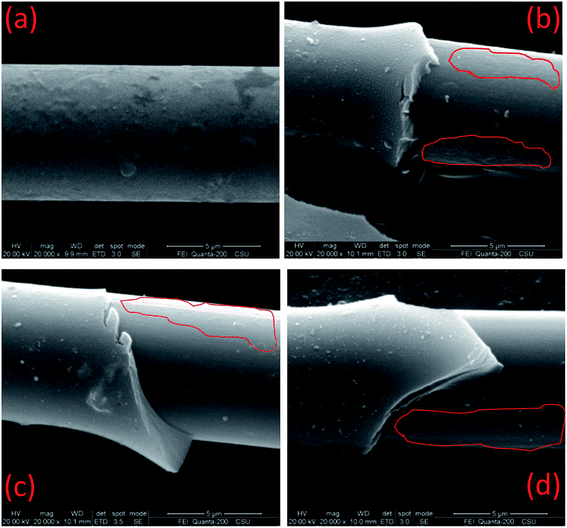
To determine the cause of the micro-droplet test results, the desized and de-bonded surfaces of carbon fiber in micro-composites were observed and compared (Fig. 3). These images show that the desized carbon fiber surfaces are covered in bumps and rugged areas and the de-bonded surfaces of Cf are smoother and more rounded than that of the desized fiber. This may be due to the fiber is surface treated through anodic electrolytic oxidation before sizing treatment.In Fig. 3(b)–(d), it shows a layer of cured resin film remaining on the de-bonded carbon fiber surface, as the red coiled part in the picture. However, the resin residues are not easy to observe. This is mainly due to the cured PN resin residues are in the form of sheet-like and are in close contact with the surface of the fiber.
As is well known, carbon fiber is normally subjected to an anodic electrolytic surface treatment after it is manufactured. The edges of the basal planes can be activated and oxidized to introduce a range of oxygen-containing functional groups, such as carboxylic acid and phenolic hydroxyl groups.11,27–33 These oxygen-containing groups can react with the epoxy resin, as shown in Fig. 4, and promote the formation of a coating layer on the carbon fiber surface. As a result, the rugged and bumped surfaces become smoother and more rounded. Therefore, the interface adhesion performance of the Cf/epoxy resin composite is enhanced.19,27
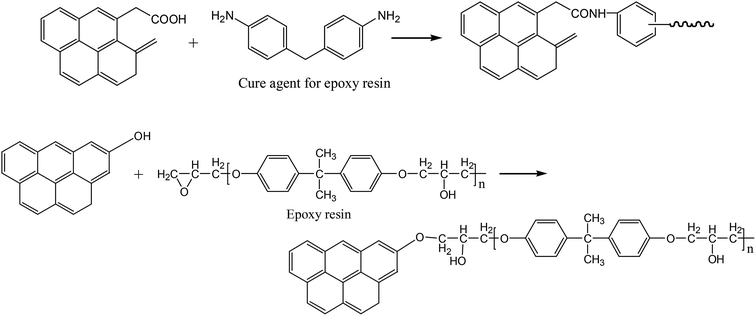 | ||
| Fig. 4 Schematic of the reactions between epoxy resin and oxygen-containing functional groups on carbon fiber. | ||
However, the carboxylic acid and phenolic hydroxyl groups on the Cf surface can also react with CN groups in the PN resin (Fig. 5).34,35 Furthermore, the amino groups in the PN resin can react with carboxylic acid groups on the Cf. Therefore, the interface performance of the Cf/PN composites should be better than that of the Cf/epoxy resin. This should be one of the reasons for the excellence interface adhesion performance of Cf/PN composites.
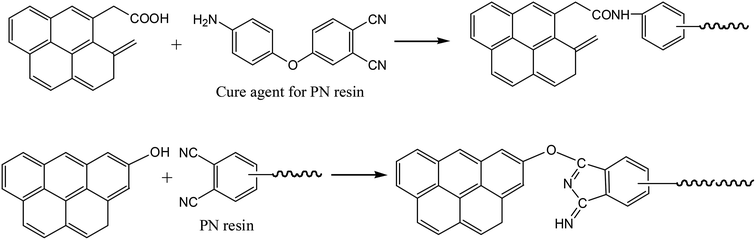 | ||
| Fig. 5 Schematic of the reactions between PN resin and oxygen-containing functional groups on carbon fiber. | ||
3.3 Elemental analysis
In order to obtain in situ information on the de-bonded surface of carbon fiber in the PN micro-composite, a combined detection technology (SEM-EDS) was employed. The micromorphology images and energy spectra were recorded. From the spectra, the elemental composition of different samples are collected (Table 3).Comparing the elemental composition of desized Cf surfaces, there is a distinct increase in overall N and O content in the de-bonded Cf surface for the PN micro-composites, regardless of the cure temperature. This suggests that there must be more residual N and O on the surface of Cf in PN composites in general. When comparing the N content on de-bonded surfaces of Cf in PN micro-composites prepared under different temperatures, a slight increase in N content is observed with the increase in cure temperature. When comparing the IFSS value in Table 2 to the N content in Table 3, we find that there is a synchronous relationship between N content increase and IFSS improvement. This demonstrates that an increasing N content on the de-bonded surface of Cf can help enhance the interface performance in PN composites.
3.4 IR spectral analysis
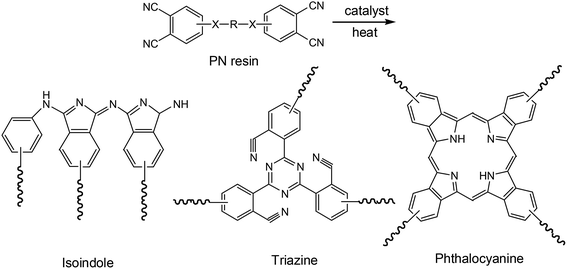
To determine the cause of the synchronous relationship between N content on the de-bonded surfaces of Cf in PN micro-composites and IFSS improvement, the structures of cured polymers derived from PN resin were examined by FTIR.As shown in Fig. 6, when the cure temperature increased, there is a decline in transmittance percentage at 2230 cm−1, which corresponds to a CN group. At the same time, an increase is observed at 1653 cm−1, 1513 cm−1 and 1018 cm−1, corresponding to isoindole, triazine, and phthalocyanine, respectively.36–38 This suggests that the main products of the cross-linking reaction of CN are isoindole, triazine, and phthalocyanine. It also indicates that the higher cure temperature results in a higher conversion of the cross-linking reaction of CN. This result is mainly due to the slow and sluggish reactivity of CN, which usually requires high temperatures and long reaction times before gelling occurs.10,39–41
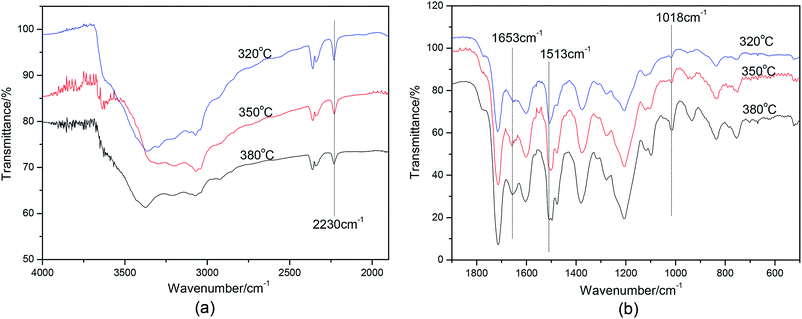 | ||
| Fig. 6 IR spectrum of PN polymers cured under different temperatures. (a) Wavenumber from 1900–4000 cm−1, (b) Wavenumber from 500–1900 cm−1. | ||
All these cross-linking products derived from the addition-polymerization reaction, have conjugated structures (Fig. 7). These cyclic conjugated structures are different from those of the epoxy polymers. The epoxy polymers are composed mainly of chained compounds derived from ring-opening reactions. The primary chemical structure of the carbon fiber surface is layered graphene, consisting of repeated and conjugated benzene rings. These conjugated structures of cross-linking products from the PN resin and graphene of carbon fiber can interact with each other through pi–pi stacking. As a result, interface conjugation can be formed, as shown in Fig. 8, which can enhance the interface adhesion in the PN composite. Because the electron withdrawing ability of N is stronger than that of C, the interfacial conjugation force between either triazine, isoindole, or phthalocyanine and a benzene ring is stronger than that between two benzene rings. Therefore, a higher N content will strengthen the interfacial conjugation force. This may be the cause for the synchronous relationship between N content increase and IFSS improvement.
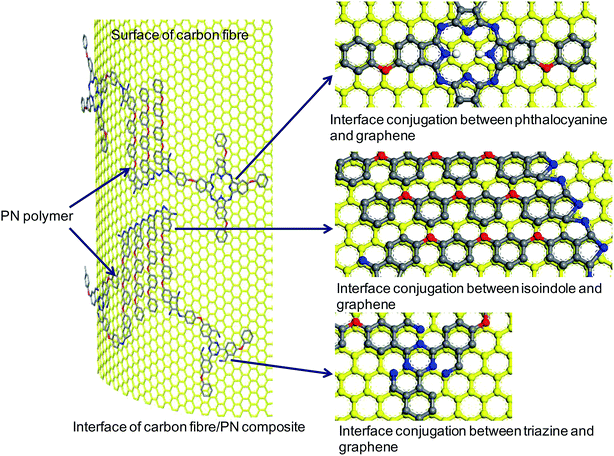 | ||
| Fig. 8 Schematic diagram of interface conjugation between a PN polymer and the surface of carbon fiber (yellow = carbon fiber surface; gray = carbon atom; blue = nitrogen atom; red = oxygen atom). | ||
The interface adhesion mechanism within Cf/epoxy composites is usually considered to be a combined contribution of the reactions of chemical functional groups (i.e., the reactions between oxygen-containing functional groups on the edges of basal planes of carbon fiber and the amine and epoxy groups in the epoxy resin) and a suitable molecular dimension of resin to micropores in the carbon fibers.27 Therefore, the interface conjugation in Cf/PN composites contains a novel and additional force, when compared to the interface adhesion mechanism in Cf/epoxy composites. This additional interface conjugation may be another cause for the excellent IFSS of Cf/PN composites. Compared to other Cf reinforced resin matrix composites, the interface conjugation function of the Cf/PN composite is also a novel interfacial binding force that may allow PN resin to have more applications in the field of interfacial modification.
3.5 Thermal stability
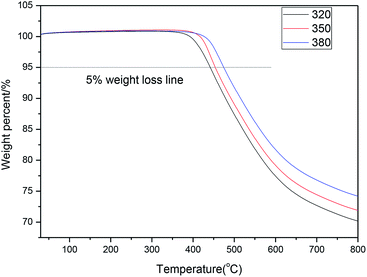
The thermal stabilities of PN polymers prepared under different cure temperatures were detected by TGA. Their TGA curves are compared in Fig. 9. As expected, the initial decomposition temperature (T5%) and char yield at 800 °C of the PN polymers are elevated at increasing cure temperatures. This is completely consistent with the conclusions inferred from the IR results. The IR spectra suggest that higher cure temperatures lead to higher yields of triazine, isoindole, and phthalocyanine. As is well known, these compounds have outstanding thermal stabilities. Thus, it can be seen that the T5% and char yield at 800 °C kept rising with the cure temperature (Fig. 9).As can be seen in Table 4, the initial decomposition temperatures of the PN polymers are between 441 °C and 474 °C, which are higher than most of the sizing resins, like epoxy,42 bismaleimides,43 polyurethane,44 and even some polyimides.44 Furthermore, the improvement in thermal stability (Table 4) is in step with the promotion of IFSS (Table 2). These results all suggest that PN resins not only have significant advantages in the field of interfacial modification, but also have excellent thermal stabilities. Thus, in the field of high temperature interface modification, PN resin will likely be more advantageous than other resins.
| Label | T5%/°C | T10%/°C | Char yield at 800 °C |
|---|---|---|---|
| PN polymer-1 | 441.5 | 478.7 | 70.2 |
| PN polymer-2 | 453.9 | 492.1 | 71.9 |
| PN polymer-3 | 474.5 | 515.4 | 74.2 |
Conclusions
To explore the interfacial performance of PN composites, micro-composites of Cf/PN and Cf/epoxy resin were prepared. Their IFSSs were examined by micro-droplet testing. By comparing their IFSSs, the Cf/PN composite shows an obvious advantage. This indicates that there should be a more effective interfacial adhesion in the Cf/PN composite. The SEM results demonstrate that there are a polymer films coating on the de-bonded surface of the carbon fiber. The in situ SEM-EDS results show that there are more N and O elements in the de-bonded area around the carbon fiber in the Cf/PN composite. The FTIR results show that conjugated compounds are produced when the PN resin is treated at high temperatures. The PN polymers with conjugated chemical structures can interact with the graphene on the surface of the carbon fiber through π–π stacking. The interaction between them promotes the formation of interface conjugation between the cured PN resin and the carbon fiber. The interface conjugation force is a new and additional interface adhesion function, when comparing to the interface adhesion mechanism of Cf/epoxy composites. Thus, this conjugation may be the primary cause for the stronger IFSS observed for the PN composite when compared to the epoxy composite. In addition, with the excellent thermal stability of the PN polymer indicated by the TGA results, the PN resin is likely to have an advantage over epoxy composites in its application to the field of high-temperature interface modification.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (51803055), Hunan Provincial Key Research and Development Program (2018GK2062), and National and Local Joint Engineering Laboratory Open Fund (KF201805). We thank LetPub (http://www.letpub.com) for its linguistic assistance during the preparation of this manuscript.References
- Y. Lin, G. Ehlert and H. A. Sodano, Adv. Funct. Mater., 2009, 19, 2654–2660 CrossRef CAS.
- M. Sharma, S. Gao, E. Mäder, H. Sharma, L. Y. Wei and J. Bijwe, Compos. Sci. Technol., 2014, 102, 35–50 CrossRef CAS.
- L. Liu, C. Jia, J. He, F. Zhao, D. Fan, L. Xing, M. Wang, F. Wang, Z. Jiang and Y. Huang, Compos. Sci. Technol., 2015, 121, 56–72 CrossRef CAS.
- K. Uetani, S. Ata, S. Tomonoh, T. Yamada, M. Yumura and K. Hata, Adv. Mater., 2014, 26, 5857–5862 CrossRef CAS PubMed.
- X. Zhang, X. Fan, C. Yan, H. Li, Y. Zhu, X. Li and L. Yu, ACS Appl. Mater. Interfaces, 2012, 4, 1543–1552 CrossRef CAS PubMed.
- M. Laskoski, M. B. Schear, A. Neal, D. D. Dominguez, H. L. Ricks-Laskoski, J. Hervey and T. M. Keller, Polymer, 2015, 67, 185–191 CrossRef CAS.
- L. Sheng, C. Yin and J. Xiao, RSC Adv., 2016, 6, 22204–22212 RSC.
- F. Zhao, R. Liu, C. Kang, X. Yu, K. Naito, X. Qu and Q. Zhang, RSC Adv., 2014, 4, 8383–8390 RSC.
- L. Zhang, M. Liu, S. Roy, E. K. Chu, K. Y. See and X. Hu, ACS Appl. Mater. Interfaces, 2016, 8, 7422–7430 CrossRef CAS PubMed.
- J. Hu, Y. Liu, Y. Jiao, S. Ji, R. Sun, P. Yuan, K. Zeng, X. Pu and G. Yang, RSC Adv., 2015, 5, 16199–16206 RSC.
- L. Ma, L. Meng, G. Wu, Y. Wang, M. Zhao, C. Zhang and Y. Huang, Compos. Sci. Technol., 2015, 117, 289–297 CrossRef CAS.
- E. Totry, J. M. Molina-Aldareguia, C. Gonzalez and J. LLorca, Compos. Sci. Technol., 2010, 70, 970–980 CrossRef CAS.
- P. Ma, S. Mo, B. Tang and J. Kim, Carbon, 2010, 48, 1824–1834 CrossRef CAS.
- J. Gu, N. Li, L. Tian, Z. Lv and Q. Zhang, RSC Adv., 2015, 5, 36334–36339 RSC.
- R. L. Zhang, Y. D. Huang, D. Su, L. Liu and Y. R. Tang, Mater. Des., 2012, 34, 649–654 CrossRef CAS.
- L. Yao, M. Li, Q. Wu, Z. Dai, Y. Gu, Y. Li and Z. Zhang, Appl. Surf. Sci., 2012, 263, 326–333 CrossRef CAS.
- Y. Ren, C. Wang and Y. Qiu, Appl. Surf. Sci., 2007, 253, 9283–9289 CrossRef CAS.
- C. C. Guo, Y. Zhao, D. Chen and M. S. Zhan, Mater. Res. Innovations, 2014, 18, S4–S997 CAS.
- C. Kozlowski and P. M. A. Sherwood, Carbon, 1987, 25, 751–760 CrossRef CAS.
- N. Dilsiz and J. P. Wightman, Colloids Surf., A, 2000, 164, 325–336 CrossRef CAS.
- P. Denison, F. R. Jones and J. F. Watts, J. Mater. Sci., 1985, 20, 4647–4656 CrossRef CAS.
- J. Schultz and L. Lavielle, Interfacial Properties of Carbon Fiber—Epoxy Matrix Composites, Ameri Chem. Soci., 1989, vol. 391, pp. 185–202 Search PubMed.
- Y. Bai, Z. Wang and L. Feng, Mater. Des., 2010, 31, 1613–1616 CrossRef CAS.
- K. M. Beggs, L. Servinis, T. R. Gengenbach, M. G. Huson, B. L. Fox and L. C. Henderson, Compos. Sci. Technol., 2015, 118, 31–38 CrossRef CAS.
- Q. Wu, R. Zhao, Q. Liu, T. Jiao, J. Zhu and F. Wang, Mater. Des., 2018, 149, 15–24 CrossRef CAS.
- B. Jiang, T. Zhang, L. Zhao and Y. Huang, Compos. Sci. Technol., 2017, 140, 39–45 CrossRef CAS.
- F. R. Jones and J. Adhes, J. Adhes. Sci. Technol., 2010, 24, 171–202 CrossRef CAS.
- J. Dong, C. Jia, M. Wang, X. Fang, H. Wei, H. Xie, T. Zhang, J. He, Z. Jiang and Y. Huang, Compos. Sci. Technol., 2017, 149, 75–80 CrossRef CAS.
- T. Wang and P. M. A. Sherwood, Chem. Mater., 1995, 7, 1020–1030 CrossRef CAS.
- T. Wang and P. M. A. Sherwood, Chem. Mater., 1995, 7, 1031–1040 CrossRef CAS.
- Y. Wang, F. Zhang and P. M. A. Sherwood, Chem. Mater., 2001, 13, 832–841 CrossRef CAS.
- Y. Wang, F. Zhang and P. M. A. Sherwood, Chem. Mater., 1999, 11, 2573–2583 CrossRef CAS.
- J. D. Schaefer, A. J. Rodriguez, M. E. Guzman, C. Lim and B. Minaie, Carbon, 2011, 49, 2750–2759 CrossRef CAS.
- D. Augustine, D. Mathew and C. P. R. Nair, Polym. Int., 2013, 62, 1068–1076 CAS.
- K. Zeng, K. Zhou, S. Zhou, H. Hong, H. Zhou, Y. Wang, P. Miao and G. Yang, Eur. Polym. J., 2009, 45, 1328–1335 CrossRef CAS.
- P. J. Burchill, J. Polym. Sci., Polym. Chem. Ed., 1994, 32, 1–8 CrossRef CAS.
- T. M. Keller and T. R. Price, J. Macromol. Sci., Part A, 1982, 6, 931–937 CrossRef.
- J. Yang, X. Yang, Y. Zou, Y. Zhan, R. Zhao and X. Liu, J. Appl. Polym. Sci., 2012, 126, 1129–1135 CrossRef CAS.
- T. Zhang, J. Wang, M. Derradji, N. Ramdani, H. Wang, Z. Lin and W. Liu, Thermochim. Acta, 2015, 602, 22–29 CrossRef CAS.
- P. Yuan, Y. Liu, K. Zeng and G. Yang, Des. Monomers Polym., 2015, 18, 343–349 CrossRef CAS.
- M. Laskoski, A. Neal, T. M. Keller, D. Dominguez, C. A. Klug and A. P. Saab, J. Polym. Sci., Polym. Chem. Ed., 2014, 52, 1662–1668 CrossRef CAS.
- X. Ma, T. Reng and J. Wang, China Text. Leader, 2017, 44–46 Search PubMed.
- C. Wang, Synthesis and Characterization of a Bismaleimide Heat-resistant Emulsion Sizing Agent for Carbon Fibers, PhD thesis, Jilin University, 2011.
- J. Wang, Z. Li and Y. Jiang, Synth. Fiber China, 2017, 36–38 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |